Abstract
Norovirus (NoV) is an emerging RNA virus that has been associated with global epidemics of gastroenteritis. Each global epidemic arises with the emergence of novel antigenic variants. While the majority of NoV infections are mild and self-limiting, in the young, elderly, and immunocompromised, severe and prolonged illness can result. As yet, there is no vaccine or therapeutic treatment to prevent or control infection. In order to design effective control strategies, it is important to understand the mechanisms and source of the new antigenic variants. In this study, we used next-generation sequencing (NGS) technology to investigate genetic diversification in three contexts: the impact of a NoV transmission event on viral diversity and the contribution to diversity of intrahost evolution over both a short period of time (10 days), in accordance with a typical acute NoV infection, and a prolonged period of time (288 days), as observed for NoV chronic infections of immunocompromised individuals. Investigations of the transmission event revealed that minor variants at frequencies as low as 0.01% were successfully transmitted, indicating that transmission is an important source of diversity at the interhost level of NoV evolution. Our results also suggest that chronically infected immunocompromised subjects represent a potential reservoir for the emergence of new viral variants. In contrast, in a typical acute NoV infection, the viral population was highly homogenous and relatively stable. These results indicate that the evolution of NoV occurs through multiple mechanisms.
INTRODUCTION
Norovirus (NoV) is a rapidly evolving RNA virus that causes global epidemics of acute gastroenteritis (8, 37, 57, 59) approximately biennially since 2002 (59). These global epidemics are associated with the emergence of novel, antigenically distinct variants of the genogroup II, genotype 4 (GII.4), lineage that cause significant morbidity, particularly in the young, elderly, and immunocompromised (37, 57).
NoV has a single-stranded RNA genome of approximately 7.5 kb that is divided into three open reading frames (ORFs) (30). ORF1 encodes all nonstructural proteins involved in viral replication (4). ORF2 is the most well-characterized region of the NoV genome, as it encodes the viral capsid protein VP1, which contains the antigenic domains and the receptors that determine viral entry. VP1 itself can be divided into three structural domains (50). A conserved shell domain exists at the N-terminus, leading into a protruding central stem, the P1 domain, which has a hypervariable insert termed the P2 domain. The P2 domain is the most surface-exposed region of the viral capsid and is therefore believed to be involved in immune escape from neutralizing antibodies (2, 18, 36–38). The P2 domain also contains residues involved in histo-blood group antigen (HBGA) binding (12, 55, 64). These polymorphic carbohydrates are thought to be attachment factors for NoV (43, 55). ORF3 encodes a small basic protein, VP2. Although the exact function of VP2 is yet to be determined, it is believed to support viral capsid assembly through the stabilization of VP1 (5).
Despite large amounts of sequence diversity, approximately 5% nucleotide differences across ORF2, arising among the global outbreak GII.4 variants, minimal diversity has been observed within a global outbreak season, which raises the question of where these new variants originate from. The interhost evolutionary trends of NoV have been frequently compared to those of influenza virus (58). However, for influenza virus, in addition to viral diversity generated from infections within the human population, new variants also emerge from zoonotic sources following reassortment events between human and avian and/or swine strains, such as with the emergence of the swine-origin H1N1 2009 pandemic strain (60). NoV strains have been identified in a wide range of animals, including pigs, cows, dogs, sheep, and mice (31, 39, 44, 67, 69). Furthermore, human NoVs have been shown to infect some nonhuman primates and pigs under experimental conditions (7, 52, 62). Despite this, no example of zoonotic transmission from an animal to a human has been reported. Therefore, current evidence suggests that the evolution of human NoV variants is confined to the human population. Analogous to reassortment in influenza viruses, NoV has a mechanism of recombination that facilitates the interchange of nonstructural and structural genomic regions at the ORF1/2 overlap when coinfection occurs (9, 11). The exchange of antigenic elements through recombination at the capsid P1/P2 domain boundaries has also been reported (38). Therefore, recombination is likely to be an important mechanism for the emergence of new NoV variants.
In addition to understanding the impact of recombination on NoV evolution, it is also important to understand NoV between-host dynamics, as transmission events will determine which variant will persist in the host population. As determined by evolutionary studies of human immunodeficiency virus (HIV) and hepatitis C virus (HCV), a strong genetic bottleneck occurs following a transmission event, where on average only 1 to 3 viruses are transmitted to the new host (10, 23, 27, 33, 53). Strong functional constraints on the transmitted variants are believed to drive this bottleneck event (reviewed in reference 32). However, both HIV and HCV are associated with chronic infection. In-depth viral population analyses of acute viral infections caused by rhinovirus and equine influenza virus revealed that transmission events were not characterized by strong genetic bottlenecks but rather by the coinfection of a cloud of closely related variants (16, 46).
Intrahost dynamics are another source of NoV genomic diversification. It has been suggested that persons chronically infected with NoV may be a source of new variants as mutations accumulate over the course of infection (56). However, currently, very little is known about the patterns of evolution at the intrahost level and how this contributes to the overall evolution at the interhost level and the subsequent emergence of new global epidemic variants. Of the studies that have examined NoV intrahost evolution to date, the majority have been performed on immunocompromised individuals with chronic NoV infection (14, 54, 56). Despite its clinical significance, the prevalence of chronic NoV infection in the population is unknown. To date, chronic NoV infections have been identified in a range of settings where the immune status of an individual was compromised, such as transplant recipients, HIV-positive individuals, and patients with leukemia (6, 13, 54, 68). In addition, all previous studies of NoV evolution have used only traditional sequencing methods, such as Sanger sequencing (1, 14, 58, 66, 71). No study has looked in depth at the intrahost evolution of an acute or chronic NoV infection at high resolution using next-generation sequencing (NGS) methods. As most NoV infections within an epidemic season will occur among healthy individuals, quantifying and understanding the extent of diversification generated by the viral population within an individual host are important for determining the source of NoV diversity. The development of NGS enables an in-depth understanding of viral population dynamics (10, 51). For example, NGS of influenza virus has revealed that drug-resistant variants are present in the viral population at low levels and emerge only when drug therapy is initiated (24). This technology can therefore be applied to test whether novel NoV variants are already present at low levels in the viral population, and under the right selection pressure, an alternative variant could emerge to dominate the interhost population.
In terms of NoV evolution, an understanding of transmission events and the potential differences between acute and chronic infections are important for vaccine development and antiviral therapy design. Given the fact that NoV vaccines have entered the initial stages of clinical trials, the need for this information is imminent. Therefore, in this study, we investigated the evolutionary dynamics of NoV during a transmission event in a typical acute NoV infection and also an atypical chronic NoV infection in an immunocompromised host.
MATERIALS AND METHODS
Cohort. (i) Transmission cohort.
Stool specimens were collected from three subjects, all from the same family, where a 5-year-old boy (subject DS) was known to infect both his father (subject RF) and grandfather (subject RG) within a week after the presentation of symptoms (Table 1).
