Abstract
Surveys were carried out to better understand the tick vector ecology and genetic diversity of Huaiyangshan virus (HYSV) in both regions of endemicity and regions of nonendemicity. Haemaphysalis longicornis ticks were dominant in regions of endemicity, while Rhipicephalus microplus is more abundant in regions of nonendemicity. HYSV RNA was found in human and both tick species, with greater prevalence in H. longicornis and lesser prevalence in R. microplus. Phylogenetic analyses indicate that HYSV is a novel species of the genus Phlebovirus.
TEXT
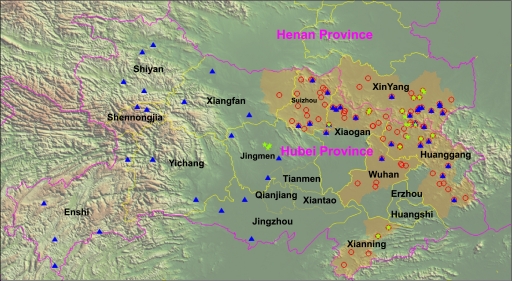
Recently, a hemorrhagic fever-like disease caused by a novel bunyavirus occurred in China (14, 16). Yu et al. reported the disease as severe fever with thrombocytopenia syndrome (SFTS) (14). As thrombocytopenia is not specific for this disease and is present in nearly all hemorrhagic fevers caused by viruses (11) or Rickettsia (15), we previously proposed naming the syndrome Huaiyangshan hemorrhagic fever (HYSHF) and the virus Huaiyangshan virus (HYSV) (16). Haemaphysalis longicornis ticks might be the vector of HYSV (14, 16). However, less is known about the arthropod vector ecology, the genetic diversity, and the phylogeny of HYSV. Thus, we performed an investigation in regions of endemicity and nonendemicity in Henan and Hubei provinces (Fig. 1).
Fig 1.
Map showing the locations of tick collection sites in regions of endemicity (orange) and regions of nonendemicity of hemorrhagic fever caused by Huaiyangshan virus (HYSV) in Henan and Hubei provinces. The red circles represent the cases diagnosed in 2009 and 2010. The blue triangles represent the sites in which ticks were collected, and the green five-pointed stars represent the sites in which HYSV RNA-positive ticks were collected.
A total of 17,731 adult ticks were collected (Table 1). After morphological examination and sequence analysis of mitochondrial 12S ribosomal DNA (rDNA) as described previously (2, 16), only H. longicornis and Rhipicephalus microplus were found. In the regions of endemicity, 4,501 ticks (3,498 H. longicornis and 1,003 R. microplus) were collected from 15 counties of Henan and Hubei. In the regions of nonendemicity, 13,230 ticks (400 H. longicornis and 12,830 R. microplus) were collected from 23 counties of Hubei. These data suggested that H. longicornis and R. microplus were the dominant species in regions of endemicity and regions of nonendemicity, respectively.
Table 1.
Species and numbers of ticks and Huaiyangshan virus-positive pools
| Species | Origin | No. of PCR-positive ticks (pool)/total no. of ticks collected |
|||
|---|---|---|---|---|---|
| Hubei province |
Henan province (region of endemicity) | Subtotal | |||
| Region of endemicity | Region of nonendemicity | ||||
| H. longicornis | Cattle, buffalo | 6/898 | 0/400 | 6/873 | 12/2,171 |
| Goat | 2/354 | 0/0 | 0/83 | 2/437 | |
| Dog | 1/85 | 0/0 | 1/24 | 2/109 | |
| Cat | 0/37 | 0/0 | 1/53 | 1/90 | |
| Grassland | 0/0 | 0/0 | 0/11 | 0/11 | |
| Tea garden | 1/1,058 | 0/0 | 0/22 | 1/1,080 | |
| Subtotal | 10/2,432 | 0/400 | 8/1,066 | 18/3898 | |
| R. microplus | Cattle, buffalo | 1/417 | 3/12,829 | 1/554 | 5/13,800 |
| Goat | 0/24 | 0/1 | 0/0 | 0/25 | |
| Dog | 0/6 | 0/0 | 0/0 | 0/6 | |
| Cat | 0/0 | 0/0 | 0/0 | 0/0 | |
| Grassland | 0/0 | 0/0 | 0/0 | 0/0 | |
| Tea garden | 0/2 | 0/0 | 0/0 | 0/2 | |
| Subtotal | 1/449 | 3/12,830 | 1/554 | 5/13,833 | |
| Total | 11/2,881 | 3/13,230 | 9/1,620 | 23/17,731 | |
