Hippo and TGF-β converge on CTGF to promote malignant mesothelioma.
Abstract
Malignant mesothelioma (MM) is an incurable malignancy that is caused by exposure to asbestos and is accompanied by severe fibrosis. Because MM is usually diagnosed at an advanced stage and clinical identification of early lesions is difficult, its molecular pathogenesis has not been completely elucidated. Nearly 75% of MM cases have inactivating mutations in the NF2 (neurofibromatosis type 2; Merlin) gene or in downstream signaling molecules of the Hippo signaling cascade, which negatively regulates the transcription factor Yes-associated protein (YAP). In this study, we demonstrate a functional interaction between the Hippo and TGF-β pathways in regulating connective tissue growth factor (CTGF). Expression of CTGF in MM cells was induced by the formation of a YAP–TEAD4–Smad3–p300 complex on the CTGF promoter. Knocking down CTGF expression in MM cells prolonged the survival of xenografted mice, and a significant association was seen between CTGF expression and extracellular matrix deposition in MM xenografts and in patient tissue specimens. We further suggest that CTGF may influence the malignancy of mesothelioma because of the different histological expression patterns observed in human MM tissues. These data suggest that CTGF is an important modulator of MM growth and pathology and represents a novel therapeutic target for this disease.
Malignant mesothelioma (MM), arising from serosal cells of the pleural, peritoneal, and pericardial cavities, has a poor prognosis because it is frequently diagnosed at advanced stages. The primary cause of this disease has often been linked to asbestos exposure, and the number of patients worldwide is predicted to peak in the next two decades (Robinson and Lake, 2005; Murayama et al., 2006). The latent period between first exposure to asbestos and onset of the disease is ∼20–40 yr, and the first symptom is insidious and may include chest pain and breathlessness. Although there has been significant recent progress in clinical treatment with combination chemotherapies, a curative therapy for MM is still unknown, with the median survival ranging between 9 and 17 mo from the first diagnosis (Tsao et al., 2009).
The involvement of tumor suppressor genes, including p16INK4a/p14ARF and NF2 (neurofibromatosis type 2), has been demonstrated to be crucial in the development of various MMs (Bianchi et al., 1995; Sekido et al., 1995). The NF2 gene, known to be responsible for NF2 syndrome, encodes Merlin, and deletions or mutations of this gene were found in 40–50% of MMs. The downstream signaling of Merlin is the mammalian Hippo cascade, which was originally identified by genetic studies in Drosophila melanogaster (Hay and Guo, 2003; Ryoo and Steller, 2003; Wu et al., 2003; Hamaratoglu et al., 2006). The Hippo signaling cascade is a critical regulator of organ size in Drosophila as well as in mammals (Dong et al., 2007). In the conditional transgenic mouse model, the dysregulation of the pathway leads to tumorigenesis (Zhang et al., 2010). Considering Merlin and downstream components of the Hippo cascade, SAV1 (Salvador 1) and LATS2 (large tumor suppressor 2), 75% of MM cell lines had genetic inactivation of at least one of these three proteins (Murakami et al., 2011). Merlin inhibits the transcriptional coactivation activity of Yes-associated protein (YAP) by inducing phosphorylation and cytoplasmic retention of YAP (Yokoyama et al., 2008). YAP accumulation in the nucleus is also observed in MMs accompanied by mutation or deletion of LATS2 (Murakami et al., 2011). YAP is a possible oncogene that associates with TEAD (TEA domain family member), a transcription factor, and exerts biological functions such as gene expression stimulation, cell growth, anchorage-independent cell growth, and epithelial-mesenchymal transition (Vassilev et al., 2001; Zhao et al., 2008, 2009).
TGF-β was originally identified as a protein that mediates the transformation of nonneoplastic rat kidney and murine AKR-2B fibroblasts (de Larco and Todaro, 1978; Moses et al., 1981; Anzano et al., 1983). TGF-β can induce extremely variable responses depending on the cell type, mainly through the Smad2/3-dependent pathway. For example, TGF-β induces growth arrest and apoptosis in epithelial cells; it can also activate fibroblasts. Subsequent studies further revealed that TGF-β acts as a tumor suppressor in premalignant cells as well as cells progressing through the early stages of carcinogenesis; furthermore, it exerts prooncogenic effects in metastatic tumors (Roberts and Wakefield, 2003; Massagué, 2008). TGF-β is a powerful cytokine produced by many different cell types, with effects on multiple cell types, and because of this complexity, signaling in each cell and context should be carefully studied.
Upon TGF-β stimulation, Smad2 and Smad3 form complexes with Smad4 and accumulate in the nucleus (Massagué et al., 2005). p300, a transcriptional co-activator, binds with Smad3 and Smad2 and enhances Smad-induced transactivation of target genes (Nishihara et al., 1998). Recruitment of p300 frequently plays a core role not only in enhancing transactivation but also in binding other proteins to stabilize protein complexes (Fujii et al., 2006).
Mesothelial cells were reported to demonstrate an increase in DNA synthesis after TGF-β stimulation (Gabrielson et al., 1988), and both normal human mesothelial cells and MM cell lines secrete TGF-β (Gerwin et al., 1987). Furthermore, a soluble TGF-β type II receptor inhibitor and a TGF-β type I receptor kinase inhibitor (SM16) were shown to inhibit the growth of murine MM tumors injected into the flanks of mice through the reactivation of antitumor immune responses (Suzuki et al., 2004, 2007).
Given the involvement of genetic inactivation of components of the Hippo pathway in 75% of mesotheliomas and previous evidence for a protumorigenic role for the TGF-β pathway, we examined the relationship between these two pathways to further understand the molecular mechanisms underlying mesothelioma genesis. Cross talk between the Hippo and TGF-β and BMP (bone morphogenetic protein) signaling pathways has been previously reported (Varelas et al., 2008, 2010; Alarcón et al., 2009). TAZ controls nucleocytoplasmic localization of Smad2/3–Smad4 complexes and regulates the nuclear accumulation of Smad complexes (Varelas et al., 2008). Furthermore, TAZ and YAP dictate the localization of active Smad complexes during mouse embryogenesis (Varelas et al., 2010). YAP is also known to strongly bind to Smad1 and support Smad1-dependent transcription and is required for BMP suppression of neural differentiation in mouse embryonic stem cells (Alarcón et al., 2009). However, whether the cross talk between Hippo and TGF-β signaling plays an important role in tumorigenesis has not been elucidated. We found that YAP and Smad3 interact at the transcription regulation level through participants of other related transcription cofactors, forming a complex in the connective tissue growth factor (CTGF) promoter region and thereby enhancing CTGF expression. This evidence shows that the cross talk between the Hippo and TGF-β pathway directly controls prooncogenic effects in malignancy. We further show that antagonism of the TGF-β pathway and CTGF expression can prolong the survival of mice with MM tumor xenografts, suggesting new approaches for the treatment of MM.
RESULTS
The TGF-β–Smad pathway is activated in clinical samples of human mesothelioma and regulates proliferation and extracellular matrix (ECM) production in MM cells in vitro
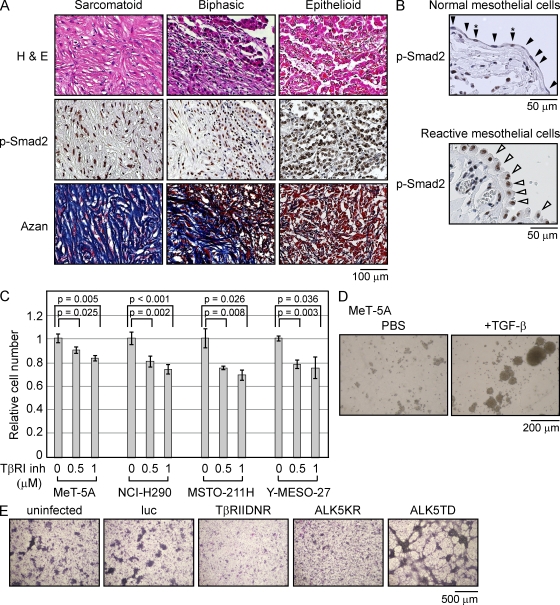
To assess the involvement of the TGF-β pathway in MM tumor growth, we performed immunohistochemical analysis in 24 tissue specimens obtained from patients to determine the p-Smad2 level and evaluate whether MM cells receive TGF-β signaling during their growth. In all 24 samples, which were subtyped into three major categories, epithelioid (12 patients), biphasic (3 patients), and sarcomatoid (7 patients), p-Smad2 staining was strongly observed in nuclei. This suggested that the constitutive activation of TGF-β is important for MM tumor development (Fig. 1 A and see Table 2). Histological subtype is a significant prognostic factor associated with longer survival because the median survival for patients with epithelioid tumors is 16.3 mo, which is longer than that for patients with biphasic tumors (9.5 mo) and sarcomatoid tumors (6.1 mo; Flores et al., 2007). Samples from all three subtypes exhibited the same level of p-Smad2 nuclear staining, indicating that the activation of the TGF-β pathway is maintained across different grades of malignancies, whereas normal pleural mesothelial cells did not exhibit significant p-Smad2 signals (Fig. 1 B, top, arrowheads). Interestingly, the nuclei of reactive pleural mesothelial cells, which were adjacent to the mesothelioma, having cuboidal appearance may be under serosal stimulation and were positively stained by p-Smad2, suggesting that the surrounding tissues were also affected by paracrine TGF-β signaling (Fig. 1 B, bottom).
Figure 1.
