Abstract
Herpes simplex virus (HSV) enters cells by fusion at plasma membranes or endosomes. Cellular factors route the virus to different pathways. αVβ3-integrin directs HSV to a lipid raft and acidic endosome pathway. We report that infection mediated by nectin1 plus αVβ3-integrin exhibits the same characteristics as entry mediated by raft-located forms of nectin. αVβ3-integrin relocalizes nectin1 to lipid rafts, independently of virus. Thus, HSV routing to the lipid raft-dependent pathway is consequent to the integrin-induced relocalization of nectin1. Inhibition by the Na+/H+ exchanger 5-(N-ethyl-N-isopropyl)amirolide suggests that αVβ3-integrin overexpression favors HSV macropinocytic uptake in some cells but not in others.
TEXT
Herpes simplex virus (HSV) enters cells by a number of different routes, i.e., fusion of the virion envelope with plasma membranes or with endosomes—which can be neutral or acidic endosomes; in specialized cells, HSV enters by macropinocytosis (5, 10, 19–23). On the virus side, entry requires four essential glycoproteins, including gD as the receptor binding protein plus gH/gL and gB as the conserved fusion apparatus present in all herpesviruses (2, 7). For HSV, the different routes of entry are dictated by the cell. So far, limited attention has been paid to the cellular determinants that route HSV to one or another entry pathway. Recently, our laboratory has shown that αVβ3-integrin serves as such a determinant and routes HSV to a pathway dependent on lipid rafts, dynamin 2, and acidic endosomes (12). Specifically, CHO cells lack β3-integrin (11), including the αVβ3-integrin heterodimer. When CHO cells express nectin1 or HVEM, two of the alternative gD receptors, they enable HSV entry, implying that αVβ3-integrin is not required for HSV to enter cells. Nonetheless, this surface molecule does affect the HSV entry pathway. Specifically, in the absence of αVβ3-integrin, HSV enters CHO-nectin1 cells through a pathway independently of cholesterol-rich membrane microdomains, commonly referred to as lipid rafts (14, 26), and of dynamin2, an endocytosis GTPase responsible for the scission of newly formed vesicles, which invaginate from the plasma membrane and are then released into cytoplasm by the action of dynamin2 (14). In the presence of αVβ3-integrin, HSV enters CHO-nectin1 cells through a pathway dependent on cholesterol-rich rafts and dynamin2. Entry into CHO cells is through acidic endosomes, irrespective of the presence of αVβ3-integrin. HSV enters J-nectin1 (6) and 293T cells via a neutral pH compartment—either the plasma membrane or nonacidic endosomes—independently of cholesterol-rich rafts and dynamin2 (10, 12). When the same cells overexpress αVβ3-integrin, HSV infection is inhibited by bafilomycin A (BFLA), implying that infection takes place through an acidic compartment and is dependent on the presence of cholesterol-rich rafts and dynamin2 and is thus similar to that seen in αVβ3-integrin-positive CHO-nectin1 cells (12).
Wild-type nectin1 (wt-nectin1) exists in two isoforms named α and δ, which differ in the cytoplasmic tail; the δ isoform carries a longer C-tail and a C-ter PDZ-binding domain (2, 6, 28). Neither isoform localizes to the lipid rafts. We hypothesized that a mechanism by which αVβ3-integrin can route HSV to lipid raft- and dynamin2-dependent entry rests on redirecting nectin1, or the complex of nectin1 plus HSV, to the lipid rafts, from which location the virus is then endocytosed. The hypothesis predicts that engineered forms of nectin1 targeted to lipid rafts, in the absence of αVβ3-integrin, mimic the entry pathway dependent on lipid rafts and dynamyn 2, mediated by nectin1 plus αVβ3-integrin. We probed this hypothesis by characterizing the entry pathway mediated by nectin1-GPI (nectin1-glycosylphosphatidylinisitol) and nectin1-EGFR (nectin1-epidermal growth factor receptor). nectin1-GPI is a form of nectin1 devoid of the natural transmembrane and cytoplasmic tail and anchored to the membranes by a GPI anchor. GPI-anchored proteins are among the best available markers of cholesterol-rich rafts (26), and nectin1-GPI indeed localizes to these microdomains (10). nectin-EGFR carries the replacement of the natural nectin1 C-tail by that of EGFR (10) and was included here because EGFR is also a typical raft-located receptor (26). The previous finding that the characteristics of HSV entry into J cells mediated by wt-nectin or nectin-GPI differed with respect to inhibition by BFLA, a compound which blocks endosome acidification, is consistent with the hypothesis. BFLA did not inhibit infection of J-nectin1 cells but did inhibit infection of J-nectin-GPI cells, implying that nectin-GPI routes HSV to an acidic endosomal pathway (10).
