Neutropenia in mice and humans results in the generation of NK cells with an immature and hyporesponsive phenotype.
Abstract
Natural killer (NK) cells are bone marrow (BM)–derived granular lymphocytes involved in immune defense against microbial infections and tumors. In an N-ethyl N-nitrosourea (ENU) mutagenesis strategy, we identified a mouse mutant with impaired NK cell reactivity both in vitro and in vivo. Dissection of this phenotype showed that mature neutrophils were required both in the BM and in the periphery for proper NK cell development. In mice lacking neutrophils, NK cells displayed hyperproliferation and poor survival and were blocked at an immature stage associated with hyporesponsiveness. The role of neutrophils as key regulators of NK cell functions was confirmed in patients with severe congenital neutropenia and autoimmune neutropenia. In addition to their direct antimicrobial activity, mature neutrophils are thus endowed with immunoregulatory functions that are conserved across species. These findings reveal novel types of cooperation between cells of the innate immune system and prompt examination of NK cell functional deficiency in patients suffering from neutropenia-associated diseases.
NK cells are innate immune lymphocytes involved in controlling microbial infections, tumor immunosurveillance, hematopoietic allograft rejection, and pregnancy (Vivier et al., 2008; Orr and Lanier, 2010; Vivier et al., 2011). NK cell effector functions include direct cytotoxicity and production of the cytokines (e.g., IFN-γ) and chemokines involved in the regulation of immune responses. NK cell activation is regulated by an array of activating and inhibitory cell surface receptors, which can detect nonself ligands or changes in the expression of self-molecules on infected or abnormal cells. NK cells were originally identified on the basis of their ability to lyse tumor cells without prior sensitization, but multiple mechanisms underlie the acquisition of their full effector functions (Moretta, 2002; Degli-Esposti and Smyth, 2005; Newman and Riley, 2007; Orr and Lanier, 2010; Vivier et al., 2011). MHC class I molecule (MHC-I) recognition by inhibitory receptors expressed on NK cells participates in the acquisition of NK cell responsiveness. Indeed, NK cells lacking self-MHC-I–specific inhibitory receptors and NK cells from MHC-I–deficient humans or mice are hyporesponsive to activating receptor stimulation (Fernandez et al., 2005; Kim et al., 2005; Anfossi et al., 2006; Raulet, 2006; Brodin et al., 2009a,b; Guia et al., 2011). This process, referred as to NK cell MHC-I–dependent education, ensures self-tolerance and immunocompetence. In addition, NK cells must be primed with cytokines, such as IL-15, IL-12, and IL-18, and by interactions with accessory cells, to achieve their full effector potential (Orr and Lanier, 2010; Vivier et al., 2011). In particular, activated DCs have been shown to prime NK cells by trans-presenting IL-15 on their IL-15Rα (Lucas et al., 2007). Interactions between NK cells and monocytes or macrophages have also been shown to be required for correct NK cell activation (Dalbeth et al., 2004; Baratin et al., 2005; Welte et al., 2006; Nedvetzki et al., 2007; Tu et al., 2008; Bellora et al., 2010; Soderquest et al., 2011). These mechanisms help to adapt NK cell function to the host environment, ensuring appropriate and regulated NK cell reactivity.
Neutrophils, like NK cells, are part of the innate immune system. They are the most abundant type of white blood cell in humans and play a key role in immunity by providing a first line of defense against pathogens. Neutrophil deficiency results in a profound immunodeficiency, leading to susceptibility to invasive bacterial infections (skin abscesses, pneumonia, and septicemia) and fungal infections (Mantovani et al., 2011). For many years, the reported short lifespan of these cells, together with their potent antimicrobial functions, including phagocytosis and pathogen killing, restricted our understanding of their role in immunity to that of effector cells. It is now becoming increasingly clear that neutrophils also directly regulate adaptive immune responses during acute and chronic microbial infections (Spörri et al., 2008; Zhang et al., 2009; Mantovani et al., 2011). We describe here a new immunoregulatory function of neutrophils in the guidance of NK cell maturation at steady state, in both humans and mice.
RESULTS
NK cells in Genista mice are hyporesponsive
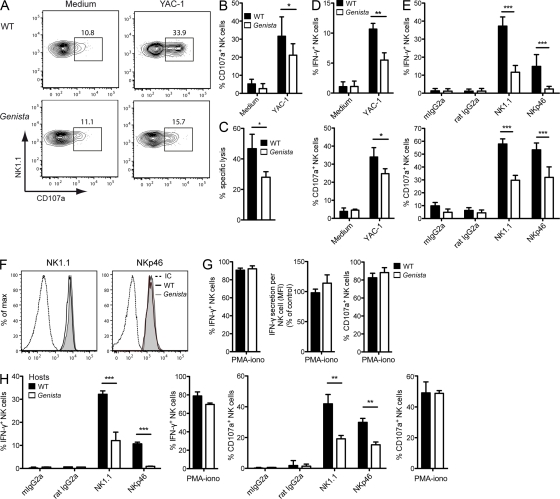
We conducted an N-ethyl N-nitrosourea (ENU) mutagenesis program in mice, using a functional test to screen and identify genes involved in NK cell responsiveness. We selected a new mouse pedigree, Genista, with hyporesponsive NK cells (Fig. 1, A and B). This hyporeactivity in vitro was revealed by testing blood NK cell degranulation (i.e., surface exposure of CD107a) after stimulation with YAC-1 target cells (Fig. 1, A and B), and was accompanied by an impaired capacity to reject MHC-I–deficient splenocytes in vivo (Fig. 1 C). We used Genista splenocytes to analyze NK cell functions in more detail. The fraction of spleen NK cells responding to stimulation with YAC-1 cells was lower than that in WT mice, as demonstrated by determinations of the percentages of CD107a+ and IFN-γ+ NK cells, confirming our initial observations on blood (Fig. 1 D). Genista NK cells also responded less strongly than WT cells to stimulation of the NK1.1-, NKp46-, or NKG2D- and Ly49D-activating receptors (Fig. 1 E and not depicted). This hyporeactivity was not caused by down-regulation of the surface expression of these receptors (Fig. 1 F and not depicted). We then used phorbol 12-myristate 13-acetate (PMA) and ionomycin stimulation, which induces NK cell activation by bypassing cell surface receptor engagement. In these conditions, the vast majority of NK cells from Genista and WT mice responded similarly, both in terms of the percentage of responding NK cells and in terms of the ability of IFN-γ production per cell (Fig. 1 G). Thus, NK cell hyporeactivity of Genista mice was not caused by a permanent inability to degranulate or to produce cytokines.
Figure 1.
An extrinsic factor contributes to NK cell hyporeactivity in Genista mice. (A) Circulating NK cells from WT (top) or Genista mice (bottom) were stimulated for 4 h with YAC-1 target cells (right) or medium alone (left). Representative FACS histograms show NK1.1 and CD107a expression gated on NK1.1+ CD3− cells. (B) Histograms representing the percentage of CD107a+ blood NK cells in WT or Genista mice upon stimulation with YAC-1 tumor cells. Data were pooled from three independent experiments. n = 3. (C) WT and β2mKO splenocytes were stained with two different doses of the fluorescent dye CFSE, mixed at a 1:1 ratio, and transferred into WT or Genista mice. The percentage of specific lysis of MHC-I–deficient cells corresponds to the ratio of the various populations before and 48 h after co-injection in the spleen. The experiment was performed twice. n = 2–3. (D and E) Splenocytes from WT (shaded bars) or Genista (open bars) mice were stimulated for 4 h with YAC-1 tumor targets or medium alone (D), or with anti-NK1.1, anti-NKp46, or isotype control mAb-coated plates (E). NK cell intracellular IFN-γ production (D and E, top) and degranulation (CD107a surface exposure; D and E, bottom) were measured by FACS on NK1.1+ CD3− or NKp46+ CD3− NK cells. Data were pooled from five independent experiments. n = 3–6. (F) Representative FACS profiles are shown for NK1.1, NKp46 surface expression, or isotype control mAb (IC, dashed) on WT (black) or Genista (gray) NK cells. (G) Splenic NK cells from WT (shaded bars) or Genista (open bars) mice were stimulated for 4 h with a mixture of PMA and ionomycin. Data show the frequencies of IFN-γ+ (left) or CD107a+ (right) NK cells among the total NK cell population in WT or Genista, or the amount of IFN-γ produced (MFI) per IFN-γ+ NK cells from WT or Genista (middle). MFI control values were calculated as the average of the IFN-γ mean fluorescence intensity (MFI) of IFN-γ+ NK cells from WT mice. (H) Purified WT spleen NK cells were transferred to WT (shaded bars) or Genista (open bars) hosts, as indicated. 7 d after transfer, the frequencies of IFN-γ–producing cells (left) and CD107a+ cells (right) among transferred WT NK cells were analyzed after stimulation with isotype control, anti-NK1.1, or anti-NKp46 mAb-coated plates. Experiments were repeated three times, with n = 2–4 transfer groups. Statistical significance was determined with a Mann-Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
NK cell defect in Genista mice is NK cell extrinsic
We further dissected the mechanisms involved in the NK cell functional defect in Genista mice by determining whether the hyporesponsive phenotype was NK cell intrinsic or extrinsic. We transferred purified spleen NK cells from CD45.1+ WT donors into CD45.2+ Genista recipients or CD45.2+ WT recipients as a control and analyzed the reactivity of CD45.1+ WT donor cells 7 d after adoptive transfer. By stimulating NK1.1 and NKp46, we showed that WT NK cells transferred into Genista mice became hyporesponsive, displaying weaker responses than WT NK cells transferred into WT recipients (Fig. 1 H). The exposure of spleen WT NK cells to a Genista environment thus modified their responsiveness, demonstrating the involvement of an NK cell–extrinsic factor inducing NK cell hyporeactivity in Genista mice. In control, when stimulated by PMA and ionomycin, WT and Genista NK cells showed similar responsiveness (Fig. 1 H). It has recently been reported that splenic WT NK cells become hyporeactive when transferred into a MHC-I–deficient environment (Elliott et al., 2010; Joncker et al., 2010). We therefore assessed the expression of MHC class I molecules on the cell surface in Genista mice and found no difference between these mice and WT mice (unpublished data).