All ticks were grouped into 1,180 pools (450 pools from a region of endemicity and 730 pools from a region of nonendemicity) according to species, host, and geographic origin. H. longicornis and R. microplus represented 365 (30.93%) and 815 (69.07%) pools, respectively. For screening HYSV and sequencing the partial S segment (nucleotides [nt] 63 to 663) or L segment (nt 2208 to 3121) and whole-genome sequences of HYSV, total RNA was extracted from ticks and human sera and was then subjected to reverse transcription-PCR (RT-PCR) as described previously (16). As a result, HYSV RNA was identified in 18 (4.93%) H. longicornis pools and in 5 (0.613%) R. microplus pools, suggesting that both species can carry HYSV. Remarkably, the HYSV RNA-positive H. longicornis ticks were found only in the regions of endemicity, whereas HYSV RNA was identified in R. microplus ticks from both the regions of endemicity (2 pools) and neighboring regions of nonendemicity (3 pools) (Fig. 1). Obviously, the prevalence of HYSV was higher in H. longicornis ticks than in R. microplus ticks and higher in the regions of endemicity than in the regions of nonendemicity. Thus, these observations suggest that H. longicornis may be the major vector of HYSV and that its geographic distribution and density may play a central role in the risk of HYSV infection in humans. As H. longicornis and R. microplus ticks exhibit a high level of genetic identity (see Fig. S1 in the supplemental material) and are widely distributed in China and even outside China, this report also reinforces the need for surveillance of local tick colonies for the presence of HYSV or HYSV-like viruses in those regions.
To investigate genetic and phylogenetic relationships between viruses from ticks and humans, 11 partial L and 17 partial S segment sequences were successfully amplified from ticks, and 14 partial L and 16 partial S segment sequences were also obtained from the serum samples collected from 16 patients infected with HYSV admitted into Union Hospital and Zhongnan Hospital in Wuhan city from July to October 2010. Genetic analyses revealed that the sequences obtained from the ticks are closely related to each other, with 98.3% to 100% nucleotide identity for the partial L segments and 96.8% to 99.8% nucleotide identity for the partial S segments. Furthermore, the sequences amplified from ticks showed a high level of identity with those obtained from humans in this study and previously (95.9% to 100% for the partial L segment sequences and 93.5% to 100% for the partial S segment sequences).
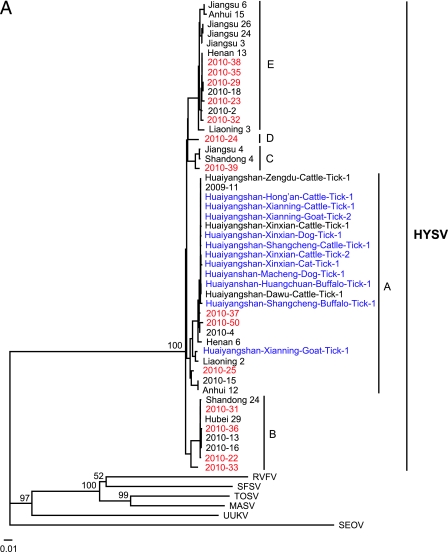
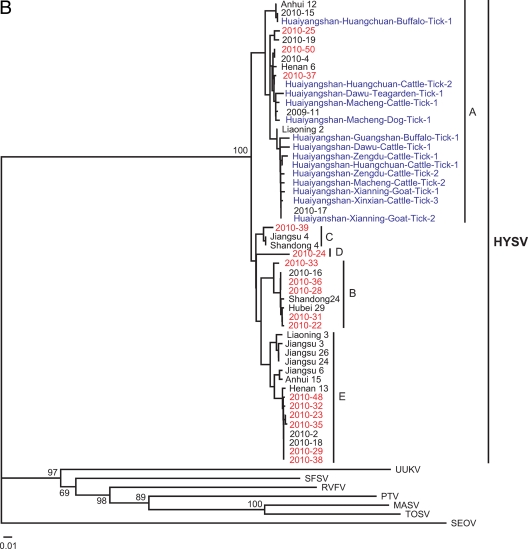
Neighbor-joining (NJ) trees were constructed based on the partial L or S segment sequences obtained in this study as well as all available sequences retrieved from GenBank. As shown in Fig. 2, all reported strains were closely related to each other, regardless of host species or geographic location of origin. Although all available HYSV strains could be grouped into five sublineages on L trees (Fig. 2A) or S trees (Fig. 2B), these sublineages were not supported well (<50% bootstrap values). Every sublineage except sublineage D included strains identified from ≥3 provinces that are approximately 1,300 to 3,200 km from the Huaiyangshan mountainous region. These data suggest that HYSV species do not exhibit a geographic clustering pattern such as is seen with zoonotic viruses such as hantavirus and rabies virus (4, 18).
