Abstract
Pluripotent human stem cells are a powerful tool for the generation of differentiated cells that can be used for the study of human disease. We recently demonstrated that neurons derived from pluripotent human embryonic stem cells (hESC) can be infected by the highly host-restricted human alphaherpesvirus varicella-zoster virus (VZV), permitting the interaction of VZV with neurons to be readily evaluated in culture. In the present study, we examine whether pluripotent hESC and neural progenitors at intermediate stages of differentiation are permissive for VZV infection. We demonstrate here that VZV infection is blocked in naïve hESC. A block to VZV replication is also seen when a bacterial artificial chromosome (BAC) containing the VZV genome is transfected into hESC. In contrast, related alphaherpesviruses herpes simplex virus 1 (HSV-1) and pseudorabies virus (PrV) productively infect naïve hESC in a cell-free manner, and PrV replicates from a BAC transfected into hESC. Neurons differentiate from hESC via neural progenitor intermediates, as is the case in the embryo. The first in vitro stage at which permissiveness of hESC-derived neural precursors to VZV replication is observed is upon formation of “neurospheres,” immediately after detachment from the inductive stromal feeder layer. These findings suggest that hESC may be useful in deciphering the yet enigmatic mechanisms of specificity of VZV infection and replication.
INTRODUCTION
Varicella-zoster virus (VZV) replication is highly host restricted, growing efficiently only in human cells. In varicella, VZV typically infects and replicates in cutaneous fibroblasts and epidermal cells as well as several types of immune cells. VZV infections of central nervous system (CNS) vasculature are also not uncommonly observed, the virus infecting smooth muscle actin-expressing cells in vessel walls (16). VZV infects effectively primarily in a cell-associated manner in vitro, and it is thought that cell-to-cell spread occurs in most tissues. Cell-free virus is made in vivo by keratinocytes and is present in cutaneous vesicles (8), and released VZV appears to be an important component of T cell-to-skin transmission in vivo (reviewed in reference 1).
VZV infection of neurons is essential for establishment of latency and the ability to reactivate to cause herpes zoster. Initial neuronal infection by VZV is via cutaneous axons and retrograde transport to peripheral somatic and autonomic ganglia and/or by infected circulating lymphocytes that infiltrate the ganglia (28). VZV replicates in both neurons and ganglionic support cells of somatic and cranial peripheral sensory ganglia both upon initial infection and upon reactivation. Importantly, VZV causes a plethora of CNS diseases (due at least in part to infection of the vasculature) (16) and ocular diseases (reviewed in reference 9). The growth of VZV in neurons and the interactions that govern latency and reactivation have proven to be difficult to study outside the human host because of the species restriction of infection and the limited availability of human neuronal tissues.
Human embryonic stem cells (hESC) are pluripotent cell lines derived from the inner cell mass of early embryos. The ability to grow theoretically unlimited numbers of these stem cells and generate normal (i.e., nontransformed) cells of the human body makes them an exceptional tool for biomedical research and candidates for cell therapy of disease. Viral infections of hESC have been performed for nearly a decade, primarily using lentiviruses and retroviruses as vectors for transgenesis, gene delivery, and expression. However, it has been reported that adenovirus does not effectively infect and replicate in some hESC lines and infection is correlated with the expression of coxsackievirus receptor (CAR) but not αv-integrin in naïve hESC (2). Others have reported that the coxsackievirus infects several lines of undifferentiated hESC (21).
We recently reported that VZV productively infects differentiated neurons derived from hESC in vitro (14) and proposed this system as a novel model for studying virus-neuron interactions of this highly human-specific neurotropic herpesvirus. Neural induction of hESC is performed in our lab by the widely used method of coculture with the murine stromal cell line PA6 originated by Sasai (11). Neurons derived from hESC differentiate from cycling neural precursors/progenitors, presumably mimicking the in utero progression from the pluripotent cells of the inner cell mass to differentiated neuronal phenotypes. Although some have succeeded in growing hESC-derived neural precursors as adherent cultures (i.e., reference 12), most laboratories differentiate and expand these neural precursors/progenitors in suspension. hESC are neurally induced using specific feeder lines (i.e. reference 18) and/or growth factors (i.e., reference 10) and maintained under nonadherent conditions to generate “neurospheres” analogous to those produced from the CNS of adult and fetal mammals.
