Bystander activation of human CD8+ T cells specific for influenza or cytomegalovirus during primary Epstein-Barr virus infection does not result in preexisting memory T cell attrition.
Abstract
Acute Epstein-Barr virus (EBV) infection results in an unusually robust CD8+ T cell response in young adults. Based on mouse studies, such a response would be predicted to result in attrition of preexisting memory to heterologous infections like influenza A (Flu) and cytomegalovirus (CMV). Furthermore, many studies have attempted to define the lymphocytosis that occurs during acute EBV infection in humans, but it is unclear whether bystander T cells contribute to it. To address these issues, we performed a longitudinal prospective study of primary EBV infection in humans. During acute EBV infection, both preexisting CMV- and Flu-specific memory CD8+ T cells showed signs of bystander activation, including up-regulation of granzyme B. However, they generally did not expand, suggesting that the profound CD8+ lymphocytosis associated with acute EBV infection is composed largely of EBV-specific T cells. Importantly, the numbers of CMV- and Flu-specific T cells were comparable before and after acute EBV infection. The data support the concept that, in humans, a robust CD8+ T cell response creates a new memory CD8+ T cell niche without substantially depleting preexisting memory for heterologous infections.
Studies in mice have provided compelling evidence that preexisting memory CD8+ T cells specific for bacteria (Smith et al., 2002), parasites (Schmidt and Harty, 2011), and especially viruses (Selin et al., 1996, 1999; Kim and Welsh, 2004) undergo attrition during sequential heterologous infections. Thus, it has been argued that robust CD8+ T cell responses and even vaccines specific for one pathogen might result in detrimental gaps in the immunological compartment, resulting in the inability to control subsequent reinfections or viral reactivation with other pathogens. In the context of human immunity, in which each individual is challenged with a broad variety of pathogens and adaptive immunity is required for long-term protection, deciphering the impact of heterologous infections on preexisting memory T cells after an acute infection is essential. Studies that have attempted to do this have come to conflicting conclusions (van Leeuwen et al., 2006; Zhang et al., 2008). Furthermore, one group examined this in the transplant setting (van Leeuwen et al., 2006), which may not mimic normal immunity, and the other only examined acute infection and not longer time points (Zhang et al., 2008).
To study the impact of acute viral infection on preexisting CD8+ T cell memory in humans, we studied the effect of natural EBV infection on preexisting memory CD8+ T cells specific for influenza A (Flu) and CMV. EBV is a herpes virus that commonly affects children and young adults and causes lifelong latent infection (Hislop et al., 2007). It is an interesting infection to study in this context for several reasons. First, primary infection of young adults results in an unusually robust CD8+ T cell expansion, called infectious mononucleosis when severe (Odumade et al., 2011). Thus, it provides a rigorous test of the passive attrition model, which proposes that newly formed memory T cells compete with preexisting memory T cells for survival niches. Second, it is associated with a strong IFN response, which was shown to mediate active attrition in animal models (Bahl et al., 2006). For these two reasons, EBV presents a likely scenario to observe attrition of preexisting memory T cells in humans. Finally, transmission of EBV occurs primarily via the oral route from an EBV-positive healthy individual to an EBV-naive person. Because of this, young adults who are EBV naive when they enter an independent and socially active era like college often experience a high rate of natural infection. This enabled us to study EBV-naive university freshmen as they experienced primary infection with EBV.
In this paper, we report the first study of bystander CD8+ T cell activation and attrition in healthy humans from prospective analysis of natural infection. Surprisingly, we did not observe attrition of preformed memory CD8+ T cell populations. Our results suggest that immunological memory is generally preserved during heterologous infections.
RESULTS AND DISCUSSION
CD8+ T cells undergo robust expansion and activation during acute primary EBV infection
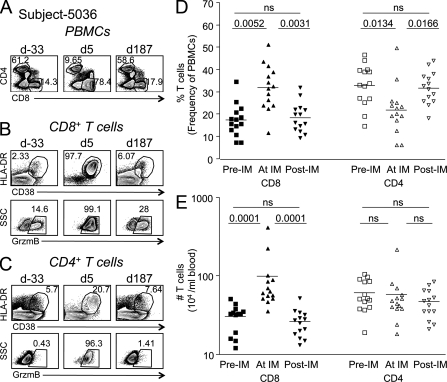
EBV-naive college freshmen were recruited and followed prospectively for 4 yr (see Materials and methods). Of 66 subjects that experienced primary EBV infection during that time, we identified 16 who had readily identifiable CMV- and/or Flu-specific memory CD8+ T cell responses before EBV infection and who had blood samples taken at least 60 d before acute infection, during acute infection, and at least 150 d after. As expected for primary infection with EBV (Hislop et al., 2007), we observed a marked increase in the frequency and number of total CD8+ but not CD4+ T cells in the blood, which returned to baseline after acute infection resolved (Fig. 1). The activation status of bulk CD8+ T cells before, during, and after primary EBV infection was assessed using the cytolytic marker granzyme B (GrzmB) and human T cell activation markers HLA-DR (DR) and CD38 (Fig. 1, B and C; Callan et al., 1998; Miller et al., 2008). We observed a mean baseline expression of 3.65 ± 4.35% CD38+/DR+ and 13.7 ± 8.49% GrzmB+CD8+ T cells before EBV infection. During acute infection, activation resulted in a mean of 65.4 ± 25.6% CD38+/DR+ and 74.1 ± 25.6% GrzmB+CD8+ T cells. In some individuals, almost all (97%) of the circulating CD8+ T cells displayed an activated phenotype via these three markers (Fig. 1 B). CD38, DR, and GrzmB expression on total CD8+ T cells returned to baseline around 150 d past acute illness (Fig. 1 B and not depicted).
