Abstract
HIV-1 requires the cellular transcription factor CBFβ to stabilize its accessory protein Vif and promote APOBEC3G degradation. Here, we demonstrate that both isoforms of CBFβ allow for increased steady-state levels of Vif, enhanced APOBEC3G degradation, and increased viral infectivity. This conserved functional interaction enhances the steady-state levels of Vif proteins from multiple HIV-1 subtypes and is required for the degradation of all human and rhesus Vif-sensitive APOBEC3 proteins by their respective lentiviral Vif proteins.
TEXT
Human immunodeficiency virus type 1 (HIV-1) and related lentiviruses require the viral accessory protein Vif to neutralize members of the APOBEC3 family of retrovirus restriction factors and render host cells permissive for productive viral replication. HIV-1 Vif neutralizes the APOBEC3 proteins by recruitment of an E3 ubiquitin ligase complex that polyubiquitinates APOBEC3 proteins and targets them for proteasomal degradation (13; reviewed in references 1, 9, and 12). Recently, the cellular transcription factor CBFβ was found to be associated with this complex and to allow for its reconstitution in vitro (6). Furthermore, CBFβ was found to be required for the stability of HIV-1IIIB Vif in vivo, allowing for efficient degradation of APOBEC3G (A3G) and increased viral infectivity (6). The current model is that HIV-1 Vif hijacks cellular CBFβ to facilitate Vif folding and/or stability, as well as nucleation of the E3 ubiquitin ligase complex. While it has been shown that rhesus macaque simian immunodeficiency virus molecular clone 239 (SIVmac239) Vif also requires CBFβ to degrade rhesus A3G (6), the generality of the CBFβ/Vif/APOBEC3 functional interplay remains to be determined. The goal of the current study was to determine which isoforms of CBFβ contribute to Vif stabilization, whether CBFβ is required to stabilize Vif proteins of multiple different HIV subtypes, and finally, if CBFβ is required by Vif to neutralize the entire repertoire of Vif-sensitive APOBEC3 proteins.
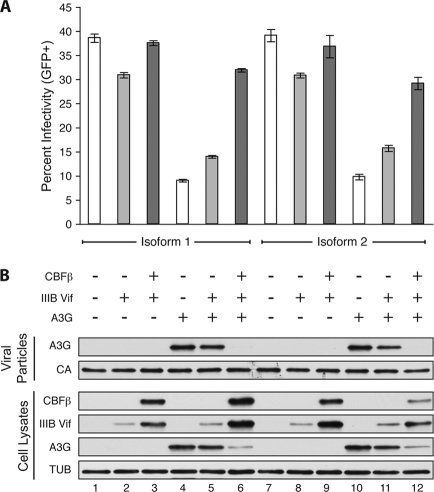
Alternative splicing generates at least two isoforms of CBFβ in human cells (GenBank accession numbers NM_022845.2 and NM_001755.2). Though they differ in size and in amino acid sequence at their C-terminal end, these splice variants share 165 N-terminal residues, including the RUNX heterodimerization domain, and a clear functional difference has yet to be delineated. To determine if HIV-1 Vif distinguishes between these CBFβ isoforms, a stable CBFβ knockdown clone of HEK293T was created using a stably integrated small hairpin RNA (shRNA) that targets both isoforms (6). This line was transiently transfected with a Vif-proficient or Vif-deficient A200C HIV-1IIIB molecular clone (3) in the presence or absence of human A3G and complemented with either the 187-amino-acid CBFβ isoform 1 (cloned from CEM cell cDNA by PCR and standard molecular biology techniques) or the shorter 182-amino-acid CBFβ isoform 2 (as used previously [6]). Forty-eight hours after transient transfection, cell lysates and viral particles were collected for immunoblotting and viral infectivity was monitored by infection of the reporter cell line CEM-GFP (5). The two isoforms resulted in comparable increases in HIV-1IIIB Vif steady-state levels, enhanced degradation of A3G, and rescue of viral infectivity (Fig. 1). In the absence of A3G, neither CBFβ isoform had an impact on viral infectivity.
Fig 1.
