Abstract
Espirito Santo virus (ESV) is a newly discovered virus recovered as contamination in a sample of a virulent strain of dengue-2 virus (strain 44/2), which was recovered from a patient in the state of Espirito Santo, Brazil, and amplified in insect cells. ESV was found to be dependent upon coinfection with a virulent strain of dengue-2 virus and to replicate in C6/36 insect cells but not in mammalian Vero cells. A sequence of the genome has been produced by de novo assembly and was not found to match to any known viral sequence. An incomplete match to the nucleotide sequence of the RNA-dependent RNA polymerase from Drosophila X virus (DXV), another birnavirus, could be detected. Mass spectrometry analysis of ESV proteins found no matches in the protein data banks. However, peptides recovered by mass spectrometry corresponded to the de novo-assembled sequence by BLAST analysis. The composition and three-dimensional structure of ESV are presented, and its sequence is compared to those of other members of the birnavirus family. Although the virus was found to belong to the family Birnaviridae, biochemical and sequence information for ESV differed from that of DXV, the representative species of the genus Entomobirnavirus. Thus, significant differences underscore the uniqueness of this infectious agent, and its relationship to the coinfecting virus is discussed.
INTRODUCTION
The birnavirus family is a distinct double-stranded RNA (dsRNA) family of viruses that infects animal species from vertebrates to mollusks, fish, as well as insects. The family is grouped into three main genera according to its hosts, the genera Aquabirnavirus, Avibirnavirus, and Entomobirnavirus (18, 29). They include viruses with a bisegmented dsRNA genome encapsidated within single-shelled, unenveloped, icosahedral particles (46). These viruses are approximately 70 nm in diameter and exhibit 260 spikes in a proposed T=13 organization (5, 14). Members of the genera Aquabirnavirus and Avibirnavirus include infectious pancreatic necrosis virus (IPNV) and infectious bursal disease virus (IBDV), respectively, which are two well-characterized viruses of economic importance to the aquaculture and poultry industries (1, 10). These members account for much of the current knowledge about the structure and function of the birnaviruses. The prototype species of the genus Entomobirnavirus is Drosophila X virus (DXV), which was first isolated as a contaminant in experiments studying insect rhabdovirus sigma (18). Drosophila X virus stands as the only known member of the genus Entomobirnavirus (18).
The birnaviruses are characterized by two dsRNA segments, segments A and B, which make up their genome (18) and which exhibit a strong degree of conservation with regard to structure (11). The size difference between the large (segment A) and smaller (segment B) genomes is the least in the case of DVX, where segment A is 3,360 bp and segment B is 3,423 bp (11). The sizes of IBDV genome segments A and B have been reported to be 3,261 bp and 2,800 bp, respectively (3), and those of IPNV are 3,097 bp and 2,855 bp, respectively (3, 18). The structural proteins of birnaviruses generally fall into three size classes (large, medium, and small), which are present in different relative proportions. The minor high-molecular-mass VP1 polypeptide is encoded by genome segment B (38), it does not undergo posttranslational cleavage, and it is the virion-associated RNA polymerase (34). The polypeptides VP2, VP3, and VP4 are encoded by genome segment A. VP2 and VP3 form the outer and inner layers, respectively, of the virions, and internally, VP3 forms a ribonucleoprotein complex with the genomic RNA (32), and VP1 is found both free and covalently attached to the genomic RNA (19). There is no description of entomobirnaviruses that infect mosquitoes or mosquito-derived cell lines. The only common contaminants of mosquito cell lines described are the small densoviruses, 20-nm viruses belonging to the family Parvoviridae (4). Among them, a new densovirus from Aedes albopictus C6/36 cells that were chronically infected was recently isolated and characterized (9). Aedes albopictus, along with Aedes aegypti, is considered to be one of the most important dengue virus (DENV) vectors, with an even higher level of susceptibility to dengue virus than A. aegypti (37). The Aedes albopictus C6/36 cell line has become very important in the study of arboviruses because of its wide range of susceptibilities to different viruses and its ability to produce plaques with a number of them (16, 44). It was described in early studies that some Aedes albopictus cell lines developed in the late 1960s presented contamination with multiple viruses, such as parvovirus, togavirus, and orbivirus-like particles (26). These persistent and innocuous viral infections were described to be common in insects and crustaceans as single, dual, or multiple coinfections (27).
Our group is interested in performing host cell penetration studies with flaviviruses such as dengue virus. For that purpose, a virulent strain of dengue virus type 2 was obtained from the Fiocruz Institute in Rio de Janeiro, Brazil. The strain, designated 44/2, originated from an isolate in the state of Espirito Santo, Brazil (22, 36), from a patient with dengue fever. During the course of our experiments and with the improvement of dengue virus purification methods, we have observed by electron microscopy particles that had a structure completely different from that described for flaviviruses. These particles could not be detected by common procedures used to identify dengue virus, such as PCR, immunofluorescence, or immunoblotting. Here we report the discovery of a new insect virus found in C6/36 mosquito cells coinfected with dengue-2 virus. The bisegmented genome size, its nature, and the size of structural proteins suggested that this new virus belongs to the birnavirus family. This was corroborated by RNA sequencing analysis, mass spectrometry, and cryo-electron microscopy (cryo-EM) reconstruction of the particles. Here we characterize the genome, structural proteins, and the capsid structure by electron cryomicroscopy of the new birnavirus, designated Espirito Santo virus (ESV). The data provide the means to detect this new virus in insect cell lines or insect vectors and will be useful for comparisons with other dsRNA viruses, specifically with birnaviruses.
MATERIALS AND METHODS
Virus growth and purification.
A sample of dengue-2 virus was obtained from the Fiocruz Institute, Brazil. This sample was isolated in Aedes albopictus C6/36 cells, and at its third passage, it was plaque purified. The selected 44/2 clone virus produces uniformly sized plaques. This sample was found to carry an unknown viral contaminant denominated ESV, which grew in C6/36 mosquito cells. However, ESV does not produce plaques and therefore cannot be plaque purified from the dengue virus sample. At 5 days postinfection, the virus was purified and concentrated by using isopycnic ultracentrifugation in iodixanol gradients (Optiprep; Sigma, St. Louis, MO). The virus was spun to equilibrium in 35% to 12% iodixanol gradients overnight at 76,000 × g in an SW28 rotor at 4°C. The visible band was collected and diluted in phosphate-buffered saline (PBS) and then layered over a second gradient (20% to 35%) and run for 3 h at 90,000 × g in an SW28.1 rotor at 4°C. For viral protein analysis, the sample was further purified in CsCl gradients (25% to 37%) as previously described (13). For cryo-EM, these samples were cross-linked with 1.5% buffered glutaraldehyde in 20 mM HEPES (pH 7.5) at room temperature. After 10 min, the reaction was stopped with 100 mM Tris (pH 8.0).