In the present study, we asked whether pluripotent hESC and neural progenitors at intermediate stages of differentiation are susceptible to VZV infection. We found that VZV did not replicate in naïve pluripotent hESC. In contrast, alphaherpesviruses HSV-1 and pseudorabies virus (PrV) readily productively infect naïve hESC. VZV is also unable to infect neural precursors adherent to the stromal cells before generation of the neurospheres, but it can infect and replicate in hESC-derived neurospheres immediately after they are placed into suspension. Study of the ontogeny of competence for infection/replication of cells generated from hESC by VZV may provide a tool to address the mechanisms of entry and permissiveness for VZV infection and for the study of intrinsic antiviral mechanisms in human pluripotent stem cells.
MATERIALS AND METHODS
Tissue culture.
H9 (WA09; US National Stem Cell Bank) or HUES9 (4) human embryonic stem cells were maintained on feeder layers of mitotically inactivated human foreskin fibroblasts (HFF) in NutriStem medium (Biological Industries, Israel) with medium replacement every other day. The cells were passaged weekly at a ratio of approximately 1:30. Human neonatal foreskin fibroblasts (HFF) and the PA6, MeWo, ARPE, and Vero lines were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS), 2 mM glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. In some experiments, a line of hESC stably expressing enhanced green fluorescent protein (EGFP) under the control of the EF1α promoter was used (Lina Gamarnik, unpublished data).
Neural differentiation of hESC to neural precursors and neurons.
Neurospheres were prepared from hESC as detailed previously (18). Briefly, hESC were seeded as a single-cell suspension on the murine stromal line PA6 and cultured for 14 days. Colonies with a specific morphology were dissected from the PA6 cells and cultured in suspension as neurospheres for 2 weeks prior to plating. Neurospheres were then broken up mechanically and seeded on laminin-coated coverslips in a differentiation medium containing 10 ng/ml nerve growth factor (NGF), 5 ng/ml brain-derived neurotrophic factor (BDNF), and 10 ng/ml neurotrophin 3 (NT3).
Viruses.
Recombinant VZV parent Oka (POka)-based viruses used in this study were derived using the bacterial artificial chromosome (BAC) recombineering methods detailed previously (7). The VZV used here contained EGFP fused to the amino terminus of open reading frame 23 (ORF23), derived using the VZV BAC (14), fused to the amino terminus of ORF66 protein kinase (derived using the cosmid systems) (6), or expressed GFP under an independent simian virus 40 (SV40) immediate early (IE) promoter (a kind gift from Hua Zhu, New Jersey Medical School) (29). A new VZV expressing monomeric red fluorescent protein (mRFP) fused to the amino terminus of ORF23 was constructed in a manner identical to that detailed previously (14), using an mRFP with an internal kanamycin cassette in an mRFP construct (a kind gift of N. Osterreider, Cornell University, Ithaca, NY). The insertion of the mRFP gene used the two-step scarless λ red recombination system (25), with kanamycin selection to obtain BACs with the mRFP gene insertion, followed by a second recombination step to remove the kanamycin cassette, following IsceI linearization of BAC at the kanamycin cassette. The bacterial strain pGS1783 was used as host for recombination (a kind gift from Gregory Smith, Northwestern University).
Generation of herpes simplex virus 1 (HSV-1) gCp-GFP, which expresses EGFP from the glycoprotein C promoter, was detailed previously, (5), and the strain was grown in Vero cells. PrV expressing GFP as a fusion to the C terminus of the IE180 protein was developed from the PrV Becker BAC (a generous gift from Lynn Enquist, Princeton University, Princeton, NJ) (23) using a PCR-amplified EGFP-kanamycin cassette engineered with 45-bp homologous flanking sequences to place GFP in frame with IE180, as detailed in the construction of VZV-IE62-GFP BACs (14). Oligonucleotide sequences will be provided upon request. The PrV BAC and resulting virus BAC and virus contain only one copy of IE180 linked to GFP. PrV expressing IE180 GFP was derived after transfection of the BAC into Vero cells and propagated in Vero cells, and plaques were picked five times based on GFP fluorescence. Supernatant was collected from HSV-1/PrV-infected cultures showing 100% fluorescence and cytopathic effect following low-multiplicity infection, and aliquots were stored at −80°C.