TGF-β signaling affects the growth of human MM cells. (A) Immunohistochemical staining for p-Smad2 of MM tissues derived from patients. Three representative sections, namely sarcomatoid, biphasic, and epithelioid subtype tumors, are shown. Azan staining was performed to visualize collagen fibers. H&E, hematoxylin and eosin. (B) Immunohistochemical staining for p-Smad2 in normal mesothelial cells (closed arrowheads) from normal lungs and reactivated normal mesothelial cells (open arrowheads) adjacent to MM tumors. Asterisks show cells with positive nuclear staining in normal mesothelial cells. (C) Cell numbers were counted 3 d after treatment with TGF-β type I receptor kinase inhibitor (SD-208). Results are expressed as mean ± SEM and are representative of three independent assays. (D) Soft agar colony formation assay was performed using MeT-5A cells treated with 4 ng/ml TGF-β for 7 d. The panel is representative of three independent assays. (E) NCI-H290 cells were infected with the indicated lentiviral expression vectors and stained with Giemsa after 14 d. HA-tagged luciferase (luc) was used as a control. The panel is representative of three independent assays. TβRIIDNR, dominant-negative form of the TGF-β type II receptor; ALK5KR, dominant-negative form of the TGF-β type I receptor; ALK5TD, constitutively activated TGF-β type I receptor.
Table 2.
CTGF expression in MM cells was dominantly observed in sarcomatoid type tissues
| No. | Pathological subtype | Features | p-Smad2 (nuclear) | YAP (nuclear) | CTGF (cytoplasm) |
| 1 | Sarcomatoid | Fibrous | + | + | + |
| 2 | Sarcomatoid | Fibrous | + | + | + |
| 3 | Sarcomatoid | Fibrous | + | + | + |
| 4 | Sarcomatoid | Fibrous | + | ± | + |
| 5 | Sarcomatoid | Fibrous | + | ± | + |
| 6 | Sarcomatoid | Fibrous | + | + | + |
| 7 | Sarcomatoid | Fibrous | + | ± | + |
| 8 | Desmoplastic (sarcomatoid) | Fibrous | + | + | + |
| 9 | Desmoplastic (sarcomatoid) | Fibrous | + | + | + |
| 10 | Biphasic | Fibrous sarcomatoid, tubulopapillary | + | S: +, E: ± | S: +, E:− |
| 11 | Biphasic | Fibrous sarcomatoid, microcystic | + | S: ±, E: ± | S: +, E: + |
| 12 | Biphasic | Fibrous sarcomatoid, solid (well-differentiated) | + | S: +, E: + | S: ±, E: ± |
| 13 | Epithelioid | Fibrous microcystic | + | + | + |
| 14 | Epithelioid | Tubulopapillary | + | − | − |
| 15 | Epithelioid | Microcystic, tubulopapillary | + | + | ± |
| 16 | Epithelioid | Tubulopapillary | + | − | − |
| 17 | Epithelioid | Tubulopapillary | + | − | − |
| 18 | Epithelioid | Tubulopapillary | + | − | − |
| 19 | Epithelioid | Tubulopapillary | + | − | − |
| 20 | Epithelioid | Microcystic | + | + | − |
| 21 | Epithelioid | Tubulopapillary | + | + | − |
| 22 | Epithelioid | Tubulopapillary | + | + | ± |
| 23 | Epithelioid | Solid (well-differentiated) | + | + | − |
| 24 | Epithelioid | Tubulopapillary | + | + | − |
Immunohistochemical staining of 24 patients was performed using p-Smad2, YAP, and CTGF antibodies. Tissues were classified based on pathological subtypes. +, positive; ±, partially positive; −, negative; S, sarcomatoid part; E, epithelioid part.
TGF-β promotes proliferation and ECM production in normal and malignant mesothelial cells
To examine whether TGF-β signaling affects monolayer cell growth, we compared cell growth by counting cell number after exposing cells to a TGF-β type I receptor kinase inhibitor, SD-208. MeT-5A is a nonmalignant human pleural mesothelial cell line immortalized by SV40 early region DNA (Ke et al., 1989). Phosphorylation of Smad3 by TGF-β peaked around 1 h and gradually decreased later in the MeT-5A cells and MM cell lines (Fig. S1 A). The proliferation and the activation of MeT-5A cells and MM cell lines, as well as the Smad-dependent pathway induced by TGF-β in these cells were effectively suppressed by TGF-β type I receptor kinase inhibitor (Fig. 1 C and Fig. S1, B and C). To further assess the biological activity of TGF-β in mesothelial cells, we performed an anchorage-independent cell proliferation assay with MeT-5A cells. After 7 d, colony formation was observed, showing the innate response of MeT-5A cells to TGF-β, which promotes colony formation similarly to that observed in fibroblasts (Fig. 1 D). Using a lentiviral vector system, we examined whether TGF-β signaling could modulate focus formation in NCI-H290 cells, an MM cell line (Fig. 1 E). Examination of the foci formed after 14 d showed a prominent decrease in the number of foci formed in cells expressing dominant-negative forms of the TGF-β type I and type II receptors. Conversely, cells with constitutive activation of the TGF-β type I receptor showed aggressive formation of foci, suggesting that TGF-β signaling affects the oncogenic property of mesothelioma cells.
To further investigate the molecular consequences of TGF-β pathway activation in mesothelioma cells, we performed cDNA microarray analysis to elucidate alterations in gene expression profiles after TGF-β treatment in MeT-5A and Y-MESO-27 cells (Table 1). Looking for overlap in the gene expression profiles, we found that 54 genes were regulated more than 1.5-fold by TGF-β in both cell types after 24 h of treatment. 42% of the commonly up-regulated genes (16/38) in TGF-β–treated MeT-5A cells and Y-MESO-27 cells were classified as ECM-related proteins. Changes in the expression profiles of representative genes (e.g., MMP2, CTGF, COL1A1, and TGF-β) were confirmed by real-time RT-PCR (Fig. S1, D and E).
Table 1.
Common genes responsive to TGF-β in MeT-5A and Y-MESO-27 cells
| Symbol | Description |
| MeT-5A and Y-MESO-27 common genes up (>1.5fold) | |
| CSF1R | colony-stimulating factor 1 receptor |
| SNAI1 | snail homologue 1 |
| LOX* | lysyl oxidase |
| RASGRP1 | RAS guanyl-releasing protein 1 |
| MMP2* | matrix metallopeptidase 2 |
| ITGA11* | integrin, alpha 11 |
| SERPINE1 | plasminogen activator inhibitor type 1 |
| BMP6 | bone morphogenetic protein 6 |
| GDF6 | growth differentiation factor 6 |
| COL1A1* | Prepro-alpha1(I) collagen |
| EDN1 | endothelin 1 |
| COL1A2* | collagen, type I, alpha 2 |
| MFAP4 | microfibrillar-associated protein 4 |
| SERPINE2 | plasminogen activator inhibitor type 1, member 2 |
| COL20A1* | collagen, type XX, alpha 1 |
| COL5A1* | collagen, type V, alpha 1 |
| ITGB3* | integrin, beta 3 |
| LTBP2 | latent transforming growth factor beta binding protein 2 |
| SMAD7 | SMAD family member 7 |
| COL7A1* | collagen, type VII, alpha 1 |
| SKIL | SKI-like oncogene |
| COL4A1* | collagen, type IV, alpha 1 |
| TGFB1 | transforming growth factor, beta 1 |
| ITGAV* | integrin, alpha V (vitronectin receptor) |
| IGFBP3 | insulin-like growth factor binding protein 3 |
| COL4A4* | collagen, type IV, alpha 4 |
| CDH11 | cadherin 11, type 2, OB-cadherin (osteoblast) |
| GADD45B | growth arrest and DNA-damage-inducible, beta |
| ADAM12 | ADAM metallopeptidase domain 12 |
| BMPR2 | bone morphogenetic protein receptor, type II |
| ITGA1* | integrin, alpha 1 |
| COL16A1* | collagen, type XVI, alpha 1 |
| MDAC1 | MDAC1 |
| TGFB2 | transforming growth factor, beta 2 |
| EGR2 | early growth response 2 |
| CTGF* | connective tissue growth factor |
| ID3 | inhibitor of DNA binding 3 |
| FN1* | fibronectin |
| MeT-5A and Y-MESO-27 common genes down (<0.67-fold) | |
| IL6R | interleukin 6 receptor |
| VCAM1 | vascular cell adhesion molecule 1 |
| CASP1 | caspase 1, apoptosis-related cysteine peptidase |
| IL12A | interleukin 12, alpha |
| IL1A | interleukin 1, alpha |
| IL7 | interleukin 7 |
| FAS | TNF receptor superfamily, member 6 |
| MMP26* | matrix metallopeptidase 26 |
| FGF23 | fibroblast growth factor 23 |
| CAMK2A | calcium/calmodulin-dependent protein kinase II alpha |
| NEDD4L | neural precursor cell expressed, developmentally down regulated 4-like |
| COL24A1* | collagen, type XXIV, alpha 1 |
| TGFA | transforming growth factor, alpha |
| CAMK2D | calcium/calmodulin-dependent protein kinase II delta |
| TLR3 | toll-like receptor 3 |
| BCL2 | B-cell CLL/lymphoma 2 |
MeT-5A and Y-MESO-27 cells were treated with TGF-β for 24 h. Total RNA was extracted and subjected to microarray analysis. The genes encoding ECM-related protein are indicated by an asterisk.