The HSV-1 entry pathway mediated by nectin1 plus αVβ3-integrin exhibits characteristics similar to those of the entry pathway mediated by raft-located nectin1-GPI and nectin1-EGFR.
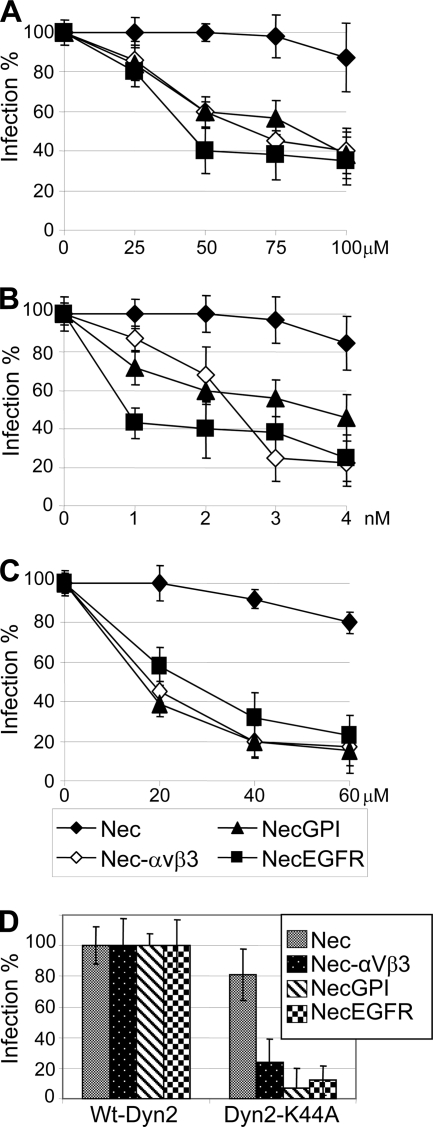
To ascertain whether entry mediated by nectin1-GPI or nectin-EGFR requires lipid rafts, CHO cells expressing nectin1 and no β3-integrin, nectin1 plus αVβ3-integrin, nectin-GPI, or nectin-EGFR (the latter two in the absence of β3-integrin) were exposed to filipin or nystatin, two cholesterol-depleting compounds, and infected with R8102, a recombinant which carries a lacZ gene under the control of the immediate early α27 promoter (6). A large body of evidence indicates that R8102 β-galactosidase (β-Gal) expression, formally used to measure α gene expression, is directly proportional to the amount of virus entered into the cell and is therefore a bona fide quantitative indicator of HSV entry (6, 8). Figure 1A and B show that HSV entry into CHO cells expressing nectin-GPI or nectin-EGFR was inhibited by nystatin or filipin in a dose-dependent fashion. The pattern of inhibition was very similar to that seen in CHO cells expressing wt-nectin1 in the presence of αVβ3-integrin and contrasted with that seen in cells expressing wt-nectin1 alone, which exhibited no inhibition. This series of results provides evidence that entry mediated by nectin plus αVβ3-integrin mimics entry mediated by nectin-GPI or nectin-EGFR into CHO cells and suggests that αVβ3-integrin may act by relocalizing nectin1 to lipid rafts.
Fig 1.