Mitotic inhibition of MeWo and ARPE cells and primary human foreskin fibroblasts.
MeWo and ARPE cells were infected with recombinant VZV and maintained until about 70% of the monolayer expressed GFP or mRFP. Cell lines infected with VZV and primary human foreskin fibroblasts used as feeders for hESC were mitotically inhibited as detailed previously (14) using 10 μg/ml mitomycin C for 3 h, washed 3 times with phosphate-buffered saline (PBS), trypsinized, and frozen in 90% FCS–10% dimethyl sulfoxide (DMSO).
Infection of stem cells and neurons.
Mitotically inhibited VZV-infected MeWo or ARPE cells were thawed and immediately seeded in dishes containing hESC, neural precursors on PA6, or just-plated neurospheres or neurons, or they were incubated with neurospheres in suspension in nonadhesive petri dishes. In one experiment, nonmitotically inhibited VZV-infected MeWo cells were seeded on naïve hESC. Figure 1 is a schematic representation of the methods used for cell-associated VZV infection. Media containing HSV-1 and PrV were added to cultures at multiplicities of infection of 0.5 and 0.01, respectively, based on plaque assays. Infection by all viruses was monitored by daily observation of fluorescence in living cultures. Immunostaining was performed as described in reference 14. The antibodies used were mouse monoclonal anti-Oct4 (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti-GFP (Molecular Probes). Secondary antibodies were Alexa 594 (anti-mouse) and Alexa 488 (anti-rabbit) conjugates (Molecular Probes). Nuclei were counterstained with Hoechst.
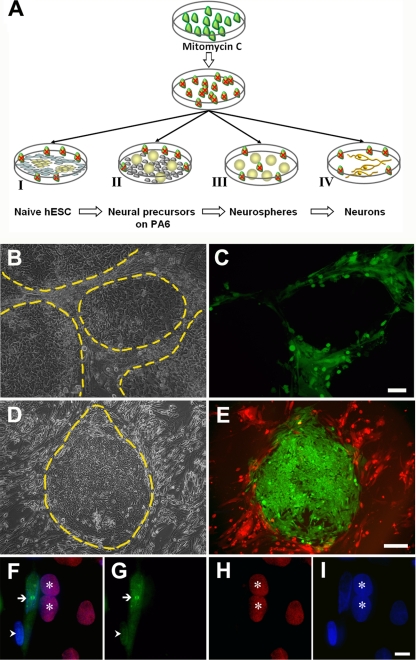
Fig 1.
Naïve hESC are not infected by VZV. (A) Schematic of the infection of hESC and their neurally differentiated derivatives. VZV-infected MeWo or ARPE cells (green) were treated with mitomycin C to prevent proliferation and overrunning the hESC cultures (indicated by red x over cells). Mitotically inhibited VZV-MeWo cells were then mixed with one of four different targets: I, pluripotent hESC cells (yellow) growing on foreskin fibroblasts (gray); II, neurally induced hESC colonies (round yellow balls) on PA6 stromal feeders (gray); III, hESC-derived neurospheres (yellow balls) in suspension; or IV, hESC-derived neural precursors differentiated by plating on a laminin substrate (dark yellow neurons). (B and C) VZV-GFP-infected MeWo cells were added to hESC grown on human fibroblasts, and the live cultures were photographed 7 days later. The fibroblast feeders are infected with the virus and express GFP, whereas the hESC colonies are devoid of GFP expression. Panel B is a phase-contrast micrograph with several large hESC colonies demarcated by dashed yellow lines adjacent to infected, GFP-expressing feeder fibroblasts. Panel C is a combined fluorescence and phase-contrast micrograph. (D and E) hESC cells stably expressing GFP under the control of the EF1α promoter grown on a human fibroblast feeder layer were infected with mitomycin-treated VZV-RFP23-containing MeWo cells and imaged 3 days later. Again, the virus infected and labeled the feeders red, and the GFP-expressing hESC are not infected. Panel D is a phase-contrast micrograph of a large hESC colony demarcated by a dashed yellow line, and panel E shows only fluorescence. Red fluorescence is only observed outside the GFP-expressing hESC colonies. (F to I) Lack of evidence for VZV virion/capsid entry into undifferentiated hESC cells. Mitotically inhibited VZV-GFP23-containing ARPE cells were added to a culture of hESC. Two days later, cultures were fixed and immunostained for GFP (green) and Oct-4, a marker for pluripotent stem cells (red), and examined with a 100× oil-immersion objective. A strongly infected fibroblast displays GFP fluorescence in both its nucleus and cytoplasm (arrow), whereas an apparently more recently infected fibroblast (arrowhead) displays GFP only in its nucleus, and no ORF23-GFP is yet apparent in its cytoplasm. Pluripotent (Oct-4 immunopositive; red) hESC (asterisks) adjacent to the fibroblast whose cytoplasm is filled with GFP are completely devoid of GFP immunofluorescence. (F) Composite of all fluorescence channels; (G) GFP (ORF23) fluorescence only; (H) Oct-4 labeling only; (I) nuclear Hoechst staining (blue). Scale bars are 100 μm in panels B and C, 200 μm in panels D and E, and 20 μm in panels F to I.