CBFβ isoform 1 and isoform 2 stabilize HIV-1 Vif to degrade A3G and increase viral infectivity. (A) Percent infectivity of HIV-1IIIB measured by duplicate infection of CEM-GFP and flow cytometry, reported as the mean ± standard deviation of the results for the technical replicate. Constant amounts of Vif-deficient or Vif-proficient A200C HIV-1IIIB molecular clone (1 μg) were cotransfected with A3G or empty plasmid (50 ng) in the presence or absence of CBFβ complementation vector (25 ng) as indicated. (B) Immunoblots of CBFβ, Vif, and hemagglutinin (HA)-tagged human A3G in cell lysates and of A3G in HIV-1 particles produced by those cells. Tubulin (TUB) and p24 (CA) served as cell and viral lysate loading controls.
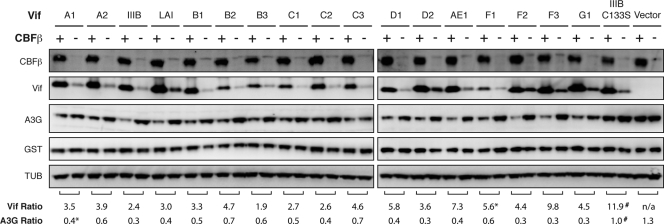
Most laboratory strains of HIV-1, including HIV-1IIIB, HIV-1NL4-3, and HIV-1LAI, are subtype B, but over 10 different HIV-1 subtypes are found worldwide, with subtype C being the most prevalent (4). To determine if CBFβ can stabilize Vif proteins from multiple subtypes, representative Vif alleles from HIV-1 subtypes A, B, C, D, AE, F, and G (as described previously [2]) were cotransfected into the HEK293T CBFβ knockdown cell line with A3G in the presence or absence of CBFβ isoform 2 and glutathione S-transferase (GST) as a transfection control. In every case, CBFβ increased the steady-state level of the Vif variant and resulted in increased degradation of A3G (Fig. 2). While basal Vif expression levels varied, CBFβ increased the steady-state level of each Vif variant by an average of approximately 4-fold. Furthermore, while each variant also differs in its ability to neutralize A3G (2), steady-state levels of A3G were decreased upon CBFβ complementation in every case by an average of 2-fold. A3G levels were not affected by CBFβ in the absence of Vif or in the presence of HIV-1IIIB Vif C133S, which fails to recruit the E3 ubiquitin ligase complex (7, 8). CBFβ did not affect the expression of the GST control. Thus, the dependency of Vif on CBFβ is broadly conserved across multiple HIV-1 subtypes.
Fig 2.
CBFβ stabilizes Vif proteins from multiple HIV-1 subtypes. Immunoblots of HA-tagged CBFβ, HIV-1 Vif, and FLAG-tagged human A3G in cell lysates. Tubulin (TUB) and V5-tagged GST served as cell lysate loading and transfection controls, respectively. Constant amounts of the indicated Vif variants (pCRV1 expression vector, 50 ng) were cotransfected with A3G (300 ng), GST (200 ng), and either CBFβ isoform 2 (100 ng) or empty vector. The untagged Vif variants were detected with a polyclonal rabbit anti-Vif antibody (NIH catalog no. 2221). One representative experiment of three independent transfections is shown. The Vif ratio represents the average ratio of Vif in the presence versus the absence of CBFβ (relative to GST) over three experiments unless otherwise noted (*, n = 2; #, n = 6). The A3G ratio was calculated analogously. Quantification was performed using Image Gauge version 4.0.
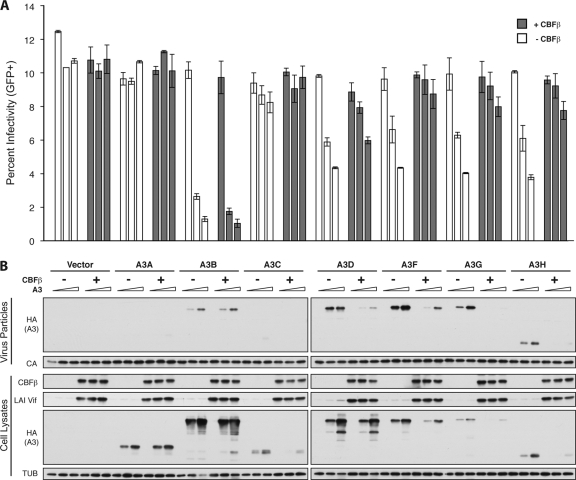
Human CD4+ T cells express six APOBEC3 proteins, of which HIV-1 Vif degrades five: A3C, A3D, A3F, A3G, and A3H (5, 10). To determine if HIV-1 Vif requires CBFβ to neutralize not only A3G but the other Vif-sensitive APOBEC3 proteins as well, the HIV-1LAI molecular clone was transfected into HEK293T CBFβ knockdown cells with increasing amounts of each human APOBEC3 protein in the presence or absence of CBFβ isoform 2. CBFβ increased Vif steady-state levels and resulted in decreased cellular levels of all Vif-sensitive APOBEC3 proteins (A3C, A3D, A3F, A3G, and A3H haplotype II) (Fig. 3). In the presence of CBFβ, packaging of A3D, A3F, A3G, and A3H was also decreased and viral infectivity increased accordingly. Neither A3A nor A3B are sensitive to HIV-1LAI Vif, and consequently, their expression, packaging, and impact on viral infectivity were not affected by CBFβ. Thus, HIV-1 Vif requires CBFβ to neutralize not only A3G but the entire repertoire of Vif-sensitive human APOBEC3 proteins.