SDS-PAGE.
SDS-polyacrylamide gel electrophoresis was performed with the NuPAGE (4 to 12%) electrophoresis system (Invitrogen). Viral proteins were disrupted in dissociation buffer containing NuPAGE reducing agent, according to manufacturer's instructions. The gel was run at 200 V until the dye marker reached the bottom. The gel was then fixed in 50% methanol with 7% acetic acid for 30 min. The staining of viral proteins was performed by using Sypro ruby red (Molecular Probes, CA). A High-Range Rainbow marker was used as a molecular standard (GE Healthcare, NJ).
RNA extraction.
Purified virus was pelleted at 240,000 × g in an SW55Ti (Beckman Coulter, Fullerton, CA) rotor for 1 h. Approximately 500 μg/ml of pelleted virus was resuspended in 300 μl of lysis buffer (100 mM Tris-Cl [pH 7.0], 20 mM EDTA, 1% SDS) at 37°C for 20 min. The virus was then treated twice with 300 μl of phenol and once with an equal volume of pure chloroform. RNA in solution was then precipitated in 100% isopropanol at −80°C overnight and resuspended in water on the following day. Approximately 10 ng/μl of purified RNA was obtained, run on a 1% agarose gel (SeaKem-GTG-agarose; Lonsa, MA), and stained with ethidium bromide.
RNA sequencing and de novo genome assembly.
Samples were prepared by the RNA extraction of gradient-purified virus, and purity was determined by using an Agilent Bioanalyzer. Libraries of purified RNA were prepared by using the TruSeq Sample Prep kit with the following adjustments: mRNA enrichment was not done; instead, purified RNA was fragmented directly, and the processed cDNA PCR products were gel purified on a 2% agarose gel. The ∼350-bp products were extracted by using a Qiagen gel (2) extraction kit. Sequencing was performed on an Illumina GAIIx instrument using 72-bp single-read conditions. A total of 42,993,210 fastq files were generated, with ∼74% of those reads passing the quality control filter. In order to perform the de novo assembly, the fastq files from the Illumina data were imported into Velvet (48), and the Velvet module was used to produce sequence nodes. This file was then imported into the Lasergene 8 Seqman module and used to assemble the contig files and the final assembled sequences of segments A and the B of the virus genome. The cDNA and translated protein products were validated by matching peptide sequences generated from the same virus using mass spectrometry analysis.
Analysis of peptides by liquid chromatography-mass spectrometry.
A small volume (100 μl) of purified virus in PBS corresponding to 50 μg of total protein was digested in solution with the endoproteinase lysine C (Lys-C). Prior to digestion, the solution was heated at 80°C for 15 min to facilitate protein denaturation. Following heating, 7 M guanidine-HCl (GuHCl) solution in 50 mM phosphate buffer was added to the virus sample in a 7:1 ratio, yielding a final concentration of 1 M GuHCl. A 1-μg/μl endoproteinase Lys-C solution was prepared in water, and 10 μl of the Lys-C solution was added to the virus sample and incubated for 24 h at 37°C. The Lys-C digest was stored at −20°C until use. An aliquot of the Lys-C-digested virus sample corresponding to 1 μg of digested protein was subjected to liquid chromatography/elevated energy mass spectrometry (LC/MSE) analysis using a nanoAcquity ultrapressure liquid chromatography (UPLC; Waters Corp., Milford, MA) coupled to a Q-Tof Premier mass spectrometer (Waters Corp.). A gradient of 2 to 40% acetonitrile (MeCN) in water containing 0.1% formic acid was used to elute peptides over a period of 1 h from a 75-μm-internal-diameter (i.d.) by 25-cm analytical column packed with 1.7-μm bridged ethyl hybrid (BEH) particles into the Nanolockspray source of the Q-Tof Premier instrument. The Q-Tof Premier instrument was operated in the LC/MSE mode of operation (43), with alternating scans of normal and elevated collision energies to provide both intact precursor and product ion data for all peptides. Alternating normal and elevated collision energy scans were acquired at a rate of 1 Hz. In addition, a solution of 600 fmol/μl Glu-fibrinopeptide B was infused into the Nanolockspray source to allow for the postacquisition “lockmass” correction of observed ion masses.
Data analysis and database searching.
LC/MSE spectra were processed, and databases were searched by using Proteinlynx Global Server 2.4 (PLGS) software (Waters Corp.). Protein sequences corresponding to the predicted open reading frames (ORFs) for ESV were formatted and appended to the Uniprot-Sprot protein database (523,151 entries) for database searching (www.uniprot.org). A variable modification for methionine oxidation was used for searching, and 1 missed Lys-C cleavage site was allowed. Other parameters included for searching were as follows: a minimum number of fragment ions per peptide of 3, a minimum number of fragment ions per protein of ≥7, and a minimum number of peptides per protein of 1. Because the data were to be subsequently loaded into the Scaffold program (Proteome Software, Portland, OR), a false-positive rate (FPR) of 100% was utilized for the PLGS searches to ensure a sufficient number of random matches in the search results needed for the proper functioning of the statistical algorithms within Scaffold. The Scaffold plug-in within PLGS was used to export database search results from PLGS, which could be imported directly into Scaffold. Protein and peptide identifications from PLGS were loaded into Scaffold for visualization and determinations of peptide and protein probability scores. For declaring a protein successfully identified, a minimum of two matching peptides and minimum protein and peptide probability scores of 95% and 90%, respectively, were required.
Electron microscopy.
ESV samples were first analyzed by negative staining with 1% uranyl acetate to assess the optimal concentration and sample homogeneity for cryo-EM. Thin sections of DENV-2- and ESV-infected cells were prepared as previously described (35). The specimen was prepared for cryo-EM by applying ESV onto holey carbon EM grids prepared according to a method described previously by Fukami and Adachi (23). The grids were vitrified in liquid ethane as previously described (21). These grids were then placed into a 300-keV FEI TF30He Polara G2 electron cryomicroscope. A total of 199 focal pairs were subsequently recorded with the EMMENU4/EMTOOLS automation package (TVIPS) at a microscope magnification of ×39,000 using an F415 4k by 4k charge-coupled device (CCD) (TVIPS), for a final size of 2.29 Å per pixel.
Image processing.
In total, 5,928 virus images were boxed out from all CCD images by using e2boxer.py in the EMAN2 image processing package (45). Once boxed out, the contrast transfer function (CTF) parameters for each particle set was determined by using ctfit in EMAN, and these were parameters used to CTF correct the images for subsequent processing. The virus images were then centered and classified by using EMAN (31). The initial refinement by EMAN classified the data into 123 class averages through 8 iterations and produced a three-dimensional (3D) structure with a 13-Å resolution based on a Fourier shell correlation (FSC) of 0.5 between two maps produced from two separate halves of the data. Three additional refinement iterations with 212 class averages produced a 12-Å 3D map similarly based on an FSC of 0.5.