Inhibition of R8102 infection by nystantin (A), filipin (B), dynasore (C), or the dominant-negative DynK44A-GFP (D) in CHO cells expressing the following receptors: nectin1 alone (Nec), nectin1 plus αVβ3-integrin (Nec-αVβ3), nectin-GPI (NecGPI), and nectin-EGFR (NecEGFR). Cells were pretreated with inhibitors at the amounts indicated in the abscissas and infected with R8102 (3 PFU/ml) for 90 min at 37°C in the same medium. Inoculum was removed, and cells were overlaid with Dulbecco's minimal essential medium (DMEM) containing or not the appropriate inhibitor for a further 8 h. For filipin, cells were preincubated with the compound at 37°C for 30 min and infected for 30 min (30 PFU/ml) in the same medium. For nystatin, cells were preincubated with nystatin for 16 h, the inhibitor was removed, and cells were washed and infected for 60 min at 37°C (3 PFU/ml) in the absence of nystatin. With both inhibitors, infected cells were overlaid without inhibitor. For dynasore, cells were preincubated for 60 min at 37°C. (D) Cells were transfected with DynK44A-GFP or wt-DynGFP (16) encoding dynamin2 fused to green fluorescent protein (GFP) in the wt or dominant-negative (DN) version (K44A substitution) 18 h prior to infection. In all assays, each point represents averages ± standard deviations (vertical bars) of the results of experiments performed in triplicate. HSV R8102 recombinant expresses lacZ under the control of the α27 promoter (6). Infection was quantified as the β-Gal level; results are expressed as percentages compared to untreated cells.
Next, we examined whether the HSV entry pathway mediated by nectin1-GPI or nectin-EGFR requires the sealing factor dynamin2. CHO cells expressing nectin1, nectin1 plus αVβ3-integrin, nectin-GPI, or nectin-EGFR were exposed to the dynamin2 inhibitor dynasore (17) and infected with R8102. As shown in Fig. 1C, infection mediated by nectin1-GPI or nectin-EGFR was dose-dependently inhibited by dynasore, in similarity to infection mediated by wt-nectin1 plus αVβ3-integrin. To strengthen these results, cells were transfected with the dominant-negative (DN) version of dynamin 2, DynK44A-GFP, or wt-DynGFP as a control prior to infection with R8102, essentially as described previously (12). DynK44A-GFP inhibited infection relative to that seen with DynGFP in nectin1 αVβ3-integrin-expressing cells, as described previously (12), as well as in cells in which entry was mediated by nectin-GPI or nectin-EGFR (Fig. 1D). The results provide evidence that infection mediated by wt-nectin1 in the presence of αVβ3-integrin closely resembles that mediated by raft-targeted nectin-GPI or nectin-EGFR with respect to the dynamin2 requirement. It was previously shown that entry into J cells mediated by nectin-GPI and nectin-EGFR becomes sensitive to bafilomycin A and is therefore similar to entry into J cells expressing both nectin1 and αVβ3-integrin (10). Cumulatively, entry mediated by nectin1 plus αVβ3-integrin and entry mediated by nectin-GPI or nectin-EGFR exhibit the same distinguishing requirements, i.e., those for lipid rafts, dynamin2, and acidic endosomes.
αVβ3-integrin relocalizes nectin1 to lipid rafts.