Table 1.
Description of the transmission cluster cohort
| Subject | Subject ID | Clinical description | Virus |
|---|---|---|---|
| Donor, son | DS | A young boy returned from child care with symptomatic acute gastroenteritis; the father and grandfather were then exposed to vomitus and stools that day within a similar time frame | GII.g/GII.12 |
| Recipient, father | RF | ||
| Recipient, grandfather | RG |
(ii) Longitudinal cohort.
Longitudinal samples were collected from two deidentified subjects through the Department of Microbiology, SEALS, Prince of Wales Hospital, Sydney, Australia. One subject had a typical acute NoV infection (subject Ac), and the other had an atypical chronic NoV infection (subject Ch). Two stool specimens were collected from the acute subject at day 1 (sample Ac_1) and day 10 (sample Ac_10), while the chronic subject had three stool specimens, collected at day 1 (sample Ch_1), day 4 (sample Ch_4), and day 288 (sample Ch_288) (Table 2).
Table 2.
Description of the longitudinal study cohort
| Type of infection | Subject | Day of specimen collection | Specimen | Clinical description of infection | Virus |
|---|---|---|---|---|---|
| Acute | Ac | 1 | Ac_1 | Nosocomial infection of immunocompetent individual | GII.4 2006b |
| 10 | Ac_10 | ||||
| Chronic | Ch | 1 | Ch_1 | Chronic infection of infant with a severe undefined immunodeficiency | GII.4 2006b |
| 4 | Ch_4 | ||||
| 288 | Ch_288 |
Day 1 was defined as the first day of collection and not the first day of disease onset. All stool specimens were collected between 2010 and 2011 and then stored at −20°C until required.
NoV capsid RT-PCR.
Viral RNA was extracted from 20% (vol/vol) suspensions using the QIAamp viral RNA minikit (Qiagen, Hilden, Germany) and initially genotyped as previously described (21). A one-step reverse transcription-PCR (RT-PCR) was then employed to amplify a region of the NoV genome that included the complete ORF2 (which encodes VP1) and partial ORF3 using the SuperScript III One-Step RT-PCR system with Platinum Taq high-fidelity polymerase (Invitrogen, Carlsbad, CA) and the primers described in Table 3. The RT-PCR products (∼1.9 kb) were gel purified by using the QIAquick gel extraction kit (Qiagen) and then quantified by using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE).
Table 3.
Oligonucleotides used in this study
| Primer | Sequence (5′–3′)a | Orientation | Positionb | Genotype |
|---|---|---|---|---|
| GV305 | CAGRCAAGAGCCAATGTTCAGATGG | Sense | 4999 | GII.4 |
| GV306 | GGCCTCAATTTGTGCTTGGAGC | Antisense | 6916 | GII.4 |
| GV308 | GCTTGGAGCATCTCTTTRTCATG | Antisense | 6903 | GII.4 |
| GV315 | CAAGGCAAGAGCCAATGTTTCGATGG | Sense | 5004 | GII.g |
| GV316 | CTGGTGATGATGTATTTACTGTCTCC | Sense | 5662 | GII.12 |
| GV317 | GTGGCTYGAATTTGAGCTTGCAGC | Antisense | 6876 | GII.12 |
DNA sequencing and analysis.
Purified PCR products were sequenced directly with an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA) using dye terminator chemistry. Pairwise alignments of DNA and protein sequences and evolutionary distances between sequences were determined by using programs within MEGA 5 (63).
Protein structure homology modeling of the viral capsid was performed by generating a Protein Data Bank (PDB) file from the amino acid sequence in FastA format using software at the Swiss-Model server of the Swiss Institute of Bioinformatics (3, 34, 48). Protein structures were then visualized and manipulated by using PyMOL v1.4.1 (Schrodinger, Portland, OR). Informative site analysis of the ORF2 coding region was performed by using the DIVEIN Web server (19).
Roche FLX sequencing.
Purified RT-PCR amplicons were submitted for library preparation before subsequent NGS (42), using a 454 Roche FLX Titanium at Murdoch University, Perth, Australia. Samples were bar coded and then combined in one lane on an eight-gasket plate. Analysis of the 454 data was conducted as previously described (10). In brief, sequence reads were removed prior to the assembly stage if they were shorter than 55 bp and had an average quality score of <20. The terminal 20 nucleotides (nt) were removed from all remaining reads. These remaining sequences were aligned with a nucleotide identity threshold of 95% against the unique consensus sequence for each subject with the alignment tool MOSAIK (http://bioinformatics.bc.edu/marthlab/Mosaik). The consensus sequence for each subject was derived from the sequencing of the gel-purified RT-PCR products on the ABI 3730 DNA analyzer. The quality of the aligned file was assessed, and reads were excluded from the alignments according to standards outlined previously (10).
Cloning and colony PCR.
The same purified ORF2/partial ORF3 RT-PCR products submitted for 454 sequencing were TA cloned by using the pGEM-T Easy vector system (Promega, Fitchburg, WI). Individual colonies were screened by PCR for inserts of the appropriate size with vector-specific primers. Five positive amplicons from each sample were then purified by using ExoSAP-IT (GE Life Science, Uppsala, Sweden) and sequenced directly.
SNP detection and haplotype reconstruction.
Single nucleotide polymorphism (SNP) detection from the aligned 454 reads was performed with the SNP caller VarScan (35). A further manual check was performed around homopolymeric regions. Additionally, since 454 sequencing has an intrinsic error rate of 1% (25), a minimum quality score threshold was assigned for SNP calling. The quality score combines a variety of information on noise, false calls, and errors from homopolymeric stretches to produce a probability-of-error value using the same phred algorithm employed for Sanger sequencing (22). The quality scores range from 0 (worst) to 40 (best). Most studies use a cutoff of 20 (reviewed in reference 47), since this score represents an accuracy of at least 99% (42). In this study, a conservative approach was taken, and the threshold was raised to 25, as this was the median score (25) of all the individual bases in the reads. Aligned 454 reads were further analyzed with a Bayesian probabilistic method implemented in the software package ShoRAH (72), as previously described (10). This software was used to reconstruct NoV haplotypes (variants) over the capsid domain (∼1,620 nt in length for the ORF2 coding region) and to estimate the frequency of the occurrence of each reassembled variant within the sample. A window size of 330 and a step size of 110 were used. The resulting population of capsid variants was compared to the available sequences obtained by standard cloning and sequencing. The ShoRAH analyses were performed in triplicate for each data set to ensure that the stochastic nature of Bayesian statistics based on Monte Carlo Markov chain simulations was not affecting the results. Only SNPs and variants detected in all the three simulation runs with a frequency of >2.5% were considered for further analyses, as it was previously shown that chimeric variants may be reconstructed below this frequency (see reference 10 for a detailed explanation of these parameters and a validation of the haplotype reconstruction method).