YAP is critical for TGF-β induction of a small number of target genes
Genetic alteration of the tumor suppressor gene NF2 and downstream components of the Hippo pathway, SAV1 and LATS2, were observed in 75% of MM tumors (Murakami et al., 2011). This suggests that the disturbance of the Hippo pathway is strongly associated with the development of mesothelioma. Merlin, a protein encoded by NF2, SAV1, or LATS, negatively regulates YAP, whose oncogenic property has been recently reported (Wang et al., 2009). Dephosphorylated YAP translocates into the nucleus, where it binds to TEAD and activates the transcription of target genes. Merlin inhibits the transcriptional coactivation activity of YAP by inducing phosphorylation and cytoplasmic retention of YAP (Yokoyama et al., 2008). Furthermore, genome-wide comprehensive genomic hybridization analysis of 22 MM specimens from patients showed that there is high copy amplification of 11q22 regions containing the YAP oncogene (Taniguchi et al., 2007). Thus, many genetic alterations in the Hippo pathway that have been seen in mesothelioma converge to increase YAP activity.
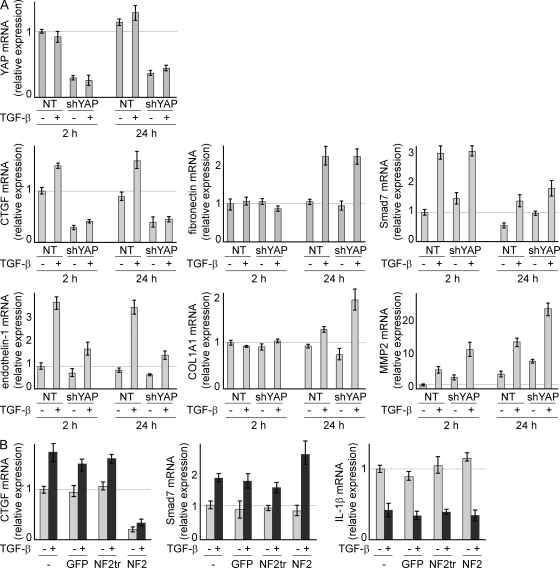
To analyze the possible alteration in TGF-β response in mesothelioma cells caused by defects in the Hippo pathway, we suppressed endogenous YAP expression using short hairpin RNA (shRNA) vectors in NCI-H290 cells, which have a genetic deletion of NF2. YAP messenger RNA (mRNA) expression was successfully down-regulated in shYAP-transfected cells without any alteration after treatment of TGF-β for either 2 or 24 h. YAP expression was not affected by TGF-β treatment in MM cells (Fig. 2 A). Because YAP is a known transcriptional modulator, we investigated the effect of YAP down-regulation on mRNA levels of genes regulated by TGF-β. Interestingly, genes regulated by TGF-β could be categorized into one of three groups. The expression of fibronectin and COL1A1 did not immediately change; however, these genes were later activated by TGF-β, suggesting that they may be the target of a secondary response to TGF-β. Smad7 and MMP2 were up-regulated by TGF-β within 2 h, but YAP depletion did not affect this activation. CTGF and endothelin-1 were activated by TGF-β within 2 h, and this activation was suppressed by knockdown of endogenous YAP. Surprisingly, the number of functionally known genes identified by expression microarray, up-regulated in control versus TGF-β by >1.5-fold and also down-regulated in nontarget shRNA with TGF-β versus shYAP with TGF-β by <0.67-fold, was limited to these two genes in NCI-H290 cells (unpublished data). The aforementioned observations suggest that YAP does not influence the expression of all TGF-β target genes but does affect the transactivation of select genes. To confirm this result, NCI-H290 cells were infected with lentivirus vector carrying GFP, full-length NF2, or NF2 with the truncated FERM (four-point-one/ezrin/radixin/moesin) domain. FERM truncation resulted in loss in the ability to phosphorylate YAP on Ser 127 (Yokoyama et al., 2008). Although neither Smad7 up-regulation nor IL-1β down-regulation was affected, irrespective of TGF-β treatment, CTGF was greatly suppressed by NF2 overexpression (Fig. 2 B).
Figure 2.
The CTGF expression level was modulated by the TGF-β and Hippo pathways in MM cells. (A) NCI-H290 cells, which show homologous deletion of NF2 with concomitant YAP translocation to the nucleus, were transfected with plasmids containing shYAP. After 48 h of puromycin selection, TGF-β was added, followed by mRNA extraction after 2 and 24 h to perform real-time RT-PCR to evaluate gene expression. A plasmid with a nontarget sequence (NT) was used as the control. (B) Lentiviral vectors containing full-length and truncated NF2 that lack the ability to phosphorylate YAP on Ser 127 were used to infect NCI-H290 cells. Real-time RT-PCR was performed using mRNA extracted 2 h after TGF-β treatment. −, untreated or uninfected; NF2tr, truncated NF2. (A and B) Results are expressed as mean ± SEM and are representative of three independent assays.
YAP associates with Smad2/3 and synergistically enhances the transactivation of CTGF
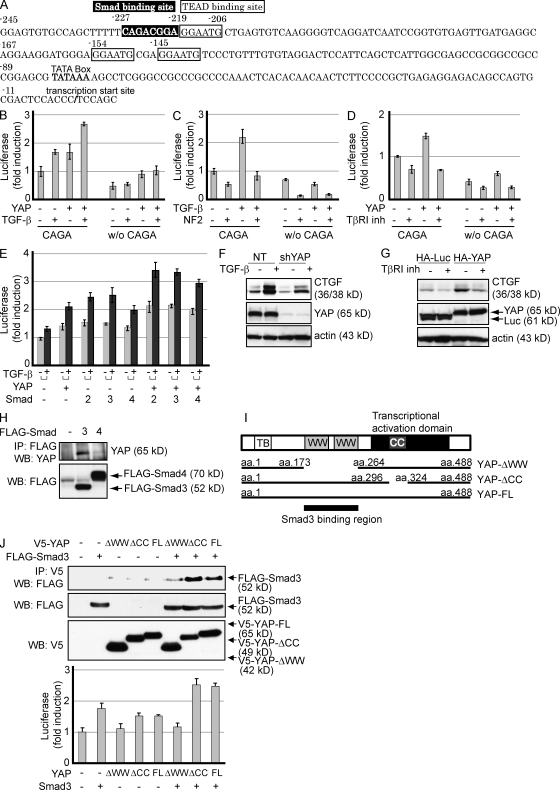
TGF-β is known to positively regulate CTGF expression through Smad activation in NIH 3T3 fibroblasts (Holmes et al., 2001) and induces fibrosis in vivo. YAP binds to TEAD, and it is recruited to the putative TEAD-binding site that resides on the CTGF promoter (Zhao et al., 2008). We found that the CTGF promoter contains both a TEAD-binding site and a consensus Smad-binding site adjacent to each other (Fig. 3 A). Smad3 is a crucial mediator of TGF-β signaling, directly activating genes through Smad3/Smad4 DNA-binding motifs in mouse embryo fibroblasts (Yang et al., 2003; Roberts et al., 2006). To examine whether the TGF-β pathway and YAP may regulate the transcriptional activity of CTGF, we generated CTGF promoters containing or lacking this Smad/TEAD-binding site linked to luciferase. Treatment with TGF-β as well as overexpression of YAP enhanced the transcriptional activity of the CTGF promoter with the Smad-binding site in NCI-H290 cells. Deletion of the Smad/TEAD-binding site weakened basal promoter activities and responses to YAP (Fig. 3 B). The slight induction by TGF-β was possibly through TEAD-binding sites and might be the effects of the complex formation described in Fig. 4. Merlin, a protein encoded by NF2, suppresses TGF-β–induced activation, indicating the involvement of the Hippo signaling pathway in CTGF promoter activation by TGF-β (Fig. 3 C). On the contrary, TGF-β type I receptor kinase inhibitor blocked the transactivation by YAP (Fig. 3 D). Transfection of vectors carrying Smad2, Smad3, and Smad4 together with YAP enhanced the transcriptional activity of the CTGF promoter, which was further activated by TGF-β treatment (Fig. 3 E). The data suggest that Smad3 and YAP can synergize to up-regulate CTGF expression.
Figure 3.
Smad3 interacts with YAP and enhances the transactivation activity of the CTGF promoter. (A) The human CTGF promoter region contains a Smad-binding site (−227 to −220) and a TEAD-binding site (−219 to −214) adjacent to each other. A luciferase reporter plasmid was linked to the CTGF promoter with (CAGA, −245 to 16) or without (w/o CAGA, −206 to 16) the Smad/TEAD-binding sites indicated. (B–D) NCI-H290 cells were transfected with CTGF-luciferase reporter plasmids with or without the Smad/TEAD-binding sites, together with the indicated plasmids. After 24 h, either the TGF-β or TβRI inhibitor was added to the medium and incubated for an additional 24 h. Fold induction of transcriptional response relative to untreated cells is shown. Results are expressed as mean ± SEM and are representative of three independent assays. (E) Effects of TGF-β treatment on the activation of the CTGF promoter by YAP and Smads in NCI-H290 cells. Fold induction of transcriptional response relative to untreated cells is shown. Results are expressed as mean ± SEM and are representative of three independent assays. (F) Y-MESO-27 cells were lentivirally infected with either nontarget (NT) or YAP shRNA (shYAP) vector, followed by treatment with TGF-β type I receptor kinase inhibitor. Protein levels were determined by Western blotting. (G) Y-MESO-27 cells were lentivirally infected to express YAP protein and were treated with TGF-β. A lentivirus vector containing HA-luciferase was used as a control. Protein levels were determined by Western blotting. (H) HEK293 cells were transiently transfected with flag-tagged Smad vectors, and lysates were subjected to immunoprecipitation (IP), followed by Western blotting (WB) with anti-YAP antibody. Expression levels of exogenous Smads were confirmed in the bottom panel. (I) Schematic representation of the deletion constructs of YAP. CC, CC domains; FL, full length; TB, TEAD-binding domain; WW, WW domains. (J) HEK293 cells were transiently transfected with the indicated plasmids and subjected to immunoprecipitation. Western blotting was performed to confirm the expression level of YAP and Smad3 (top). NCI-H290 cells were transfected with the CTGF-luciferase reporter plasmid and combinations of Smad3 and YAP deletion constructs (bottom). Results are mean ± SEM and are representative of three independent assays.