Figure 1.
Extensive CD8+ T cell activation and expansion occur during primary EBV infection. PBMCs from each subject were stained with antibodies against CD3, CD4, CD8, CD38, CD45RA, DR, and GrzmB. Panels A–C show sample flow cytometric analysis from subject 5036 before, during, and after acute infection with EBV. d, day relative to symptom onset. (A) CD4 and CD8 expression on CD3+ T cells over time. (B and C) Dot plots showing activation markers CD38, HLA-DR (DR), or GrzmB expression on total CD3+CD8+ T cells (B) or total CD3+CD4+ T cells (C). (D and E) The frequency (D) or number (E) of CD8+ or CD4+ T cells before (Pre-IM), during (At IM), and after (Post-IM) acute primary EBV infection (infectious mononucleosis) is shown. Wilcoxon signed rank test, P < 0.05 considered statistically significant (n = 14). Horizontal bars indicate the mean.
EBV infection increases the overall memory CD8 pool slightly but, unlike CMV, does not cause phenotypic changes
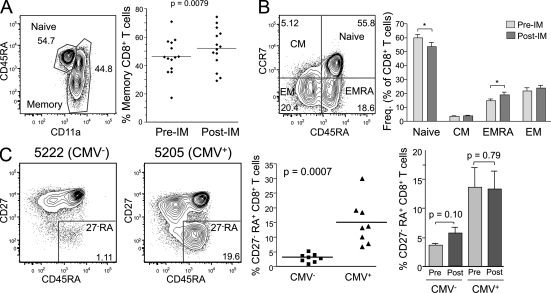
Because of the magnitude of the CD8+ T cell response to EBV, we wanted to determine whether EBV infection changed the size or composition of the memory CD8 pool. The percentage of memory CD8+ T cells varied among different individuals, yet increased a mean of 1.13-fold after primary EBV (from a mean of 46% to 52% memory CD8+ T cells, with samples analyzed at least 1 yr after; Fig. 2 A). The proportion of cells in the central, effector, and effector-RA subsets did not change significantly except for a small increase in the percentage of effector-RA cells (Fig. 2 B). CMV infection was reported to increase the proportion of CD8+ T cells with a CD27−CD45RA+ phenotype (Kuijpers et al., 2003). We observed this phenomenon in our cohort as well (Fig. 2 C, scatter plot). However, primary EBV infection did not change this proportion (Fig. 2 C, bar graph). Further work will be needed to understand why one common herpes virus infection (CMV) has this effect on CD8+ T cells yet another one (EBV) does not.
Figure 2.
Primary EBV infection results in a slight increase in effector memory RA+, but not CD27−RA+, CD8+ T cells. PBMCs from each subject were stained with antibodies against CD3, CD4, CD8, CD11a, CD45RA, CCR7, CD62L, and CD27. All panels show analysis of CD3+CD8+ T cells. (A) Memory phenotype CD8+ T cells were defined as CD11ahi. The percentage of cells in this gate was analyzed in 14 subjects before (Pre-IM) or at least 350 d after primary EBV infection (Post-IM). The mean day after infection was 750 (the p-value was determined using a paired Student’s t test). (B) CCR7 and CD45RA expression was examined on CD8+ T cells from subjects before or at least 350 d after primary EBV infection. Central memory (CM), effector memory (EM), and effector memory RA (EMRA) subsets were defined as shown. Statistically significant changes are indicated with an asterisk (paired Student’s t test: *, P < 0.05). Error bars indicate standard deviation. (C) CD27 and CD45RA expression was examined on CD8+ T cells in 16 subjects. Eight of these were CMV seropositive at enrollment, and the other eight were CMV seronegative and remained negative during the duration of the study. Scatter plot compares the percentage of CD27 negative, CD45RA+ (CD27−RA) CD8+ T cells in CMV seropositive or seronegative individuals (n = 8/group; unpaired Student’s t test, significant). The bar graph shows the mean and standard deviation of CD27−RA CD8+ T cells before versus after primary EBV infection (n = 6/group; paired Student’s t test, not significant for either). (A and C) Horizontal bars indicate the mean.
EBV-specific T cells are highly activated during acute primary EBV infection
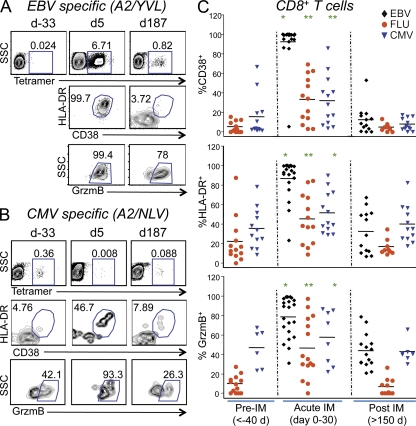
The robust activation and expansion of total CD8+ T cells during acute EBV infection has been suggested to be largely or entirely composed of EBV-specific T cells (Hislop et al., 2007). To test this, we evaluated CD8+ T cells specific to EBV and other viruses using peptide–MHCI (pMHCI) tetramers. EBV-specific T cells expressed high levels of CD38, DR, and GrzmB during acute infection, as expected (Fig. 3 A). This was observed with both lytic and latent antigens and was consistent between individuals with different MHC alleles (not depicted). The level of expression of activation markers on EBV-specific T cells returned to baseline more slowly than on total CD8+ T cells (Figs. 1 B and 3 A). This discrepancy might reflect different kinetics between CD8+ T cells specific for different viral epitopes. Alternatively, it might reflect different kinetics between CD8+ T cells specific for EBV versus those specific for non-EBV epitopes (bystander CD8+ T cells) because it has been reported that IFN can cause T cell activation of bystander T cells (McNally et al., 2001; Kohlmeier et al., 2010). Thus, we considered the possibility that activation of CD8+ T cells specific for non-EBV antigens also occurred during primary EBV infection.