Immunofluorescence.
Infected and mock-infected C6/36 cells and Vero cells were fixed in 6-well plates with 4% paraformaldehyde in 0.1 M cacodylate buffer at 2 days postinfection. Cells were permeabilized with 2% NP-40 and blocked with 50 mM ammonium chloride and 3% bovine serum albumin (BSA). Monoclonal antibody 4G2 (25) against the flavivirus E protein (1:50 dilution) was added to the cells and then incubated at room temperature for 2 h. Cells were washed and blocked again with 3% BSA. Goat anti-mouse antibody conjugated with Alexa Fluor 488 (Molecular Probes) was added (20 μg), and the mixture was incubated for another 50 min at room temperature. Cells were also stained with 4′,6-diamidino-2-phenylindole (DAPI) (1 μg/ml) for 5 min. The infected cells were observed under a Zeiss LSM 710 laser scanning confocal microscope.
Accession numbers.
The nucleotide and deduced amino acid sequence data reported in this study have been deposited in GenBank under the following accession numbers: JN589003 for ESV segment A and JN589002 for ESV segment B. Sequence comparisons with other birnaviruses were performed by using the following accession numbers: D00867 for IBDV, AJ622822 for IPNV, and U60650 for DXV. The map of ESV was deposited into the EMDB database (reference number EMD-24848) and is available under accession number 5353.
RESULTS
Characterization of the unknown virus.
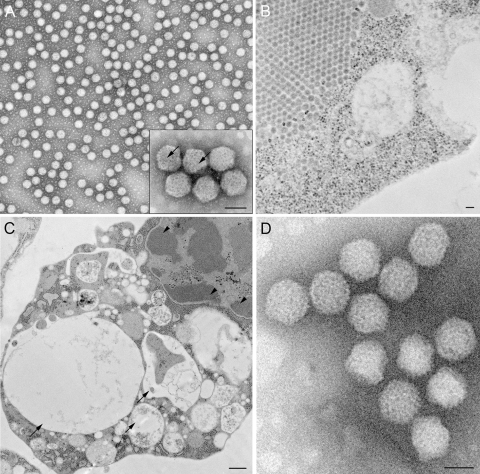
Viruses harvested from mosquito cells and purified in iodixanol gradients resulted in a virus band that was different in density from that of dengue virus. Virus banding at the density of dengue virus was not seen, as ESV suppresses dengue virus assembly (see below). Observations of ultrathin sections of infected mosquito cells revealed particles that did not resemble dengue virus in both structure and size. After negative staining with uranyl acetate, a predominant shape was identified, consisting of obvious icosahedral particles (Fig. 1A and B), with a distinct lattice structure on the surface (Fig. 1A, inset). The virus was named Espirito Santo virus (ESV) after the state in Brazil where the original dengue virus sample was collected. In thin sections of infected mosquito cells, ESV virions appeared icosahedral in shape and unenveloped, with a diameter of between 68 and 72 nm. In the cytoplasm of heavily infected cells, complete virus particles associated to form paracrystalline arrays (Fig. 1B). The average EM-measured diameter for the particles in negative stains was 70 ± 2 nm. Curiously, there was only one homogeneous population of particles in the preparations, with no evidence of the presence of dengue virus-like particles according to its previously described size and overall structure. In addition, when C6/36 cells were infected with ESV, cytopathic effects such as extensive vacuolization and chromatin segregation to the nuclear periphery could be observed (Fig. 1B and C). Virions that were purified from the infected mosquito cells appeared to be nonenveloped and icosahedral when analyzed by negative staining, and the presence of protruding spikes on the virus surface was evident (Fig. 1A). This size range and these structural characteristics were further confirmed by the observation of particles embedded in vitreous ice and examined by cryo-EM (Fig. 1D) (described in detail below). Although ESV grows to a high concentration in insect cells with DENV-2 (44/2), no virus could be identified in gradients or by EM when the insect-cell-produced virus was used to infect Vero cells.
Fig 1.
Electron microscopy of purified viral particles and infected mosquito cells. (A) TEM of purified viral particles after negative staining with uranyl acetate. Note the icosahedral symmetry and homogeneity of the sample population. At a higher magnification, particles show a distinct surface structure (inset). (B) Thin section of a virus-infected A. albopictus C6/36 cell, where a paracrystalline array of particles can be observed. (C) Ultrathin section of an infected C6/36 cell showing cytopathic effects characterized by extensive vacuolization (arrows) and segregation of the chromatin (arrowheads). (D) Cryo-electron micrograph of the purified particles, confirming the icosahedral symmetry, emphasized by the ability to detect the particles in different orientations and the protrusions on the surface of the particles. Bars, 500 nm (A), 1 μm (B), 2 mm (C), and 500 nm (D).
Virus-specific polypeptides.
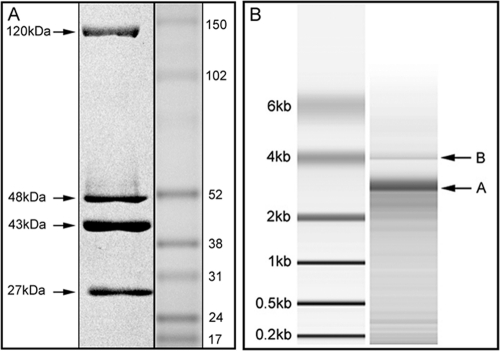
SDS-PAGE analysis was performed to estimate the molecular masses of the ESV capsid proteins. The electrophoresis of the viral proteins revealed a profile of four major protein bands of approximately 120 kDa, 48 kDa, 43 kDa, and 27 kDa. These polypeptides are likely to represent VP1, VP2, VP3, and VP4 (Fig. 2A), according to the molecular mass range of VPs of the birnaviruses. In some samples, additional protein bands of around 17 kDa and 54 kDa could be seen when the sample was not purified in CsCl gradients (not shown). This could be a result of the protein degradation of the stored sample, mosquito cell proteins, or even dengue virus proteins. Previous studies of DXV with pulse-chase experiments and peptide mapping showed that the posttranslational cleavage of a 67-kDa polypeptide gives rise to a 49-kDa protein, which undergoes a slow maturation-associated cleavage to generate the 45-kDa final product (38, 39). As shown in Fig. 2, the cleavage of a polypeptide in the 45- to 55-kDa range seemed to be complete, since only one product could be found in that molecular mass range. The protein sizes not only were in agreement with the size range expected for members of the birnavirus family but also seemed to be most closely related to the sizes described for DVX (Table 1). Figure 2 presents pVP2 and VP2 as the 49-kDa and 45-kDa proteins, respectively. A protein with a molecular mass corresponding to VP5 did not seem to be present in the virion in sufficient amounts to be stained by Coomassie blue or Sypro ruby red staining.