Figure 4.
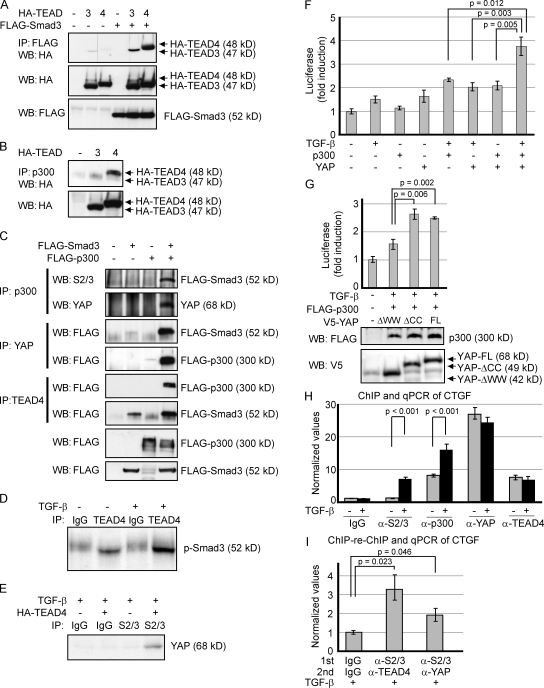
Smad3, YAP, TEAD, and p300 are components of a functional complex on the CTGF promoter. (A and B) HEK293 cells were transiently cotransfected with indicated plasmids, and lysates were subjected to immunoprecipitation (IP) followed by Western blotting (WB). (C) HEK293 cells were transfected with expression vectors as indicated. Cell lysates were divided and subjected to immunoprecipitation using p300, YAP, and TEAD4 antibodies. Samples subjected to Western blot are shown in the lowest panels. (D) Interaction between endogenous TEAD4 and p-Smad3 was examined using TGF-β–treated Y-MESO-27 cells. (E) Y-MESO-27 cells were infected with a TEAD4 lentiviral vector. After 4 d, the cells were treated with TGF-β, and lysates were immunoprecipitated with Smad2/3 antibodies and detected by YAP antibodies. (F) NCI-H290 cells were transfected with the CTGF-luciferase reporter plasmid and combinations of TGF-β, YAP, and p300. Results are expressed as mean ± SEM and are representative of three independent assays. (G) NCI-H290 cells were transfected with the CTGF-luciferase reporter together with the indicated plasmids. Luciferase activity (top) and protein levels (bottom) are shown. Results are expressed as mean ± SEM and are representative of three independent assays. (H) ChIP analysis was performed using MSTO-211H cells by pulling down endogenous Smad2/3, p300, YAP, and TEAD4. CTGF promoter with Smad- and TEAD-binding adjacent regions was amplified by PCR. The value was normalized by input. The results shown are representative of three independent assays. (I) ChIP-reChIP assay was performed using TGF-β–treated MSTO-211H cell lysates, followed by quantitative PCR (qPCR). The first and second primary antibodies used for immunoprecipitation are indicated. (H and I) Results are expressed as mean ± SEM and are representative of three independent assays.
To confirm these results at the protein level, we overexpressed YAP in MM cells and investigated whether CTGF protein expression was modulated by the TGF-β pathway and YAP. Depletion of endogenous YAP suppressed CTGF protein expression in both cell lines, irrespective of TGF-β treatment (Fig. 3 F). Conversely, TGF-β type I receptor kinase inhibitor suppressed YAP-enhanced CTGF expression (Fig. 3 G). These results further support the possible involvement of two distinct pathways in CTGF regulation.
Functional and physical associations between Smads and WW domain–containing proteins such as TAZ and YAP have been demonstrated, and these associations were implicated in the transcription of multiple target genes (Varelas et al., 2008; Alarcón et al., 2009). We found that YAP binds to Smad3 but not to Smad4 (Fig. 3 H). To identify the requisite domain in the Smad3–YAP interaction, we used YAP deletion constructs, which lacked either WW or coiled-coil (CC) domains (Fig. 3 I). Smad3 could be coimmunoprecipitated with YAP-ΔCC, but a prominent decrease in binding was observed with YAP-ΔWW, suggesting that the WW domain is important for YAP binding to Smad3 (Fig. 3 J, top). In agreement with this result, although YAP-ΔCC could enhance the transcriptional activity of the CTGF promoter, YAP-ΔWW failed to augment this activity (Fig. 3 J, bottom). These data suggest the functional interaction through the YAP-WW domain with Smad3 in regulating CTGF expression.
YAP, Smad3, TEAD, and p300 comprise a common complex
Although the aforementioned results suggest physical binding and functional interactions between YAP and Smad3, the affinity of these proteins are not strong compared with the interaction of YAP with Smad1, as previously shown in HEK293T cells (Alarcón et al., 2009). To address this issue, we assessed the possible involvement of components in complex formation to further demonstrate both binding and functional activity.
Transient transfection experiments with HEK293 cells revealed that Smad3, p300, and YAP coprecipitate with both TEAD3 and TEAD4, although binding affinity was much stronger for TEAD4, compared with TEAD3 (Fig. 4, A and B). An immunoprecipitation assay using HEK293T cells with ectopic expression of Smad3 and p300 showed that the binding between ectopic p300 and endogenous YAP was enhanced in the presence of ectopic Smad3 (Fig. 4 C, second panel). The interaction of endogenous YAP with ectopic Smad3 was enhanced in the presence of ectopic p300 (Fig. 4 C, third panel), and on the contrary, the binding of ectopic p300 to endogenous YAP was much stronger in the presence of Smad3 (Fig. 4 C, fourth panel). Furthermore, endogenous TEAD4 bound to ectopic p300 in the presence of ectopic Smad3 and with ectopic Smad3 in the presence of ectopic p300 (Fig. 4 C, fifth and sixth panels). Using Y-MESO-27 cells, an MM cell line in which LATS2 has been deleted (Murakami et al., 2011), we confirmed the endogenous interactions between TEAD4 and phosphorylated Smad3. TGF-β treatment further increased the amount of p-Smad3 that was immunoprecipitated with TEAD4 (Fig. 4 D). Consistent with the aforementioned results, interaction between endogenous Smad2/3 and YAP was observed under the existence of TEAD4 protein in Y-MESO-27 cells (Fig. 4 E). We also examined whether Smad3 and p300 augment YAP–TEAD4 complex formation and found that neither Smad3 nor p300 affects the interaction between YAP and TEAD4 (unpublished data). These data suggest that YAP, Smad3, p300, and TEAD4 mutually assist each other to strengthen the formation of a complex based on the stable binding between YAP and TEAD4 on the CTGF promoter.
Maximum transcriptional activation of CTGF promoter was observed when two proteins were overexpressed under TGF-β treatment (Fig. 4 F). Transfection of TEAD4 instead of YAP did not enhance the transactivation (unpublished data), suggesting that YAP is a prerequisite for YAP–TEAD4–Smad3–p300 complex formation and CTGF activation. We then assessed the significance of the YAP–Smad3 association in this complex. YAP-ΔCC enhanced the transactivation of the CTGF promoter to the same extent of full-length YAP, whereas YAP-ΔWW substantially reduced this activity, indicating that YAP–Smad3 binding plays a functionally important role in this complex (Fig. 4 G). Consistent with the presented results, the chromatin immunoprecipitation (ChIP) assay of CTGF promoter demonstrates that TGF-β stimulates the binding of endogenous Smad2/3 and p300 to the CTGF promoter but not the binding of YAP and TEAD4, which perhaps constitutively reside on the promoter region in MSTO-211H cells (Fig. 4 H). Furthermore, ChIP-reChIP assay using TGF-β–treated MSTO-211H cells showed that YAP, Smad2/3, and TEAD4 reside on the same CTGF promoter site (Fig. 4 I). Collectively, these data demonstrate that YAP–TEAD4–Smad3–p300 complex formation on the CTGF promoter is crucial for CTGF gene expression in MM cells.
CTGF regulates proliferation and ECM production in MM cells in vitro
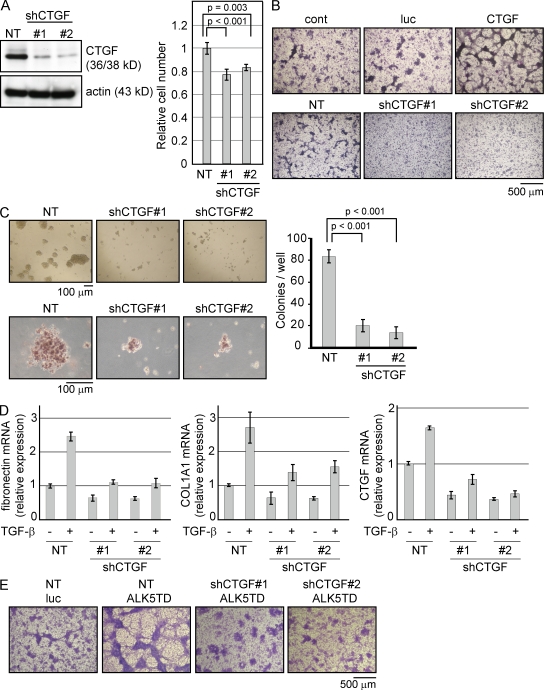
Given the synergistic activation of the CTGF gene by both the Hippo pathway and TGF-β signaling in MM cells, we investigated whether CTGF is essential for the oncogenic properties of these cells. Using an shRNA lentiviral vector system, we examined whether CTGF is required for cell growth in NCI-H290 cells. Knockdown of CTGF suppressed NCI-H290 cell growth to a mean of 77.2% (ShCTGF#1) and 84% (ShCTGF#2), respectively, compared with the growth of the nontarget control (Fig. 5 A). We examined whether the expression of CTGF could modulate foci formation in NCI-H290 cells. Interestingly, CTGF expression showed the same response as the constitutive activation of the TGF-β type I receptor, which showed aggressive formation of foci, whereas the knockdown of CTGF reduced the number of foci (Fig. 5 B). Furthermore, the soft agar colony formation assay showed a reduction in size (Fig. 5 C, left) and the number of colonies in CTGF–knocked down NCI-H290 cells (Fig. 5 C, right). We then assessed whether CTGF expression affects the level of ECM proteins.