Phylogenetic analyses.
Sequences from standard cloning and reconstructed haplotypes were visualized and curated with the MEGA 5 and R packages. Phylogenetic and evolutionary analyses, including the detection of recombination, were performed with PhyML (28), MEGA 5, and RDP3 (45). Trees were constructed from sequences by using the best-fit model determined by JModelTEST according to the Akaike Information Criterion with Correction (AICc) (49). Trees were visualized with FigTree. Group mean amino acid distances were calculated with MEGA 5 using the Poisson model with uniform rates. Sequence alignments of reconstructed haplotypes and clonal data can be obtained from the authors upon request.
RESULTS
Subjects.
Five subjects were selected for a detailed analysis of the intrahost NoV population (Tables 1 and 2). Three of these subjects were infected with a recombinant GII.g/GII.12 NoV (St. George virus, NSW199U/2008/AU; GenBank accession number GQ845370) and were a transmission cluster, where the son, the donor (subject DS), infected two recipients, the father (subject RF) and grandfather (subject RG). A single sample was collected for each of these three subjects (Table 1). The remaining two subjects, subjects Ac and Ch, were infected with a NoV GII.4 2006b variant (Fig. 1). Subject Ac had a typical acute infection, and two samples were collected 9 days apart, designated samples Ac_1 and Ac_10 in this study (Table 2). In contrast, subject Ch was an infant (<2 years of age) persistently infected with the same NoV GII.4 2006b variant (Fig. 1). This child had an underlying immune deficiency of an unknown type, manifesting clinically with hepatomegaly and anemia and treated long term with adalimumab (a tumor necrosis factor alpha [TNF-α] inhibitor) for the control of inflammation-induced hepatitis. Three samples from subject Ch were collected, at day 1 (sample Ch_1), day 4 (sample Ch_4), and day 288 (sample Ch_288) (Table 2).
Fig 1.
Phylogenetic comparison of the GII.4 ORF2 nucleotide sequence isolated longitudinally from subjects with acute and chronic NoV infections. The full-length ORF2 consensus sequences, determined by bulk sequencing, were generated at two time points (ranging 10 days) for the subject with acute infection (green) and at three time points (ranging 288 days) for the subject with chronic infection (blue). In addition, ORF2 sequences from GII.4 epidemic variants detected in NSW, Australia, during 2006 to 2011 and reference sequences derived from GenBank were included in the analysis (n = 248). Each major GII.4 clade was described previously (59), except for the 2009 and 2010 GII.4 variants, which recently emerged. Both subjects were infected with variants that clustered within the GII.4 2006b variant clade (red). For the subject with acute infection, the consensus sequence was identical at both time points. For the subject with chronic infection, the ORF2 sequence at the third time point differed by 4.1% compared to the sequence from the first two time points. The tree shows that subject Ch was persistently infected with the same variant and not reinfected with another circulating 2006b variant. The distance scale represents the number of nucleotide substitutions per position.
Sequence analysis.
NGS was performed on the amplicons generated from the RT-PCR amplification of the ORF2/partial ORF3 region of the NoV genome for each of the eight samples. The NGS run was performed twice on the samples, and the two runs combined produced a total of 69,154 reads (28.5 Mb), with a median read length of 412 bp and a median average quality score per site of 25. Any reads that were shorter than 55 bp were automatically removed and excluded from further analyses. The remaining reads were aligned with a sequence identity threshold of >95% against the consensus sequence for each sample, which was generated by the bulk sequencing of the RT-PCR product. This resulted in the alignment of 16.7 Mb, with an average number of bases aligned per site of 950 (range, 231 to 1,892). For comparison, regions amplified from the eight samples were also cloned, and five colonies were isolated and sequenced.
Intrahost population diversity.
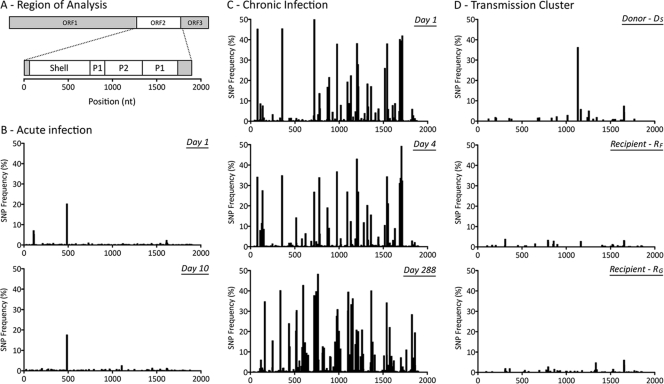
SNP analysis was performed across the full-length ORF2 and partial ORF3 regions for all eight samples isolated from the five subjects (Fig. 2). The analysis revealed that the viral population was highly homogenous within the four subjects with typical acute NoV infections (subjects DS, RF, and RG and both time points for subject Ac, i.e., samples Ac_1 and Ac_10) (Fig. 2B and D). For these four subjects, only 5 to 8 SNPs occurred at frequencies above 2%. Approximately half, 46 to 64%, of the SNPs detected were nonsynonymous.
Fig 2.
Distribution of single nucleotide polymorphisms (SNPs) detected from the 3′ end of ORF1 to the 5′ end of ORF3. (A) The NoV genomic region analyzed and domains of interest within ORF2, which are shown across the x axes of panels B to D. (B) Distribution of SNPs for subject Ac (acute infection), measured 9 days apart. (C) Distribution of SNPs for subject Ch (chronic infection) at days 1, 4, and 288. (D) Distribution of SNPs for the transmission cluster, involving three acutely infected family members. The donor (subject DS) transmitted the virus to two recipients, subjects RF and RG. A single sample was collected from each infected subject at each time point during the acute stage. The distribution of SNPs, portrayed as a percentage of the viral population, indicates that intrahost viral populations were homogenous for the four acutely infected subjects, with only a few prevalent SNPs (>10%) (B and D). In contrast, subject Ch (C) presented a heterogeneous intrahost population over the course of the infection. Multiple SNPs with a frequency of >10% in the viral population were distributed across the entire length analyzed.
In contrast, for the fifth subject, subject Ch, who had an atypical chronic NoV infection, the population had greater heterogeneity, with 48, 59, and 109 SNPs being detected above a frequency of 2% for the three time points, in samples Ch_1, Ch_4, and Ch_288, respectively (Fig. 2C). Almost half, 34 to 48%, of these SNPs detected were nonsynonymous. The high-frequency SNPs (>2%) were randomly distributed and did not localize to any particular region within ORF2 or ORF3 (Fig. 2).
Transmission-driven NoV evolution.
A transmission cluster was analyzed to investigate the impact of the transmission event on NoV evolution. The transmission cluster consisted of a son (subject DS), who infected his father (subject RF) and grandfather (subject RG) with a NoV GII.g/GII.12 strain (Table 1).