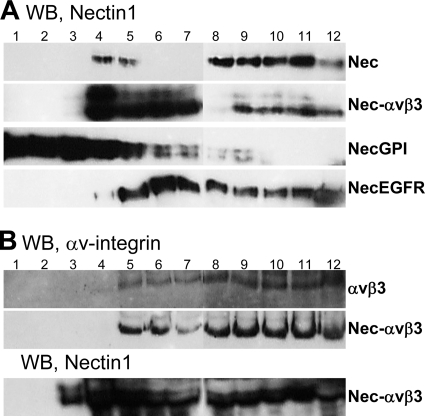
The finding that entry mediated by nectin1 plus αVβ3-integrin mimics entry mediated by raft-located forms of nectin1 raises the possibility that integrin induces a relocalization of nectin1 to lipid rafts. To verify this, we overexpressed nectin1 alone, αVβ3-integrin alone, or the combination of nectin1 plus αVβ3-integrin in 293T cells (9) and analyzed their subdomain membrane localization by means of a floatation experiment. The use of 293T cells, which were preferred because of their high level of transgene expression following transfection, was legitimate based on the previous finding that the entry pathway into 293 cells overexpressing αVβ3-integrin closely resembles that mediated by nectin1 plus integrin into CHO cells with respect to the requirement for lipid rafts, dynamin2, and acidic endosomes (12). In the floatation experiment, the cell membranes, prepared as described in reference 10, were layered at the bottom of a preformed discontinuous (5%–35%–42%) sucrose gradient. The lighter membrane fractions, which include lipid rafts, float to the medial-upper fractions, whereas the heavier membrane fractions partition toward the bottom of the gradient. The results presented in Fig. 2A show that, in cells overexpressing nectin1 alone, nectin1 partitioned predominantly to the denser, bottom fractions of the gradient (fractions 8 to 12), whereas in cells overexpressing nectin1 plus αVβ3-integrin, nectin partitioned predominantly to the medial fractions (fractions 4 to 7) and in smaller amounts to the denser fractions. In a replicate experiment (Fig. 2B), αVβ3-integrin partitioned to the medial-bottom fractions of the gradient, independently of whether it was overexpressed alone or in combination with nectin1; nectin1 partioned to the same fractions as αVβ3-integrin. The presence of a high proportion of nectin at the medial fractions was similar to that shown in panel A. nectin-GPI partitioned to the medial-upper fractions of the gradient (Fig. 2A), in agreement with a previous report (10). nectin-EGFR partitioned to the medial-bottom fractions, in similarity to the results seen when nectin1 was coexpressed with αVβ3-integrin. The electrophoretic mobility of nectin1 as a doublet was reported previously and likely reflects heterogeneity in oligosaccharide composition and maturation (27). Of note, the relocalization of nectin1 in αVβ3-integrin-overexpressing cells was prevalent, not absolute; furthermore, a small amount of nectin1 in 293T cells overexpressing nectin1 alone partitioned to the medial fractions (fractions 4 and 5), likely because 293T cells express endogenous αVβ3-integrin. Altogether, the results show that, when coexpressed with αVβ3-integrin, a large fraction of nectin1 was relocalized to the less dense membrane fractions. We note that membrane microdomains are highly dynamic structures, ranging from nanoscale assemblies to the more stable clustered platforms to complete micrometer-scale phase separations (26). The nectin-GPI marker appears to associate to the very light assemblies, whereas nectin1 plus αVβ3-integrin, similarly to nectin-EGFR, appears to associate to coalescing microdomains. Coalescence of lipid rafts is a frequent phenomenon in integrin-guided redistribution of cell surface proteins (15).
Fig 2.
Floatation of membranes from cells expressing nectin1 alone or in combination with αVβ3-integrin. 293T cells overexpressing nectin1 alone (Nec), αVβ3-integrin alone (αVβ3), nectin1 plus αVβ3-integrin (Nec-αVβ3), nectin1-GPI (NecGPI), or nectin1-EGFR (necEGFR) were solubilized in TNE buffer (10 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.036 mg/ml of each of the protease inhibitors Nα-p-tosyl-l-lysine chloromethyl ketone [TLCK; Sigma-Aldrich] and N-tosyl-l-phenylalanine chloromethyl ketone [TPCK; Sigma-Aldrich]) and layered at the bottom of a preformed 5%–35%–42% sucrose gradient, as detailed in reference 10. After centrifugation at 200,000 × g for 20 h at 4°C in a SW41 swing-out rotor, 12 fractions (1 ml) were collected from the top. Aliquots from each fraction were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). nectin1 was visualized by Western blotting (WB) with CK6 monoclonal antibody (MAb; Santa Cruz Biotechnologies) directed to the ectodomain, followed by peroxidase-conjugated anti-mouse antibody and enhanced chemiluminescence (ECL). For panel B, samples were denatured in the absence of β-mercaptoethanol to enable integrin visualization by polyclonal antibody (PAb) 1930 (Chemicon, Milan, Italy) to αV-integrin. Numbers indicate the numbers of the fractions.