Figure 5.
CTGF expression affects the growth and malignancy of MM cells. (A) NCI-H290 cells were lentivirally infected with shRNA against CTGF (shCTGF). Cell proliferation analysis was performed 4 d after lentiviral shRNA transduction. The endogenous protein level of CTGF was confirmed by Western blotting (left). Cell number was counted and normalized to the nontarget (NT) control (right). (B) NCI-H290 cells were infected with the indicated lentivirus expression vectors and stained with Giemsa after 14 d. HA-tagged luciferase (luc) was used as a control for CTGF and nontarget control for shCTGF. The results shown are representative of three independent assays. (C) Soft agar colony formation assay was performed using shRNA lentivirus–transduced NCI-H290 cells and stained with 0.5 mg/ml p-iodonitrotetrazolium after 10 d. The lower panel shows the mean size of colonies in each well. Colony number was counted in a range with a >100-µm diameter. (D) Real-time RT-PCR was performed using NCI-H290 cells infected by shRNA lentivirus 24 h after the treatment of TGF-β. (A, C, and D) Results are expressed as mean ± SEM and are representative of three independent assays. (E) NCI-H290 cells infected with the shCTGF lentivirus were kept under puromycin selection. Cells were then infected with the ALK5 lentivirus and stained using Giemsa after 14 d. The results shown are representative of three independent assays.
There is a study that shows the CTGF protein involvement in attenuation of fibronectin and Collagen 1 production induced by TGF-β in cultured human peritoneal mesothelial cells (Xiao et al., 2010). To confirm whether this CTGF function was also observed in MM cells, we used NCI-H290 cells and found that the CTGF expression affected the mRNA level of fibronectin and COL1A1 in 24 h. As shown in Fig. 2, these two genes were late response genes to TGF-β, showing that CTGF modulates the expression level of ECM proteins as a consequence of a series of signaling (Fig. 5 D). The knockdown of CTGF expression abolished the aggressive formation of foci induced by the constitutively activated TGF-β type I receptor (Fig. 5 E).
CTGF is an important modulator of MM cell growth and deposition of ECM
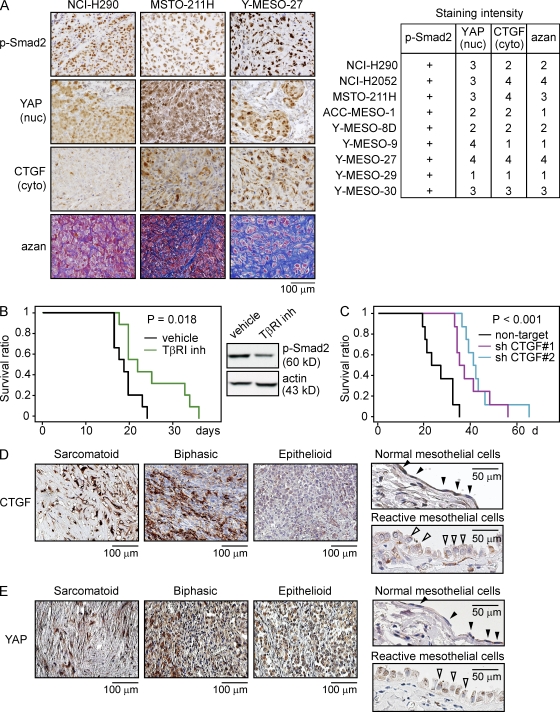
Immunohistochemical analysis using nine human MM cells implanted into the thoracic cavity of nude mice again showed clear nuclear staining of p-Smad2, with little staining in stromal or normal tissues (Fig. 6 A, left). Nuclear staining of YAP was also observed in most tissues, although staining varied widely from weak to strong and showed a moderate correlation with the cytoplasmic staining of CTGF in MM cells (Fig. 6 A, right). Of note, the amount of stroma, which plays an important role in the cancer microenvironment, also appeared to strongly correlate with CTGF expression in MMs, suggesting that CTGF regulates the growth of MMs and also leads to a tumor environment suitable for their growth.
Figure 6.
CTGF expression associates with the deposition of ECM protein in MM tumors. (A) Immunohistochemical staining of MM cells implanted in the thoracic cavities of athymic nude mice using p-Smad2, YAP, and CTGF antibodies. Collagen fibers were visualized in blue by azan staining (left). The right panel shows the scoring of the immunohistochemical staining. Staining intensity was scored as follows: 1, normal; 2, mild; 3, moderate; and 4, severe. (B) The Kaplan-Meier method was used to monitor the survival of athymic nude mice after the thoracic implantation of the NCI-H290 cells, followed by initiation of treatment with the TβRI inhibitor SD-208 (60 mg/kg) 2 d later (left). SD-208 was administered daily by oral gavage for 10 d (n = 9), and then tumor tissues were excised and subjected to Western blotting (right). The results shown are representative of two independent experiments. (C) NCI-H290 cells lentivirally transduced with shRNA constructs were implanted into the thoracic cavities of athymic nude mice, and survival was monitored (n = 8). The results shown are representative of three independent experiments. (D and E) Immunohistochemical staining of CTGF and YAP in MM tissues derived from patients with sarcomatoid as well as biphasic and epithelioid subtypes. Normal mesothelial cells (closed arrowheads) and reactivated normal mesothelial cells (open arrowheads) are shown in the right panels.
Based on the aforementioned findings implicating TGF-β signaling and CTGF in MM cell and tumor growth, we determined whether inhibition of TGF-β type I receptor signaling and CTGF expression induces growth suppression of MM tumors in a mouse model. SD-208 is an orally bioactive TGF-β type I receptor kinase inhibitor previously shown to significantly reduce osteolytic lesions in breast cancer bone metastasis (Dunn et al., 2009). Oral gavage with 60 mg/kg SD-208 decreased the p-Smad2 level in NCI-H290 tissues implanted in the thoracic cavities of nude mice and further prolonged their survival (Fig. 6 B). Knockdown of CTGF expression in NCI-H290 cells also facilitated longer survival of mice (Fig. 6 C). These data demonstrate that the blockade of TGF-β signaling and suppression of CTGF protein expression impair MM tumor growth.
To further examine the contribution of CTGF expression in MM tumor growth, immunohistochemical staining was again performed by staining the 24 tissue specimens used earlier (Fig. 1 A) with YAP and CTGF antibodies. Although p-Smad2 nuclear staining was observed in all MM tumor tissues, strong CTGF expression in the cytoplasm of MM cells was dominantly observed in sarcomatoid tumors but was weak in epithelioid tumors and normal mesothelial cells (Fig. 6 D and Table 2). YAP nuclear staining was also observed in sarcomatoid tumors but was not dominant in epithelioid and normal cells, although there was a strong staining in cytoplasm in epithelioid tumor cells (Fig. 6 E and Table 2). Sporadic nuclear staining of YAP was observed in reactive mesothelioma, but compatible with the previous study which shows the amplification of YAP oncogene (Yokoyama et al., 2008). YAP staining in mesothelioma tissues was much stronger than normal tissues. 7 out of 12 epithelioid tissues were positively stained for YAP, suggesting that some additional factor that is present in sarcomatoid tumors might be required for CTGF expression.
Sarcomatoid mesotheliomas are composed of spindle cells with abundant stroma that resemble the histological appearance of Y-MESO-27 and NCI-H2052 cells implanted in mice (Figs. 1 A and 6 A). In MM tumor patients, histological subtype is one of the most important predictors of survival and in the selection of appropriate treatment. Our findings revealed a strong association between MM cell growth and TGF-β signaling, partially through synergistic enhancement of CTGF expression with YAP. Furthermore, we identified the involvement of CTGF in subsequent induction of stroma in MM tumors and its possible association with malignancy. Based on these findings, TGF-β and CTGF are strong candidates for targeting therapies that may be effective for both MM cells and the surrounding stroma.
DISCUSSION
MM is a cancer that often shows dissemination and progression in the thoracic or peritoneal cavity at diagnosis, and many problems remain unresolved regarding its early diagnosis and effective treatment. MM shows resistance to conventional chemotherapies probably because it does not originate from the epithelial cells and does not have the same characteristics as these cells, which can be treated with drugs. Although patients with MM usually have a poor clinical prognosis, both basic and clinical studies are lacking compared with the studies on other malignancies. Thus, there is an urgent need to develop new therapies. Based on a previous study concerning TGF-β secretion from MMs into pleural fluid and the sensitivity of murine mesothelioma to TGF-β type I receptor kinase inhibitor (Suzuki et al., 2007), we speculated a strong association between MM growth and TGF-β signaling. Moreover, MM is usually accompanied by a thick fibrotic layer that may cause thickening of the pleura and subsequent functional disorder of the lung. Because TGF-β is known to contribute to fibrosis through the Smad3-dependent pathway (Roberts et al., 2006), this fibrotic change in MM tissues could also be primarily induced by TGF-β activation. If TGF-β was continuously produced by MM tumors, TGF-β might further affect the surrounding cancer environment including fibrosis, vascularization, and suppression of immune responses. In our study, TGF-β treatment could successfully activate the Smad2/3 pathway in both normal MeT-5A and malignant MM cells, suggesting that TGF-β signaling is intact during the progression of malignancy.