NK cell functions are impaired in the absence of neutrophils
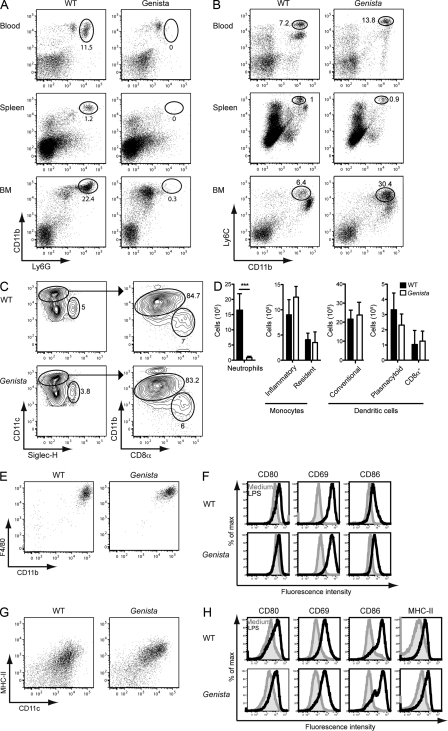
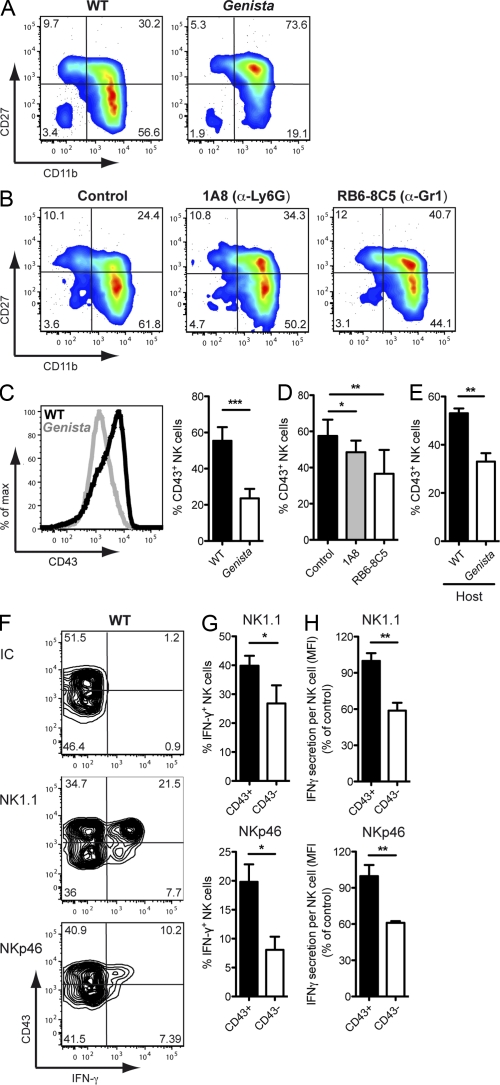
In studies performed in parallel to our NK cell–oriented screen, Genista mice were found to lack mature neutrophils (unpublished data). Analysis of the neutrophil compartment in the blood, spleen, and BM (Fig. 2 A), as well as in the liver, lungs, and lymph nodes (not depicted) showed that mature CD11b+Ly6Ghigh neutrophils were selectively absent from Genista mice. The NK cell hyporesponsive phenotype and the neutropenia were perfectly correlated in the colony of F2 animals obtained from the cross of Genista and WT mice. Genetic analysis identified a point mutation leading to an amino-acid substitution in the third zinc finger of the growth factor–independent-1 (Gfi-1) transcription factor in Genista mice (unpublished data). Gfi-1 has already been implicated in neutrophil development, as patients with mutations in GFI-1 and Gfi-1 KO mice are severely neutropenic (Karsunky et al., 2002; Zarebski et al., 2008). As previously observed in Gfi-1 KO mice (Karsunky et al., 2002), Genista mice display an accumulation of atypical myeloid precursors (Ly6Glow, Ly6Chigh, CD11b+) in the BM, but we did not detect any major modification in the percentages of monocytes at the periphery (Fig. 2, A and B). The dissection of the monocyte compartment in the spleen of Genista mice showed normal numbers of inflammatory (CD115+, CD11b+, Ly6C+) and resident (CD115+, CD11b+, Ly6C−) monocytes as compared with WT (Fig. 2 D). In addition, percentages and numbers of DC subpopulations (conventional, plasmacytoid and CD8α) were comparable between WT and Genista in the spleen as well as in the cutaneous lymph nodes (Fig. 2, C and D; and not depicted). As Gfi-1 has also been described as a critical regulator of DC versus macrophage differentiation (Rathinam et al., 2005), we sought to test the ability of BM cells from Genista mice to differentiate into DCs or macrophages in vitro. After 7 d in culture with M-CSF, Genista BM cells normally differentiated into BM-derived macrophages (BMMs) as judged by the up-regulation of F4/80 and CD11b (Fig. 2 E). The overnight stimulation with LPS induced a comparable up-regulation of the co-stimulatory molecules CD80, CD69, and CD86 at the surface of BMMs from WT and Genista (Fig. 2 F). In vitro DC differentiation assays lead to the generation of normal MHC-II+ CD11c+ BM-derived DCs (BMDCs) from WT and Genista BM cells (Fig. 2 G). Overnight activation with LPS showed a comparable up-regulation of CD80, CD69, CD86, and MHC-II on WT and Genista-derived BMDCs (Fig. 2 H). Collectively, these data show that, beside the lack of neutrophils, Genista mice do not display any major defect in NK cell accessory cells.
Figure 2.
Genista mice lack mature neutrophils. (A and B) Representative FACS profiles of blood, spleen, and BM cells from WT or Genista mice stained with anti-CD11b and anti-Ly6G mAb (A) or anti-CD11b and anti-Ly6C mAb (B). Percentage of neutrophils (CD11bhigh Ly6Ghigh; A) and monocytes (CD11binter Ly6Chigh; B) are indicated. Data are representative of 6–10 independent experiments. n = 3–4. (C) Gating strategy for identification of DC populations in the spleen of WT (top) or Genista (bottom) mice. Live, single, CD11c+ Lin− (CD3, CD19, NK1.1) spleen cells were plotted for CD11c and Siglec-H expression, with numbers indicating the percentage of pDCs (CD11c+ Siglec-H+). CD11chigh DCs were then subdivided in CD11bhigh CD8α− (conventional DCs) and CD11b− CD8α+ (CD8α DCs) cells. Numbers indicate the percentage of each population. Data represent two experiments. n = 4. (D) Histograms representing the total numbers of neutrophils, monocytes, and DCs subpopulations in the spleen of WT (black) or Genista (white) mice. Live, single, Lin− (CD3, CD19 NK1.1, Ly6G), CD115+ CD11b+ monocytes were further subdivided into inflammatory (Ly6C+) and resident (Ly6C−) monocytes. Experiments were performed two to five times. n = 3–4. Statistical significance was determined with a Mann-Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (E) Representative FACS profiles of F4/80 and CD11b expression on BMMs from WT or Genista mice obtained after 8 d of in vitro differentiation of BM cells with GM-CSF. (F) CD80, CD69, and CD86 cell surface expression of BMMs from WT (top) or Genista (bottom) mice after overnight incubation with LPS (black histograms) or medium (filled gray histograms). (G) Representative FACS profiles of MHC-II and CD11c expression on BMDCs from WT or Genista mice obtained after 8 d of in vitro differentiation of BM cells with GM-CSF. (H) CD80, CD69, CD86, and MHC-II cell surface expression on BMDCs from WT (top) or Genista (bottom) after overnight incubation with LPS (black histograms) or medium (filled gray histograms). Experiments were performed twice. n = 3.
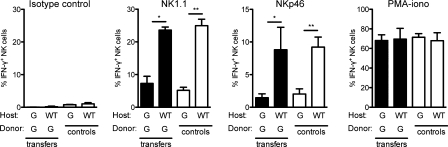
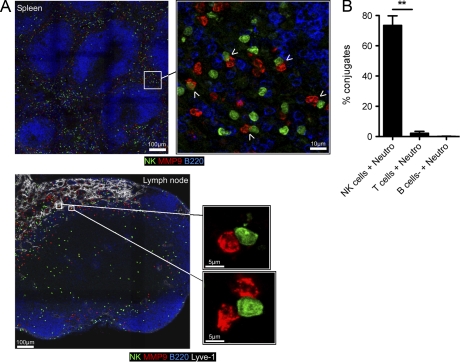
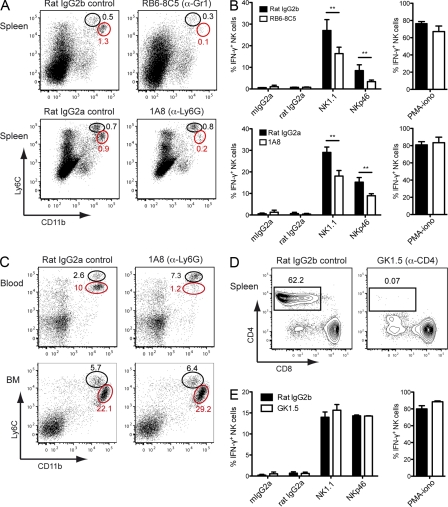
Gfi-1 is expressed in mouse NK cells, and might thus play a role in NK cell function. To address this question, we transferred purified spleen NK cells from Genista mice in WT recipients and monitored NK cell reactivity 7 d after transfer. In a WT environment, Genista NK cells became as responsive as host WT NK cells (Fig. 3 A), showing that the Gfi-1 mutation in NK cells was not sufficient to affect their functional capacities at steady-state. We thus focused on the extrinsic factors present or absent in Genista mice that led to NK cell hyporesponsiveness. With the exception of their NK cell phenotype, the only apparent defect in Genista mice was neutropenia. In WT animals, immunohistological analysis of spleen and lymph node sections showed that the two cell types were localized in close proximity in the red pulp of the spleen or in the medulla next to the lymphatic vessels of the lymph nodes (Fig. 4 A). Furthermore, in vitro experiments assessing the ability of NK cells to form conjugates with neutrophils revealed the strong propensity of these two cell types to interact, as compared with the lack of conjugates detected between neutrophils and T or B cells (Fig. 4 B). We thus directly tested the hypothesis that neutrophils represented the NK cell extrinsic factor required to promote NK cell reactivity by using mAbs to deplete neutrophils from WT animals. As the use of neutrophil-depleting mAbs is still a matter of debate (Daley et al., 2008), we performed these depletion experiments using anti-Gr1 (RB6-8C5) or anti-Ly6G (1A8) mAb. 6 d after mAb injection, RB6-8C5 treatment resulted in the depletion of neutrophils, but also of some monocytes (Ly6ChighCD11blow), whereas 1A8 treatment had induced the selective depletion of neutrophils, as previously reported (Daley et al., 2008; Fig. 5 A). Remarkably, the depletion of neutrophils by either treatment was sufficient to induce NK cell hyporeactivity in WT mice (Fig. 5 B). The intensity of the NK cell hyporesponsiveness in these conditions was lower than that in Genista mice, which is consistent with the lower severity of the neutropenia induced by mAb treatment than of that observed in Genista mice. Indeed, anti-Gr1 (RB6-8C5) or anti-Ly6G (1A8) mAb treatment induced neutrophil depletion in the blood and in the spleen, but not in the BM (Fig. 5 C and not depicted). A CD4 cell-depleting control mAb had no effect on NK cell function, indicating that the hyporeactivity induced by the injection of neutrophil-depleting mAb resulted directly from the lack of neutrophils, rather than from eventual side effects of the depleting antibody treatment (Fig. 5, D and E). Neutrophils are thus essential for NK cell function at steady state.