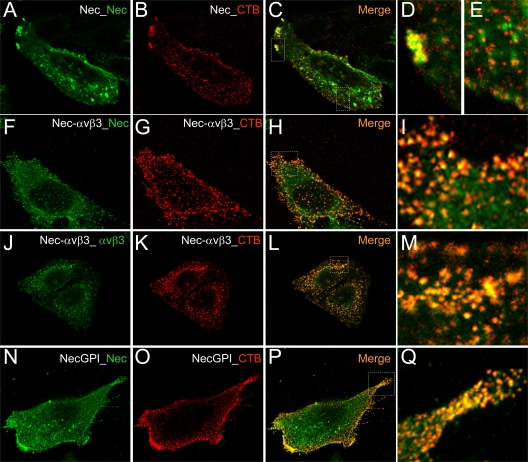
The lipid raft localization of nectin1 expressed in combination with αVβ3-integrin was further validated by confocal microscopy, with the aid of a typical raft marker, the antibody cross-linked cholera toxin B (CT-B). CT-B binds the pentasaccharide chain of ganglioside GM1, a constituent of cholesterol-rich microdomains. As shown in Fig. 3A to E, in the absence of αVβ3-integrin overexpression, a very small fraction of nectin1 colocalized with CT-B (Fig. 3E), mainly at the growing edge of the cell (Fig. 3D). In cells overexpressing αVβ3-integrin, a large fraction of nectin1 (Fig. 3F to I) as well as of αVβ3-integrin (Fig. 3J to M) colocalized with CT-B, in agreement with the floatation results. The vast majority of the surface-expressed nectin-GPI colocalized with CT-B (Fig. 3N to Q), in agreement with the floatation experiment. Overall, we observed heterogeneity in the extent to which cells bind CT-B; for heuristic purposes, we show here cells with high CT-B uptake.
Fig 3.
Localization of nectin1 and αVβ3-integrin at lipid rafts, as detected by colocalization with CT-B (cholera toxin B) by confocal microscopy. CHO cells transiently overexpressing nectin1 alone (Nec) (A to E), nectin1 plus αVβ3-integrin (Nec-αVβ3) (F to M), or nectin-GPI (NecGPI) (N to Q) were labeled using a Vybrant lipid raft-labeling kit (Invitrogen). Live cells were incubated for 10 min at 4°C with recombinant CT-B, rinsed several times with chilled phosphate-buffered saline, and incubated with anti-CT-B rabbit antibody for 15 min at 4°C. After several rinses, cells were paraformaldehyde fixed, permeabilized with Triton X-100, and then stained for the presence of nectin1 (green) (A, F, and N) by means of MAbs R1.302 and CK35 for nectin1 or MAb L230 for αVβ3-integrin (J), followed by anti-mouse fluorescein isothiocyanate-coupled antibody. CT-B was stained with anti-rabbit DyLight 549 (red) (B, G, K, and O). Cells were mounted with Fluoromount and observed with a Leica TCS-SL confocal microscope. Images were collected with a 63× oil immersion objective (numerical aperture, 1.62); confocal slices were 0.7 to 1.5 μm thick; confocal microscopy was performed as previously detailed (1). Panels C, H, L, and P show merged images. Panels D, E, I, M, and Q show enlargements of the white dotted rectangles in panels C, H, L, and P, respectively.