Figure 3.
Both EBV-specific and bystander (CMV and Flu specific) CD8+ T cells were activated during acute EBV infection. PBMCs from each subject were stained with antibodies against CD3, CD4, CD8, CD38, CD45RA, DR, and GrzmB, as well as pMHCI tetramers. Panels A and B show sample flow cytometric analysis of PBMCs from subject 5036 before, during, and after acute infection with EBV. (A) HLA-A2/YVL (EBV) tetramer staining on CD8+ T cells. (B) HLA-A2/NLV (CMV) tetramer staining on CD8+ T cells. (A and B) Lower dot plots show CD38, HLA-DR (DR), and GrzmB staining of tetramer+ cells. (C) Percentage of tetramer+CD8+ T cells that expressed CD38 (top), DR (middle), or GrzmB (bottom) before (>40 d prior), during (0–30 d), and after (>150 d) acute primary EBV infection. EBV-specific CD8+ T cells were identified with HLA-A2/YVL, A3/RVR, or B8/RAK tetramers; Flu-specific CD8+ T cells were identified with HLA-A2/GIL tetramers; and CMV-specific CD8+ T cells were identified with HLA-A2/NLV, B7/TPR, B8/ELR, or B35/IPS tetramers (n = 6–19 per virus-specific T cell; ANOVA: *, P < 0.05; **, P < 0.001). Horizontal bars indicate the mean.
Preexisting bystander memory CD8+ T cells are activated but not expanded during acute primary EBV infection
We used pMHCI tetramers to detect T cells specific for Flu and CMV. To ensure we were evaluating preexisting memory CD8+ T cells and not co-infection, for CMV, we analyzed individuals who were CMV seropositive before EBV infection. For Flu, we selected individuals that had a readily detectable population of tetramer-positive cells that displayed a memory phenotype in samples taken at least 60 d before EBV infection. When we gated on the tetramer+ population before EBV infection, we observed relatively low levels of activation markers on Flu- and CMV-specific memory T cells, as expected (Fig. 3 B). Nonetheless, CMV-specific T cells (Fig. 3 C, blue triangles) tended to express more GrzmB at baseline than Flu-specific T cells (Fig. 3 C, red circles).
Interestingly, both Flu- and CMV-specific T cells showed marked up-regulation of CD38, DR, and GrzmB during acute EBV infection (Fig. 3 C). In some cases, this activation occurred at levels nearly equivalent to those of EBV-specific T cells. It is possible that the activated phenotype of CMV-specific T cells might reflect alterations in the viral load of CMV (i.e., viral reactivation) during primary EBV infection, such as was observed in primary Hantavirus infection (Tuuminen et al., 2007). This would be particularly likely if bystander activation were to be associated with impaired function, as proposed based on in vitro experiments (Zhang et al., 2008). However, we did not detect CMV DNA in the blood by PCR during acute infection with any of the subjects in this study. Furthermore, alterations in viral load cannot explain the activated phenotype of Flu-specific T cells. The fact that 97–99% of total blood CD8+ T cells expressed an activated phenotype in some individuals implies that even naive T cells may be subject to bystander activation, as shown in the mouse (Kohlmeier et al., 2010). Put together, these data suggest that the presence of cognate antigen is not necessary for bystander activation of T cells during an acute viral infection. By day 150, the proportion of activated Flu- and CMV-specific CD8+ T cells had returned to baseline (Fig. 3 C).
Our results are consistent with data from two other studies showing bystander activation during HIV (Doisne et al., 2004) and Hepatitis B (Sandalova et al., 2010) infection in humans. In those studies, the small numbers of patients were not directly compared with controls (Sandalova et al., 2010) or to samples taken before infection (Doisne et al., 2004). Nonetheless, collectively with our study, it would seem that bystander activation of T cells is a common occurrence during acute infection in humans and clearly demonstrates that not all activated CD8+ T cells observed in peripheral blood during acute viral infection are virus specific.
Both IFN and IL-15 have been suggested to mediate bystander activation in mouse studies (McNally et al., 2001; Kohlmeier et al., 2010; Marshall et al., 2010). Furthermore, both cytokines were also shown to up-regulate activation markers on human memory CD8+ T cells when given alone at high concentrations in vitro (Kohlmeier et al., 2010; Sandalova et al., 2010). Type I IFNs up-regulated GrzmB in Flu-specific T cells (Kohlmeier et al., 2010), and IL-15 up-regulated DR and CD38 on Flu, CMV, and EBV memory T cells (Sandalova et al., 2010). Therefore, the contribution of each cytokine to the bystander T cell activation observed in this study may not be exclusive. To determine whether elevated cytokine levels were associated with bystander activation, we measured IFN-α, IFN-β, and IFN-γ levels in the plasma. Similar to previous studies, we detected elevated levels of IFN-β and IFN-γ (Linde et al., 1992; Schuster et al., 1993; Hornef et al., 1995; Biglino et al., 1996; Prabhu et al., 1996; Corsi et al., 2004; Williams et al., 2005); however, the levels of each were not significantly correlated with the level of bystander activation (not depicted). We did not measure IL-15 serum levels because it has been observed to be stable during acute infectious mononucleosis (Williams et al., 2005) and the properties of IL-15 make ex vivo detection difficult. Therefore, the mechanism of bystander T cell activation in humans remains to be determined.