Fig 2.
Electrophoretic profile of ESV polypeptides and dsRNA. (A) The polypeptides were separated by use of the NuPAGE system and stained by Sypro ruby red. The estimated molecular masses of four viral proteins are indicated as 120 kDa, 48 kDa, 43 kDa, and 27 kDa. (B) The ESV bipartite RNA genome is shown with two distinctive bands.
Table 1.
Comparison of birnavirus protein sizes
| Putative protein | Molecular mass (kDa) |
|||
|---|---|---|---|---|
| ESV | DXV | IBDV | IPNV | |
| VP1 | 120 | 112 (VP1) | 98 | 94 |
| VP2 | 48 | 49 (pVP2) | 47 | 54 |
| VP3 | 43 | 45 (VP2) | 29 | 31 |
| VP4 | 27 | 27 | 27 | 29 |
ESV sequencing and de novo assembly.
To characterize the virus genome, viral nucleic acid was extracted from virus purified by using CsCl gradients to exclude possible flavivirus particles. The electrophoresis results indicated a bipartite genome that had an approximate size of 3.2 kb (Fig. 2B). The extracted RNA was sequenced, and the resulting nucleotide sequences of ESV were determined to be 3,250 nucleotides (nt) for segment B and 3430 nt for segment A (Table 2). The resulting sizes of the bipartite genome are in agreement with the average size for other birnaviruses, particularly DXV (11, 38).
Table 2.
Comparison of birnavirus genome sequences
| Birnavirus | Genome sequence size (nt) |
|
|---|---|---|
| Segment A | Segment B | |
| ESV | 3,430 | 3,250 |
| DXV | 3,360 | 3,243 |
| IBDV | 3,261 | 2,827 |
| IPNV | 3,097 | 2,855 |
ESV genome organization.
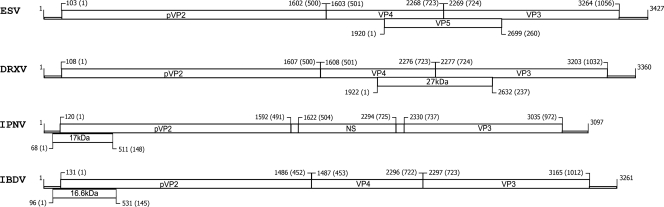
One large open reading frame (ORF) was found on each segment, spanning nucleotides 97 to 3093 on segment B and nucleotides 59 to 3158 on segment A. In addition, a small putative ORF was also found to be capable of encoding a 27-kDa polypeptide of 260 amino acids (aa) in segment A (Fig. 3). The polyprotein ORF encompasses most of the genome of segment A in the case of ESV as well as other members of the birnaviruses. The large ORF in segment B encodes VP1, which has been described to represent the viral RNA-dependent RNA polymerase (RdRp). The genomic arrangement of ESV therefore resembles that of other members of the birnavirus family. The expression of the ESV genes during the replication of the virus is likely to involve the polycistronic expression of the large polypeptide from the primary ORF of segment A, which in this case is capable of encoding a 120-kDa polypeptide (1,050 aa). This large ORF should be processed into VP2, VP3, and VP4, as occurs with DXV and other family members. The putative small polypeptide ORF is likely to represent VP5; however, it could not be detected by SDS-PAGE. Further sequence analysis using BLASTn demonstrated that the viral bisegmented RNA presented approximately 70% homology to the DXV RdRp that resulted from the 3,200-bp segment B (Fig. 4). These results indicate that the newly identified ESV is indeed a member of the birnavirus family. The putative protein of 27 kDa of ESV is larger than the VP5 proteins of IPNV and IBDV, as is also the case for DXV, in which it was described to be a nonstructural protein. In our study, this protein was not confirmed, as there is no start codon in that region; however, there is a putative single mutation, G1920A, that would express this protein by creating a start codon for it. Since this is likely to be a nonstructural protein, its presence would be rather difficult to confirm. Furthermore, there may not be an NS/VP5 protein in ESV that is expressed regularly.
Fig 3.
ESV and birnaviruses genome organization. Shown are a schematic representation of the gene organization of genome segment A of ESV and a comparison with its DRXV (Drosophila X virus), IPNV (infectious pancreatic necrosis virus), and IBDV (infectious bursal disease virus) homologs. Lines represent untranslated regions, boxes represent ORFs, and numbers above the ORFs indicate the first nucleotide involved in the initiation codon. Numbers in parentheses indicate the respective amino acids.
Fig 4.
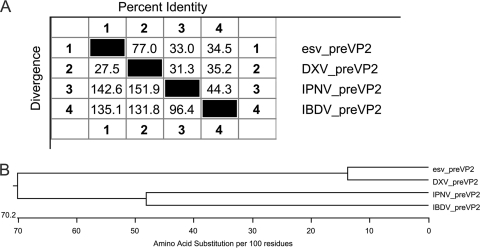
Phylogenetic relationship with birnaviruses. (A) Pairwise distances of ESV, DXV, IPNV, and IBDV based on the nucleotide sequence and deduced amino acid sequences of VP2. (B) Cladogram representing phylogenetic relationships of ESV and other members of the birnavirus family based on deduced amino acid sequences of the VP2 capsid protein.
ESV sequence comparisons with other birnaviruses.
The putative ESV RdRp nucleotide sequence obtained was compared with all nucleotide sequences in the NCBI database by using BLAST. The deduced amino acid sequences of ESV segments A and B were compared to those of DXV, IPNV, and IBDV, which represent three different genera of birnaviruses. Interestingly, no high degree of similarity was found for RdRp or other viral proteins between ESV and other members of the birnavirus family or any other known virus. When the nucleotide sequences were compared, ESV RdRp and the Drosophila X virus segment B putative RNA-dependent RNA polymerase VP1 gene (GenBank accession number AF196645.2) shared approximately 71% similarity, while ESV VP1 shared approximately 4% similarity with IPNV. Segment A of ESV has 72% identity (E value of approximately 0.0/1,170 total score, the sum of the scores of all high-scoring pairs from the same database sequence) for the Drosophila X virus polyprotein gene (accession number U60650.1), and it was found to have 72% identity (E value of 2e−04/59.0 total score) with the infectious pancreatic necrosis virus strain Reno segment A VP2 structural protein (accession number AY026345.1).