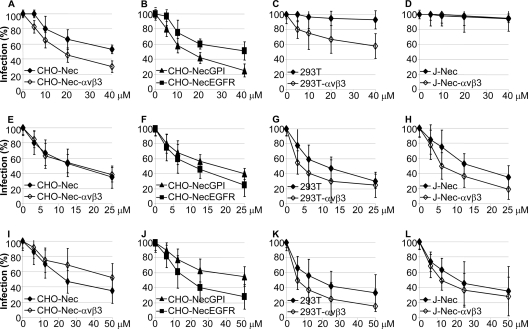
An open question is whether the integrin-mediated HSV entry via lipid rafts occurs by macropynocytosis. One argument against this possibility is the observed requirement for dynamin2, as this GTPase does not generally participate in macropinocytosis-mediated virus entry (18). To address this issue, we measured the effect of EIPA [5-(N-ethyl-N-isopropyl)amirolide], a Na+/H+ exchanger considered to a be a diagnostic inhibitor. Rottlerin and genistein, inhibitors of protein kinase Cδ (PKCδ) and of tyrosine kinases, respectively, were included in these assays, as entry by macropinocytosis usually requires these activities (18). CHO cells expressing nectin1 with or without αVβ3-integrin, nectin1-GPI, and nectin-EGFR, 293T cells (wt or overexpressing αVβ3-integrin), and J-nectin1 cells (wt or overexpressing αVβ3-integrin) were exposed to increasing concentrations of inhibitors. The results presented in Fig. 4 show that EIPA inhibited HSV infection to a great extent in CHO-nectin1 cells, i.e., in the absence of αVβ3-integrin; the presence of integrin increased inhibition to a small extent. In J-nectin1 cells, EIPA did not exert any inhibition, irrespective of the presence or absence of integrin. In 293T cells, αVβ3-integrin overexpression conferred about 40% inhibition by EIPA; this percentage is in contrast to the dramatic changes induced by αVβ3-integrin overexpression with respect to cholesterol depletion and dynamin2 dependency (12). The extent of EIPA inhibition in αVβ3-integrin-overexpressing 293T cells was similar to that reported for keratinocyte cultures (25). Inhibition by genistein and rottlerin highlighted a requirement for cellular tyrosine phosphorylation and PKCδ, was scarcely affected by the presence or absence of αVβ3-integrin, and took place also in J-nectin cells, which were unaffected by EIPA. Based on these results, αVβ3-integrin may favor partial HSV uptake by macropinocytosis in some cells but not in others; some cells exhibit EIPA sensitivity even in the absence of αVβ3-integrin.
Fig 4.
Effect of EIPA (A to D), rottlerin (E to H), and genistein (I to L) on R8102 infection of CHO or J cells expressing nectin1α with or without αVβ3-integrin, nectin-GPI, and nectin-EGFR and of 293T cells (wt or overexpressing αVβ3-integrin). Cells were exposed to the indicated concentrations of inhibitors from 1 h prior to infection with R8102 (3 PFU/ml) and harvested at 6 h after infection. For all assays, each point represents the averages ± standard deviations (vertical bars) of the results of experiments performed in triplicate. Infection was quantified as β-Gal levels and is expressed as a percentage compared to untreated cells, as detailed in the legend to Fig. 1. For all inhibitors, toxicity was measured simultaneously with the effect of the inhibitors on virus infection by adding 10% AlamarBlue (Invitrogen) to replicate specimens in DMEM lacking phenol red from time zero until harvesting and optical reading at 570 and 600 nm, as described previously (12). Cytotoxicity, expressed as the percentage of nonviable cells at 25 μM concentration, ranged between 1% and 5%.
Conclusions.
αVβ3-integrin routes HSV to a entry pathway dependent on the presence of lipid rafts, dynamin2, and acidic endosomes. Here, we provide evidence that the underlying mechanism is relocalization of nectin1 to lipid rafts. The evidence is 3-fold. First, the nectin1- and αVβ3-integrin-dependent pathway exhibits the same requirements as the pathway mediated by nectin-GPI or nectin-EGFR, i.e., dependency on lipid rafts, dynamin2 and acidic endosomes. Previously, it was shown that nectin-GPI indeed localizes to lipid rafts, a typical feature of GPI-anchored proteins (10); here we confirm that nectin-EGFR also localizes to lipid rafts. Second, in floatation experiments, when expressed together with αVβ3-integrin, a large portion of nectin1 partitioned with the less dense membrane fractions, which typically included the lipid rafts. In contrast, when αVβ3-integrin was not overexpressed, nectin1 partitioned to the denser fractions of the gradient. Third, when expressed with αVβ3-integrin, part of the nectin1 colocalized with the lipid raft marker CT-B, a molecule known to bind GM1, a lipid constituent of lipid rafts. Importantly, the αVβ3-integrin-dependent relocalization of nectin1 to lipid rafts was observed in uninfected cells, and therefore it is a cell-dependent phenomenon, induced by αVβ3-integrin, that is independent of HSV-1 binding to nectin1.