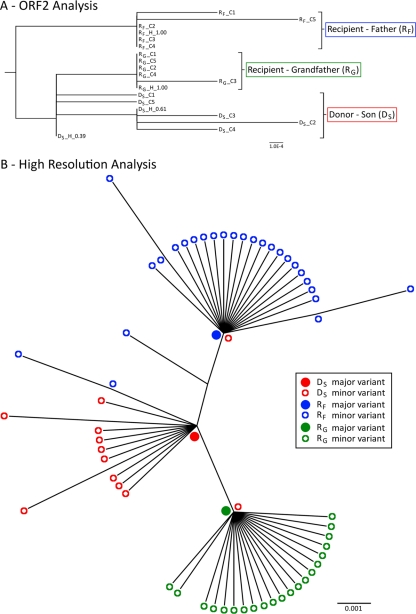
NGS analysis was performed at a single time point with an isolate from each of the three subjects. As shown in Fig. 2D, these three subjects had a highly homogenous population, with very low numbers of SNPs detected. In order to compare the infecting strain among the three subjects, the variants spanning the full-length ORF2 and the first 102 nucleotides of ORF3 with a frequency above 2.5% were reassembled from NGS reads. In the donor subject, two ORF2/3 variants were reconstructed with frequencies of ∼61% and ∼38%. These two variants differed by only one synonymous substitution (nt 1048 with reference to the start site of ORF2). For the two recipients, distinct ORF2/3 variants with frequencies of ∼99% were found. A phylogenetic analysis of the interhost sequences from the NGS and clonal data revealed that the recipients, subjects RF and RG, were not infected with either of the two major variants present in the donor (Fig. 3A). The dominant variants present in the two recipients, subjects RF and RG, were most similar to the minor variant (39%) in donor subject DS but differed at two and one nucleotide sites, respectively. The nucleotide difference between subjects DS and RG was located in ORF3 and was nonsynonymous (residue 26). The two nucleotide differences between isolates from subjects DS and RF were both located in ORF2 and were also nonsynonymous. One caused a mutation within the base of the VP1 protein, residue 509, and the other caused a mutation in residue 347, which is located within the P2 domain and lies adjacent to the HBGA binding site.
Fig 3.
Phylogenetic analysis of sequences from the NoV transmission cluster. (A) Phylogenetic tree of the full-length ORF2 sequences generated from reassembled short NGS reads and from cloning. Sequences are labeled first by their subject name, followed by whether they were generated by cloning (C) or NGS haplotype reconstructions (H). The haplotype frequency is also included at the end of the name. In this cluster, the donor (subject DS) had two closely related variants that were present at high frequencies (38 and 61%) and were identified by both NGS and cloning. However, neither of these two donor variants were found in the viral populations of the two recipients, and each subject's sequences clustered separately. The distance scale represents the number of nucleotide substitutions per position. (B) High-resolution phylogenetic analysis of a region spanning the 3′-terminal 171 nucleotides of ORF2 to the 5′-terminal 133 nucleotides of ORF3. This analysis was performed with NGS data and revealed substantial interhost diversity. For each subject (subjects DS, RF, and RG), the major variant was located at the node of the branch, with minor variants branching from it, indicating that the minor variants had evolved from the dominant variant. In addition, each recipient's major variant was found to be identical to a unique minor variant (<0.01%) isolated from the donor (subject DS). The donor's major variant was not identified in any of the recipient variants at frequencies as low as 0.01%. Filled circles represent major variants within a population, and open circles represent minor variants. Red indicates the donor son (subject DS), while blue represents the recipient father (subject RF) and green represents the recipient grandfather (subject RG). The distance scale represents the number of nucleotide substitutions per position.
Due to a sensitivity limitation of 2.5% for the ShoRAH software for variant reassembly, the raw reads were manually searched for (i) the presence of the recipient's major variant in the donor and (ii) the presence of the donor's major variant in the recipient. This analysis focused on the 3′-terminal 171 nucleotides of ORF2 and the 5′-terminal 133 nucleotides of ORF3, as this region displayed the greatest interhost diversity. In this high-resolution analysis, each recipient's major variant was found to be 100% identical to a unique minor variant (<0.01%) isolated from the donor (Fig. 3B). Surprisingly, the major variant present in the donor was not identified in either of the two recipients, even at a low frequency (<0.01%).
Longitudinal evolution of the intrahost viral population in a typical acute NoV infection.
To examine the evolutionary dynamics of a typical acute NoV infection, two samples isolated 9 days apart from subject Ac were analyzed via NGS of the ORF2 and partial ORF3 regions. The SNPs were calculated at each time point (Fig. 2B), and their variation in frequency between the time points was determined. This analysis revealed that the distribution of viral variants being excreted by subject Ac remained reasonably constant over time. The SNPs varied only by a maximum of 2.6% and by an average of 0.07%. Mutations did emerge in reported antigenic regions of the capsid but at frequencies below 2.5% (data not shown).
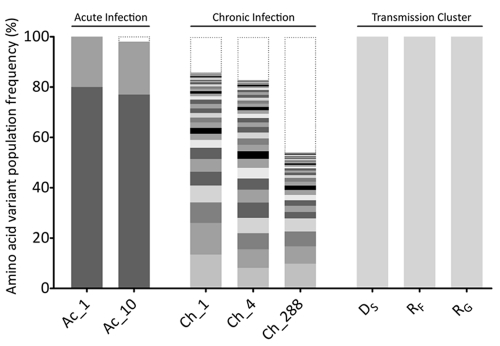
In order to understand the population diversity at the variant level, a Bayesian statistical tool (ShoRAH) was used to reconstruct the ORF2 variants (1,620 nt) present in the population at a frequency of >2.5%. For both time points, two variants were reconstructed. These two variants differed at one nucleotide site, which resulted in a nonsynonymous substitution (S to G) at residue 134 within VP1. The major variant, VP1-134S, represented 80% and 77% of the population at the first time point and the second time point, respectively. The second minor variant, VP1-134G, represented about 20% and 21% of the population at the two time points, respectively (Fig. 4). Other minor variants did exist within the population but were present at a frequency below 2.5%, below the reconstruction threshold established for this type of analysis, and were therefore not reported (10). The individual SNPs contributing to these minor variants are presented in Fig. 2B.
Fig 4.
Comparison of the intrahost distributions of NoV variants in all subjects. Full-length NoV variants of the ORF2 region were reassembled from NGS reads and translated into amino acid sequences, and each unique variant is represented by alternate gray shading. The histogram shows the frequency distribution of unique NoV variants in each sample analyzed. Low-frequency variants, with an estimated frequency of occurrence below the detection threshold (2%), are indicated by black dotted lines. For the subject with acute infection (subject Ac), only two variants were detected, with frequencies of occurrence of ∼79% and ∼20%, respectively. These variants remained stable over the 9 days of infection. For the subject with chronic infection (subject Ch), no dominant variant was observed. Instead, a distribution of low-frequency variants coexisted, and their prevalences varied over the course of the infection. A single variant was identified in each subject within the transmission cluster cohort (subjects DS, RF, and RG).