Flu- and CMV-specific CD8+ T cell numbers were unchanged or increased slightly during acute EBV infection
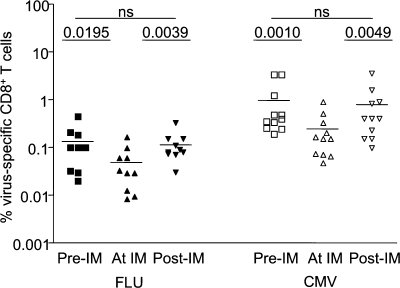
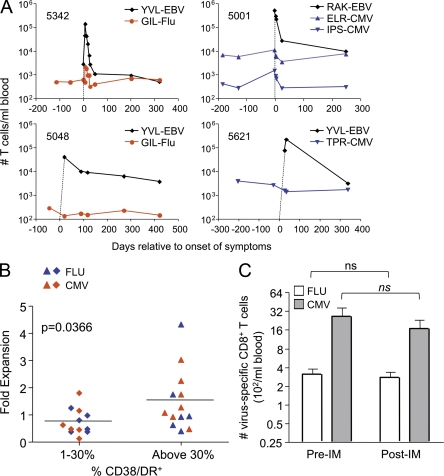
Fig. 3 C shows that T cells specific for other viruses became activated during EBV infection. However, it was unclear whether such cells expanded and contributed to the CD8+ lymphocytosis that characterizes acute EBV infection or underwent apoptosis, as occurs in some animal models. To address this, we calculated the total numbers of Flu- and CMV antigen–specific CD8+ T cells per milliliter of blood before, during, and after acute EBV infection. We observed a significantly reduced frequency of both Flu- and CMV-specific T cells during acute EBV (Fig. 4). The frequency of CD8+ T cells that bind a given tetramer is what is typically reported in the human immunology literature. However, because the total CD8+ T cell numbers increase dramatically during primary EBV infection (Fig. 1 E), it was essential to determine the total number of CMV- and Flu-specific T cells. Interestingly, these remained steady or slightly increased (Fig. 5, A and B). An increase in the number of Flu-specific T cells (Fig. 5 A, subject 5342) was observed in 4 of 15 individuals and of CMV-specific T cells (Fig. 5 A, subject 5001) in 2 of 12 individuals studied. The other individuals typically showed no increase or decrease of Flu-specific (Fig. 5 A, subject 5048) or CMV-specific (Fig. 5 A, subject 5621) CD8 T cells during after primary EBV infection.
Figure 4.
There is a transient reduction in the frequency of Flu- and CMV-specific T cells during primary EBV infection. PBMCs were stained with antibodies against CD3, CD4, CD8, and pMHCI tetramers. The graph represents the frequency of Flu (left) and CMV (right) tetramer+CD3+CD8+ T cells before (squares), during (triangles), and after (inverted triangles) acute primary EBV infection (n = 10 for Flu and n = 11 for CMV; paired Student’s t test, P > 0.05 was considered not significant). Horizontal bars indicate the mean.
Figure 5.
There is no attrition of preexisting memory CD8+ T cells after primary EBV infection. (A) Panels show data from four individual subjects depicting the total number of CD8+ T cells per milliliter of blood that stained with the indicated tetramers for EBV (black diamond), Flu (red circle), or CMV (blue inverted triangle) over time. Dotted lines indicate that EBV-specific T cells were not detectable before primary infection. (B) The fold expansion of CMV- and Flu-specific CD8+ T cells in subjects that displayed a more activated phenotype (based on >30% expression of CD38 and DR) or less activated phenotype (1–30% expression of CD38 and DR). Student’s t test, P < 0.05 (n = 26). Horizontal bars indicate the mean. (C) Flu- and CMV-specific CD8+ T cell numbers before and >150 d after primary EBV infection. Paired Student’s t test, P > 0.05 (n = 10 for Flu and n = 11 for CMV). Error bars indicate standard deviation.
To determine whether the cells that underwent the most extreme bystander activation also underwent apoptosis, as suggested for CMV-specific cells during Hepatitis B infection (Zhang et al., 2008), we examined the relationship between bystander activation and T cell numbers. The number of Flu- or CMV-specific T cells per milliliter of blood during acute EBV infection was normalized relative to the preexisting virus-specific cell numbers (set to 1) for each individual, and this value was designated as fold expansion (Fig. 5 B). When we separated the subjects into two groups based on whether Flu/CMV-specific T cells underwent more (>30% CD38/DR+) or less (<30% CD38/DR+) bystander activation, we found that the cells that underwent a greater extent of bystander activation showed, if anything, a modest (1.6-fold) increase in total numbers of bystander memory CD8+ T cells in peripheral blood (Fig. 5 B). These data suggest that memory CD8+ T cells undergoing bystander activation do not necessarily undergo apoptosis and may even contribute to the increase in CD8+ T cell numbers in the blood, albeit slightly. During acute Hepatitis B infection, it is possible that other factors besides those driving bystander T cell activation cause apoptosis of CMV-specific T cells.