Fig 3.
CBFβ is required for HIV-1 Vif to degrade all Vif-sensitive human APOBEC3 proteins. (A) Percent infectivity of HIV-1LAI measured by duplicate infection of CEM-GFP cells and flow cytometry, reported as the mean ± standard deviation of the results for the technical replicate. Constant amounts of Vif-proficient HIV-1LAI proviral construct (1 μg) were cotransfected with increasing concentrations of each human HA-tagged APOBEC3 protein (0, 50, or 100 ng) in the presence or absence of CBFβ isoform 2 complementation vector (25 ng) as indicated. (B) Immunoblots of CBFβ, Vif, and the HA-tagged human APOBEC3 proteins in cell lysates and of the APOBEC3 proteins in HIV particles produced by those cells. Tubulin (TUB) and p24 (CA) served as cell and viral lysate loading controls.
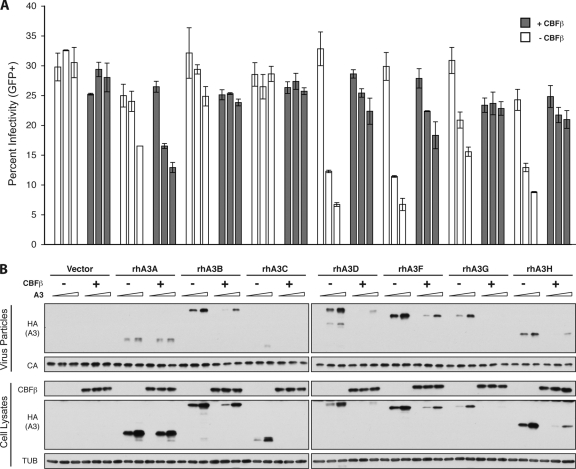
Rhesus macaques also encode seven distinct APOBEC3 proteins, of which rhesus A3D, A3F, A3G, and A3H can restrict Vif-deficient HIV-1 and SIV (5, 11). SIVmac239 Vif neutralizes all four restrictive rhesus APOBEC3 proteins and also degrades rhesus A3B and rhesus A3C (5). To determine if SIVmac239 Vif requires CBFβ to neutralize the rhesus APOBEC3 proteins, the Vif-deficient HIV-1IIIB molecular clone was transfected into HEK293T CBFβ knockdown cells alongside SIVmac239 Vif and increasing amounts of each rhesus APOBEC3 protein in the presence or absence of CBFβ isoform 2. Human CBFβ isoforms 1 and 2 are identical at the amino acid level to rhesus CBFβ isoforms 1 and 2, respectively. While there is no antibody for SIVmac239 Vif, the addition of CBFβ resulted in decreased cellular levels of all Vif-sensitive rhesus APOBEC3 proteins (rhesus A3B, A3C, A3D, A3F, A3G, and A3H) (Fig. 4). In the presence of CBFβ, packaging of rhesus A3D, A3F, A3G, and A3H was also decreased and viral infectivity consequently increased. Rhesus A3A is not sensitive to SIVmac239 Vif, and so its expression, packaging, and effect on viral infectivity were unaltered by CBFβ. Thus, SIV Vif demonstrates a conserved requirement for CBFβ to neutralize the rhesus repertoire of APOBEC3 proteins.
Fig 4.