Longitudinal evolution of the intrahost viral population in an atypical chronic NoV infection.
For subject Ch, an infant with an underlying immune deficiency and persistent NoV infection, three samples were collected, at day 1, day 4, and day 288. Samples from all three time points were analyzed by NGS. There was significant variation in synonymous and nonsynonymous SNPs observed over time, particularly at the third time point, where 18 SNPs reached fixation (defined as >98% of the population), 8 of which resulted in an amino acid substitution. The fixation of multiple SNPs indicated that a selective sweep had occurred between day 4 and day 288 and that the variants present at the first two time points were replaced by new variants. Samples from the first two time points had a mean group genetic distance of 0.7%, while the sample from third time point had a mean group genetic distance of 4.1% compared to samples from the first two time points and appeared to be genetically distinct. To confirm that the variants present at the third time point were indeed progeny from the early time points and not due to reinfection by a new variant, a phylogenetic analysis was performed on the variants determined by using the majority consensus sequence from samples from all three time points, and the sequence was compared to those of other GII.4 variants in circulation across New South Wales (NSW), Australia, and globally (Fig. 1). The viruses identified in the chronic subject clustered together for all three time points and away from the other GII.4 2006b variants in circulation, suggesting that subject Ch had been continuously infected with the same virus.
In order to understand how the population was evolving at the variant level, the ORF2 variants were reconstructed with ShoRAH. Surprisingly, unlike the four subjects with acute infection, there was no dominant (i.e., >50%) ORF2 variant isolated from the viral population for any of the three samples collected from subject Ch. Six main variants with a frequency of >2.5% were detected at day 1, and five main variants were detected at day 4. None of these variants were present at both day 1 and day 4. The most common variant had a frequency of 6.1% at day 1. At day 288, no ORF2 variants were reconstructed at a frequency greater than the threshold of 2.5%. The lack of variants reconstructed for the third time point and the low frequency of those that were reconstructed for the first two time points indicated that the viral population in subject Ch was highly heterogeneous, with the circulation of many (>50) minor variants at all three time points. This was also supported by the fact that all five clones, for each time point for subject Ch, were nonidentical sequences (data not shown).
The distribution of ORF2 variants at the amino acid level was also determined for subject Ch. In this analysis, more variants with a higher frequency were detected than with the nucleotide analysis. Despite the increase, the overall frequency of the individual variants remained low (Fig. 4). At day 1, only eight variants were reconstructed at a frequency of >2.5%, with the most common variant being present at a frequency of 13.4%. Similarly, at day 4 and day 288, only 12 and 6 variants were reconstructed at a frequency of >2.5%, respectively; however, no variants were detected above a frequency of 10% (Fig. 4).
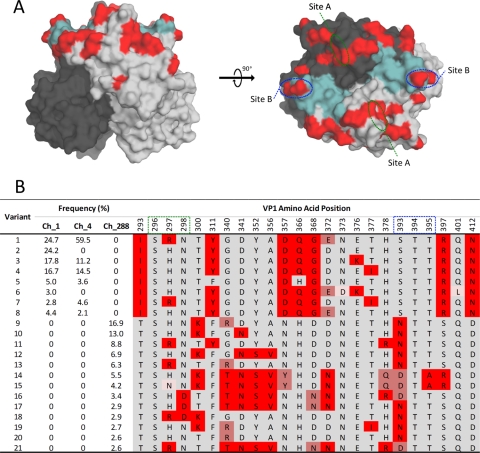
Individuals persistently infected with NoV may act as a reservoir for novel antigenic variants (56). To explore this possibility, we examined the frequency of variants within the viral populations and the distribution of polymorphic positions across the antigenic P2 domain of the viral capsid (amino acids [aa] 275 to 417) in subject Ch (Fig. 5). Reconstructions of the P2 domain amino acid sequence (at a frequency of >2.5%) identified 21 distinct P2 variants present throughout the infection. Eight, six, and 13 distinct variants were present at days 1, 4, and 288, respectively (Fig. 5B). The frequencies of variants present at each time point varied significantly. At day 1, the two most common variants were present at frequencies of 24.7% and 24.2%. By day 4, a shift in variant dominance occurred, where the first variant (present initially at a frequency of 24.7% at day 1) increased in frequency to 59.5%, while the second variant (present at a frequency of 24.2% at day 1) could no longer be detected (Fig. 5B). Similarly, two other variants present at day 1 had disappeared by day 4. By day 288, 13 P2 domain variants that were distinct from those previously identified at days 1 and 4 were identified. This transition of viral variants identified at day 288 was defined by substitutions at positions 293, 357, 368, 393, and 412 (Fig. 5B). The most common variant was present at a frequency of 16.9%, and similar to the analysis of the entire ORF2 region, the P2 domain from sample Ch_288 appeared to be highly heterogeneous (Fig. 5B).
Fig 5.
Analysis of amino acid variants in the P2 region for subject Ch (chronic NoV infection). (A) Positions of evolving sites (highlighted in red) on the surface of a P2 domain dimer. Each P2 monomer is distinguished by light or dark gray shading. Key antibody binding sites (site A and site B) as well as the histo-blood group antigen (HBGA) binding pocket (highlighted in aqua) are shown. Two orientations are provided: a side view (left) and a top view (right). (B) Amino acid sequence of each NoV variant at 24 amino acid sites, 22 of which were identified as being evolving and polymorphic. The frequency of each variant at days 1, 4, and 288 is provided for subject Ch. Residues that varied between subjects and over time are highlighted by shades of red.
Due to the coexistence of genetically diverse variants in the subject with a chronic infection, it is possible that additional diversity could occur through recombination, further increasing the evolution rate of NoV. In this study, the viral variants isolated from the chronically infected subject were screened for recombination events (data not shown); however, due to the closely related nature of the population and the low frequency of the variants in the population, it was not possible to call any recombination events with confidence.
DISCUSSION
The genetic diversity of NoV occurs at the interhost level and results in new antigenic variants and subsequent escape from herd immunity (37). However, it is not known when and how this genetic change occurs. By studying NoV intrahost evolution in acute and chronic NoV infections and by monitoring a documented transmission cluster, we address these issues in this study.