Persistence of preexisting memory CD8 T cells
Finally, we compared both the frequency and the numbers of Flu- and CMV-specific T cells before EBV infection with those after resolution of the acute phase of infection. Although acute EBV infection transiently alters the CD8+ T cell compartment, both the frequency and total numbers of CD8+ and CD4+ T cells >150 d after acute infection are similar to baseline (Fig. 1, D and E), indicating that homeostasis of the peripheral immune compartment is not grossly altered by infectious mononucleosis. Consistent with this, there was no significant loss of either CMV- or Flu- specific memory T cells at later time points after EBV infection (Fig. 5 C). Altogether, these data suggest there is no attrition of peripheral blood memory CD8+ T cells during or after heterologous infections in young adults.
This was surprising because studies in animal models showed attrition of preexisting memory CD8+ T cells in many infections (Selin et al., 1996, 1999; Varga et al., 2001; Smith et al., 2002; Liu et al., 2003; Kim and Welsh, 2004; Dudani et al., 2008; Schmidt and Harty, 2011). Theoretically, attrition could be mediated by competition for cellular resources (passive model). EBV represents an extremely rigorous test of this model in humans, as acute infection with this virus causes an extraordinary expansion in the total numbers of CD8+ T cells (increasing 12 times above baseline in one of our subjects). Nonetheless, we did not observe the loss of T cells specific for heterologous infections in this context. These results are consistent with Vezys et al. (2009), who used a prime-boost immunization model in mice to demonstrate that the CD8+ T cell compartment could expand substantially without depleting preexisting memory T cells.
An alternative model suggests that attrition is mediated by type I or type II IFNs (active model). During bacterial infection of mice, attrition was dependent on IFN-γ (Dudani et al., 2008), whereas in viral studies, bystander activation was dependent on type I IFN and accompanied by apoptosis (McNally et al., 2001; Jiang et al., 2005; Bahl et al., 2006). Interestingly, elevations in type I IFN (Linde et al., 1992; Hornef et al., 1995; Prabhu et al., 1996; Williams et al., 2005) and in IFN-γ (Linde et al., 1992; Schuster et al., 1993; Hornef et al., 1995; Biglino et al., 1996; Corsi et al., 2004; Williams et al., 2005) have been well documented during acute EBV infection. Additionally, microarray data demonstrated both strong type I IFN and IFN-γ gene signatures during acute EBV infection in these subjects (unpublished data), suggesting again that EBV should be a prime candidate for active attrition. The fact that we did not observe attrition suggests that potent induction of an IFN response by itself may not be sufficient to cause apoptosis of bystander memory CD8+ T cells.
We considered the possibility that CMV-specific memory T cells may be retained by reactivation of the latent virus. Although we did not detect CMV DNA in the blood of any of the subjects during acute EBV infection (unpublished data), this possibility cannot be ruled out. Nonetheless, this should not be the case with influenza infection, which is cleared. None of the subjects in this study displayed symptoms of influenza infection during the time period between acute EBV and when postinfection samples were taken for analysis. Another possible explanation was suggested by the report that rare Flu-specific CD8+ T cells can cross-react with EBV (Clute et al., 2005). Thus, EBV might expand a minor population of dual reactive T cells, masking an overall decline in the broad Flu-specific CD8+ population. However, we did not detect dual tetramer-binding T cells (GIL/GLC or GIL/YVL) after EBV infection in any of the 12 A2+ subjects in this study (unpublished data), suggesting that this is an unlikely explanation for the persistence of Flu-specific memory.
It is interesting that infection with MHV-68 (mouse gammaherpesvirus 68), considered the closest mouse model for EBV infection, was shown to result in partial depletion of preexisting Flu-specific T cells (Liu et al., 2003). There is some evidence that central memory T cells (more likely to reside in lymphoid organs) are more sensitive to IFN-induced apoptosis than effector memory T cells (more likely to circulate in blood; Wang et al., 2003). This is one potential reason the findings described here may be at odds with animal studies. Although there are studies that indicate attrition is a comprehensive process that occurs in both lymphoid and peripheral tissues in mice, in this human study, we measured T cell numbers in the blood, in contrast to most animal studies, which focus on secondary lymphoid organs (Kim and Welsh, 2004; Dudani et al., 2008; Schmidt and Harty, 2011). An alternative explanation for why our findings may be at odds with animal studies has to do with time. Multiple studies have shown that attrition mediated by infection or type I IFN is less pronounced in older mice than younger mice (Jiang et al., 2003, 2005). We studied young adults, which might be considered developmentally equivalent to young mice. However, it is possible that when it comes to immunity, age in real time (4–6 wk vs. 18 yr) may be more important than developmental age. Overall, these results emphasize the importance of studying immunity in humans, as well as in animal models (Davis, 2008).
In summary, we studied natural infection with a common virus that induces robust innate and adaptive immune responses in humans. Our findings provide definitive data that bystander activation of preexisting memory T cells occurs in this context but does not result in attrition. Thus, we propose that the preservation of immunological memory through heterologous infections in humans is the rule, although attrition may occur under as yet undefined circumstances.
MATERIALS AND METHODS
Design of prospective study.
We performed a longitudinal study that followed a cohort of EBV-naive college students for 4 yr. We recruited healthy volunteers from the University of Minnesota undergraduate residence halls in 2006 and 2007. EBV seronegative exposure status was confirmed by the lack of IgG antibodies against EBV viral capsid antigen (EBV VCA IgG). Subjects who enrolled in the prospective study donated blood at least every 8 wk during the academic year, and an electronic journal was continuously monitored for symptoms.