One of the major categories of the TGF-β–responsive genes commonly up-regulated in both MeT-5A and Y-MESO-27 cells were ECM-related proteins that may provide anchorage for cells, making malignant cancer cells more aggressive (Radisky and Radisky, 2007; Levental et al., 2009). Furthermore, p-Smad2 nuclear staining was observed in all human MM tissue specimens, regardless of histological subtype, and also in reactivated normal mesothelial cells in MM patients, which may be evidence that constitutive activation of TGF-β signaling is a common feature during tumor development. Blockade of the TGF-β pathway in MM cells resulted in growth suppression both in vitro and in vivo. These data strongly support the hypothesis that TGF-β–stimulated growth is an innate property of mesothelial cells, which is conserved during the progression of malignancy.
Because at least 75% of MM tumors have a disturbance in the Hippo signaling pathway, this type of tumor mostly relies on this pathway for oncogenesis, whereas only 20–25% of MM tumors have a p53 mutation, which is the most frequently inactivated tumor suppressor gene in human malignancies (Sekido, 2010). Although many types of cancer cells have been recently reported to exhibit a disturbance in the Hippo signaling pathway, MM tumors have an extremely high frequency of disturbance in this pathway, indicating that the Hippo signaling pathway is the main tumor suppressor in these cells. Therefore, we investigated a possible functional link between the TGF-β and Hippo signaling pathways in mesothelioma genesis. Determining the cross talk between distinct pathways is important when searching for suitable targets of molecular target therapies because a blockade in one pathway might be insufficient to obtain the maximum effect. If there are some strong growth-driving target genes that overlap the two distinct pathways, these genes could play an important role in mesothelioma cells.
A previous study has shown that TAZ/YAP regulates the localization of Smad2/3 in response to cell density during embryogenesis (Varelas et al., 2008). TAZ/YAP dephosphorylation drives the nuclear accumulation of TAZ/YAP and Smad2/3 in the nucleus. Our data show that the two pathways also converge to regulate the transcription of disease-related target genes in MM and further relate to promote malignancy. Expression of CTGF was controlled by the presence of the YAP–TEAD4–Smad3–p300 complex in the nucleus of the MM, which we identify as a novel therapeutic target in MM.
Consistent with previous data showing that inactivation of the NF2 and Hippo pathway is the most frequent defect in mesothelioma, immunohistochemical analysis of MM specimens revealed overexpression and nuclear accumulation of YAP, whereas no signals were observed in normal pleural mesothelial cells (Yokoyama et al., 2008). Nuclear accumulation of YAP is responsible for MM cells undergoing oncogenic events, and normal mesothelial cells might have less YAP-dependent growth because of down-regulation by the intact Hippo pathway.
In looking for synergy between the inactivation of the Hippo pathway and TGF-β signaling, we found that knocking down YAP, which predominantly resides in the nucleus of NCI-H290 cells, affected only a small portion of TGF-β–responsive genes, suggesting that functional association between YAP and Smad2/3 only occurs under a specific situation. CTGF is a gene carrying both a TEAD-binding site and a consensus Smad-binding site adjacent to each other on its promoter. Although the binding between YAP and Smad3 is not overly strong, it results in an obvious synergistic activation in the reporter assay or CTGF protein expression assay. Even in the immunoprecipitation assay using HEK293 cells, the exogenous overexpression of Smad3 showed weak binding between YAP and Smad3, and we could not detect endogenous binding in mesothelioma cells. This suggests that other components may be responsible for strengthening the YAP–Smad3 binding and promoting transactivation. We found that p300 and TEAD4 are components of the Smad complex, and Smad3 preferentially binds to TEAD4 rather than to YAP. This hypothesis enabled us to determine not only the physical interactions but also the functional meanings of Smad3–TEAD4 and Smad3–YAP bindings at the endogenous level.
CTGF is a 36/38-kD cysteine-rich protein whose expression is often observed in stroma, which might reflect an active tumor–stromal interaction (Wahab and Mason, 2006). Enhancement of tumor–stromal interactions can potentially promote cancer cell invasion and metastasis. In addition to recent studies that indicate that tumor cell–derived CTGF plays an important role in the proliferation of breast cancer cells (Zhao et al., 2008) and growth of pancreatic tumors (Bennewith et al., 2009), CTGF also affects vascularization, migration, and epithelial-mesenchymal transition in the context of oncogenic properties (Wahab and Mason, 2006). Our immunohistochemical staining of MM cells implanted in the thoracic cavities of nude mice revealed that p-Smad2 constantly resided in the nucleus, whereas the level of YAP in the nucleus varied among cells. CTGF expression was moderately correlated to YAP localization in the nucleus, consistent with our result that maximum CTGF expression was achieved by activation of TGF-β signaling and inactivation of the NF2 tumor suppressor pathway. The high levels of YAP nuclear staining and low levels of CTGF staining seen in Y-MESO-9 cells and in some epithelioid type human MM tissues were the exception to this relationship, suggesting that there might be additional undiscovered mechanisms participating in the regulation and of CTGF expression.
Importantly, there was a strong association between MM CTGF expression and the amount of stroma surrounding MM cells. As noted in Y-MESO-29 cells, the cells that grew in solid/nodular form with thin connective tissues surrounding the mass of tumor cells tended to express rather low levels of CTGF. In contrast, NCI-H2052 and Y-MESO-27 cells, which have high levels of CTGF staining, showed extensive accumulation of collagen fibers. Approximately 60% of MM tumors show histologically epithelioid subtype, whereas sarcomatoid and biphasic types each account for 20% (Flores et al., 2007). From our findings which showed that sarcomatoid type MM tumors exhibit strong CTGF staining, we speculate that tumor-derived CTGF has a strong correlation with the deposition of surrounding connective tissue in MM tumors, further linking CTGF to MM malignancy. Our data suggest that TGF-β signaling is active in both the normal and transformed mesothelium. However, in the transformed mesothelium, the activation of the Hippo pathway synergizes with the TGF-β pathway signaling to increase CTGF production and thereby amplify the profibrotic and colony-stimulating effects of TGF-β and potentially inducing other protumorigenic effects in the microenvironment.
Determining the proper way to target CTGF expression is critical for clinical applications. To directly target CTGF, one must first decide whether to use antibody or antisense therapies. Our data also suggest that there might be additional undiscovered mechanisms that participate in the regulation of CTGF expression. Furthermore, the mechanism that allows CTGF to exert its effects has not yet been clarified. Further research regarding CTGF expression and its functions might lead to the discovery of new targets that could be used to regulate CTGF expression. The number of available targets could be increased if TGF-β and Hippo signaling pathways were included. There are some studies indicating that systemic administration of TGF-β antagonists can suppress the growth of mesotheliomas primarily through the reactivation of antitumor immune responses (Suzuki et al., 2004, 2007). In this study, we demonstrated that these antagonists have an important mechanistic contribution to the tumor parenchyma as well. The TGF-β type I receptor inhibitor or TGF-β antibody might be suitable for suppressing the activation of the TGF-β pathway and further attenuate CTGF expression in MM. Regarding the Hippo signaling pathway, drugs that inhibit YAP translocation into the nucleus or activate Hippo signaling are expected to be developed. Simultaneous suppression of both the TGF-β and Hippo signaling pathways may considerably reduce CTGF expression. Our findings propose Smad3 and YAP to be the factors influencing expression of CTGF, and we show for the first time that CTGF might be a strong candidate for molecularly targeted therapy, affecting both mesothelioma cell growth and the tumor microenvironment.
MATERIALS AND METHODS
Cell culture and reagents.
HEK293 cells were grown in Dulbecco’s Modified Eagle’s Medium (Invitrogen) supplemented with 10% FBS. MeT-5A, NCI-H2052, and MSTO-211H cells were purchased from the American Type Culture Collection. NCI-H290 cells were gifts from A.F. Gazdar (University of Texas Southwestern Medical Center, Dallas, TX). ACC-MESO-1, Y-MESO-8D, 9, 14, 27, 29, and 30 cells were established in our laboratory, as reported previously (Taniguchi et al., 2007). All MM cell lines were cultured in RPMI 1640 (Invitrogen) supplemented with 10% FBS. Human recombinant TGF-β1 was purchased from R&D Systems. SD-208 (2,4-disubstituted pteridine; an ATP-competitive inhibitor of TGF-β RI kinase) was purchased from Sigma-Aldrich or synthesized by Epichem Pty Ltd. and dissolved in DMSO as 10-µM stocks or at 7.5 mg/ml with 0.5% (wt/vol) methylcellulose.
Soft agar colony formation assay.
The soft agar colony formation assay was performed using standard techniques. MeT-5A cells were trypsinized, and 2 × 104 cells were plated in 0.3% top agarose and cultured for 7 d.
Western blot and immunoprecipitation analysis.