A comparison of the genomic relationships of the different birnaviruses based on the nucleotide sequences of the VP2 coding region was also performed. According to the sequence analysis and pairwise alignment of ESV and birnaviruses (Fig. 4A), a cladogram was constructed, using parsimony analysis to describe the phylogenetic relationship of these viruses (Fig. 4B). This was based on the deduced amino acid similarities of the polyprotein encoded by the large ORF (Clustal W method with DNAStar; Lasergene). Branch lengths were drawn proportional to the number of deduced amino acid differences by using the Clustal W method. Among the virus-specific polypeptides, pVP2 exhibited the highest level of amino acid conservation among members of the birnavirus family (11), and therefore, it is a good candidate protein for comparisons. The phylogenetic and sequence analysis revealed that ESV is more closely related to DXV than any other member of the birnavirus family and therefore may constitute another member of the genus Entomobirnavirus.
Mass spectrometry analysis of ESV proteins.
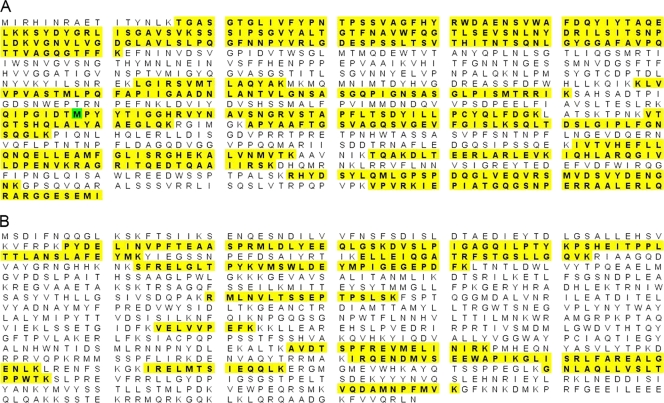
Isolated virus particles were digested with the endoproteinase Lys-C and subjected to LC/MSE analysis using a Waters Q-Tof Premier mass spectrometer. Following digestion, product ion spectra were searched against the Uniprot-Sprot database, to which two predicted protein sequences derived from the ORFs from segments A and B of ESV had been added. These two predicted protein sequences correspond to the segment A polyprotein (1,030 amino acids) and RNA RdRp (998 amino acids) of ESV. Twenty-seven proteins were identified in the sample with high confidence, corresponding primarily to growth medium components and other expected sample component proteins. In addition, high-quality, multipeptide matches to the predicted ESV proteins from ORFs in segments A and B were obtained. The segment A polyprotein was observed to have 19 unique peptide matches corresponding to a 50% amino acid sequence coverage, with a protein probability score of 100% (Fig. 5A). RdRp of ESV was observed to have 14 unique peptide matches corresponding to a 24% amino acid sequence coverage, with a protein probability score of 100% in Scaffold (Fig. 5B). The identification of these peptide sequences by mass spectrometry confirmed the expression of these two ORFs corresponding to the segment A polyprotein and RdRp in ESV. The peptide sequences identified against these two proteins were unique to ESV in the database and were not matched to other protein components in the samples.
Fig 5.
Mass spectrometry analysis. Amino acid sequence coverage was obtained for the ESV segment A polyprotein (A) and ESV segment B RdRp (B) by LC/MSE analysis. Peptide matches are highlighted in yellow, and an oxidized Met is highlighted in green.
ESV coinfection with other viruses.
Observations of DENV-2 44/2-infected cells demonstrated that ESV grows to a higher number of particles (observed by transmission electron microscopy [TEM]) during coinfection with this strain of dengue-2 virus. Coinfection controls, where Sindbis virus was inoculated with ESV, confirmed that ESV could grow only when mosquito cells were infected with dengue-2 virus, since no ESV was recovered with Sindbis virus. When this experiment was performed with less virulent strains of dengue virus, such as DENV-2 NGC 16681 and DENV-2 16803, we observed a small amount of ESV by electron microscopy. However, when ESV infection was carried out in the presence of DENV-2 44/2, large amounts of ESV were recovered from the samples, as shown in Fig. 1. Therefore, the presence of dengue virus seems to be necessary for significant ESV replication. We believe that the origin of the new virus was from the dengue virus 44/2 sample isolated in Brazil, although the precise origin of the virus remains unclear at this point. We were unable to estimate the number of PFU of ESV, because a plaque assay for the virus has not been established. Interestingly, when coinfected cells were analyzed, dengue virus proteins could be detected by immunofluorescence (Fig. 6), while no particle that resembles dengue virus could be observed by electron microscopy. The presence of dengue-2 virus in the samples was confirmed by reverse transcription (RT)-PCR analysis, immunofluorescence of infected cells with DENV-2-specific polyclonal and monoclonal antibodies (Fig. 6), and Western blotting of the virus E protein with DENV-2-specific antibody (not shown).
Fig 6.
Immunofluorescence of mosquito cells infected with dengue-2 virus and ESV. Shown are dengue-2 virus 44/2 (plus ESV)-infected mosquito cells at 2 days postinfection, immunolabeled with monoclonal antibody 4G2 against the dengue virus envelope protein (green) and DAPI (blue).
Three-dimensional structure of ESV.
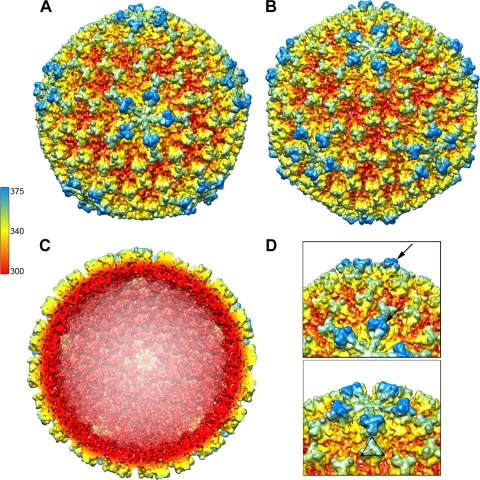
The 3D structure of ESV was determined from images obtained by cryo-EM to a resolution of 13 Å (Fig. 7). The reconstruction revealed an icosahedron-shaped particle with pentameric protrusions on each 5-fold vertex (Fig. 7). The overall features of the structure are shown in Fig. 7, which displays the surface rendering of the cryo-EM reconstruction along the 5-fold (Fig. 7A) and 3-fold (Fig. 7C) axes of symmetry, including the protrusions on the viral surface (spikes). These protrusions have 3-fold rotational symmetry (Fig. 7D), and each protrusion extends approximately 45 Å from the virus surface, giving the particle an average cross-sectional diameter ranging from 700 to 750 Å (Fig. 7A and C). A closer look at the cryo-EM structure reveals that the 5-fold-to-5-fold distance measures 750 Å, while the 3-fold-to-3-fold distance measures 700 Å (Fig. 7D). The VP2 proteins appear to be organized in 260 trimers, with 60 surrounding the 5-fold vertices and 200 organized in hexamers, making up the faces and edges of the icosahedron (Fig. 7A to C). These hexamers are ∼130 Å apart from center to center. The spikes that form the 5-fold arrays at the vertices are the most prominent structures on the virus surface; although they look structurally similar to those in 6-fold arrays, they appear to have extra density at their tips (Fig. 7D), protruding to a distance of 20 to 25 Å above the trimers of the 3-fold axes (Fig. 7C and D). The 12 pentamers and 120 hexamers that make up the surface of the virus are seen organized in the characteristic pattern expected for an external shell of 780 protein subunits and a T=13 quasiequivalent organization (Fig. 7). Such an icosahedral lattice is a skewed lattice with handedness, in which a 5-fold axis is reached from its neighboring 5-fold axis by stepping over three six-coordinated positions and taking a left or a right turn. Lattices of a different hand, laevo and dextro, have been reported for different birnaviruses (40). In particular, IBDV and IPNV were reported to have opposite hands despite their high level of amino acid conservation for VP2. However, their handedness was recently revisited (15) and was demonstrated to be organized in a T=13 laevo lattice. In addition, another group confirmed the laevo lattice of IBDV and IPNV by using heavy metal shadowing techniques (41). Therefore, it is likely that ESV has the same laevo handedness previously established for birnaviruses. Interestingly, no tubular forms of the virions were observed in the samples, as commonly observed for IBDV and DXV.