Figure 3.
Genista NK cells regain full reactivity in a WT environment. Purified Genista spleen NK cells were transferred to WT or Genista (G) hosts, as indicated. 7 d after transfer, the frequency of IFN-γ–producing cells among transferred Genista NK cells (shaded bars) was analyzed after stimulation with isotype control, anti-NK1.1, and anti-NKp46 mAb-coated plates or a mix of PMA and ionomycin. The reactivity of NK cells from WT or Genista hosts was indicated as controls (open bars). Experiments were repeated three times. n = 2–3. Statistical significance was determined with a Mann-Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 4.
NK cells and neutrophils are localized in the same areas of the spleen and the lymph nodes and form conjugates. (A) Immunostaining on NKp46iCre/wtR26ReYFP/wt spleen (top) or lymph node (bottom) sections at steady state showing neutrophils (anti-MMP9, red), NK cells (anti-GFP, green), B cells (anti-B220, blue), and the lymphatic vessels (Lyve-1, white in the lymph node image). Adjacent overlapping confocal images of spleen and lymph node sections were stitched to generate panoramic images. White arrowheads show contact between neutrophils and NK cells in the spleen. (B) Percentages of conjugates formed between neutrophils and NK cells (NK cells + Neutro), CD3+ T cells (T cells + Neutro), or CD3− NK1.1− cells (B cells + Neutro) after 10 min incubation together in vitro at 37°C. Data are representative of three independent experiments. Statistical significance was determined with a Mann-Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 5.
Neutrophils are essential for NK cell function at the steady state. (A) Representative FACS profiles of spleen cells from Rat IgG2b- or RB6-8C5–treated mice (top) or, Rat IgG2a-, or 1A8-treated mice (bottom), stained with anti-Ly6C and anti-CD11b mAb. 1A8 is specific for Ly6G, whereas RB6-8C5 recognizes Ly6G and, with a lower affinity, Ly6C (Fleming et al., 1993). As RB6-8C5 recognizes a different epitope on Ly6C than the monoclonal anti-Ly6C (AL-21) antibody (Ribechini et al., 2009), we used CD11b and Ly6C to monitor the percentage of both neutrophils (CD11bhigh Ly6Cinter, red circle) and monocytes (CD11binter Ly6Chigh, black circle). (B) 6 d after treatment, splenocytes from rat IgG2b- and RB6-8C5–treated (top) or, Rat IgG2a- or 1A8-treated mice (bottom), were stimulated by incubation for 4 h on anti-NK1.1, anti-NKp46, or isotype control mAb-coated plates or with a mixture of PMA and ionomycin. NK cell intracellular IFN-γ production was measured by FACS. Experiments were repeated 6–20 times. n = 3–6 mice per group. Statistical significance was determined in a Mann-Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Percentages of neutrophils (red circles) and monocytes (black circles) in the blood and the BM of rat IgG2a- or 1A8-treated mice. Data are representative of 6–10 independent experiments. n = 3–4. (D) 6 d after anti-CD4 antibody (GK1.5) or isotype control antibody (rat IgG2b) injection, the presence of CD4+ cells among CD3+ T cells was monitored in the spleen of rat IgG2b- or GK1.5-treated mice. (E) 6 d after depletion, splenic NK cells were stimulated for 4 h by incubation in plates coated with NK1.1, NKp46, or isotype control mAb, or with a mixture of PMA and ionomycin. The percentages of NK cells expressing intracellular IFN-γ are shown for control isotype-treated mice (shaded bars) and CD4-depleted mice (open bars). Experiments were performed twice. n = 2–3.
Neutrophils are required for proper NK cell maturation
We next investigated the mechanisms by which neutrophils affect NK cell function by analyzing the effects of these cells on NK cell maturation, a process that is classically studied by monitoring the cell surface expression of CD27 and CD11b. Immature CD27−CD11b− NK cells first acquire CD27 expression, to become CD27+CD11b−, and then acquire CD11b, yielding double-positive (DP) CD27+CD11b+ NK cells, which eventually lose their CD27 expression to become fully mature CD27−CD11b+ NK cells (Kim et al., 2002; Hayakawa and Smyth, 2006; Chiossone et al., 2009). An investigation of these markers showed that NK cell maturation was severely affected in Genista mice, with most NK cells blocked at the DP stage (Fig. 6 A). An accumulation of DP NK cells was also observed in WT animals depleted of neutrophils (Fig. 6 B). Neutrophils therefore appeared to be required for the final stages of NK cell maturation, corresponding to the transition from the DP stage to the most mature CD27−CD11b+ stage. We characterized these maturation stages further by studying CD43, which is up-regulated during the down-regulation of CD27 in fully mature WT NK cells (Kim et al., 2002; Yokoyama et al., 2004). The percentage of NK cells expressing CD43 was much lower in Genista mice than in WT mice (Fig. 6 C). These findings were confirmed in studies in which mAbs were used to deplete neutrophils in WT mice (Fig. 6 D). We interpreted the lower decrease in CD43 expression in WT mice depleted of neutrophils as compared with Genista mice as a consequence of the milder severity of the neutropenia induced by mAb treatment, as mentioned in the previous paragraph. Importantly, the percentage of CD43+ WT NK cells 7 d after transfer into Genista mice was also lower than that monitored after transfer into a WT recipient (Fig. 6 E). We investigated the relationship between this impaired maturation in the absence of neutrophils and the hyporesponsive phenotype of NK cells by comparing the responsiveness of CD43− and CD43+ NK cells in WT mice. After the stimulation of NK1.1 and NKp46, both the percentage of IFN-γ+ NK cells and the mean fluorescence intensity of IFN-γ+ NK cells were lower in the CD43− NK cell population than in the CD43+ NK cell population (Fig. 6, F–H). CD43-expressing NK cells thus responded more strongly than CD43− NK cells in these conditions of stimulation. The lower percentage of CD43-expressing NK cells may thus account for the hyporeactivity of NK cells in neutropenic mice.
Figure 6.
Neutrophils are required for terminal NK cell maturation. (A and B) Representative FACS profiles of splenic NK cells stained with anti-CD27 and anti-CD11b mAb from WT and Genista mice (A) or 1A8, RB6-8C5, or isotype control mAb–treated mice (B). Experiments were performed 6–20 times. n = 3–6. (C) Representative FACS histograms of CD43 expression of splenic NK cells from WT (black line) or Genista (gray line). Tinted histogram shows isotype control staining. Data represent at least 20 independent experiments. (C–E) Percentages of splenic NK cells positive for CD43, for WT and Genista mice (C, right); isotype control-, 1A8-, or RB6-8C5–treated mice (D); or donor WT NK cells 7 d after transfer into WT or Genista hosts (E). Experiments were performed three to six times. n = 3–4. Statistical significance was determined in a Mann-Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (F) Representative FACS profiles of splenic NK cells (gated on CD3− NKp46+ or NK1.1+) from WT mice stained for CD43 and intracellular IFN-γ, 4 h after stimulation by incubation in isotype control, anti-NK1.1, or anti-NKp46 mAb-coated plates. (G) Frequencies of IFN-γ–producing splenic NK cells generated from CD43+ or CD43− WT NK cells by stimulation on anti-NK1.1 or anti-NKp46 mAb-coated plates. (H) Amount of IFN-γ secreted per CD43+ (shaded bars) or CD43− (open bars) NK cell in response to stimulation by incubation in anti-NK1.1 (top) or anti-NKp46 (bottom) mAb-coated plates. The MFI for IFN-γ of IFN-γ+ CD43− NK cells was normalized with respect to that for IFN-γ+ CD43+ NK cells. Experiments were performed twice. n = 5–8. Statistical significance was determined in a Mann-Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
NK cell homeostasis is modified in mice lacking neutrophils
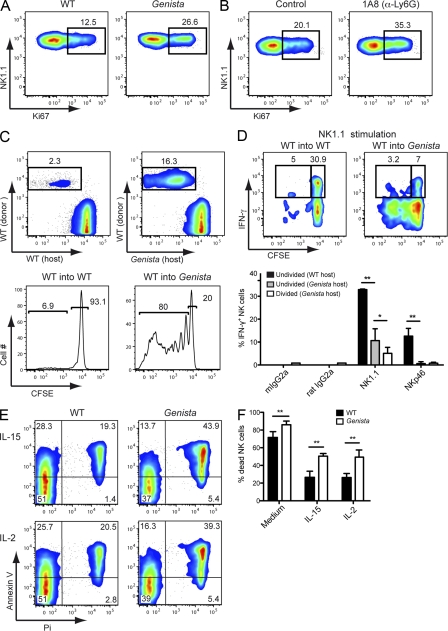
In addition to their defective maturation, NK cells proliferated more in the absence of neutrophils. Indeed, the percentage of Ki67+ NK cells in Genista was significantly higher than that in WT mice, whereas there was no difference in the T and B cell compartments (Fig. 7 A and not depicted). This phenotype was neutrophil-dependent, as neutrophil depletion in WT mice led to the specific proliferation of NK cells (Fig. 7 B). This conclusion was also supported by experiments involving the adoptive transfer of WT NK cells. Indeed, 7 d after the transfer of CFSE-labeled WT NK cells into Genista recipients, 80 ± 13.2% of the WT NK cells had proliferated, whereas no proliferation was observed after transfer into WT recipients (Fig. 7 C). Given this extensive proliferation of WT NK cells after transfer into Genista, we hypothesized that the hyporeactive NK cell phenotype might be displayed only by new NK cells generated by the division of donor cells. We tested this hypothesis by comparing the responsiveness of undivided (CFSEhigh) WT NK cells after transfer into WT or Genista mice. We showed, by stimulation of NK1.1 and NKp46, that there was a change in NK cell responsiveness after transfer of WT cells in Genista mice, even for NK cells that did not divide (Fig. 7 D), ruling out the possibility that NK cell hyporeactivity in the absence of neutrophils was caused by NK cell proliferation alone. Nevertheless, NK cells that had divided (CFSEdiluted) had lower levels of activity than undivided (CFSEhigh) cells (Fig. 7 D). NK cell proliferation in the absence of neutrophils was therefore associated with NK cell hyporeactivity, but did not fully account for the phenotype. The higher level of NK cell proliferation in Genista mice was not associated with an increase in the number of NK cells. Indeed, the overall percentages of NK cells in the blood and spleen were unchanged (blood, 3.71 ± 0.85% for WT and 3.05 ± 1.04% for Genista, P = 0.3417; spleen, 2.98% ± 0.87 for WT and 2.4% ± 1.01 for Genista, P = 0.3002), whereas it was significantly reduced in the BM of Genista mice as compared with WT mice (BM, 0.93 ± 0.35 for WT and 0.49 ± 0.33% for Genista, P = 0.0021), suggesting that NK cell proliferation was accompanied by a decrease in NK cell survival. Indeed, Genista NK cells displayed higher levels of cell death in vitro than WT NK cells, and this difference was not abolished by overnight treatment with IL-15 or IL-2 (Fig. 7 E). Thus, in mice lacking neutrophils, NK cells displayed hyperproliferation and poor survival and were blocked at an immature stage associated with hyporesponsiveness.