Transfections.
BACs containing VZV-ORF23-GFP or PrV-GFP were transfected into human neonatal foreskin fibroblasts, MeWo, ARPE, and hESC, using Trans-IT transfection reagent (Mirus Bio, WI) according to the manufacturer's recommendations.
Microscopy and photography.
Preparations were viewed with Olympus IX70 (live cultures) and BX51 (immunostainings) microscopes, photographed using digital cameras (Scion, Frederick, MD, and Jenoptiks, Jena, Germany), and processed using ImageJ software. Images were enhanced using ImageJ and Paint-Shop-Pro software with all changes in the images (i.e., contrast, brightness, gamma, and sharpening) made evenly across the entire field, and no features were removed or added digitally.
RESULTS
VZV does not infect naïve human embryonic stem cells.
VZV were shown recently to infect and replicate efficiently in neurons derived from human embryonic stem cells (hESC), leading to release of infectious progeny virus into the extracellular milieu (14). In that study, neurons were terminally differentiated for at least 10 days from neural precursor-containing “neurospheres” (18). We asked here whether VZV was able to infect the cells that give rise to these neurons: hESC before beginning their differentiation as well as hESC-derived cells at intermediate steps of differentiation to neurons.
hESC are propagated in a pluripotent “undifferentiated” state, usually on feeder cells. Seeding MeWo cells containing VZV expressing GFP as a fusion protein with capsid protein ORF23 (VZV-GFP23) (14) or under an independent SV40 early promoter (VZV-GFP) (29) resulted in infection of the supporting foreskin fibroblast (HFF) feeder cells (as indicated by GFP expression) 2 days after addition of VZV-infected MeWo cells. In contrast, EGFP expression was not detected in hESC cells, even those in direct contact with GFP-positive fibroblasts at the edges of colonies (Fig. 1B and C). The fluorescence in the HFF spread with time, in spite of the fact that these feeder cells were mitotically inhibited prior to use as hESC feeders, suggesting productive infection. This experiment was performed more than 10 times using both MeWo and ARPE cells, each experiment with at least three coverslips. Even after incubation periods of up to a week with MeWo/ARPE containing VZV, no hESC colonies expressing GFP were ever observed.
In order to demonstrate this in a visually more direct manner, MeWo cells containing VZV expressing mRFP as a fusion with ORF23 (VZV-mRFP23) were seeded on a line of hESC stably expressing GFP (Fig. 1D and E). Three days after addition of the input cells, most HFF were infected by VZV, as indicated by mRFP expression, while the EGFP-fluorescing hESC colonies were devoid of red fluorescence. Again, even those hESC at the periphery of colonies adjacent to the input cells did not express mRFP after prolonged direct contact. These results were obtained using the very widely used hESC line H9. Since there is variability in infectivity of different hESC lines by adenovirus (2), we repeated the experiments with an unrelated and non-genetically modified hESC line, HUES9 (4), using MeWo cells containing VZV expressing GFP. Again, the hESC were completely devoid of GFP expression even after massive infection of the supporting HFF (data not shown). These observations are consistent with the inability of VZV to infect pluripotent hESC.