Cells were washed with ice-cold PBS, lysed on ice for 30 min in lysis buffer (10 mM Hepes, 200 mM NaCl, 30 mM sodium pyrophosphate, 50 mM NaF, 5 µM ZnCl2, and 1.0% Triton X-100, pH 7.5) supplemented with protease inhibitor cocktail (Roche), and centrifuged at 12,000 g for 20 min. Supernatants were immunoprecipitated with Immunoprecipitation kit–Dynabeads Protein G (Invitrogen) according to the manufacturer’s instructions, using anti-YAP (63.7; Santa Cruz Biotechnology, Inc.), p300 (C-20; Santa Cruz Biotechnology, Inc.), TEAD4 (aa 151–260; Abnova), Smad2/3 (BD and Cell Signaling Technology), HA (Y-11; Santa Cruz Biotechnology, Inc.), or FLAG (M2; Sigma-Aldrich) antibody. Immunoprecipitated proteins were resolved by 10% Tris-glycine SDS-PAGE (Invitrogen), transferred to Immobilon-P membranes (Millipore), and detected with the appropriate primary antibody. Western blots were prepared by standard procedures using anti–p-Smad2/3 (Cell Signaling Technology), YAP1 (EP1674Y; Abcam), CTGF (L-20; Santa Cruz Biotechnology, Inc.), actin monoclonal (Millipore), and other antibodies described above. Immunoreactivity was detected by ECL (GE Healthcare).
Transcriptional reporter assays.
Luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega) in which Renilla luciferase plasmids were cotransfected as a control to standardize the transfection efficiency. All activity results are normalized to Renilla expression and are representative of three independent assays.
RNA interference vectors in human MM cells.
To generate lentivirus that transcribes shRNA, short hairpin oligonucleotides were inserted into pLentiLox 3.7 containing the U6 promoter and PLKO.1 (Sigma-Aldrich) and transfected into HEK293FT cells together with VSVG, RSV-REV, and pMDLg/pRRE. Short hairpin oligonucleotides for YAP (Yokoyama et al., 2008) and CTGF (Zhao et al., 2008) were designed as described previously. A plasmid vector containing the U6 promoter and puromycin-resistance gene was used for transient expression of shYAP (Yokoyama et al., 2008).
Expression profiling with microarrays.
Extracted mRNA was subjected to generate cRNA, which was labeled with Cy3 or Cy5 (GE Healthcare) using a low RNA Fluorescent Linear Amplification kit (Agilent Technologies) according to the manufacturer’s protocol. Labeled cRNA was then hybridized to an Agilent 44K Whole Human Genome Microarray, followed by confocal laser scanning (Agilent Technologies). The microarray data have been deposited in ArrayExpress under accession no. E-TABM-1144.
Real-time RT-PCR.
Quantitative real-time RT-PCR was performed using first-strand complementary DNA with the TaqMan Universal PCR Master Mix (Applied Biosystems), and amplification was performed with an ABI 7500 Real-time PCR System (Applied Biosystems) according to the manufacturer’s instructions. Quantification of GAPDH transcripts as an internal control for the amount and quality of cDNA was performed for all samples.
ChIP.
The ChIP assay was performed using the ChIP-IT Express Magnetic Chromatin Immunoprecipitation kit (Active Motif) according to the manufacturer’s instructions, followed by real-time RT-PCR with the forward primer 5′-ATATGAATCAGGAGTGGTGCGA-3′ and reverse primer 5′-CAACTCACACCGGATTGATCC-3′. The antibodies used were anti-YAP (H-125; Santa Cruz Biotechnology, Inc.), p300 (C-20; Santa Cruz Biotechnology, Inc.), and Smad2/3 (BD). Re-ChIP-IT Magnetic Chromatin Re-Immunoprecipitation kit (Active Motif) was used for the ChIP-reChIP experiment.
Animal experiments.
7–8-wk-old female athymic nude mice of KSN strain (Shizuoka Laboratory Animal Center) were weighed and randomly assigned to different treatment groups. Lentivirally infected or uninfected NCI-H290 cells were then orthotopically injected into the right thoracic cavity of each mouse. To examine the effect of TGF-β type I receptor kinase inhibition, mice (n = 8) were daily treated with single 0.2-ml doses of 0.5% (wt/vol) methylcellulose as a vehicle or 60 mg/kg SD-208 by oral gavage as described previously (Uhl et al., 2004) for 10 d, starting 2 d after tumor cell inoculation. The experimental design was approved by the Animal Care Committee of the Aichi Cancer Center Research Institute, and the animals were cared for in accordance with institutional guidelines as well as the Guidelines for Proper Conduct of Animal Experiments (Science Council of Japan, June 1, 2006).
Immunohistochemistry.
The tumor-bearing mice were sacrificed under deep anesthesia and excised the intrathoracic tumor tissue, which was fixed in 10% neutral-buffered formalin and processed for histopathological (hematoxylin and eosin and azan staining) and immunohistochemical examination using an indirect immunoperoxidase method. The antibodies used for immunohistochemical staining were anti–p-Smad2, p-specific (Ser 463/467; Millipore), YAP (H-125; Santa Cruz Biotechnology, Inc.), and CTGF (L-20; Santa Cruz Biotechnology, Inc.).
Statistical analysis.
Survival period was analyzed by the Kaplan-Meier method and compared using the log-rank test. All reported p-values were two-sided, with P < 0.05 considered statistically significant. Calculations were performed with StatView software version 5.0 (Abacus Concepts).
Online supplemental material.
Fig. S1 shows that TGF-β affects the signaling of MM cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20111653/DC1.
Acknowledgments
We thank L.M. Wakefield and A. Hata for comments on the manuscript and K.C. Flanders, K. Kawaguchi, T. Mizuno, F. Ishiguro, K. Shinjo, and T. Matsuki for useful discussions. We thank M. Kizuki, N. Saito, and M. Tsuji for excellent technical assistance.
This work was supported by funds from the 24th General Assembly of the Japanese Association of Medical Sciences (to M. Fujii), funds from the Aichi Cancer Research Foundation (to M. Fujii), and Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (20590420 and 23592788 to M. Fujii) and partly by a Special Coordination Fund for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology of Japan (H18-1-3-3-1 to Y. Sekido), Grants-in-Aid for Scientific Research (22300338 to Y. Sekido), Grants-in-aid for Third-Term Comprehensive Control Research for Cancer from the Ministry of Health, Labor and Welfare of Japan (to Y. Sekido), the Takeda Science Foundation (Y. Sekido), and the Kobayashi Foundation for Cancer Research (Y. Sekido).
The authors have no additional financial interests.
Footnotes
Abbreviation used:
- CC
- coiled-coil
- ChIP
- chromatin immunoprecipitation
- CTGF
- connective tissue growth factor
- ECM
- extracellular matrix
- MM
- malignant mesothelioma
- mRNA
- messenger RNA
- shRNA
- short hairpin RNA
- TEAD
- TEA domain family member
- YAP
- Yes-associated protein
References
- Alarcón C., Zaromytidou A.I., Xi Q., Gao S., Yu J., Fujisawa S., Barlas A., Miller A.N., Manova-Todorova K., Macias M.J., et al. 2009. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell. 139:757–769 10.1016/j.cell.2009.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzano M.A., Roberts A.B., Smith J.M., Sporn M.B., De Larco J.E. 1983. Sarcoma growth factor from conditioned medium of virally transformed cells is composed of both type α and type β transforming growth factors. Proc. Natl. Acad. Sci. USA. 80:6264–6268 10.1073/pnas.80.20.6264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennewith K.L., Huang X., Ham C.M., Graves E.E., Erler J.T., Kambham N., Feazell J., Yang G.P., Koong A., Giaccia A.J. 2009. The role of tumor cell-derived connective tissue growth factor (CTGF/CCN2) in pancreatic tumor growth. Cancer Res. 69:775–784 10.1158/0008-5472.CAN-08-0987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A.B., Mitsunaga S.I., Cheng J.Q., Klein W.M., Jhanwar S.C., Seizinger B., Kley N., Klein-Szanto A.J., Testa J.R. 1995. High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc. Natl. Acad. Sci. USA. 92:10854–10858 10.1073/pnas.92.24.10854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Larco J.E., Todaro G.J. 1978. Growth factors from murine sarcoma virus-transformed cells. Proc. Natl. Acad. Sci. USA. 75:4001–4005 10.1073/pnas.75.8.4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Feldmann G., Huang J., Wu S., Zhang N., Comerford S.A., Gayyed M.F., Anders R.A., Maitra A., Pan D. 2007. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 130:1120–1133 10.1016/j.cell.2007.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L.K., Mohammad K.S., Fournier P.G., McKenna C.R., Davis H.W., Niewolna M., Peng X.H., Chirgwin J.M., Guise T.A. 2009. Hypoxia and TGF-β drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS ONE. 4:e6896 10.1371/journal.pone.0006896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores R.M., Zakowski M., Venkatraman E., Krug L., Rosenzweig K., Dycoco J., Lee C., Yeoh C., Bains M., Rusch V. 2007. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J. Thorac. Oncol. 2:957–965 10.1097/JTO.0b013e31815608d9 [DOI] [PubMed] [Google Scholar]