The cell-guided relocalization of nectin to lipid rafts in a αVβ3-integrin-dependent fashion is a novelty. The properties of nectin and integrins may well account for this phenomenon. Indeed, in the well-documented system of formation of adherens junctions, nectin1 affects the localization of αVβ3-integrin and vice versa (28). Specifically, nectin1 is a constituent of and guides the formation of adherens junctions in polarized epithelial cells. nectin1 homo- and heterotransdimerizes with other nectins in opposing cells, plays an important role in the integration of integrin at the cell-cell adhesion sites of contacting cells and at the leading edges of moving cells, and is thus involved in fundamental cellular functions together with integrin. With respect to the ability of integrins to organize and concentrate receptors at the lipid raft platforms, a notable example is the stabilization of the immunological synapse. In that example, T cells first interact via the T-cell receptor with major histocompatibility complex class II (MHC-II) on antigen-presenting cells; the β2 integrin LFA-1 (lymphocyte function-associated antigen 1; also named antigen CD11A) expressed on T cells is then activated through an inside-out signaling process that increases LFA-1's avidity for its ICAM (intercellular adhesion molecule) ligand on the adjacent antigen-presenting cell, thus leading to the formation of stable conjugates (26, 29). A further consideration is the fact that a number of viruses make use of lipid rafts as gateways to the cell. In general, this reflects the localization of the viral receptors. With respect to herpesviruses, the involvement of integrin, not as a primary HSV receptor but as a routing factor, and the concomitant role of lipid rafts as the site of entry differ from the role of integrins and lipid rafts in the infection of Kaposi's sarcoma-associated herpesvirus (KSHV) and Epstein-Barr virus (EBV). Both of those viruses exploit integrins as entry receptors, interacting with gB and gB/gL, respectively (3, 4). Interestingly, KSHV infection is inhibited by drugs that affect lipid raft integrity in a cell line-dependent manner; inhibition occurs in some but not all endothelial cells and not in fibroblasts (3). This route of entry does not require dynamin2. EBV entry into B cells is inhibited by lipid raft disruption through methyl-β-cyclodextrin and nystatin (13). Whether this reflects a requirement for integrin localization at lipid rafts remains to be investigated. With respect to viruses other than herpesviruses, an interesting case in point is human immunodeficiency virus (HIV). The raft-located CD4 interacts with and induces conformational changes to virion gp120. The coreceptor CXCR4 is not located at the raft. By lateral mobility, the CD4-modified gp120 recruits CXCR4 to the periphery of the raft, and the unburied V3 loop of gp120 interacts with it (24). In this example, the coreceptor becomes recruited to the periphery of the raft by the virion glycoprotein. The notable difference from the mechanism described here with nectin1 is that the relocalization of nectin1 enabled by αVβ3-integrin is independent of the presence of HSV.
ACKNOWLEDGMENTS
This work was supported by Investigator grants to G.C.-F. from AIRC (Associazione Italiana per la Ricerca sul Cancro) Milan, by Fondi Roberto and Cornelia Pallotti from the Department of Experimental Pathology, University of Bologna, by PRIN projects from the Italian Ministry for University and Research, and by the University of Bologna RFO (Ricerca Fondamentale Orientata).