Subjects with symptoms consistent with acute primary EBV infection were encouraged to come in to the clinical virology research clinic for both a physical exam and laboratory confirmation of primary EBV infection. Healthcare service personnel made the initial diagnosis of infectious mononucleosis. Primary EBV infection was defined as development of the typical EBV-specific antibody profile and documentation of EBV DNA in the oral and/or blood compartments (Balfour et al., 2005). Subjects continued prescheduled follow-up visits after seroconversion. All participants gave informed consent, and the University of Minnesota Institutional Review Board approved all protocols used.
Sample collection and handling.
Peripheral blood samples were obtained from subjects via venipuncture and collected in 10-ml purple-top EDTA Vacutainer tubes (Thermo Fisher Scientific). 200 µl of blood was used for DNA extraction and HLA typing. PBMCs were isolated by ACCUSPIN System-Histopaque-1077 (Sigma-Aldrich) density gradient centrifugation per the manufacturer’s instructions. PBMC counts were recorded after Ficoll purification of whole blood during each collection and stored as detailed in the next paragraph. Once pelleted, cells were frozen in 107 cells/ml aliquots in a cryopreservative solution containing 90% FBS and 10% DMSO (Sigma-Aldrich).
Samples were allowed to slowly freeze at −80°C overnight and then transferred to liquid nitrogen for storage until needed. Cells were rapidly thawed in a 37°C water bath, diluted to 10 ml in RPNK media supplemented with 50 U/ml Benzonase (EMD; RPNK media: RPMI 1640 [Cellgro] supplemented with 10% FBS [Atlanta Biologicals], 2% penicillin–streptomycin [5,000 U/ml and 5,000 µg/ml, respectively; Invitrogen], and 1% l-glutamine [29.2 mg/ml; Invitrogen]). Cells were then counted using a hemocytometer and divided into separate fractions for flow cytometry or RNA processing.
Plasma cytokine measurements.
For plasma collection, laboratory personnel centrifuged whole blood for 10 min. Once separated from the cellular components, a sterile serological pipette was used to create 0.5- to 1-ml aliquots. These were cryopreserved until later use. Samples before EBV infection and manufacturer-supplied controls were used as patients’ baselines and low-middle-high positive controls, respectively.
Peptide MHC class I tetramers reagents.
Commercial tetramer reagents were acquired from Beckman Coulter: human CMV pp65495–503 (NLVPMVATV)-A*0201, CMV pp65417–426 (TPRVTGGGAM)-B*0702, CMV pp65123–131 (IPSINVHHY)-B*3501, CMV IE188–96 (ELRRKMMYM)-B*0801, influenza A Matrix-158–66 (GILGFVFTL)-A*02, and EBV BMLF1259–267 (GLCTLVAML)-A*0201 ready to use. Biotinylated MHC-peptide monomers from the National Institutes of Health (NIH) tetramer facility were obtained for the following: EBV BRLF1109–117 (YVLDHLIVV)-A*0201, EBV BRLF1147–155 (RVRAYTYSK)-A*03, EBV BZLF1190–197 (RAKFKQLL)-B*08, EBV EBNA3A325–333 (FLRGRAYGL)-B*08, EBNA3A379–387 (RPPIFIRRL)-B*07, and EBNA3A603–611 (RLRAEAQVK)-A*03. Before use, we added PE-, APC-streptavidin (Invitrogen) to the monomers at a 4:1 molar ratio overnight in the dark at 4°C to generate fluorescent pMHCI tetrameric complexes. All tetramers were stored in the dark at 4°C.
Determination of preexisting immunity to CMV and influenza.
To determine the presence of preexisting immunity to human CMV, serum samples were tested for virus-specific IgG antibody responses using an ELISA (Diamedix). For influenza, we limited analysis to only individuals who were HLA-A2 positive to use available pMHCI tetramers (defined in the previous section). A2+ individuals with a detectable population of Flu-specific, memory phenotype T cells in samples taken before EBV infection were selected (16/17 individuals tested).
Flow cytometry analysis.
PBMCs from each subject were stained with antibodies (e-Bioscience, BioLegend, or Invitrogen) against CD3, CD4, CD8, CD56, CD38, HLA-DR, CD11a, CD45RA, CCR7, or CD27 and pMHCI tetramers (NIH tetramer facility or Beckman Coulter), washed, permeabilized, and then stained with an antibody to GrzmB using the Cytofix/Cytoperm kit according to the manufacturer’s instructions (BD). Samples were analyzed on an LSR II (BD), and all data were processed using FlowJo software (Tree Star). For each analysis, more than four independent experiments were performed.
Statistical analysis.
All statistical analysis was performed using Prism software (GraphPad Software) or Excel (Microsoft). Comparisons between groups were performed with an unpaired two-tailed Student’s t test, a paired two-tailed Student’s t test, or a one-way ANOVA (analysis of variance; as indicated in the figure legend) with a p-value <0.05 as the cutoff for statistical significance.
Acknowledgments
We thank Jess Norman, Jane Ding, and Steve Peery for technical support and Vaiva Vezys, Janelle Olson, Steve Jameson, and Minelva Nanton for helpful discussion and for reviewing this manuscript. We also thank the Center for Immunology FlowCore facilities and the staff and nurses (Beth Mullan and Julie Ed) in the University of Minnesota Clinical Virology Research Program.
This work was supported in part by the University of Minnesota International Center for Antiviral Research and Epidemiology and the National Institutes of Health (grants T32-CA009138 and F31AI084524 to O.A. Odumade).
The authors declare no competing commercial or financial interests.