Figure 7.
Higher rates of proliferation and poorer survival of NK cells in the absence of neutrophils. (A and B) Representative FACS profiles of splenic NK cells from WT and Genista (A, or isotype control– and 1A8-treated mice (B) stained with anti-NK1.1 and anti-Ki67 mAb. Data a representative of three independent experiments. n = 3–4. (C and D) CFSE-labeled CD45.1+ WT spleen NK cells (donor) were transferred into CD45.2+ WT or Genista hosts for 7 d. (C) Representative FACS profiles show the percentage of donor WT NK cells (rectangle) after transfer into WT (top left) or Genista (top right) mice. Histograms of CFSE expression gated on donor CD45.1+ WT NK cells after transfer into WT (bottom left) or Genista (bottom right) mice. (D) 7 d after transfer into WT (left) or Genista (right) mice, the frequency of undivided (CFSEhigh) or divided (CFSEdiluted) donor WT NK cells producing IFN-γ in response to stimulation with isotype control, anti-NK1.1, or anti-NKp46–coated plates was determined. (top) Representative FACS profile upon NK1.1 activation. (bottom) Statistical comparison of NK cell responsiveness in undivided (CFSEhigh) and divided (CFSEdiluted) donor NK cells. Experiments were performed three times. n = 2–4. (E) Representative FACS profiles of WT or Genista NK cells (gated on CD3− NKp46+ or NK1.1+) stained with anti–Annexin V and propidium iodine (Pi) after overnight incubation with medium, 1,000 U/ml IL-2 or 15 ng/ml IL-15. (F) Frequencies of Pi+ NK cells from WT (shaded bars) or Genista (open bars) mice after overnight incubation with medium, 1,000 U/ml IL-2 or 15 ng/ml IL-15. Experiments were repeated three times. n = 7–10. Statistical significance was determined in a Mann-Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.005.
Impaired NK cell maturation and function in neutropenic patients
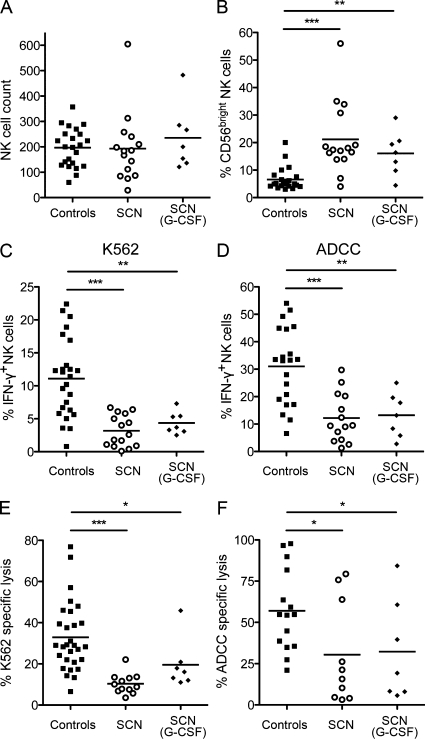
These data revealing a new role for neutrophils in NK cell function in the mouse prompted us to assess NK cell maturation and function in patients suffering from neutropenia. Patients with severe congenital neutropenia (SCN) have no terminally differentiated neutrophils because of the blockage of maturation at the promyelocyte stage in the BM (Donadieu et al., 2011; Klein, 2011). Mutations of the neutrophil ELANE gene, encoding the serine protease elastase, are the most common cause of SCN (Bellanné-Chantelot et al., 2004; Horwitz et al., 2007), but mutations targeting the gene involved in Wiskott-Aldrich syndrome (Ancliff et al., 2006; Devriendt et al., 2001), HCLS1-associated protein X-1 (Klein et al., 2007), G6PC3 (Boztug et al., 2009), or GFI-1 (Person et al., 2003) have also been described. We analyzed a cohort of 22 SCN patients, 11 of whom had a mutation in ELANE; the mutations in the other 11 patients were unidentified (Table 1). Some of them were treated with granulocyte colony-stimulating factor (G-CSF) to increase neutrophil counts. Blood NK cell counts were similar in SCN patients and healthy controls (Fig. 8 A), but using CD56 down-regulation as a marker of terminal NK cell maturation, the frequency of CD56bright NK cells was higher in SCN patients than in controls, indicating that NK cells of SCN patients were less mature (Fig. 8 B). We assessed the possible functional impairment of NK cells from SCN patients, by monitoring the percentage of IFN-γ+ NK cells generated in response to stimulation with K562 tumor target cells or antibody-coated P815 cells, as a means to evaluate antibody-dependent cell-mediated cytotoxicity (ADCC). Under both types of stimulation, the percentage of responding NK cells was much lower in SCN patients than in healthy controls (Fig. 8, C and D). This hyporesponsive phenotype was confirmed by determining the percentage of lysed target cells (Fig. 8, E–F). NK cells from patients lacking neutrophils thus displayed a maturation and functional defect similar to that observed in neutropenic mice, demonstrating that the key role of neutrophils in NK cell maturation and function is conserved between humans and mice.
Table 1.
Patient characteristics
| Patient | Age | Sex | Mutations | Neutrophil count | NK cell count | Treatment with G-CSF |
| yr | cells/µl | cells/µl | ||||
| 1 | 24 | F | SCN ELANE | 860 | 136 | + |
| 2 | 2 | F | SCN ELANE | 58 | 121 | + |
| 3 | 3 | F | SCN ELANE | ND | 167 | − |
| 4 | 34 | F | SCN ELANE | ND | 210 | − |
| 5 | 5 | F | SCN ELANE | 1,400 | 267 | + |
| 6 | 5 | F | SCN ELANE | 2,500 | 285 | + |
| 7 | 2 | F | SCN ELANE | 100 | 483 | + |
| 8 | 30 | F | SCN ELANE | 1,300 | 198 | − |
| 9 | 33 | F | SCN ELANE | 360 | 85 | − |
| 10 | 3 | M | SCN ELANE | 590 | 186 | − |
| 11 | 36 | F | SCN ELANE | 60 | 144 | − |
| 12 | 21 | F | SCN unidentified | 4,624 | 154 | + |
| 13 | 4 | F | SCN unidentified | 425 | 258 | − |
| 14 | 19 | F | SCN unidentified | 1363 | 200 | + |
| 15 | 9 | F | SCN unidentified | 264 | 184 | − |
| 16 | 4 | M | SCN unidentified | 440 | 112 | − |
| 17 | 0.4 | F | SCN unidentified | 920 | 240 | − |
| 18 | 0.5 | M | SCN unidentified | 490 | 87 | − |
| 19 | 60 | M | SCN unidentified | 540 | 29 | − |
| 20 | 3 | M | SCN unidentified | 230 | 313 | − |
| 21 | 22 | F | SCN unidentified | 170 | 75 | − |
| 22 | 1 | F | SCN unidentified | 460 | 605 | − |
| 23 | 1 | F | AIN | 0 | 384 | − |
| 24 | 1.2 | F | AIN | 370 | 943 | − |
| 25 | 3 | F | AIN | 1010 | 104 | − |
| 26 | 3 | F | AIN | 1530 | 276 | − |
| 27 | 5 | F | AIN | 160 | 207 | − |
| 28 | 2 | M | AIN | 550 | 339 | − |
The SCN patients (n = 22; median age = 5 yr; range = 0.4–60) and AIN patients (n = 6; median age = 2.6 yr; range = 1–5) described were analyzed and compared with healthy control individuals (n = 25; median = 30 yr; range = 22–55). ND, not determined.
Figure 8.
Impaired NK cell maturation and function in patients with SCN. Total NK cell (CD3−CD56+; A) and percentages of CD56bright NK cells (B) in peripheral blood of healthy controls (black squares), SCN patients not treated with G-CSF (open circles) or SCN patient treated with G-CSF (black diamonds). (C-F) PBMCs from healthy control individuals (black squares), SCN patients not treated with G-CSF (open circles), or SCN patient treated with G-CSF (black diamonds) were stimulated for 4 h with K562 target cells (C–E) or antibody-coated cells (ADCC; D-F) at an effector/target ratio of 5:2 and 50:1, respectively. Frequencies of IFN-γ–producing NK cells (C and D) and NK cell cytotoxic activity (E and F) were determined by FACS. Statistical significance was determined in a Mann-Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.005.
SCN is currently treated by G-CSF injections, which increase the rate of granulopoiesis and result in the mobilization of neutrophils to the periphery. Neutrophil count restoration is variable among patients and depends on the dose of G-CSF used (Donadieu et al., 2011). In some patients, these neutrophils have been shown to be only partially functional (Donini et al., 2007). Some studies have indicated a potential role of G-CSF in NK cell function (Miller et al., 1997). We therefore compared the reactivity of NK cells between SCN patients with and without G-CSF treatment. NK cell reactivity did not differ between G-CSF–treated and untreated patients (Table 1; and Fig. 8, C–F). An increase in the frequency of CD56bright cells was also observed in SCN patients treated with G-CSF compared with controls (Fig. 8 B). Thus, G-CSF treatment is not sufficient to restore NK cell reactivity and maturation in SCN patients.