Footnotes
Published ahead of print 14 December 2011
REFERENCES
- 1. Avitabile E, Forghieri C, Campadelli-Fiume G. 2009. Cross talk among the glycoproteins involved in herpes simplex virus entry and fusion: the interaction between gB and gH/gL does not necessarily require gD. J. Virol. 83:10752–10760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campadelli-Fiume G, et al. 2007. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev. Med. Virol. 17:313–326 [DOI] [PubMed] [Google Scholar]
- 3. Chandran B. 2010. Early events in Kaposi's sarcoma-associated herpesvirus infection of target cells. J. Virol. 84:2188–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. 2009. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins {alpha}v{beta}6 or {alpha}v{beta}8. Proc. Natl. Acad. Sci. U. S. A. 106:20464–20469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clement C, et al. 2006. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J. Cell Biol. 174:1009–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992–10002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. 2011. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 9:369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618–1620 [DOI] [PubMed] [Google Scholar]
- 9. Gianni T, Amasio M, Campadelli-Fiume G. 2009. Herpes simplex virus gD forms distinct complexes with fusion executors gB and gH/gL through the C-terminal profusion. J. Biol. Chem. 284:17370–17382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gianni T, Campadelli-Fiume G, Menotti L. 2004. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J. Virol. 78:12268–12276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gianni T, et al. 2010. Herpes simplex virus glycoproteins H/L bind to cells independently of {alpha}V{beta}3 integrin and inhibit virus entry, and their constitutive expression restricts infection. J. Virol. 84:4013–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gianni T, Gatta V, Campadelli-Fiume G. 2010. {alpha}V{beta}3-integrin routes herpes simplex virus to an entry pathway dependent on cholesterol-rich lipid rafts and dynamin2. Proc. Natl. Acad. Sci. U. S. A. 107:22260–22265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katzman RB, Longnecker R. 2003. Cholesterol-dependent infection of Burkitt's lymphoma cell lines by Epstein-Barr virus. J. Gen. Virol. 84:2987–2992 [DOI] [PubMed] [Google Scholar]
- 14. Lajoie P, Nabi IR. 2010. Lipid rafts, caveolae, and their endocytosis. Int. Rev. Cell Mol. Biol. 282:135–163 [DOI] [PubMed] [Google Scholar]
- 15. Lee JL, Wang MJ, Sudhir PR, Chen JY. 2008. CD44 engagement promotes matrix-derived survival through the CD44-SRC-integrin axis in lipid rafts. Mol. Cell. Biol. 28:5710–5723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Llorente A, Rapak A, Schmid SL, van Deurs B, Sandvig K. 1998. Expression of mutant dynamin inhibits toxicity and transport of endocytosed ricin to the Golgi apparatus. J. Cell Biol. 140:553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Macia E, et al. 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10:839–850 [DOI] [PubMed] [Google Scholar]
- 18. Mercer J, Helenius A. 2009. Virus entry by macropinocytosis. Nat. Cell Biol. 11:510–520 [DOI] [PubMed] [Google Scholar]
- 19. Mercer J, Schelhaas M, Helenius A. 2010. Virus entry by endocytosis. Annu. Rev. Biochem. 79:803–833 [DOI] [PubMed] [Google Scholar]
- 20. Milne RS, Nicola AV, Whitbeck JC, Eisenberg RJ, Cohen GH. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J. Virol. 79:6655–6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicola AV, Hou J, Major EO, Straus SE. 2005. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J. Virol. 79:7609–7616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicola AV, McEvoy AM, Straus SE. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324–5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicola AV, Straus SE. 2004. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J. Virol. 78:7508–7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Popik W, Alce TM, Au WC. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J. Virol. 76:4709–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rahn E, Petermann P, Hsu MJ, Rixon FJ, Knebel-Morsdorf D. 2011. Entry pathways of herpes simplex virus type 1 into human keratinocytes are dynamin- and cholesterol-dependent. PLoS One 6:e25464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simons K, Gerl MJ. 2010. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 11:688–699 [DOI] [PubMed] [Google Scholar]
- 27. Stiles KM, Milne RS, Cohen GH, Eisenberg RJ, Krummenacher C. 2008. The herpes simplex virus receptor nectin-1 is down-regulated after trans-interaction with glycoprotein D. Virology 373:98–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takai Y, Miyoshi J, Ikeda W, Ogita H. 2008. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat. Rev. Mol. Cell Biol. 9:603–615 [DOI] [PubMed] [Google Scholar]
- 29. Yokosuka T, et al. 2005. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 6:1253–1262 [DOI] [PubMed] [Google Scholar]