CBFβ is required for SIV Vif to degrade all Vif-sensitive rhesus APOBEC3 proteins. (A) Percent infectivity of Vif-deficient HIV-1IIIB supplemented with SIVmac239 Vif measured by duplicate infection of CEM-GFP and flow cytometry, reported as the mean ± standard deviation of the results for the technical replicate. Constant amounts of Vif-deficient A200C HIV-1IIIB proviral construct (1 μg) were cotransfected with untagged SIVmac239 Vif (pVR1012 expression vector, 50 ng) and increasing concentrations of each HA-tagged rhesus (rh) APOBEC3 protein (0, 50, or 100 ng) in the presence or absence of CBFβ isoform 2 complementation vector (25 ng) as indicated. (B) Immunoblots of CBFβ, Vif, and the HA-tagged rhesus APOBEC3 proteins in cell lysates and of the rhesus APOBEC3 proteins in HIV particles produced by those cells. Tubulin (TUB) and p24 (CA) served as cell and viral lysate loading controls.
HIV-1IIIB Vif was previously shown to require CBFβ isoform 2 for stable expression and neutralization of A3G (6). Here, we demonstrate that both CBFβ isoform 1 and isoform 2 may be hijacked to stabilize HIV-1IIIB Vif and degrade A3G. This functional interaction was conserved across all tested HIV-1 subtypes and was required for the neutralization of not only A3G but all Vif-sensitive human APOBEC3 proteins. SIVmac239 Vif also required CBFβ to neutralize all restrictive rhesus APOBEC3 proteins. Taken together, the CBFβ-Vif interaction appears to be broadly conserved and essential for Vif function, implicating this interface as a candidate for disruption by small-molecule therapeutics that would alleviate the repression of multiple restrictive APOBEC3 proteins.
ACKNOWLEDGMENTS
We thank N. Krogan and J. Gross for discussions and data sharing prior to publication and the NIH AIDS Research and Reference Reagent Program for materials.
This research was funded by grants NIH R01 AI064046 and NIH P01 GM091743 to R.S.H. and NIH R01 AI064001 and NIH R01 AI089246 to V.S. J.F.H. was supported by an NSF predoctoral fellowship.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 28 December 2011
REFERENCES
- 1. Albin JS, Harris RS. 2010. Interactions of host APOBEC3 restriction factors with HIV-1 in vivo: implications for therapeutics. Expert Rev. Mol. Med. 12:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Binka M, Ooms M, Steward M, Simon V. 2012. The activity spectrum of Vif from multiple HIV-1 subtypes against APOBEC3G, APOBEC3F, and APOBEC3H. J. Virol. 86:49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haché G, Abbink TE, Berkhout B, Harris RS. 2009. Optimal translation initiation enables Vif-deficient human immunodeficiency virus type 1 to escape restriction by APOBEC3G. J. Virol. 83:5956–5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hemelaar J, Gouws E, Ghys PD, Osmanov S. 2011. Global trends in molecular epidemiology of HIV-1 during 2000–2007. AIDS 25:679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hultquist JF, et al. 2011. Human and rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H demonstrate a conserved capacity to restrict Vif-deficient HIV-1. J. Virol. 85:11220–11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jäger S, et al. 21 December 2011. Vif hijacks CBFβ to degrade APOBEC3G and promote HIV-1 infection. Nature [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kobayashi M, Takaori-Kondo A, Miyauchi Y, Iwai K, Uchiyama T. 2005. Ubiquitination of APOBEC3G by an HIV-1 Vif-Cullin5-Elongin B-Elongin C complex is essential for Vif function. J. Biol. Chem. 280:18573–18578 [DOI] [PubMed] [Google Scholar]
- 8. Luo K, et al. 2005. Primate lentiviral virion infectivity factors are substrate receptors that assemble with cullin 5-E3 ligase through a HCCH motif to suppress APOBEC3G. Proc. Natl. Acad. Sci. U. S. A. 102:11444–11449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Malim MH, Emerman M. 2008. HIV-1 accessory proteins—ensuring viral survival in a hostile environment. Cell Host Microbe 3:388–398 [DOI] [PubMed] [Google Scholar]
- 10. Refsland EW, et al. 2010. Quantitative profiling of the full APOBEC3 mRNA repertoire in lymphocytes and tissues: implications for HIV-1 restriction. Nucleic Acids Res. 38:4274–4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Virgen CA, Hatziioannou T. 2007. Antiretroviral activity and Vif sensitivity of rhesus macaque APOBEC3 proteins. J. Virol. 81:13932–13937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolf D, Goff SP. 2008. Host restriction factors blocking retroviral replication. Annu. Rev. Genet. 42:143–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu X, et al. 2003. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science 302:1056–1060 [DOI] [PubMed] [Google Scholar]