Fig 7.
Three-dimensional reconstruction and surface organization of ESV. (A and B) The surface rendering is colored radially, red (275 Å) to blue (375 Å), along the 5-fold (A) and along the 3-fold (B) axes, respectively. The particle size is ∼700 Å and shows 260 trimeric protrusions that extend ∼45 to 50 Å from the viral surface. (C) Cross-section of the reconstruction along the 5-fold axis. Most of the internal map densities corresponding to the RNA are at different contour levels and were excised for clarity. (D) Close views of the VP2 protein trimers with areas where an extra density on the pentamer trimers can be seen extending (arrows) on the surface. The extra density gives the virion a diameter of 750 Å from vertex to vertex. Trimer dimensions are ∼80 Å at the triangular edge. The bottom panel shows a top view of one of the 12 pentamers and adjacent hexamers.
DISCUSSION
In recent years, there has been considerable interest in insect cell lines in which viruses can both survive and disseminate. Viruses of public health and veterinary importance usually receive the most attention, although other viruses have also been extensively studied (29). Aedes albopictus is one of the most important vectors of emergent infectious diseases such as dengue fever and yellow fever worldwide (37). So far, there is not an effective method of controlling the population of mosquitoes that transmit these diseases, which is one of the main reasons for their prevalence in tropical areas. In this research, a novel dsRNA virus with characteristics of the birnavirus family was isolated in mosquito cells from a dengue-2 virus strain sample originating from a dengue fever patient from Espirito Santo, Brazil. It is important to point out that it is unclear at what point the sample became contaminated with ESV. Furthermore, none of the laboratories involved have had projects involving birnaviruses, and for the decades that we have been working with these insect cell lines, we have never identified a contaminating virus in spite of many years of study involving electron microscopy. The cytopathic observations and the unique biochemical and structural characteristics of the purified virus led us to conclude that ESV was an undescribed virus. ESV appears to share morphological features with members of the birnavirus family such as infectious pancreatic necrosis of trout (12), infectious bursal disease of poultry (24), and, especially, Drosophila X virus (DXV). Accordingly, it is an unenveloped single capsid with a diameter of approximately 70 nm and a suggested T=13 triangulation number. Even though no three-dimensional structure has been reported for other entomobirnaviruses such as DXV, there have been structures described and modeled for other genera, such as IBDV (Avibirnavirus) and IPNV (Aquabirnavirus) (5, 15, 33).
In addition to these structural characteristics, the genetic organization of genome segment A of ESV is similar to those of other birnaviruses (DVX, IPNV, and IBDV) belonging to different genera (11). A well-known feature of the birnavirus architecture is the presence of 260 trimeric spikes formed by VP2, projecting radially from the capsid (41). Internally, VP3 forms a ribonucleoprotein complex with the genomic RNA (32). VP2 together with VP3 are the major components of the particles (30). Like most of the birnaviruses, ESV contains four viral polypeptides, with VP2 and VP3 possibly being the major capsid components. However, the ordered density in previous cryo-EM reconstructions and models corresponds exclusively to VP2, while the icosahedral capsid contains VP3 molecules that do not follow icosahedral symmetry, although VP3 seems to play a key role in the assembly of the virion (34a). It was shown previously that VP2 relies on a transient scaffold provided by inner proteins to reach its T=13 laevo architecture (15). In the case of the Reoviridae,VP2 homologs can form such a lattice only over an inner capsid, acting as a permanent scaffold (20). For ESV and other birnaviruses, more structural information is needed to clarify the role of VP3 as a possible inner capsid scaffold for VP2.