- Fujii M., Lyakh L.A., Bracken C.P., Fukuoka J., Hayakawa M., Tsukiyama T., Soll S.J., Harris M., Rocha S., Roche K.C., et al. 2006. SNIP1 is a candidate modifier of the transcriptional activity of c-Myc on E box-dependent target genes. Mol. Cell. 24:771–783 10.1016/j.molcel.2006.11.006 [DOI] [PubMed] [Google Scholar]
- Gabrielson E.W., Gerwin B.I., Harris C.C., Roberts A.B., Sporn M.B., Lechner J.F. 1988. Stimulation of DNA synthesis in cultured primary human mesothelial cells by specific growth factors. FASEB J. 2:2717–2721 [DOI] [PubMed] [Google Scholar]
- Gerwin B.I., Lechner J.F., Reddel R.R., Roberts A.B., Robbins K.C., Gabrielson E.W., Harris C.C. 1987. Comparison of production of transforming growth factor-β and platelet-derived growth factor by normal human mesothelial cells and mesothelioma cell lines. Cancer Res. 47:6180–6184 [PubMed] [Google Scholar]
- Hamaratoglu F., Willecke M., Kango-Singh M., Nolo R., Hyun E., Tao C., Jafar-Nejad H., Halder G. 2006. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 8:27–36 10.1038/ncb1339 [DOI] [PubMed] [Google Scholar]
- Hay B.A., Guo M. 2003. Coupling cell growth, proliferation, and death. Hippo weighs in. Dev. Cell. 5:361–363 10.1016/S1534-5807(03)00270-3 [DOI] [PubMed] [Google Scholar]
- Holmes A., Abraham D.J., Sa S., Shiwen X., Black C.M., Leask A. 2001. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J. Biol. Chem. 276:10594–10601 10.1074/jbc.M010149200 [DOI] [PubMed] [Google Scholar]
- Ke Y., Reddel R.R., Gerwin B.I., Reddel H.K., Somers A.N., McMenamin M.G., LaVeck M.A., Stahel R.A., Lechner J.F., Harris C.C. 1989. Establishment of a human in vitro mesothelial cell model system for investigating mechanisms of asbestos-induced mesothelioma. Am. J. Pathol. 134:979–991 [PMC free article] [PubMed] [Google Scholar]
- Levental K.R., Yu H., Kass L., Lakins J.N., Egeblad M., Erler J.T., Fong S.F., Csiszar K., Giaccia A., Weninger W., et al. 2009. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 139:891–906 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. 2008. TGFbeta in cancer. Cell. 134:215–230 10.1016/j.cell.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Seoane J., Wotton D. 2005. Smad transcription factors. Genes Dev. 19:2783–2810 10.1101/gad.1350705 [DOI] [PubMed] [Google Scholar]
- Moses H.L., Branum E.L., Proper J.A., Robinson R.A. 1981. Transforming growth factor production by chemically transformed cells. Cancer Res. 41:2842–2848 [PubMed] [Google Scholar]
- Murakami H., Mizuno T., Taniguchi T., Fujii M., Ishiguro F., Fukui T., Akatsuka S., Horio Y., Hida T., Kondo Y., et al. 2011. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 71:873–883 10.1158/0008-5472.CAN-10-2164 [DOI] [PubMed] [Google Scholar]
- Murayama T., Takahashi K., Natori Y., Kurumatani N. 2006. Estimation of future mortality from pleural malignant mesothelioma in Japan based on an age-cohort model. Am. J. Ind. Med. 49:1–7 10.1002/ajim.20246 [DOI] [PubMed] [Google Scholar]
- Nishihara A., Hanai J.I., Okamoto N., Yanagisawa J., Kato S., Miyazono K., Kawabata M. 1998. Role of p300, a transcriptional coactivator, in signalling of TGF-β. Genes Cells. 3:613–623 10.1046/j.1365-2443.1998.00217.x [DOI] [PubMed] [Google Scholar]
- Radisky E.S., Radisky D.C. 2007. Stromal induction of breast cancer: inflammation and invasion. Rev. Endocr. Metab. Disord. 8:279–287 10.1007/s11154-007-9037-1 [DOI] [PubMed] [Google Scholar]
- Roberts A.B., Wakefield L.M. 2003. The two faces of transforming growth factor β in carcinogenesis. Proc. Natl. Acad. Sci. USA. 100:8621–8623 10.1073/pnas.1633291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.B., Tian F., Byfield S.D., Stuelten C., Ooshima A., Saika S., Flanders K.C. 2006. Smad3 is key to TGF-β-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev. 17:19–27 10.1016/j.cytogfr.2005.09.008 [DOI] [PubMed] [Google Scholar]
- Robinson B.W., Lake R.A. 2005. Advances in malignant mesothelioma. N. Engl. J. Med. 353:1591–1603 10.1056/NEJMra050152 [DOI] [PubMed] [Google Scholar]
- Ryoo H.D., Steller H. 2003. Hippo and its mission for growth control. Nat. Cell Biol. 5:853–855 10.1038/ncb1003-853 [DOI] [PubMed] [Google Scholar]
- Sekido Y. 2010. Genomic abnormalities and signal transduction dysregulation in malignant mesothelioma cells. Cancer Sci. 101:1–6 10.1111/j.1349-7006.2009.01336.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekido Y., Pass H.I., Bader S., Mew D.J., Christman M.F., Gazdar A.F., Minna J.D. 1995. Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res. 55:1227–1231 [PubMed] [Google Scholar]
- Suzuki E., Kapoor V., Cheung H.K., Ling L.E., DeLong P.A., Kaiser L.R., Albelda S.M. 2004. Soluble type II transforming growth factor-β receptor inhibits established murine malignant mesothelioma tumor growth by augmenting host antitumor immunity. Clin. Cancer Res. 10:5907–5918 10.1158/1078-0432.CCR-03-0611 [DOI] [PubMed] [Google Scholar]
- Suzuki E., Kim S., Cheung H.K., Corbley M.J., Zhang X., Sun L., Shan F., Singh J., Lee W.C., Albelda S.M., Ling L.E. 2007. A novel small-molecule inhibitor of transforming growth factor β type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res. 67:2351–2359 10.1158/0008-5472.CAN-06-2389 [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Karnan S., Fukui T., Yokoyama T., Tagawa H., Yokoi K., Ueda Y., Mitsudomi T., Horio Y., Hida T., et al. 2007. Genomic profiling of malignant pleural mesothelioma with array-based comparative genomic hybridization shows frequent non-random chromosomal alteration regions including JUN amplification on 1p32. Cancer Sci. 98:438–446 10.1111/j.1349-7006.2006.00386.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao A.S., Wistuba I., Roth J.A., Kindler H.L. 2009. Malignant pleural mesothelioma. J. Clin. Oncol. 27:2081–2090 10.1200/JCO.2008.19.8523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl M., Aulwurm S., Wischhusen J., Weiler M., Ma J.Y., Almirez R., Mangadu R., Liu Y.W., Platten M., Herrlinger U., et al. 2004. SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 64:7954–7961 10.1158/0008-5472.CAN-04-1013 [DOI] [PubMed] [Google Scholar]
- Varelas X., Sakuma R., Samavarchi-Tehrani P., Peerani R., Rao B.M., Dembowy J., Yaffe M.B., Zandstra P.W., Wrana J.L. 2008. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat. Cell Biol. 10:837–848 10.1038/ncb1748 [DOI] [PubMed] [Google Scholar]
- Varelas X., Samavarchi-Tehrani P., Narimatsu M., Weiss A., Cockburn K., Larsen B.G., Rossant J., Wrana J.L. 2010. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev. Cell. 19:831–844 10.1016/j.devcel.2010.11.012 [DOI] [PubMed] [Google Scholar]
- Vassilev A., Kaneko K.J., Shu H., Zhao Y., DePamphilis M.L. 2001. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 15:1229–1241 10.1101/gad.888601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab N.A., Mason R.M. 2006. A critical look at growth factors and epithelial-to-mesenchymal transition in the adult kidney. Interrelationships between growth factors that regulate EMT in the adult kidney. Nephron, Exp. Nephrol. 104:e129–e134 10.1159/000094963 [DOI] [PubMed] [Google Scholar]
- Wang K., Degerny C., Xu M., Yang X.J. 2009. YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem. Cell Biol. 87:77–91 10.1139/O08-114 [DOI] [PubMed] [Google Scholar]
- Wu S., Huang J., Dong J., Pan D. 2003. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 114:445–456 10.1016/S0092-8674(03)00549-X [DOI] [PubMed] [Google Scholar]
- Xiao L., Sun L., Liu F.Y., Peng Y.M., Duan S.B. 2010. Connective tissue growth factor knockdown attenuated matrix protein production and vascular endothelial growth factor expression induced by transforming growth factor-β1 in cultured human peritoneal mesothelial cells. Ther. Apher. Dial. 14:27–34 10.1111/j.1744-9987.2009.00701.x [DOI] [PubMed] [Google Scholar]
- Yang Y.C., Piek E., Zavadil J., Liang D., Xie D., Heyer J., Pavlidis P., Kucherlapati R., Roberts A.B., Böttinger E.P. 2003. Hierarchical model of gene regulation by transforming growth factor β. Proc. Natl. Acad. Sci. USA. 100:10269–10274 10.1073/pnas.1834070100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T., Osada H., Murakami H., Tatematsu Y., Taniguchi T., Kondo Y., Yatabe Y., Hasegawa Y., Shimokata K., Horio Y., et al. 2008. YAP1 is involved in mesothelioma development and negatively regulated by Merlin through phosphorylation. Carcinogenesis. 29:2139–2146 10.1093/carcin/bgn200 [DOI] [PubMed] [Google Scholar]
- Zhang N., Bai H., David K.K., Dong J., Zheng Y., Cai J., Giovannini M., Liu P., Anders R.A., Pan D. 2010. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell. 19:27–38 10.1016/j.devcel.2010.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Ye X., Yu J., Li L., Li W., Li S., Yu J., Lin J.D., Wang C.Y., Chinnaiyan A.M., et al. 2008. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22:1962–1971 10.1101/gad.1664408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Kim J., Ye X., Lai Z.C., Guan K.L. 2009. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 69:1089–1098 10.1158/0008-5472.CAN-08-2997 [DOI] [PubMed] [Google Scholar]