Fig 2.
Phylogenetic relationship of Huaiyangshan viruses (HYSV) from humans and ticks. The trees were constructed based on the partial L segment sequences (A) and the partial S segment sequences (B) by using the neighbor-joining (NJ) method. Seoul virus (SEOV) was used as the outgroup. Numbers (>50%) at the branch nodes indicate bootstrap values obtained by NJ analysis. Red and blue colors highlight HYSV strains identified from humans and ticks obtained in this study, respectively. The GenBank accession numbers of the L and S segment sequences of other viruses are listed in parentheses as follows: Massilia virus (MASV; EU725771 and EU725773, respectively), Toscana virus (TOSV; NC_006319 and NC_006318), Rift Valley fever virus (RVFV; DQ375406 and DQ380149), sandfly fever Sicilian virus (SFSV; GQ847513 and GQ847511), and Uukuniemi virus (UUKV; D10759 and M33551). HYSV (or SFTSV) strains isolated by Yu et al. (14) include Shandong 4 (HM802202 and HM802204), Shandong 24 (HM802205 and HM802200), Liaoning 2 (HQ141607 and HQ141609), Liaoning 3 (HQ141610 and HQ141612), Henan 6 (HQ141595 and HQ141597), Henan 13 (HQ141598 and HQ141600), Anhui 12 (HQ116417 and HQ141591), Anhui 15 (HQ141592 and HQ141594), Hubei 29 (HM745930 and HM745932), Jiangsu 3 (HQ141601 and HQ141603), Jiangsu 4 (HQ141604 and HQ141606), Jiangsu 6 (HQ830169 and HQ830171), Jiangsu 24 (HQ830163 and HQ830165), and Jiangsu 26 (HQ830166 and HQ830168). HYSV strains recovered from patients in our previous study are 2009-11 (HQ171186 and HQ171191), 2010-2 (HQ171190 and HQ171195), 2010-4 (HQ171187 and HQ171192), 2010-13 (HQ419226; no S sequence was used), 2010-15 (HQ171189 and HQ171194), 2010-16 (HQ171188 and HQ171193), 2010-17 (HQ179737; no L sequence was used), 2010-18 (HQ179708 and HQ179940), and 2010-19 (HQ179744; no L sequence was used). The GenBank accession numbers of the L segment sequences of HYSV from ticks from our previous study are HQ419223 to HQ419225. These sequences are described in more detail in Table S1 in the supplemental material.
The whole-genome sequences were also successfully obtained from ticks and human sera. Like those of other bunyaviruses (3, 12), the L segment encodes RNA-dependent RNA polymerase (see Fig. S2A in the supplemental material) and the M segment has an open reading frame (ORF) coding for a GnGc precursor in the order Gn-Gc. In similarity to Uukuniemi virus but not other species of the Phlebovirus genus, HYSV does not encode NSm (see Fig. S2B in the supplemental material). The S segment uses ambisense coding to express two proteins; one is a nucleocapsid (N) protein encoded by the 5′ half of viral complementary sense S RNA, and the other is a nonstructural (NS) protein encoded by viral sense S RNA (see Fig. S2C in the supplemental material). Although HYSV has the closest evolutionary relationship with viruses of the genus Phlebovirus, they appear genetically quite distinct (>71.43% amino acid differences) (16). In addition, both the L and M segments of HYSV have one nucleotide difference in the conserved 3′ and 5′ termini, which is the typical genetic feature of the family Bunyaviridae (12). Thus, our data suggest that HYSV shares a common ancestor with Phleboviruses but represents a novel species of the genus Phlebovirus.