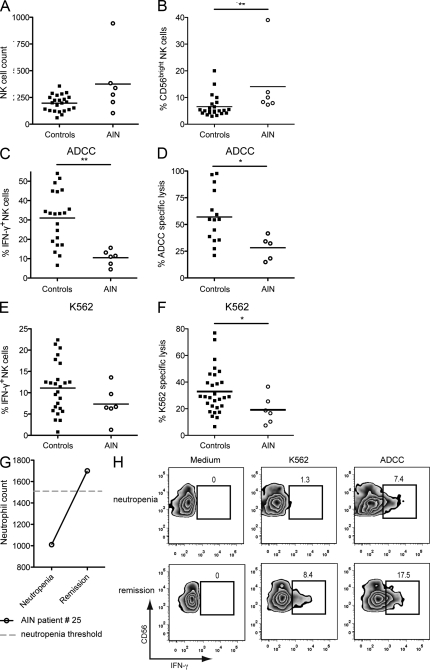
The BM is the main reservoir for neutrophils at steady state. We found that neutrophils were still present in the BM of neutrophil-depleted WT mice in which NK cells were hyporesponsive (Fig. 2 A; and Fig. 5, B and C). In this model, the NK cell hyporesponsiveness was milder than that in Genista mice, which lack neutrophils in both the BM and the periphery, suggesting a contribution from, but not a requirement for, BM neutrophils (Fig. 1, E and F; and Fig. 5 B). We therefore investigated whether BM neutrophils or neutrophils circulating at the periphery were required for NK cell function in humans. We addressed this question by analyzing a second cohort of patients with autoimmune neutropenia (AIN; Table 1). AIN patients have circulating antibodies targeting neutrophils, leading to a lack of mature neutrophils at the periphery. Unlike SCN patients, AIN patient display normal neutrophil development in the BM. In AIN patients, NK cell counts were not statistically different from the control individuals (Fig. 9 A), whereas NK cell maturation was affected with a trend toward an accumulation of immature CD56bright NK cells (Fig. 9 B). Despite the high variability among individuals and the limited number of AIN patients, NK cells from these patients were also less reactive to antibody-coated cells, as shown by the percentage of IFN-γ+ cells (Fig. 9 C) or direct target cytotoxicity (Fig. 9 D). A down-regulation of NK cell reactivity in response to K562 target cells was also observed in blood samples from AIN patients (Fig. 9, E and F), but this trend was not statistically significant for IFN-γ secretion, probably because of the small size of our cohort of AIN patients. Thus, despite the presence of neutrophils in the BM, peripheral NK cells from AIN patients were hyporesponsive, like NK cells from SCN patients, although the observed defect was milder. Interestingly, one of the AIN patients recovered from neutropenia 8 mo after the first analysis. This increase in neutrophil counts was associated with the recovery of NK cell functions (Fig. 9, G and H). Altogether, these results indicate that neutrophils contribute to NK cell development and functions in humans.
Figure 9.
Impaired NK cell maturation and function in patients with AIN. Total NK cell (CD3−CD56+; A) and percentages of CD56bright NK cells (B) in peripheral blood of healthy control individuals (black squares) and AIN patients (open circles). (C–F) PBMCs from healthy controls (black squares) or AIN patients (open circles) were stimulated for 4 h with K562 target cells (C–E) or ADCC (D–F) at an effector/target ratio of 5:2 and 50:1, respectively. (C and D) Frequencies of IFN-γ–producing NK cells and (E and F) NK cell cytotoxic activity were measured by FACS. Statistical significance was determined in a Mann-Whitney test. *, P < 0.05; **, P < 0.01; ***, P < 0.005. (G) Representation of the neutrophil count (cells/microliter) in peripheral blood of AIN patient #25 during neutropenia and after remission. Dashed line indicates the neutropenia threshold of 1,500 neutrophils/µl. (H) PBMCs from AIN patient #25 during neutropenia (top) or after remission (bottom) were stimulated for 4 h with K562 target cells, ADCC, or medium. FACS profiles show IFN-γ+ NK cells among (CD3− CD56+) NK cells. Percentages of IFN-γ+ NK cells are indicated.
DISCUSSION
We report here a new role for neutrophils as nonredundant regulatory cells ensuring the terminal maturation of NK cells and the acquisition of their full effector functions in steady-state conditions in both humans and mice.
Using a forward genetic approach we identified a mutant mouse, Genista, with hyporesponsive NK cells coupled to neutropenia. The hyporeactivity of Genista NK cells was observed in vivo against MHC-I–deficient splenocytes and in vitro upon stimulation with the tumor cells YAC-1 or after triggering of the NK1.1, NKp46, NKG2D, and Ly49 receptors. The surface expression of these receptors was unchanged, suggesting that a wide range of activating pathways coupled to various signaling adaptor molecules, such as FcRγ, DAP12, and DAP10, are affected in NK cells in the absence of neutrophils. The hyporesponsive phenotype was associated with a block in NK cell maturation before the up-regulation of cell surface CD43, a marker that accompanies NK cell responsiveness in WT mice.
In humans, we studied several subtypes of neutropenia with distinct clinical profiles and pathophysiology backgrounds. First, we analyzed SNC patients. 11 of them presented a mutation in the ELANE gene, whereas the genetic etiology was unidentified for the others. Like in the mouse model, NK cell maturation and responsiveness were affected in these patients. These results first established the conservation of the major role of neutrophils on NK cell functions across species. In addition, they also indicated that the mechanism of NK cell/neutrophil cellular cooperation was independent of the gene involved in neutropenia (Gfi-1 in the mouse, ELANE in humans). The role of neutrophils in NK cell biology was further confirmed by the depletion of neutrophils with antibodies in WT mice and by studying AIN patients. In these patients, AIN is transient and is related to the presence of antibodies directed against epitopes of neutrophil membranes: NA1a, b, and c in most cases (Bux and Stroncek, 2002). In conclusion, whatever the cause of the neutropenia is, the phenotype of NK cell hyporesponsiveness is present both in humans and in mice.
Importantly, one of the AIN patients of our cohort spontaneously resolved his neutropenia and NK cell functions were restored in parallel, reinforcing the intimate link between the two cell subsets. In contrast, no restoration of NK cell reactivity was detected in patients responding to G-CSF treatment by an increase in neutrophil count (above the threshold of 1,500 cells/µl). Similarly, 1 wk of G-CSF injections in Genista mice mobilized immature, Ly6G− neutrophils to the periphery, but did not restore NK cell function (unpublished data). Thus, neutrophils that are induced by G-CSF treatment do not acquire the capacity to potentiate NK cell function, prompting further dissection of the differences between these neutrophil populations and normal neutrophils. Along this line, it was shown in some patients that G-CSF treatment reverses neutropenia without correcting all the functional deficiencies of neutrophils (Donini et al., 2007). This issue remains to be revisited in larger cohorts of SCN patients with different genetic etiology.
NK cell homeostasis was also modified in mice lacking neutrophils. We observed an increased sensitivity to cell death ex vivo and an increased proliferation of NK cells in vivo. As we did not observe any accumulation of NK cells in neutropenic mice, it could indicate that NK cell proliferation is counterbalanced in vivo by NK cell death. NK cell proliferation induced in neutropenic mice differed from NK cell homeostatic proliferation occurring in a lymphopenic environment, which is associated with higher NK cell reactivity (Sun et al., 2011). We have shown in transfer experiments of WT NK cells in neutropenic recipients that the acquisition of the hyporesponsive NK cell phenotype did not require proliferation, even if hyporesponsiveness was increased in proliferating cells. It is possible that NK cells and neutrophils make use of the same trophic factors and that the absence of neutrophils modifies the concentration of these molecules, with an impact on the NK cell compartment. However, we do not favor this hypothesis because a large number of neutrophils remained present in the BM when peripheral neutrophils were depleted in mAb-treated WT animals and in AIN patients. In the two species, the presence of BM neutrophils was not sufficient to promote NK cell functions even if, in these conditions, the NK cell defect was milder. These findings support a model in which neutrophils are mandatory for NK cell maturation from the CD43− CD27+CD11b+ toward the CD43+ CD27−CD11b+ stage. A similar block in NK cell maturation has been previously reported in T-bet KO mice (Townsend et al., 2004; Soderquest et al., 2011), but we did not detect any change in T-bet or eomesodermin expression by intracellular staining on Genista NK cells as compared with WT (unpublished data). The lack of neutrophils may induce an increased turnover of NK cells that cannot reach a mature functional state. Neutrophil-induced NK cell maturation may occur not only in the BM, in which NK cells develop, but also at the periphery, suggesting a constant requirement for neutrophils, to promote NK cell function. Along this line, we also showed that spleen NK cells from neutropenic Genista mice reacquire a functional phenotype 7 d after transfer in a WT environment.
To further dissect the mechanisms at work in this cellular cooperation, we described direct NK cells/neutrophils interactions in vitro and in vivo. The two cell subsets are located in the same compartments in lymphoid organs (spleen and LN) of uninfected mice and have a high propensity to form conjugates in vitro. Yet, the short-term co-culture of purified WT neutrophils with Genista NK cells was not sufficient to restore NK cell function (unpublished data). In addition, we were not able to restore NK cell functions in Genista mice by injecting purified WT BM neutrophils. However, despite repeated injections (every day for 6 d), the number of neutrophils in recipient mice remained well below that observed in WT mice (unpublished data). Both in vivo and in vitro, these experimental conditions are limited by the fragility and very short half-life of neutrophils. Therefore, these negative results do not rule out a direct role of neutrophils on NK cells. Alternatively, neutrophils might cooperate with a third cell type to potentiate NK cell activity. Along this line, cross talk between human neutrophils and NK cells has been reported after stimulation with LPS and IL-2 or IL-15/IL-18 (Costantini et al., 2011). This crosstalk involves 6-sulfo LacNAc+ DCs (slanDC), through both direct cell–cell interactions dependent on CD18, ICAM-1, and ICAM-3, and indirect interactions mediated by cell-derived cytokines, such as IL-12 and IFN-γ (Costantini et al., 2011). It is impossible to address the role of slanDC in vivo, as no counterparts of these cells have been identified in the mouse (Costantini et al., 2011). Interestingly, we previously found that CD18 on NK cells was required for NK cell maturation and function (Crozat et al., 2011). CD18 associates with CD11a, CD11b, and CD11c proteins to generate functional integrin receptors (Luo and Springer, 2006). CD11/CD18 integrins bind to several receptors, including ICAM-1, ICAM-2, ICAM-3, inactivated C3b, and fibrinogen (Luo and Springer, 2006). We investigated the involvement of ICAM-1 in the acquisition of NK cell functions. In ICAM-1–deficient mice, NK cell functions, assessed by determining CD107a exposure and IFN-γ production upon stimulation with YAC-1 target, NK1.1, and NKp46, was found to be similar to that in WT controls (unpublished data). Moreover, NK cell maturation was not affected in ICAM-1 KO mice, as shown by the expression of CD43, CD11b, and CD27 markers (unpublished data). Thus, ICAM-1 is not required for neutrophil-induced NK cell maturation, indicating that if CD18 is involved in this process in vivo, it can act through interactions with other ligands. The mechanisms at work in steady-state conditions may therefore at least partially differ from the NK-neutrophil-slanDC “ménage à trois” described as being initiated by neutrophil activation with human cells in vitro (Costantini et al., 2011).