An analysis of NGS data from three subjects with known epidemiological clustering revealed that the transmission of NoV is characterized by a strong genetic bottleneck, with only minor variants present in the source population being successfully transmitted to the recipient hosts for subsequent infection. This result was found despite the fact that all subjects with acute infection studied had a highly homogenous viral population, with a single variant representing >60% of the total viral population. Interestingly, the minor variants transmitted differed from the dominant donor variant at the amino acid level, with one recipient, subject RF, carrying an amino acid change in VP1 and the other recipient, subject RG, carrying an amino acid change in VP2. These findings are not unique to NoV, as similar patterns of transmission have been identified for HCV and HIV (15, 20, 40). For HIV, the variants responsible for establishing infection in the new host have been reported to have unique phenotypic properties that are likely to contribute to the establishment of infection, for example, specific amino acids that increase viral entry efficiency as well as unique glycosylation patterns that are hypothesized to help stabilize the structural proteins and aid in the attachment to host receptors (26, 32).
Since NoV is known to bind to HBGA as attachment factors (12, 55, 64), it is possible that the transmission of NoV variants may be based on structural constraints similar to those seen for HIV. The S347G amino acid change identified in the GII.12 VP1 of the recipient's (subject RF) variants may provide evidence of HBGA structural selective pressure, as residue 347 sits directly adjacent to the primary HBGA binding site in these viruses (29). Alternatively, the transmission of a minor variant could simply be a random event, as NoV has a low infectious dose, approximately 18 virions (65); therefore, it is likely that only a small number of variants are transmitted. In addition, the transmission route could influence which variant is able to establish infection. For example, for foot-and-mouth disease virus (FMDV), different evolutionary trajectories of the transmitted viral variants have been observed for isolated body compartments of the same host (70). Therefore, the NoV population excreted in the vomitus could be genetically different from the virus excreted in the feces. Unfortunately, we were not able to test this theory, as no sample of the vomitus was available. Whether stochastic or deterministic in nature, this study suggests that transmission is an important contributor to genetic changes in the NoV population.
A longitudinal analysis of intrahost NoV evolution revealed that limited diversity was observed for the capsid region of the immunocompetent individual with a typical acute NoV infection, in contrast to chronic infection, where VP1 diversity significantly increased over the course of the infection. The observed variation of the intrahost NoV population is in accordance with data from previous bulk sequencing studies on chronic NoV infection (14, 54). However, this study revealed that chronic NoV infection generated a very diverse viral population, with the cocirculation of many (>50) minor variants (<13.4% frequency at the amino acid level). This result contrasts with the limited diversification observed for subjects who resolved infection during the acute phase and where a single major variant dominated the population.
Viral escape from neutralizing antibodies has been mapped to the hypervariable P2 domain, which sits on the protruding surface of the viral capsid (41). Based on the previous suggestion that chronic shedders may be an important source of novel antigenic variants (61), an in-depth analysis of the NoV population at the VP1 P2 domain was performed. This analysis revealed 22 polymorphic positions (Fig. 5), 7 of which occurred at sites previously determined to be functionally significant (2, 18, 36, 55). For example, residues at positions 296 to 298 and positions 393 to 395 (termed site A and site B, respectively) form a variant-specific epitope involved in neutralizing antibody binding (2). At days 1 and 4, variation was observed at site A, residue 297, for patient Ch, where both arginine and histidine residues were identified (Fig. 5B). Interestingly, the frequency of variants with an arginine increased between days 1 and 4, while variants with a histidine decreased in frequency within the same time frame. By day 288, substitutions were identified at residues 297 and 298 of site A and residues 393 and 395 of site B. More recently, two studies identified additional residues involved in antibody binding (18, 36). Debbink et al. identified epitope A that expanded site A to include amino acids 294, 368, and 372. Of these residues, the latter two were shown to evolve across all time points in the patient with a chronic infection (18). Furthermore, Lindesmith et al. showed that residues 407, 412, and 413 formed an alternate blockade epitope with an asparagine-aspartic acid transition observed for patient Ch between day 4 and day 288 at position 412 (36). Therefore, despite the severe immunodeficiency of subject Ch, some weak antibody response may have driven selection and variation at these antigenic sites. The P2 domain also contains two sites involved in HBGA binding (55) (Fig. 5A). Site I (residues 344 to 346, 374, and 440 to 444) is the primary HBGA binding site and is highly conserved across GII.4 variants. For subject Ch, no substitutions were observed at site I in the reconstructed P2 variants. At site II (residues 387 to 396), which stabilizes HBGA interactions and modulates binding specificity, substitutions were identified in variants at day 288; however, these substitutions corresponded to the same shared residues of antigenic site B (residues 393 and 395). Therefore, these changes observed at residues 393 and 395 may actually be explained by HBGA selection and host receptor adaptation rather than immune-driven selection.
Of the 22 polymorphic positions identified in the P2 domain, 10 were found to contain substitutions that matched previously identified transitions between different GII.4 variants (residues 297, 298, 340, 341, 352, 356, 372, 393, 395, and 412). For example, at position 298, P2 domain variants with either aspartic acid or asparagine residues were identified. Most ancestral GII.4 variants (pre-2001), such as Bristol, Camberwell, and US-1995/96, have an aspartic acid at this position, while modern GII.4 variants, such as Hunter (2004), 2006b (2006 to 2007), Apeldoorn (2008), and New Orleans (2010), have an asparagine at this position. For the chronically infected subject, position 340 was another example where the P2 variants were toggling between residues that defined GII.4 variants, with the amino acids glycine, arginine, and threonine all being identified in the reconstructed P2 variants. Glycine was a defining residue of the Farmington Hills (2002), Asia 2003 (2003), 2006b (2006 to 2007), and Cairo (2007) GII.4 variants, while arginine was present in the Hunter (2004) and 2006a (2006) GII.4 variants. Lastly, a threonine at position 340 defines the recent Apeldoorn (2008) and New Orleans (2010) GII.4 variants. This provides some evidence that individuals chronically infected with NoV may act as reservoirs for new variants, as a range of known antigenic variants was identified in the chronically infected subject. However, toggling between these residues may also simply reflect the virus exploring its sequence space and functionally permitted changes. Due to the unavailability of serum samples, we were not able to investigate the driving force of the residue toggling in the subjects with chronic infection. The subject with chronic infection analyzed here was severely immunocompromised; therefore, the evolution in the P2 domain could be a consequence either of a weak humoral immune response or of a greater capacity of the specific virus to generate novel variants at these sites.
It is also important to consider the role that recombination plays in the generation of antigenic diversity. We did not detect the presence of ORF2 recombination in patient Ch with any statistical significance. The short read lengths of 454 data created a reliance on bioinformatic tools (ShoRAH) to estimate the full-length ORF2 sequence. We have previously shown that variant sequences with lengths similar to that of ORF2 can be reliably reconstructed from 454 data when the variant is present at a frequency above 2.5% (10). Below this frequency, there is a risk of recombinant variants being reconstructed in silico. Given the low frequency of the variants in the chronically infected subject and the high sequence similarity of intrahost viral populations, we could not report any recombination events with confidence, even if detected. Therefore, the extent of recombination in intrahost evolution remains to be determined.