Ecosystem changes can promote the emergence of novel diseases by increasing exposure of people to the natural reservoir or vectors (1, 5, 7, 8–10). As a consequence of extensive changes in the socioeconomy and in agriculture production, the populations and ranges of ticks expanded following the increases in the numbers of domestic and wild animals over the last decades (13, 17). For example, more than 1,000 ticks could be collected in a tea garden of approximately 40 square meters, in which one patient with confirmed HYSHF had picked tea leaves (Table 1). The seasonal peak of the illnesses correlated well with the high densities of ticks during the spring and summer (6). Thus, ecological changes may have caused the emergence of the novel disease by placing people in increasing contact with ticks and potential animal reservoirs.
Nucleotide sequence accession numbers.
The GenBank accession numbers of the L segment sequences of HYSV obtained from ticks in this study are JF906030 to JF906039 and JF906056; those of the S segment sequences from ticks are JF906040 to JF906055 and JF906058. The GenBank accession numbers of the L segment sequences of HYSV obtained from humans are HQ179713 to HQ179715, HQ179717 to HQ179723, and HQ179725 to HQ179728; those of the S segment sequences from humans are HQ179745 to 179747, HQ179750 to HQ179757, and HQ179759 to HQ179763.
Supplementary Material
ACKNOWLEDGMENTS
We sincerely thank Alexander Plyusnin of Department of Virology, Infection Biology Research Program, Haartman Institute, University of Helsinki, for the help with this study.
This study was supported by a grant (2008ZX10004-001) from major project for infectious diseases from State Council of China, by a grant (2011SKLID101) from State Key Laboratory for Infectious Diseases Prevention and Control, and by a grant from National Institute of Communicable Diseases Control and Prevention.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 21 December 2011
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Barbour AG, Fish D. 1993. The biological and social phenomenon of Lyme disease. Science 260:1610–1616 [DOI] [PubMed] [Google Scholar]
- 2. Beati L, Keirans JE. 2001. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J. Parasitol. 87:32–48 [DOI] [PubMed] [Google Scholar]
- 3. Bouloy M. 2011. Molecular biology of phleboviruses, p 103–104 In Plyusnin A, Elliott RM. (ed), Bunyaviridae: molecular and cellulary biology. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 4. Bourhy H, et al. 1999. Ecology and evolution of rabies virus in Europe. J. Gen. Virol. 80:2545–2557 [DOI] [PubMed] [Google Scholar]
- 5. Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287:443–449 [DOI] [PubMed] [Google Scholar]
- 6. Deng GF. 1990. Ticks, p 397–422 In Liu ZY, Lu ML. (ed), Medical entomology. Science Press, Beijing, China: (In Chinese) [Google Scholar]
- 7. Jonsson CB, Figueiredo LT, Vapalahti O. 2010. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 23:412–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morens DM, Folkers GK, Fauci AS. 2004. The challenge of emerging and re-emerging infectious diseases. Nature 430:242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morse SS. 1995. Factors in the emergence of infectious diseases. Emerg. Infect. Dis. 1:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neumann G, Noda T, Kawaoka Y. 2009. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nichol ST. 2001. Bunyaviridae, p 1603–1633 In Knipe DM, Howley P. (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 12. Schmaljohn CS, Hooper JW. 2001. Bunyaviridae: the viruses and their replication, p 1581–1602 In Knipe DM, Howley P. (ed), Fields virology, 4th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 13. Wu X, Hu R, Zhang Y, Dong G, Rupprecht CE. 2009. Reemerging rabies and lack of systemic surveillance in People's Republic of China. Emerg. Infect. Dis. 15:1159–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu XJ, et al. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364:1523–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L, et al. 2008. Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA 300:2263–2270 [DOI] [PubMed] [Google Scholar]
- 16. Zhang YZ, et al. 2011. Hemorrhagic fever caused by a novel tick-borne bunyavirus in Huaiyangshan, China. Zhonghua Liu Xing Bing Xue Za Zhi 32:209–220 (In Chinese.) [PubMed] [Google Scholar]
- 17. Zhang YZ, Zou Y, Fu ZF, Plyusnin A. 2010. Hantavirus infection in humans and animals, China. Emerg. Infect. Dis. 16:1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zou Y, et al. 2008. Molecular diversity of Hantanviruses in Guizhou, China: evidence for origin of Hantaan virus from Guizhou. J. Gen. Virol. 89:1987–1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.