Neutrophils have been described as critical activators of NK cells in mice, acting in an IL-18/MyD88-dependent manner against Legionella pneumophila infection (Spörri et al., 2008), supporting our data on the implication of neutrophils in NK cell reactivity. Yet, our present findings extend the role of these cells to steady-state conditions in humans and mice. In addition, the mechanisms involved during the course of this microbial infection are different from those at work at steady state, as we previously showed that NK cells in MyD88 KO mice display no defect in IFN-γ secretion upon stimulation of NK1.1, NKp46, or YAC-1 (Chaix et al., 2008), in contrast to the situation in mice lacking neutrophils.
Altogether, our data thus rule out the requirement of signaling via IL-18–dependent and other MyD88-dependent receptors in neutrophil-induced NK cell maturation at steady state.
It has been reported recently that a subset of Ly6C− myeloid-derived suppressor cells that develop during chronic inflammation associated to tumors was capable of impairing NK cell development and function (Elkabets et al., 2010). We observed an increased number of Ly6C− atypical myeloid precursors in the BM of Genista mice (Fig. 2, A and B). We do not know if these cells are related to those appearing in such pathological conditions, but we cannot exclude that they could contribute in the induction of the NK cell phenotype in Genista mice. However, such an accumulation is not observed in the BM of WT mice depleted of neutrophils, excluding their major role in the function on NK cells that we report here.
NK cells cooperation with monocytes/macrophages and DCs is well documented (Dalbeth et al., 2004; Baratin et al., 2005; Welte et al., 2006; Lucas et al., 2007; Nedvetzki et al., 2007; Tu et al., 2008; Bellora et al., 2010; Soderquest et al., 2011). Our present study reveals a novel aspect of the role of neutrophils on NK cells. The possibility of a reciprocal cross talk between the two cell types could be envisaged. Along this line, it has been reported that IFN-γ regulates survival of neutrophils in the context of Mycobacterium tuberculosis infections (Nandi and Behar, 2011). As NK cells are important producers of IFN-γ, it would be interesting to analyze neutrophils in NK-deficient mice both in steady-state and inflammatory conditions.
Finally, high incidence of myelodysplasia and acute myeloid leukemia has been reported in neutropenic patients (Donadieu et al., 2005; Rosenberg et al., 2006). Risk factors for myelodysplasia and acute myeloid leukemia include the severity of neutropenia and the exposure of high doses of G-CSF (Donadieu et al., 2005). In light of the data presented here, the defect in NK cell immunosurveillance in these patients might also impact on the development of these malignancies. Thus, our data not only reveal a new regulatory pathway in innate immunity, but also provide insight into the previously unappreciated role of NK cell functional deficiency in patients suffering from neutropenia-associated diseases. This should prompt a reanalysis of immune deficiencies involving neutrophils or NK cells to further unravel the role of the cross talk between these cells in immunity.
MATERIALS AND METHODS
Mice and ENU mutagenesis.
The ENU mutagenesis leading to the isolation of the Genista pedigree was performed in the C57BL/6J (Charles River) background (Georgel et al., 2008). C57BL/6J CD45.1 mice were purchased from Charles River. C57BL/6J, Genista, and NKp46iCre/wtR26ReYFP/wt (Narni-Mancinelli et al., 2011) mice were bred and maintained under specific pathogen–free conditions at the animal facility of CIML and the Centre d’Explorations Physio-pathologiques Avancées RIO platform in Marseilles. Experiments were conducted in accordance with institutional guidelines for animal care and use. Protocols were approved by the Direction Départementales des Services Vétérinaires des Bouches du Rhône.
Antibodies.
The monoclonal antibodies used for flow cytometry and activation were: purified anti-NKp46 (29A1.4) conjugated with Alexa Fluor 647 or PE; anti-NK1.1 (PK136) conjugated with APC and PerCP-Cy5.5 and purified; anti-CD3 (145-2C11) conjugated with PE, FITC, PerCP-Cy5.5, and APC; anti-CD11b (M1/70) conjugated with V450; anti-CD27 (LG.3A10) conjugated with PE; anti-CD43 (S7) conjugated with PE or FITC; anti-CD45.1 (A20) conjugated with Pacific blue and Pe-Cy7; CD45.2 (104) conjugated with PerCP; anti–IFN-γ (XMG1.2) conjugated with APC and Alexa Fluor 647; anti-CD107a (1D4B) conjugated with FITC; Annexin V-FITC; anti-CD80 (16-10A1); anti-CD86 (GL1); anti-CD69 (H1.2F3); anti–mI-A/I-E (M5/114.15.2); and anti-CD19 (AD3). All antibodies were purchased from BD. Anti-Ki67 (20Raj1) antibody conjugated with PE and anti-CD45.2 (104) antibody conjugated with Alexa Fluor 700 were purchased from eBioscience. Anti-CD115 (AFS98) was purchased from BioLegend. Samples were analyzed with FACSCanto II, LSRII (BD), and FlowJo software (Tree Star).
In vitro NK cell stimulation.
Blood lymphocytes isolated with a Lympholyte gradient for mammalian cells (TEBU) or from spleen cell suspensions after red blood cell lysis were incubated with YAC-1 tumor targets or dispensed into a 96-well 2HB Immulon plate coated with purified antibody against NK1.1 (25 µg/ml), NKp46 (10 µg/ml), or isotype controls. Cells were activated in the presence of monensin (GolgiStop; BD), GolgiPlug (BD), and anti-CD107a antibody conjugated with FITC (where indicated) in complete medium (RPMI-1640 [Invitrogen] supplemented with 10% fetal calf serum, 1 mM sodium pyruvate, 10 mM Hepes, 100 U/ml penicillin, and 100 µg/ml streptomycin). As a positive control, we stimulated the cells with a mixture of 200 µg/ml PMA and 5 µg/ml ionomycin. The cells were incubated for 4 h at 37°C, and then cell surface staining was performed. For intracellular IFN-γ staining, cells were fixed with 2% paraformaldehyde (PFA) and permeabilized with Perm/Wash solution (BD).
In vivo rejection of target cells.
This method for the quantitative assessment of in vivo killing was adapted from a method described in a previous study (Oberg et al., 2004). In brief, splenocytes from WT or β2mKO mice were labeled with 2 and 0.2 µM CFSE (Invitrogen), respectively, and mixed at a 1:1 ratio. The ratio of the various populations before injection was determined by FACS analysis and compared with the ratio in the spleen 48 h after injection.
Adoptive transfer of NK cells.
Splenocyte suspensions from CD45.2+ WT or Genista donor mice were obtained by mechanical disruption of the spleen of the mouse, in complete medium, on a cell strainer with 70-µm pores (BD). Red blood cells were lysed in RBC lysis buffer (eBioscience). The preparation was then enriched in NK cells by negative depletion with mAbs against CD4, CD5, CD8, Ter119, and IA/IE, as previously described (Chiossone et al., 2009). NK cell purity was ∼85%. Donor NK cells were labeled with 1 µM CFSE (Invitrogen) and 2–3 × 106 NK cells/mouse were injected i.v. into WT or Genista CD45.1+ recipient mice.
In vitro DC and macrophage differentiation.
BM cells were flushed from tibiae and femurs of WT or Genista mice and cultured in complete RPMI medium supplemented with M-CSF at 10 ng/ml. Adherent BMMs were obtained after 7 d in culture. BMDCs were obtained and cultured, as previously described (Inaba et al., 1992). 7 d after culture of BMDCs or BMMs, 10 ng/ml of LPS was added to the culture and up-regulation of co-stimulatory molecule expression was monitored by FACS.
In vivo depletion.
Neutrophil depletion was achieved by injecting 500 µg of 1A8/mouse (BioXCell), 100 µg of RB6-8C5/mouse (BioXCell) or isotype controls i.p. into mice on days 0 and 2. CD4+ T cells were depleted by two i.p. injections of 200 µg of GK1.5/mouse (BD) on days 0 and 1. The analysis was performed on day 6.
Conjugate assay.
The BM was flushed from the tibia and femur of WT mice and separated on a 65% Percoll (GE Healthcare) gradient. The pellet was retained and the red blood cells were lysed in RBC lysis buffer (eBioscience). The resulting preparation contained >80% neutrophils, which were then stained with PKH26 (Sigma-Aldrich), according to the manufacturer’s protocol. Purified neutrophils were then incubated at a 1:1 ratio, with WT splenocytes previously stained with anti-NK1.1 and anti-CD3 antibodies. After 10 min at 37°C, cells were fixed with 1% paraformaldehyde and analyzed by FACS. The conjugates correspond to PKH26+ NK1.1+ CD3− cell doublets.
Immunostaining.
Organs were harvested and fixed by incubation in 0.05 M phosphate buffer supplemented with 0.1 M l-lysine, pH 7.4, 2 mg/ml NaIO4, and 10 mg/ml paraformaldehyde for 12 h. They were then washed in phosphate buffer and dehydrated by incubation in 30% sucrose in phosphate buffer. Spleens from NKp46iCre/wtR26ReYFP/wt mice were snap-frozen in Tissue-Tek (Sakura Finetek). We cut 20-µm frozen sections and stained them as previously described (Bajénoff et al., 2003). Anti-B220 (RA3-6B2) antibody conjugated with Alexa Fluor 647 (BD), anti-MMP9 rabbit polyclonal antibody (Abcam) detected with a Pacific blue–conjugated anti–rabbit antibody (Life Technologies), anti-Lyve1 rabbit polyclonal antibody (Acris GmbH) detected with A647-conjugated anti–rabbit antibody (Invitrogen), and an anti-GFP Alexa Fluor 488 antibody (Invitrogen) were used for staining. Immunofluorescence confocal microscopy was performed with a Leica SP5 confocal microscope. Separate images were collected for each fluorochrome and overlaid to obtain a multicolor image. Final image processing was performed with Imaris software (Bitplane) and Photoshop software (Adobe).