Author contributions: O.A. Odumade performed all cell-based assays, analyzed data, and wrote the manuscript. J.A. Knight helped with total cell number analysis. H.H. Balfour Jr. and K.A. Hogquist conceptualized the research. D. Masopust reviewed the manuscript and helped with project design, and K.A. Hogquist directed the study, analyzed data, and edited the manuscript. D.O. Schmeling prepared samples and performed viral detection assays.
Footnotes
Abbreviations used:
- GrzmB
- granzyme B
- pMHCI
- peptide–MHCI
References
- Bahl K., Kim S.K., Calcagno C., Ghersi D., Puzone R., Celada F., Selin L.K., Welsh R.M. 2006. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J. Immunol. 176:4284–4295 [DOI] [PubMed] [Google Scholar]
- Balfour H.H., Jr, Holman C.J., Hokanson K.M., Lelonek M.M., Giesbrecht J.E., White D.R., Schmeling D.O., Webb C.H., Cavert W., Wang D.H., Brundage R.C. 2005. A prospective clinical study of Epstein-Barr virus and host interactions during acute infectious mononucleosis. J. Infect. Dis. 192:1505–1512 10.1086/491740 [DOI] [PubMed] [Google Scholar]
- Biglino A., Sinicco A., Forno B., Pollono A.M., Sciandra M., Martini C., Pich P., Gioannini P. 1996. Serum cytokine profiles in acute primary HIV-1 infection and in infectious mononucleosis. Clin. Immunol. Immunopathol. 78:61–69 10.1006/clin.1996.0009 [DOI] [PubMed] [Google Scholar]
- Callan M.F., Tan L., Annels N., Ogg G.S., Wilson J.D., O’Callaghan C.A., Steven N., McMichael A.J., Rickinson A.B. 1998. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 187:1395–1402 10.1084/jem.187.9.1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clute S.C., Watkin L.B., Cornberg M., Naumov Y.N., Sullivan J.L., Luzuriaga K., Welsh R.M., Selin L.K. 2005. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J. Clin. Invest. 115:3602–3612 10.1172/JCI25078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi M.M., Ruscica M., Passoni D., Scarmozzino M.G., Gulletta E. 2004. High Th1-type cytokine serum levels in patients with infectious mononucleosis. Acta Virol. 48:263–266 [PubMed] [Google Scholar]
- Davis M.M. 2008. A prescription for human immunology. Immunity. 29:835–838 10.1016/j.immuni.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doisne J.M., Urrutia A., Lacabaratz-Porret C., Goujard C., Meyer L., Chaix M.L., Sinet M., Venet A. 2004. CD8+ T cells specific for EBV, cytomegalovirus, and influenza virus are activated during primary HIV infection. J. Immunol. 173:2410–2418 [DOI] [PubMed] [Google Scholar]
- Dudani R., Murali-Krishna K., Krishnan L., Sad S. 2008. IFN-gamma induces the erosion of preexisting CD8 T cell memory during infection with a heterologous intracellular bacterium. J. Immunol. 181:1700–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop A.D., Taylor G.S., Sauce D., Rickinson A.B. 2007. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 25:587–617 10.1146/annurev.immunol.25.022106.141553 [DOI] [PubMed] [Google Scholar]
- Hornef M.W., Wagner H.J., Kruse A., Kirchner H. 1995. Cytokine production in a whole-blood assay after Epstein-Barr virus infection in vivo. Clin. Diagn. Lab. Immunol. 2:209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Anaraki F., Blank K.J., Murasko D.M. 2003. Cutting edge: T cells from aged mice are resistant to depletion early during virus infection. J. Immunol. 171:3353–3357 [DOI] [PubMed] [Google Scholar]
- Jiang J., Gross D., Nogusa S., Elbaum P., Murasko D.M. 2005. Depletion of T cells by type I interferon: differences between young and aged mice. J. Immunol. 175:1820–1826 [DOI] [PubMed] [Google Scholar]
- Kim S.K., Welsh R.M. 2004. Comprehensive early and lasting loss of memory CD8 T cells and functional memory during acute and persistent viral infections. J. Immunol. 172:3139–3150 [DOI] [PubMed] [Google Scholar]
- Kohlmeier J.E., Cookenham T., Roberts A.D., Miller S.C., Woodland D.L. 2010. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 33:96–105 10.1016/j.immuni.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers T.W., Vossen M.T., Gent M.R., Davin J.C., Roos M.T., Wertheim-van Dillen P.M., Weel J.F., Baars P.A., van Lier R.A. 2003. Frequencies of circulating cytolytic, CD45RA+CD27-, CD8+ T lymphocytes depend on infection with CMV. J. Immunol. 170:4342–4348 [DOI] [PubMed] [Google Scholar]
- Linde A., Andersson B., Svenson S.B., Ahrne H., Carlsson M., Forsberg P., Hugo H., Karstorp A., Lenkei R., Lindwall A., et al. 1992. Serum levels of lymphokines and soluble cellular receptors in primary Epstein-Barr virus infection and in patients with chronic fatigue syndrome. J. Infect. Dis. 165:994–1000 10.1093/infdis/165.6.994 [DOI] [PubMed] [Google Scholar]