There is also a significant variance among members regarding viral protein sizes (Table 1); however, ESV proteins remained within the size range common for all birnaviruses. Most of these properties correspond to the essential characteristics of the family Birnaviridae defined previously by Dobos et al. (17, 18). In order to further characterize ESV and establish its relationship to the birnaviruses, the viral RNA was sequenced and compared. When analyzing the relationship of viruses and comparing their genomes, sequences from the RNA-dependent polymerases (RdRps) are generally the most useful because they are conserved and occur in most viral genomes (6, 7). In addition, the active domains of the RdRps are highly homologous among the viruses of different species belonging to different families. RdRp is a genome-linked protein located inside the viral capsid and thus is not exposed to host immune pressure, compared to the capsid protein VP2 or VP3 (49). This might be another reason why the RdRp is conserved. Sequence analysis based on the RdRp provided a higher degree of similarity with DXV (∼70%) but failed to show any significant relationship with other birnaviruses. In fact, this similarity is much less pronounced when structural proteins are considered. In previous studies, among all of the specific polypeptides analyzed, pVP2 was reported to exhibit the highest level of amino acid conservation between DXV, IBDV, and IPNV (11), although the level of amino acid sequence identity among the VP2 proteins of these viruses is still quite low. It is possible that, as was described previously for DXV (11), the differences in their amino acid sequences are not reflected in a largely altered secondary structure, and the folded polypeptides may have a similar functionality. The morphologies of these viruses are remarkably similar, except for the clear absence of tubular forms in ESV electron microscopy preparations. Several invertebrate viruses have been isolated from insect cells and other arthropod hosts. However, only one genus of the birnaviruses has been described to be present infecting insects, the entomobirnavirus DXV. The structural variations observed among these viruses suggest that entomobirnaviruses such as ESV in C6/36 cells have a capsid structure with a similar overall architecture but with underlying differences in the trimer contacts (14). By using the structure of VP2 from IPNV, previous reports described a model of the T=13 capsid of the IPNV virion (14, 33) by superimposing the VP2 trimer onto the previously described structure of IBDV (15). This forms a model that assumes that the intertrimer interactions that form the IPNV are similar to those observed for the IBDV particle. The most notable differences among the structures of the birnaviruses are at the spike-spike contacts between adjacent trimmers in the T=13 shell (concave by convex). Unfortunately, comparisons cannot be inferred from the structure of ESV because of the large difference in the resolution of its cryo-EM reconstruction compared to the crystal structures of VP2 proteins from IBDV and IPNV. This variance in capsid proteins, even though slight among some members, is probably responsible for host ranges, tissue tropisms, and differences in virulence among the birnaviruses (14, 33). In our experiments with flaviviruses, preliminary observations demonstrated that ESV grows to a higher number of particles only during coinfection with DENV-2 44/2. ESV could be isolated from this dengue virus strain but not from other dengue virus strains that we used, such as DENV-2 16801 and DENV-2 NGC 16681. Previous studies described similar effects on virus growth during coinfections with different viruses (42), suggesting that it may be caused by interfering with viral replication or competition for cell surface receptors. It was reported previously that infection of A. albopictus mosquitoes and the C6/36 cell line with a densovirus greatly reduced their susceptibility to DENV-2 (8). Natural dual or multiple infections were also reported to occur in mosquitoes infected with dengue virus and densovirus (8, 47). Wei et al. (47) obtained results that suggested that infection with DENV-2 can stimulate the production of latent C6/36 densoviruses in mosquitoes and prevent the growth of dengue virus particles. However, this mechanism of interaction was not well understood. Consequently, the presence of antigens in the cytoplasm of infected cells cannot be equated with the presence of DENV-2 particles. This phenomenon seems to be in accordance with a previous report where dengue virus particles were not seen in dual coinfections by Aedes albopictus densovirus (AalDNV) and DENV-2 (28). The fact that mosquito cells may carry persistent viral infections without cytopathic effects, as previously described (8, 9), raises the possibility that insect cell lines may be infected with other unknown viruses. The existence of unknown latent viruses in cell cultures could present serious complications in experimental studies involving arboviruses. Such contamination would be even more serious in the case of vaccine development, where an unknown and therefore undetected virus could affect the growth of the target virus. Furthermore, if these two viruses can coexist in the same cells for long periods of time, it may be an indication that there may be an opportunity for genetic exchange (27). This may have significant medical and epidemiological implications for arboviruses. Further studies are needed in order to answer questions concerning the nature of ESV and possible interactions with other viruses. The relationship of ESV with dengue virus is the subject of an ongoing investigation.
ACKNOWLEDGMENTS
This research was supported by a grant from the Foundation for Research, Carson City, NV, and by the North Carolina Agricultural Research Service at North Carolina State University. Molecular graphics images were produced by using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH grant P41 RR001081).
We are thankful to Marcos Freire at the Fiocruz Institute, Brazil, for providing the original DENV-2 44/2 strain.
Footnotes
Published ahead of print 14 December 2011
REFERENCES
- 1. Abdel-Alim G, Awaad MH, Saif YM. 2003. Characterization of Egyptian field strains of infectious bursal disease virus. Avian Dis. 47:1452–1457 [DOI] [PubMed] [Google Scholar]
- 2. Angelastro JM, Klimaschewski LP, Vitolo OV. 2000. Improved NlaIII digestion of PAGE-purified 102 bp ditags by addition of a single purification step in both the SAGE and microSAGE protocols. Nucleic Acids Res. 28:E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azad AA, Barrett SA, Fahey KJ. 1985. The characterization and molecular cloning of the double-stranded RNA genome of an Australian strain of infectious bursal disease virus. Virology 143:35–44 [DOI] [PubMed] [Google Scholar]
- 4. Barreau C, Jousset FX, Bergoin M. 1996. Pathogenicity of the Aedes albopictus parvovirus (AaPV), a denso-like virus, for Aedes aegypti mosquitoes. J. Invertebr. Pathol. 68:299–309 [DOI] [PubMed] [Google Scholar]
- 5. Bottcher B, et al. 1997. Three-dimensional structure of infectious bursal disease virus determined by electron cryomicroscopy. J. Virol. 71:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruenn JA. 1991. Relationships among the positive strand and double-strand RNA viruses as viewed through their RNA-dependent RNA polymerases. Nucleic Acids Res. 19:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruenn JA. 2003. A structural and primary sequence comparison of the viral RNA-dependent RNA polymerases. Nucleic Acids Res. 31:1821–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burivong P, Pattanakitsakul SN, Thongrungkiat S, Malasit P, Flegel TW. 2004. Markedly reduced severity of dengue virus infection in mosquito cell cultures persistently infected with Aedes albopictus densovirus (AalDNV). Virology 329:261–269 [DOI] [PubMed] [Google Scholar]