Patients and controls.
PBMCs were isolated by the centrifugation on Ficoll (EuroBio) gradients of whole blood samples obtained from healthy volunteer donors and from the SCN and AIN patients described in Table 1. Patients were recruited from departments of Pediatric Hematology of Trousseau Hospital (Paris), Necker Enfants Malades Hospital (Paris), and La Timone Hospital (Marseille) if they present a chronic neutropenia classified according to the literature (Donadieu et al., 2011). Patients with congenital neutropenia were included in the French Severe Chronic Neutropenia cohort, which has been reported elsewhere (Donadieu et al., 2005). The patients or their parents provided their written informed consent for genetic testing and inclusion in the register approved by the Commission Nationale de l’Informatique et des Libertés (number 01–1084). Genomic DNA was extracted from blood with standard procedures and ELANE, G6PC3, HAX1, WASP, and SBDS mutations were screened (Bellanné-Chantelot et al., 2004; Donadieu et al., 2011). In addition to patients with congenital neutropenia, AIN patients, diagnosed if autoantibodies against neutrophils were detected (Bux et al., 1998), were also included in the study.
In vitro activation of human NK cells.
PBMCs were incubated in the presence of GolgiStop (1/1500; BD) with MHC class I− human erythroleukemic K562 target cells or P815 mouse mastocytoma cells coated with rabbit anti–mouse lymphocyte antibodies (BioValley). After incubation for 4 h at 37°C, cells were stained with anti-CD3 (SK7), PercPCy5.5-conjugated antibody (BD) and with anti-CD56 (NKH-1) APC-conjugated antibody (Immunotech). Intracellular IFN-γ staining was performed after fixation in 2% paraformaldehyde and permeabilization in Perm/Wash solution (BD). For NK cytotoxicity analysis, K562 or P815 cells incubated with rabbit anti–mouse lymphocyte antibodies were stained with 0.5 µM CFSE (Invitrogen) for 10 min, washed, and incubated with PBMCs at a 50:1 ratio. After 4 h at 37°C, cells were stained with 2.5 µg/ml propidium iodide (Invitrogen) and analyzed by flow cytometry.
Statistics.
All P values were determined with Prism software (GraphPad Software Inc.) for nonparametric unpaired or paired tests (two-tailed), as indicated. We considered p-values <0.05 to be significant and the degree of significance is indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Acknowledgments
We thank Nicolas Chevrier for experiments on ICAM-1 KO mice, and the CIML animal facility and flow cytometry facilities.
This work was supported by European Research Council advanced grant (E. Vivier and S. Ugolini); MUGEN Network of Excellence and MASTERSWITCH Integrating Project from European Communities (B. Malissen and M. Malissen); Fondation pour la Recherche Médicale and ARC (D. Ordoñez-Rueda); Agence Nationale de la Recherche (S. Ugolini); Ligue Nationale contre le Cancer (E. Vivier); Axa Research Fund (B.N. Jaeger); and institutional grants from Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique and Université de la Méditerranée to the CIML.
E. Vivier is a cofounder and shareholder of Innate-Pharma. The other authors have no competing financial interests.
Author contributions: B.N. Jaeger, E. Vivier, and S. Ugolini designed and analyzed the experiments and wrote the paper. M. Malissen and B. Malissen initiated and conducted the early phases of the ENU mutagenesis screen, isolated the neutropenic Genista mice, and determined the corresponding genetic defect. D. Ordoñez-Rueda analyzed the neutropenic Genista phenotype; C. Bernat identified the NK cell functional defect present in Genista; B.N. Jaeger performed and analyzed the experiments on mice; C. Cognet performed and analyzed the experiments on human patients; M. Bajénoff performed immunostaining experiments; A. Fenis was responsible for mouse handling and breeding; E. Narni-Mancinelli participated in the experimental design; J. Donadieu, N. Mahlaoui, and V. Barlogis provided blood samples from neutropenic patients. J. Donadieu is the scientific secretary of the French SCN register. C. Bellanné-Chantelot was responsible of the human genetic analysis, B. Beaupain monitored the French SCN register.
Footnotes
Abbreviations used:
- ADCC
- antibody-dependent cell-mediated cytotoxicity
- AIN
- autoimmune neutropenia
- BMDC
- BM-derived DC
- BMM
- BM-derived macrophage
- DP
- double positive
- ENU
- N-ethyl N-nitrosourea
- G-CSF
- granulocyte colony-stimulating factor
- Gfi-1
- growth factor–independent-1
- PMA
- phorbol 12-myristate 13-acetate
- SCN
- severe congenital neutropenia
References
- Ancliff P.J., Blundell M.P., Cory G.O., Calle Y., Worth A., Kempski H., Burns S., Jones G.E., Sinclair J., Kinnon C., et al. 2006. Two novel activating mutations in the Wiskott-Aldrich syndrome protein result in congenital neutropenia. Blood. 108:2182–2189 10.1182/blood-2006-01-010249 [DOI] [PubMed] [Google Scholar]
- Anfossi N., André P., Guia S., Falk C.S., Roetynck S., Stewart C.A., Breso V., Frassati C., Reviron D., Middleton D., et al. 2006. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 25:331–342 10.1016/j.immuni.2006.06.013 [DOI] [PubMed] [Google Scholar]
- Bajénoff M., Granjeaud S., Guerder S. 2003. The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J. Exp. Med. 198:715–724 10.1084/jem.20030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratin M., Roetynck S., Lépolard C., Falk C., Sawadogo S., Uematsu S., Akira S., Ryffel B., Tiraby J.G., Alexopoulou L., et al. 2005. Natural killer cell and macrophage cooperation in MyD88-dependent innate responses to Plasmodium falciparum. Proc. Natl. Acad. Sci. USA. 102:14747–14752 10.1073/pnas.0507355102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellanné-Chantelot C., Clauin S., Leblanc T., Cassinat B., Rodrigues-Lima F., Beaufils S., Vaury C., Barkaoui M., Fenneteau O., Maier-Redelsperger M., et al. 2004. Mutations in the ELA2 gene correlate with more severe expression of neutropenia: a study of 81 patients from the French Neutropenia Register. Blood. 103:4119–4125 10.1182/blood-2003-10-3518 [DOI] [PubMed] [Google Scholar]
- Bellora F., Castriconi R., Dondero A., Reggiardo G., Moretta L., Mantovani A., Moretta A., Bottino C. 2010. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proc. Natl. Acad. Sci. USA. 107:21659–21664 10.1073/pnas.1007654108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boztug K., Appaswamy G., Ashikov A., Schäffer A.A., Salzer U., Diestelhorst J., Germeshausen M., Brandes G., Lee-Gossler J., Noyan F., et al. 2009. A syndrome with congenital neutropenia and mutations in G6PC3. N. Engl. J. Med. 360:32–43 10.1056/NEJMoa0805051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P., Kärre K., Höglund P. 2009a. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 30:143–149 10.1016/j.it.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Brodin P., Lakshmikanth T., Johansson S., Kärre K., Höglund P. 2009b. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 113:2434–2441 10.1182/blood-2008-05-156836 [DOI] [PubMed] [Google Scholar]
- Bux J., Stroncek D. 2002. Human neutrophil antigens. Transfusion. 42:1523 10.1046/j.1537-2995.2002.00265.x [DOI] [PubMed] [Google Scholar]
- Bux J., Behrens G., Jaeger G., Welte K. 1998. Diagnosis and clinical course of autoimmune neutropenia in infancy: analysis of 240 cases. Blood. 91:181–186 [PubMed] [Google Scholar]
- Chaix J., Tessmer M.S., Hoebe K., Fuséri N., Ryffel B., Dalod M., Alexopoulou L., Beutler B., Brossay L., Vivier E., Walzer T. 2008. Cutting edge: Priming of NK cells by IL-18. J. Immunol. 181:1627–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiossone L., Chaix J., Fuseri N., Roth C., Vivier E., Walzer T. 2009. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 113:5488–5496 10.1182/blood-2008-10-187179 [DOI] [PubMed] [Google Scholar]
- Costantini C., Calzetti F., Perbellini O., Micheletti A., Scarponi C., Lonardi S., Pelletier M., Schakel K., Pizzolo G., Facchetti F., et al. 2011. Human neutrophils interact with both 6-sulfo LacNAc+ DC and NK cells to amplify NK-derived IFNgamma: role of CD18, ICAM-1, and ICAM-3. Blood. 117:1677–1686 10.1182/blood-2010-06-287243 [DOI] [PubMed] [Google Scholar]
- Crozat K., Eidenschenk C., Jaeger B.N., Krebs P., Guia S., Beutler B., Vivier E., Ugolini S. 2011. Impact of β2 integrin deficiency on mouse natural killer cell development and function. Blood. 117:2874–2882 10.1182/blood-2010-10-315457 [DOI] [PubMed] [Google Scholar]
- Dalbeth N., Gundle R., Davies R.J., Lee Y.C., McMichael A.J., Callan M.F. 2004. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J. Immunol. 173:6418–6426 [DOI] [PubMed] [Google Scholar]
- Daley J.M., Thomay A.A., Connolly M.D., Reichner J.S., Albina J.E. 2008. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 83:64–70 10.1189/jlb.0407247 [DOI] [PubMed] [Google Scholar]
- Degli-Esposti M.A., Smyth M.J. 2005. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat. Rev. Immunol. 5:112–124 10.1038/nri1549 [DOI] [PubMed] [Google Scholar]
- Devriendt K., Kim A.S., Mathijs G., Frints S.G., Schwartz M., Van Den Oord J.J., Verhoef G.E., Boogaerts M.A., Fryns J.P., You D., et al. 2001. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat. Genet. 27:313–317 10.1038/85886 [DOI] [PubMed] [Google Scholar]
- Donadieu J., Leblanc T., Bader Meunier B., Barkaoui M., Fenneteau O., Bertrand Y., Maier-Redelsperger M., Micheau M., Stephan J.L., Phillipe N., et al. ; French Severe Chronic Neutropenia Study Group; Experience of the French Severe Chronic Neutropenia Study Group 2005. Analysis of risk factors for myelodysplasias, leukemias and death from infection among patients with congenital neutropenia. Haematologica. 90:45–53 [PubMed] [Google Scholar]