In several experiments, we attempted to find VZV-GFP23 virions/capsids entering hESC at high magnification using wide-field fluorescence (Fig. 1F to I) or confocal microscopy (not shown). In order to distinguish the fibroblasts from the hESC at high magnification, coverslips were stained with antibodies to markers for pluripotent hESC (i.e., Oct-4 [Fig. 1F to I] or SSEA-4 [not shown]) and GFP to enhance the fluorescence of the VZV. Although newly infected fibroblasts were observed adjacent to strongly GFP fluorescent fibroblasts, no GFP was observed in the adjacent hESC cells that expressed pluripotency markers (Fig. 1F to I).
In more than 10 independent experiments using mitotically inhibited input cells, we did not observe any infected hESC. Mitotically inhibited VZV-infected cells are somewhat less “infective” than uninhibited ones, so we repeated this experiment with VZV-GFP-containing MeWo input cells that had not been mitotically inhibited. The MeWo cells rapidly overgrew the hESC cultures and infected the fibroblast feeders. Focusing through the overlying MeWo revealed that the hESC colonies that were in contact with VZV-infected MeWo cells were not infected, but it was not possible to visualize individual hESC due the density of the MeWo cells. We again repeated this experiment with nonmitotically inhibited ARPE cells that divide more slowly than MeWo cells and found that only a few, rare hESC at the edges of colonies expressed GFP (data not shown). It is well known that 5 to 10% of cells at the edges of hESC colonies undergo differentiation under normal culture conditions and that stress causes hESC differentiation. It is therefore likely that these few infected hESC had begun to differentiate with enhanced stress due to the input cells that overgrew the culture and were being killed by the VZV.
Alphaherpesviruses HSV-1 and PrV readily infect naïve hESC.
We then asked whether the lack of infection of pluripotent hESC was a general characteristic of alphaherpesviruses or was specific to VZV. This was performed by attempting to infect the stem cells with fluorescent recombinant HSV-1 and PrV, allowing visualization of infection in living cultures. Both HSV-1 and PrV have a much wider host range and infect in both cell-free and cell-associated manners in vivo and in vitro. Strikingly, pluripotent hESC expressed abundant levels of EGFP by 3 days postinfection by recombinant cell-free HSV-1 and PrV (Fig. 2A to D), in contrast to the experiments with VZV described above. HSV-1 infection was apparent in both hESC and feeder fibroblasts less than 1 day after incubation with virus, with the stem cells at the periphery of colonies showing higher expression of GFP. PrV appeared to infect the hESC even more efficiently than the surrounding feeders and produced plaques in the centers of the colonies.
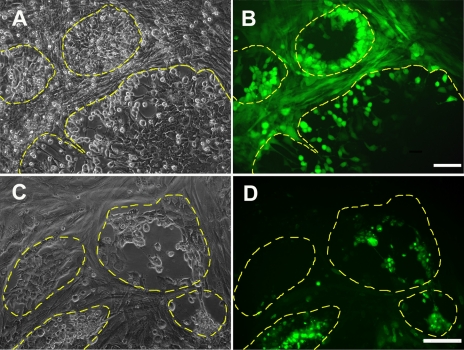
Fig 2.
Alphaherpesviruses HSV-1 and PrV productively infect naïve hESC. hESC grown on a feeder layer of mitotically inhibited human foreskin fibroblasts were infected with cell-free GFP-expressing HSV-1 (A and B) or PrV (C and D) and photographed 2 and 4 days after introduction of virus, respectively. Both the hESC (surrounded by dashed lines) and the surrounding feeder cells express GFP, indicating infection by the viruses. Many hESC show cytopathic effects after infection with these viruses, and cell-free plaques left by dead and detached hESC are seen in the middle of colonies infected with PrV. In panels A and C, micrographs are phase contrast only, whereas, in panels B and D, GFP fluorescence is shown. Scale bars, 100 μm.