- 9. Chen S, et al. 2004. Genetic, biochemical, and structural characterization of a new densovirus isolated from a chronically infected Aedes albopictus C6/36 cell line. Virology 318:123–133 [DOI] [PubMed] [Google Scholar]
- 10. Christie KE, Havarstein LS, Djupvik HO, Ness S, Endresen C. 1988. Characterization of a new serotype of infectious pancreatic necrosis virus isolated from Atlantic salmon. Arch. Virol. 103:167–177 [DOI] [PubMed] [Google Scholar]
- 11. Chung HK, Kordyban S, Cameron L, Dobos P. 1996. Sequence analysis of the bicistronic Drosophila X virus genome segment A and its encoded polypeptides. Virology 225:359–368 [DOI] [PubMed] [Google Scholar]
- 12. Cohen J, Poinsard A, Scherrer R. 1973. Physico-chemical and morphological features of infectious pancreatic necrosis virus. J. Gen. Virol. 21:485–498 [DOI] [PubMed] [Google Scholar]
- 13. Comps M, Mari J, Poisson F, Bonami JR. 1991. Biophysical and biochemical properties of an unusual birnavirus pathogenic for rotifers. J. Gen. Virol. 72(Pt 6):1229–1236 [DOI] [PubMed] [Google Scholar]
- 14. Coulibaly F, Chevalier C, Delmas B, Rey FA. 2010. Crystal structure of an aquabirnavirus particle: insights into antigenic diversity and virulence determinism. J. Virol. 84:1792–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coulibaly F, et al. 2005. The birnavirus crystal structure reveals structural relationships among icosahedral viruses. Cell 120:761–772 [DOI] [PubMed] [Google Scholar]
- 16. Davey MW, Dennett DP, Dalgarno L. 1973. The growth of two togaviruses in cultured mosquito and vertebrate cells. J. Gen. Virol. 20:225–232 [DOI] [PubMed] [Google Scholar]
- 17. Dobos P. 1979. Peptide map comparison of the proteins of infectious bursal disease virus. J. Virol. 32:1047–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dobos P, et al. 1979. Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J. Virol. 32:593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dobos P, Roberts TE. 1983. The molecular biology of infectious pancreatic necrosis virus: a review. Can. J. Microbiol. 29:377–384 [DOI] [PubMed] [Google Scholar]
- 20. Dryden KA, et al. 1993. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J. Cell Biol. 122:1023–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dubochet J, et al. 1988. Cryo-electron microscopy of vitrified specimens. Q. Rev. Biophys. 21:129–228 [DOI] [PubMed] [Google Scholar]
- 22. Freire MS, et al. 2007. Wild dengue virus types 1, 2 and 3 viremia in rhesus monkeys. Mem. Inst. Oswaldo Cruz 102:203–208 [DOI] [PubMed] [Google Scholar]
- 23. Fukami A, Adachi K. 1965. A new method of preparation of a self-perforated micro plastic grid and its application. J. Electron Microsc. (Tokyo) 14:112–118 [PubMed] [Google Scholar]
- 24. Harkness JW, Alexander DJ, Pattison M, Scott AC. 1975. Infectious bursal disease agent: morphology by negative stain electron microscopy. Arch. Virol. 48:63–73 [DOI] [PubMed] [Google Scholar]
- 25. Henchal EA, Gentry MK, McCown JM, Brandt WE. 1982. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am. J. Trop. Med. Hyg. 31:830–836 [DOI] [PubMed] [Google Scholar]
- 26. Hirumi H, et al. 1976. Viral contamination of a mosquito cell line, Aedes albopictus, associated with syncytium formation. In Vitro 12:83–97 [DOI] [PubMed] [Google Scholar]
- 27. Kanthong N, Khemnu N, Pattanakitsakul SN, Malasit P, Flegel TW. 2010. Persistent, triple-virus co-infections in mosquito cells. BMC Microbiol. 10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanthong N, et al. 2008. Mosquito cells accommodate balanced, persistent co-infections with a densovirus and dengue virus. Dev. Comp. Immunol. 32:1063–1075 [DOI] [PubMed] [Google Scholar]
- 29. Kelly DC, et al. 1982. Isolation of a bisegmented double-stranded RNA virus from Thirlmere reservoir. J. Gen. Virol. 62(Pt 2):313–322 [DOI] [PubMed] [Google Scholar]
- 30. Lombardo E, et al. 1999. VP1, the putative RNA-dependent RNA polymerase of infectious bursal disease virus, forms complexes with the capsid protein VP3, leading to efficient encapsidation into virus-like particles. J. Virol. 73:6973–6983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ludtke SJ, Baldwin PR, Chiu W. 1999. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128:82–97 [DOI] [PubMed] [Google Scholar]
- 32. Luque D, et al. 2009. Infectious bursal disease virus: ribonucleoprotein complexes of a double-stranded RNA virus. J. Mol. Biol. 386:891–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luque D, et al. 2007. Infectious bursal disease virus capsid assembly and maturation by structural rearrangements of a transient molecular switch. J. Virol. 81:6869–6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Macdonald RD, Dobos P. 1981. Identification of the proteins encoded by each genome segment of infectious pancreatic necrosis virus. Virology 114:414–422 [DOI] [PubMed] [Google Scholar]
- 34a. Maraver A, et al. 2003. The oligomerization domain of VP3, the scaffolding protein of infectious bursal disease virus, plays a critical role in capsid assembly. J. Virol. 77:6438–6449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mariante RM, Vancini RG, Benchimol M. 2006. Cell death in trichomonads: new insights. Histochem. Cell Biol. 125:545–556 [DOI] [PubMed] [Google Scholar]
- 36. Miagostovich MP, et al. 2003. Molecular typing of dengue virus type 2 in Brazil. Rev. Inst. Med. Trop. Sao Paulo 45:17–21 [DOI] [PubMed] [Google Scholar]
- 37. Mitchell CJ. 1995. The role of Aedes albopictus as an arbovirus vector. Parassitologia 37:109–113 [PubMed] [Google Scholar]
- 38. Nagy E, Dobos P. 1984. Coding assignments of Drosophila X virus genome segments: in vitro translation of native and denatured virion dsRNA. Virology 137:58–66 [DOI] [PubMed] [Google Scholar]
- 39. Nagy E, Dobos P. 1984. Synthesis of Drosophila X virus proteins in cultured Drosophila cells. Virology 134:358–367 [DOI] [PubMed] [Google Scholar]
- 40. Ozel M, Gelderblom H. 1985. Capsid symmetry of viruses of the proposed Birnavirus group. Arch. Virol. 84:149–161 [DOI] [PubMed] [Google Scholar]
- 41. Pous J, et al. 2005. Structure of birnavirus-like particles determined by combined electron cryomicroscopy and X-ray crystallography. J. Gen. Virol. 86:2339–2346 [DOI] [PubMed] [Google Scholar]
- 42. Rhode SL., III 1978. Defective interfering particles of parvovirus H-1. J. Virol. 27:347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Silva JC, et al. 2006. Simultaneous qualitative and quantitative analysis of the Escherichia coli proteome: a sweet tale. Mol. Cell. Proteomics 5:589–607 [DOI] [PubMed] [Google Scholar]
- 44. Suitor EC, Jr, Paul FJ. 1969. Syncytia formation of mosquito cell cultures mediated by type 2 dengue virus. Virology 38:482–485 [DOI] [PubMed] [Google Scholar]
- 45. Tang G, et al. 2007. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157:38–46 [DOI] [PubMed] [Google Scholar]
- 46. Villanueva RA, Galaz JL, Valdes JA, Jashes MM, Sandino AM. 2004. Genome assembly and particle maturation of the birnavirus infectious pancreatic necrosis virus. J. Virol. 78:13829–13838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wei W, et al. 2006. The pathogenicity of mosquito densovirus (C6/36DNV) and its interaction with dengue virus type II in Aedes albopictus. Am. J. Trop. Med. Hyg. 75:1118–1126 [PubMed] [Google Scholar]
- 48. Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang CX, Suzuki S. 2003. Comparison of the RNA polymerase genes of marine birnavirus strains and other birnaviruses. Arch. Virol. 148:745–758 [DOI] [PubMed] [Google Scholar]