- Donadieu J., Fenneteau O., Beaupain B., Mahlaoui N., Chantelot C.B. 2011. Congenital neutropenia: diagnosis, molecular bases and patient management. Orphanet J. Rare Dis. 6:26 10.1186/1750-1172-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donini M., Fontana S., Savoldi G., Vermi W., Tassone L., Gentili F., Zenaro E., Ferrari D., Notarangelo L.D., Porta F., et al. 2007. G-CSF treatment of severe congenital neutropenia reverses neutropenia but does not correct the underlying functional deficiency of the neutrophil in defending against microorganisms. Blood. 109:4716–4723 10.1182/blood-2006-09-045427 [DOI] [PubMed] [Google Scholar]
- Elkabets M., Ribeiro V.S., Dinarello C.A., Ostrand-Rosenberg S., Di Santo J.P., Apte R.N., Vosshenrich C.A. 2010. IL-1β regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur. J. Immunol. 40:3347–3357 10.1002/eji.201041037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J.M., Wahle J.A., Yokoyama W.M. 2010. MHC class I–deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I–sufficient environment. J. Exp. Med. 207:2073–2079 10.1084/jem.20100986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez N.C., Treiner E., Vance R.E., Jamieson A.M., Lemieux S., Raulet D.H. 2005. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 105:4416–4423 10.1182/blood-2004-08-3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T.J., Fleming M.L., Malek T.R. 1993. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 151:2399–2408 [PubMed] [Google Scholar]
- Georgel P., Du X., Hoebe K., Beutler B. 2008. ENU mutagenesis in mice. Methods Mol. Biol. 415:1–16 [DOI] [PubMed] [Google Scholar]
- Guia S., Jaeger B.N., Piatek S., Mailfert S., Trombik T., Fenis A., Chevrier N., Walzer T., Kerdiles Y.M., Marguet D., et al. 2011. Confinement of activating receptors at the plasma membrane controls natural killer cell tolerance. Sci. Signal. 4:ra21 10.1126/scisignal.2001608 [DOI] [PubMed] [Google Scholar]
- Hayakawa Y., Smyth M.J. 2006. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J. Immunol. 176:1517–1524 [DOI] [PubMed] [Google Scholar]
- Horwitz M.S., Duan Z., Korkmaz B., Lee H.H., Mealiffe M.E., Salipante S.J. 2007. Neutrophil elastase in cyclic and severe congenital neutropenia. Blood. 109:1817–1824 10.1182/blood-2006-08-019166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R.M. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693–1702 10.1084/jem.176.6.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joncker N.T., Shifrin N., Delebecque F., Raulet D.H. 2010. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J. Exp. Med. 207:2065–2072 10.1084/jem.20100570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsunky H., Zeng H., Schmidt T., Zevnik B., Kluge R., Schmid K.W., Dührsen U., Möröy T. 2002. Inflammatory reactions and severe neutropenia in mice lacking the transcriptional repressor Gfi1. Nat. Genet. 30:295–300 10.1038/ng831 [DOI] [PubMed] [Google Scholar]
- Kim S., Iizuka K., Kang H.S., Dokun A., French A.R., Greco S., Yokoyama W.M. 2002. In vivo developmental stages in murine natural killer cell maturation. Nat. Immunol. 3:523–528 10.1038/ni796 [DOI] [PubMed] [Google Scholar]
- Kim S., Poursine-Laurent J., Truscott S.M., Lybarger L., Song Y.J., Yang L., French A.R., Sunwoo J.B., Lemieux S., Hansen T.H., Yokoyama W.M. 2005. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 436:709–713 10.1038/nature03847 [DOI] [PubMed] [Google Scholar]
- Klein C. 2011. Genetic defects in severe congenital neutropenia: emerging insights into life and death of human neutrophil granulocytes. Annu. Rev. Immunol. 29:399–413 10.1146/annurev-immunol-030409-101259 [DOI] [PubMed] [Google Scholar]
- Klein C., Grudzien M., Appaswamy G., Germeshausen M., Sandrock I., Schäffer A.A., Rathinam C., Boztug K., Schwinzer B., Rezaei N., et al. 2007. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat. Genet. 39:86–92 10.1038/ng1940 [DOI] [PubMed] [Google Scholar]
- Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A. 2007. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 26:503–517 10.1016/j.immuni.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B.H., Springer T.A. 2006. Integrin structures and conformational signaling. Curr. Opin. Cell Biol. 18:579–586 10.1016/j.ceb.2006.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Cassatella M.A., Costantini C., Jaillon S. 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11:519–531 10.1038/nri3024 [DOI] [PubMed] [Google Scholar]
- Miller J.S., Prosper F., McCullar V. 1997. Natural killer (NK) cells are functionally abnormal and NK cell progenitors are diminished in granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cell collections. Blood. 90:3098–3105 [PubMed] [Google Scholar]
- Moretta A. 2002. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat. Rev. Immunol. 2:957–964 10.1038/nri956 [DOI] [PubMed] [Google Scholar]
- Nandi B., Behar S.M. 2011. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J. Exp. Med. 208:2251–2262 10.1084/jem.20110919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narni-Mancinelli E., Chaix J., Fenis A., Kerdiles Y.M., Yessaad N., Reynders A., Gregoire C., Luche H., Ugolini S., Tomasello E., et al. 2011. Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc. Natl. Acad. Sci. USA. 108:18324–18329 10.1073/pnas.1112064108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedvetzki S., Sowinski S., Eagle R.A., Harris J., Vély F., Pende D., Trowsdale J., Vivier E., Gordon S., Davis D.M. 2007. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 109:3776–3785 10.1182/blood-2006-10-052977 [DOI] [PubMed] [Google Scholar]
- Newman K.C., Riley E.M. 2007. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat. Rev. Immunol. 7:279–291 10.1038/nri2057 [DOI] [PubMed] [Google Scholar]
- Oberg L., Johansson S., Michaëlsson J., Tomasello E., Vivier E., Kärre K., Höglund P. 2004. Loss or mismatch of MHC class I is sufficient to trigger NK cell-mediated rejection of resting lymphocytes in vivo - role of KARAP/DAP12-dependent and -independent pathways. Eur. J. Immunol. 34:1646–1653 10.1002/eji.200424913 [DOI] [PubMed] [Google Scholar]
- Orr M.T., Lanier L.L. 2010. Natural killer cell education and tolerance. Cell. 142:847–856 10.1016/j.cell.2010.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person R.E., Li F.Q., Duan Z., Benson K.F., Wechsler J., Papadaki H.A., Eliopoulos G., Kaufman C., Bertolone S.J., Nakamoto B., et al. 2003. Mutations in proto-oncogene GFI1 cause human neutropenia and target ELA2. Nat. Genet. 34:308–312 10.1038/ng1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam C., Geffers R., Yücel R., Buer J., Welte K., Möröy T., Klein C. 2005. The transcriptional repressor Gfi1 controls STAT3-dependent dendritic cell development and function. Immunity. 22:717–728 [DOI] [PubMed] [Google Scholar]
- Raulet D.H. 2006. Missing self recognition and self tolerance of natural killer (NK) cells. Semin. Immunol. 18:145–150 10.1016/j.smim.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Ribechini E., Leenen P.J., Lutz M.B. 2009. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur. J. Immunol. 39:3538–3551 10.1002/eji.200939530 [DOI] [PubMed] [Google Scholar]
- Rosenberg P.S., Alter B.P., Bolyard A.A., Bonilla M.A., Boxer L.A., Cham B., Fier C., Freedman M., Kannourakis G., Kinsey S., et al. ; Severe Chronic Neutropenia International Registry 2006. The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood. 107:4628–4635 10.1182/blood-2005-11-4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderquest K., Powell N., Luci C., van Rooijen N., Hidalgo A., Geissmann F., Walzer T., Lord G.M., Martín-Fontecha A. 2011. Monocytes control natural killer cell differentiation to effector phenotypes. Blood. 117:4511–4518 10.1182/blood-2010-10-312264 [DOI] [PubMed] [Google Scholar]
- Spörri R., Joller N., Hilbi H., Oxenius A. 2008. A novel role for neutrophils as critical activators of NK cells. J. Immunol. 181:7121–7130 [DOI] [PubMed] [Google Scholar]
- Sun J.C., Beilke J.N., Bezman N.A., Lanier L.L. 2011. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J. Exp. Med. 208:357–368 10.1084/jem.20100479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M.J., Weinmann A.S., Matsuda J.L., Salomon R., Farnham P.J., Biron C.A., Gapin L., Glimcher L.H. 2004. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 20:477–494 10.1016/S1074-7613(04)00076-7 [DOI] [PubMed] [Google Scholar]
- Tu Z., Bozorgzadeh A., Pierce R.H., Kurtis J., Crispe I.N., Orloff M.S. 2008. TLR-dependent cross talk between human Kupffer cells and NK cells. J. Exp. Med. 205:233–244 10.1084/jem.20072195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. 2008. Functions of natural killer cells. Nat. Immunol. 9:503–510 10.1038/ni1582 [DOI] [PubMed] [Google Scholar]
- Vivier E., Raulet D.H., Moretta A., Caligiuri M.A., Zitvogel L., Lanier L.L., Yokoyama W.M., Ugolini S. 2011. Innate or adaptive immunity? The example of natural killer cells. Science. 331:44–49 10.1126/science.1198687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte S., Kuttruff S., Waldhauer I., Steinle A. 2006. Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat. Immunol. 7:1334–1342 10.1038/ni1402 [DOI] [PubMed] [Google Scholar]
- Yokoyama W.M., Kim S., French A.R. 2004. The dynamic life of natural killer cells. Annu. Rev. Immunol. 22:405–429 10.1146/annurev.immunol.22.012703.104711 [DOI] [PubMed] [Google Scholar]
- Zarebski A., Velu C.S., Baktula A.M., Bourdeau T., Horman S.R., Basu S., Bertolone S.J., Horwitz M., Hildeman D.A., Trent J.O., Grimes H.L. 2008. Mutations in growth factor independent-1 associated with human neutropenia block murine granulopoiesis through colony stimulating factor-1. Immunity. 28:370–380 10.1016/j.immuni.2007.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Majlessi L., Deriaud E., Leclerc C., Lo-Man R. 2009. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 31:761–771 10.1016/j.immuni.2009.09.016 [DOI] [PubMed] [Google Scholar]