VZV does not replicate in naïve human embryonic stem cells.
Possible reasons for the failure to observe EGFP fluorescence in hESC after seeding of VZV-infected cells include (i) the inability of the virus to enter the stem cells and (ii) the inability of the virus to replicate in the hESC and express viral proteins. Viral genomes may be silenced in hESC, as suggested by low EGFP expression from the cytomegalovirus promoter (26; personal observations). We therefore asked whether hESC support viral replication independent of our observed lack of infection. This possibility was addressed by introducing BAC-derived VZV genomes into hESC via transfection, thus “bypassing” restrictions to viral entry. Recombinant BACs containing GFP fused to the VZV IE62 (VZV-GFP62) (14) were cotransfected into hESC with a plasmid expressing the IE62 VZV transactivator to enhance VZV transduction (15). In parallel, the BAC and plasmids were transfected into cells of the ARPE retinal pigmented epithelial line. Transfection of hESC cells with the VZV BAC did not result in GFP-expressing cells after 5 days, while ARPE cells transfected in parallel with the same DNA yielded many GFP+ foci (40 and 97 foci in two separate experiments) whose size increased over time (Fig. 3A to D). This experiment was repeated with a different BAC, VZV-GFP23, with both MeWo and ARPE cells as controls. Once again, the hESC colonies did not express GFP, in contrast to the many fluorescent foci indicating VZV capsid expression present in MeWo and ARPE cultures. The lack of GFP fluorescence in hESC was not due to their inability to be transfected by viral-genome-containing BACs, since when hESC were transfected under the same conditions used in the previous experiments with a BAC containing PrV-GFP, colonies of fluorescent hESC displaying cytopathic effects surrounding cell-free plaques were observed (Fig. 3E to H). These experiments strongly suggest that VZV replication is blocked in hESC.
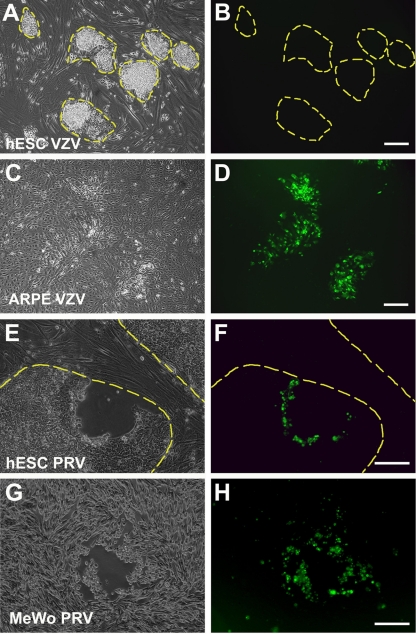
Fig 3.
VZV is unable to replicate in hESC when infection is bypassed by transfection of BAC DNA containing the VZV genome. hESC and ARPE cells were transfected with BACs containing VZV-GFP62 or PrV-GFP and photographed 6 days later. None of the hESC colonies displayed GFP, indicating VZV-GFP62 infection (A and B) in contrast to the many fluorescent foci resulting from viral replication in the ARPE cells (C and D). Transfection of PrV genome-containing BACs into hESC results in viral replication, as demonstrated by the GFP fluorescence after transfection with PrV-GFP (E and F). Transfection of PrV-GFP BACs in parallel to MeWo cells also leads to viral replication (G and H). Panels A, C, E, and G are phase-contrast micrographs, and panels B, D, F, and H are fluorescence photomicrographs of the same fields, with hESC colonies delineated by yellow dashed lines. Scale bars, 100 μm.
VZV infects and replicates in neural precursors within neurospheres in suspension, but not while still attached to inductive stromal cells.