While this study has shown that chronic variants have the propensity to rapidly generate novel variants, the contribution of this diversity to the evolution of NoV at the interhost population level is still unclear. However, it is likely to be significant, as one study showed that chronic shedders can act as a source for nosocomial outbreaks (61). For feline calicivirus (FCV), chronic infections are considered to play an important role in the epidemiology of the disease (17). However, they occur with a much higher frequency (15 to 91%) than persistent NoV infections in humans; therefore, it is difficult to assess the contribution of this diversity to the overall evolution of NoV at the interhost level.
In summary, we revealed that following NoV transmission, only minor variants successfully established a new infection. This is the first study to investigate the evolutionary impact of transmission on NoV evolution, and this study indicates that a significant bottleneck is likely to at least in part drive genetic diversification in NoV and could impact the effectiveness of future vaccines. Further studies are needed to determine whether the transmission of minor variants is a common phenomenon for NoV, as has been observed for HIV and HCV (10, 23, 53). It is also important to establish the biological relevance of this transmission bottleneck. It may be the case that NoV attachment factors such as HBGA may provide a structural barrier that reduces the number of viruses that can establish infection. This study also showed that in a typical acute NoV infection, the viral population is highly homogenous and relatively stable. In contrast, immunocompromised subjects with chronic NoV infection had a rapidly evolving and dynamic viral population. This suggests that subjects with chronic NoV infection could represent a potential reservoir for the emergence of new antigenic variants; however, the contribution of the genetically diverse repertoire to the overall evolution of NoV at the interhost level has yet to be determined. It would be beneficial to study a larger cohort of subjects chronically infected with NoV and with more frequent collection time points to determine if the rapid diversification and turnover of variants observed in this study are common phenomena. Given the fact that NoV vaccines have entered the initial stages of clinical trials, the need for information on NoV evolution is imminent, and this information will help predict the components needed for an effective vaccine and guide sequence predictions of future NoV pandemic variants.
ACKNOWLEDGMENTS
R.A.B. and F.L. were both supported by a National Health and Medical Research Council postdoctoral fellowship. J.-S.E. and K.M. were supported by Australian postgraduate awards.
We thank Abha Chopra, Juan Merif, and Son Pham for technical support.
Footnotes
Published ahead of print 28 December 2011
REFERENCES
- 1. Allen DJ, Gray JJ, Gallimore CI, Xerry J, Iturriza-Gomara M. 2008. Analysis of amino acid variation in the P2 domain of the GII-4 norovirus VP1 protein reveals putative variant-specific epitopes. PLoS One 3:e1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen DJ, et al. 2009. Characterisation of a GII-4 norovirus variant-specific surface-exposed site involved in antibody binding. Virol. J. 6:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnold K, Bordoli L, Kopp J, Schwede T. 2006. The SWISS-MODEL workspace: a Web-based environment for protein structure homology modelling. Bioinformatics 22:195–201 [DOI] [PubMed] [Google Scholar]
- 4. Belliot G, et al. 2003. In vitro proteolytic processing of the MD145 norovirus ORF1 nonstructural polyprotein yields stable precursors and products similar to those detected in calicivirus-infected cells. J. Virol. 77:10957–10974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertolotti-Ciarlet A, Crawford SE, Hutson AM, Estes MK. 2003. The 3′ end of Norwalk virus mRNA contains determinants that regulate the expression and stability of the viral capsid protein VP1: a novel function for the VP2 protein. J. Virol. 77:11603–11615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boillat Blanco N, et al. 2011. Chronic norovirus gastroenteritis in a double hematopoietic stem cell and lung transplant recipient. Transpl. Infect. Dis. 13:213–215 [DOI] [PubMed] [Google Scholar]
- 7. Bok K, et al. 2011. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc. Natl. Acad. Sci. U. S. A. 108:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bull RA, Eden JS, Rawlinson WD, White PA. 2010. Rapid evolution of pandemic noroviruses of the GII.4 lineage. PLoS Pathog. 6:e1000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bull RA, et al. 2005. Norovirus recombination in ORF1/ORF2 overlap. Emerg. Infect. Dis. 11:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bull RA, et al. 2011. Sequential bottlenecks drive viral evolution in early acute hepatitis C virus infection. PLoS Pathog. 7:e1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bull RA, Tanaka MM, White PA. 2007. Norovirus recombination. J. Gen. Virol. 88:3347–3359 [DOI] [PubMed] [Google Scholar]
- 12. Cao S, et al. 2007. Structural basis for the recognition of blood group trisaccharides by norovirus. J. Virol. 81:5949–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Capizzi T, Makari-Judson G, Steingart R, Mertens WC. 2011. Chronic diarrhea associated with persistent norovirus excretion in patients with chronic lymphocytic leukemia: report of two cases. BMC Infect. Dis. 11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carlsson B, et al. 2009. Quasispecies dynamics and molecular evolution of human norovirus capsid P region during chronic infection. J. Gen. Virol. 90:432–441 [DOI] [PubMed] [Google Scholar]
- 15. Ceballos A, et al. 2008. Lack of viral selection in human immunodeficiency virus type 1 mother-to-child transmission with primary infection during late pregnancy and/or breastfeeding. J. Gen. Virol. 89:2773–2782 [DOI] [PubMed] [Google Scholar]
- 16. Cordey S, et al. 2010. Rhinovirus genome evolution during experimental human infection. PLoS One 5:e10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coyne KP, Gaskell RM, Dawson S, Porter CJ, Radford AD. 2007. Evolutionary mechanisms of persistence and diversification of a calicivirus within endemically infected natural host populations. J. Virol. 81:1961–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Debbink K, Donaldson EF, Lindesmith LC, Baric RS. 2012. Genetic mapping of a highly variable norovirus GII.4 blockade epitope: potential role in contribution in escape from human herd immunity. J. Virol. 86:1214–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deng W, et al. 2010. DIVEIN: a Web server to analyze phylogenies, sequence divergence, diversity, and informative sites. Biotechniques 48:405–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dickover RE, Garratty EM, Plaeger S, Bryson YJ. 2001. Perinatal transmission of major, minor, and multiple maternal human immunodeficiency virus type 1 variants in utero and intrapartum. J. Virol. 75:2194–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eden JS, et al. 2010. Norovirus GII.4 variant 2006b caused epidemics of acute gastroenteritis in Australia during 2007 and 2008. J. Clin. Virol. 49:265–271 [DOI] [PubMed] [Google Scholar]
- 22. Ewing B, Green P. 1998. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 8:186–194 [PubMed] [Google Scholar]