- Liu H., Andreansky S., Diaz G., Turner S.J., Wodarz D., Doherty P.C. 2003. Quantitative analysis of long-term virus-specific CD8+-T-cell memory in mice challenged with unrelated pathogens. J. Virol. 77:7756–7763 10.1128/JVI.77.14.7756-7763.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H.D., Prince A.L., Berg L.J., Welsh R.M. 2010. IFN-alpha beta and self-MHC divert CD8 T cells into a distinct differentiation pathway characterized by rapid acquisition of effector functions. J. Immunol. 185:1419–1428 10.4049/jimmunol.1001140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally J.M., Zarozinski C.C., Lin M.Y., Brehm M.A., Chen H.D., Welsh R.M. 2001. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. J. Virol. 75:5965–5976 10.1128/JVI.75.13.5965-5976.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.D., van der Most R.G., Akondy R.S., Glidewell J.T., Albott S., Masopust D., Murali-Krishna K., Mahar P.L., Edupuganti S., Lalor S., et al. 2008. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 28:710–722 10.1016/j.immuni.2008.02.020 [DOI] [PubMed] [Google Scholar]
- Odumade O.A., Hogquist K.A., Balfour H.H., Jr 2011. Progress and problems in understanding and managing primary Epstein-Barr virus infections. Clin. Microbiol. Rev. 24:193–209 10.1128/CMR.00044-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu A., Warwick M., Mathur A. 1996. Decreased levels of circulating IFN-alpha and increased sCD23 in patients with acute infectious mononucleosis. Viral Immunol. 9:45–50 10.1089/vim.1996.9.45 [DOI] [PubMed] [Google Scholar]
- Sandalova E., Laccabue D., Boni C., Tan A.T., Fink K., Ooi E.E., Chua R., Shafaeddin Schreve B., Ferrari C., Bertoletti A. 2010. Contribution of herpesvirus specific CD8 T cells to anti-viral T cell response in humans. PLoS Pathog. 6:e1001051 10.1371/journal.ppat.1001051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N.W., Harty J.T. 2011. Cutting edge: Attrition of Plasmodium-specific memory CD8 T cells results in decreased protection that is rescued by booster immunization. J. Immunol. 186:3836–3840 10.4049/jimmunol.1003949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster V., Herold M., Wachter H., Reibnegger G. 1993. Serum concentrations of interferon gamma, interleukin-6 and neopterin in patients with infectious mononucleosis and other Epstein-Barr virus-related lymphoproliferative diseases. Infection. 21:210–213 10.1007/BF01728890 [DOI] [PubMed] [Google Scholar]
- Selin L.K., Vergilis K., Welsh R.M., Nahill S.R. 1996. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J. Exp. Med. 183:2489–2499 10.1084/jem.183.6.2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin L.K., Lin M.Y., Kraemer K.A., Pardoll D.M., Schneck J.P., Varga S.M., Santolucito P.A., Pinto A.K., Welsh R.M. 1999. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 11:733–742 10.1016/S1074-7613(00)80147-8 [DOI] [PubMed] [Google Scholar]
- Smith D.K., Dudani R., Pedras-Vasconcelos J.A., Chapdelaine Y., van Faassen H., Sad S. 2002. Cross-reactive antigen is required to prevent erosion of established T cell memory and tumor immunity: A heterologous bacterial model of attrition. J. Immunol. 169:1197–1206 [DOI] [PubMed] [Google Scholar]
- Tuuminen T., Kekäläinen E., Mäkelä S., Ala-Houhala I., Ennis F.A., Hedman K., Mustonen J., Vaheri A., Arstila T.P. 2007. Human CD8+ T cell memory generation in Puumala hantavirus infection occurs after the acute phase and is associated with boosting of EBV-specific CD8+ memory T cells. J. Immunol. 179:1988–1995 [DOI] [PubMed] [Google Scholar]
- van Leeuwen E.M., Koning J.J., Remmerswaal E.B., van Baarle D., van Lier R.A., ten Berge I.J. 2006. Differential usage of cellular niches by cytomegalovirus versus EBV- and influenza virus-specific CD8+ T cells. J. Immunol. 177:4998–5005 [DOI] [PubMed] [Google Scholar]
- Varga S.M., Selin L.K., Welsh R.M. 2001. Independent regulation of lymphocytic choriomeningitis virus-specific T cell memory pools: relative stability of CD4 memory under conditions of CD8 memory T cell loss. J. Immunol. 166:1554–1561 [DOI] [PubMed] [Google Scholar]
- Vezys V., Yates A., Casey K.A., Lanier G., Ahmed R., Antia R., Masopust D. 2009. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 457:196–199 10.1038/nature07486 [DOI] [PubMed] [Google Scholar]
- Wang X.Z., Stepp S.E., Brehm M.A., Chen H.D., Selin L.K., Welsh R.M. 2003. Virus-specific CD8 T cells in peripheral tissues are more resistant to apoptosis than those in lymphoid organs. Immunity. 18:631–642 10.1016/S1074-7613(03)00116-X [DOI] [PubMed] [Google Scholar]
- Williams H., McAulay K., Macsween K.F., Gallacher N.J., Higgins C.D., Harrison N., Swerdlow A.J., Crawford D.H. 2005. The immune response to primary EBV infection: a role for natural killer cells. Br. J. Haematol. 129:266–274 10.1111/j.1365-2141.2005.05452.x [DOI] [PubMed] [Google Scholar]
- Zhang J.Y., Zhang Z., Jin B., Zhang S.Y., Zhou C.B., Fu J.L., Wang F.S. 2008. Cutting edge: Programmed death-1 up-regulation is involved in the attrition of cytomegalovirus-specific CD8+ T cells in acute self-limited hepatitis B virus infection. J. Immunol. 181:3741–3744 [DOI] [PubMed] [Google Scholar]