- 23. Fischer W, et al. 2010. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS One 5:e12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghedin E, et al. 2011. Deep sequencing reveals mixed infection with 2009 pandemic influenza A (H1N1) virus strains and the emergence of oseltamivir resistance. J. Infect. Dis. 203:168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gilles A, et al. 2011. Accuracy and quality assessment of 454 GS-FLX Titanium pyrosequencing. BMC Genomics 12:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Go EP, et al. 2011. Characterization of glycosylation profiles of HIV-1 transmitted/founder envelopes by mass spectrometry. J. Virol. 85:8270–8284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goonetilleke N, et al. 2009. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J. Exp. Med. 206:1253–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guindon S, Gascuel O. 2003. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52:696–704 [DOI] [PubMed] [Google Scholar]
- 29. Hansman GS, et al. 2011. Crystal structures of GII.10 and GII.12 norovirus protruding domains in complex with histo-blood group antigens reveal details for a potential site of vulnerability. J. Virol. 85:6687–6701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang X, Wang M, Wang K, Estes MK. 1993. Sequence and genomic organization of Norwalk virus. Virology 195:51–61 [DOI] [PubMed] [Google Scholar]
- 31. Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW., IV 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578 [DOI] [PubMed] [Google Scholar]
- 32. Keele BF. 2010. Identifying and characterizing recently transmitted viruses. Curr. Opin. HIV AIDS 5:327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keele BF, et al. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. 2009. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 37:D387–D392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koboldt DC, et al. 2009. VarScan: variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics 25:2283–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lindesmith LC, et al. 2012. Monoclonal antibody-based antigenic mapping of norovirus GII.4-2002. J. Virol. 86:873–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lindesmith LC, Donaldson EF, Baric RS. 2011. Norovirus GII.4 strain antigenic variation. J. Virol. 85:231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lindesmith LC, et al. 2008. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 5:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu BL, et al. 1999. Molecular characterization of a bovine enteric calicivirus: relationship to the Norwalk-like viruses. J. Virol. 73:819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu CH, et al. 2006. Selective transmission of hepatitis C virus quasi species through a needlestick accident in acute resolving hepatitis. Clin. Infect. Dis. 42:1254–1259 [DOI] [PubMed] [Google Scholar]
- 41. Lochridge VP, Hardy ME. 2007. A single-amino-acid substitution in the P2 domain of VP1 of murine norovirus is sufficient for escape from antibody neutralization. J. Virol. 81:12316–12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Margulies M, et al. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marionneau S, et al. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martella V, et al. 2008. Detection and molecular characterization of a canine norovirus. Emerg. Infect. Dis. 14:1306–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martin DP, et al. 2010. RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics 26:2462–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murcia PR, et al. 2010. Intra- and interhost evolutionary dynamics of equine influenza virus. J. Virol. 84:6943–6954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nielsen R, Paul JS, Albrechtsen A, Song YS. 2011. Genotype and SNP calling from next-generation sequencing data. Nat. Rev. Genet. 12:443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peitsch MC. 1995. Protein modeling by e-mail. Nat. Biotechnol. 13:658–660 [Google Scholar]
- 49. Posada D, Buckley TR. 2004. Model selection and model averaging in phylogenetics: advantages of akaike information criterion and bayesian approaches over likelihood ratio tests. Syst. Biol. 53:793–808 [DOI] [PubMed] [Google Scholar]
- 50. Prasad BV, et al. 1999. X-ray crystallographic structure of the Norwalk virus capsid. Science 286:287–290 [DOI] [PubMed] [Google Scholar]
- 51. Ramachandran S, et al. 2011. Temporal variations in the hepatitis C virus intrahost population during chronic infection. J. Virol. 85:6369–6380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rockx BH, Bogers WM, Heeney JL, van Amerongen G, Koopmans MP. 2005. Experimental norovirus infections in non-human primates. J. Med. Virol. 75:313–320 [DOI] [PubMed] [Google Scholar]
- 53. Salazar-Gonzalez JF, et al. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 206:1273–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schorn R, et al. 2010. Chronic norovirus infection after kidney transplantation: molecular evidence for immune-driven viral evolution. Clin. Infect. Dis. 51:307–314 [DOI] [PubMed] [Google Scholar]
- 55. Shanker S, et al. 2011. Structural analysis of histo-blood group antigen binding specificity in a norovirus GII.4 epidemic variant: implications for epochal evolution. J. Virol. 85:8635–8645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Siebenga JJ, et al. 2008. High prevalence of prolonged norovirus shedding and illness among hospitalized patients: a model for in vivo molecular evolution. J. Infect. Dis. 198:994–1001 [DOI] [PubMed] [Google Scholar]
- 57. Siebenga JJ, et al. 2010. Phylodynamic reconstruction reveals norovirus GII.4 epidemic expansions and their molecular determinants. PLoS Pathog. 6:e1000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Siebenga JJ, et al. 2007. Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J. Virol. 81:9932–9941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Siebenga JJ, et al. 2009. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J. Infect. Dis. 200:802–812 [DOI] [PubMed] [Google Scholar]
- 60. Smith GJ, et al. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459:1122–1125 [DOI] [PubMed] [Google Scholar]
- 61. Sukhrie FH, Siebenga JJ, Beersma MF, Koopmans M. 2010. Chronic shedders as reservoir for nosocomial transmission of norovirus. J. Clin. Microbiol. 48:4303–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Takanashi S, et al. 2011. Characterization of emerging GII.g/GII.12 noroviruses from a gastroenteritis outbreak in the United States in 2010. J. Clin. Microbiol. 49:3234–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tan M, et al. 2008. Elucidation of strain-specific interaction of a GII-4 norovirus with HBGA receptors by site-directed mutagenesis study. Virology 379:324–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Teunis PF, et al. 2008. Norwalk virus: how infectious is it? J. Med. Virol. 80:1468–1476 [DOI] [PubMed] [Google Scholar]
- 66. Tu ET, et al. 2008. Norovirus excretion in an aged-care setting. J. Clin. Microbiol. 46:2119–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang QH, et al. 2005. Porcine noroviruses related to human noroviruses. Emerg. Infect. Dis. 11:1874–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wingfield T, et al. 2010. Chronic norovirus infection in an HIV-positive patient with persistent diarrhoea: a novel cause. J. Clin. Virol. 49:219–222 [DOI] [PubMed] [Google Scholar]
- 69. Wolf S, et al. 2009. Molecular detection of norovirus in sheep and pigs in New Zealand farms. Vet. Microbiol. 133:184–189 [DOI] [PubMed] [Google Scholar]
- 70. Wright CF, et al. 2011. Beyond the consensus: dissecting within-host viral population diversity of foot-and-mouth disease virus by using next-generation genome sequencing. J. Virol. 85:2266–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xerry J, Gallimore CI, Iturriza-Gomara M, Allen DJ, Gray JJ. 2008. Transmission events within outbreaks of gastroenteritis determined through analysis of nucleotide sequences of the P2 domain of genogroup II noroviruses. J. Clin. Microbiol. 46:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zagordi O, Bhattacharya A, Eriksson N, Beerenwinkel N. 2011. ShoRAH: estimating the genetic diversity of a mixed sample from next-generation sequencing data. BMC Bioinformatics 12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]