Taken together with our published observations (14), the results presented above suggest that hESC cells are not infected by VZV, while neurons differentiated from them are infected by and replicate and release VZV. The method used in our laboratory for generating neurons from hESC involves (i) induction by coculture with the murine stromal cell line PA6, (ii) mechanical dissection of specific colonies from the stromal cells and culture in suspension resulting in the generation of neurospheres, and (iii) plating the neurospheres in medium lacking mitogens but containing neuronal survival factors (14, 18, 30). We therefore tested hESC-derived neural progenitors at each of these stages for their ability to be infected by VZV. MeWo containing one of two types of EGFP-expressing VZV (VZV-GFP and VZV-GFP23) were seeded on hESC-PA6 cultures at the end of the induction period (14 days after addition of the hESC) (as shown in the diagram in Fig. 1AII). Even at 4 days after addition of virus-containing cells, no infection of either the murine stromal cells or the hESC-derived colonies was observed (Fig. 4A and B). In striking contrast, as soon as 1 day after colonies were removed from the PA6 and allowed to form spheres and mixed in suspension with VZV-GFP (Fig. 1AIII), the spheres were highly permissive for VZV infection and replication, as shown by extensive EGFP fluorescence (Fig. 4C and D) that appeared 1 day after input cells were added. As expected, neurospheres plated for 1 day to induce terminal differentiation were also permissive to infection and replication (Fig. 4E and F), as we have shown for terminally differentiated hESC-derived neurons 10 days to 2 weeks after plating (14). Together, these results indicate that pluripotent hESC and early stage hESC-derived neural precursors are unable to support VZV replication, and this block is released upon transfer to suspension culture and generation of neurospheres.
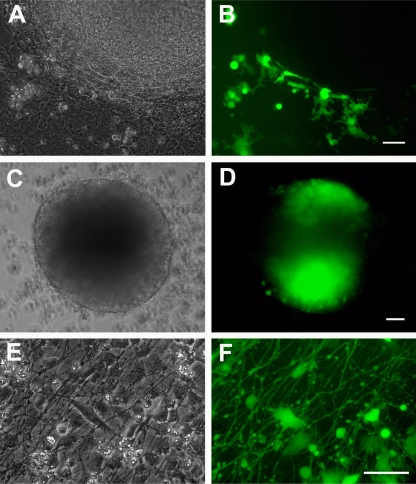
Fig 4.
Neurospheres in suspension are the first stage of neural differentiation of hESC that support infection by VZV. (A and B) hESC cells were plated on PA6 stromal cells and differentiated for 14 days (shown diagrammatically in Fig. 1II). Mitomycin-treated VZV-GFP-infected MeWo cells were added to the coculture 10 to 12 days after hESC plating. Panels A and B show an hESC colony from a 14-day coculture with adjacent GFP-expressing MeWo cells. (The PA6 cells are murine and are not infected by VZV.) The neurally differentiating hESC colony is devoid of fluorescence. (C and D) hESC were cocultured with PA6 for 14 days as in panels A and B, and colonies were cut out and placed in suspension culture with VZV-GFP-infected MeWo cells (Fig. 1III). Three days postinfection, most of the neurospheres express GFP, indicating infection. (E and F) Neurospheres generated as described above were plated on laminin-coated coverslips and VZV-GFP-infected MeWo cells were added to the cultures immediately after adhesion of the neurosphere cells (Fig. 1IV). Three days after plating, the culture is a mixture of neural precursors and differentiated neurons bearing an extensive plexus of neurites. VZV infection of the neural cells is readily observed by GFP expression driven by an SV40 promoter. GFP diffusely fills the neurites and neuronal cell bodies. Panels A, C, and E are phase-contrast images, and panels B, D, and E are fluorescence images. Scale bars, 100 μm.
DISCUSSION
We present evidence here that naïve hESC are not permissive to VZV infection and replication. The data obtained from three different fluorescent protein-expressing VZV strains suggest that the lack of productive infection of pluripotent hESC is due at least partially to the lack of the ability of the virus to bind and/or enter hESC. Attempting to infect hESC with VZV-GFP, a virus in which the expression of GFP is driven by an independent SV40 promoter (and not a VZV protein fusion), also did not result in GFP expression, consistent with lack of entry of VZV. Although VZV infection of several cellular phenotypes has been studied in the laboratory in the past decade or so (neurons, fibroblasts, immune cells, and keratinocytes), the virus has been suggested to infect several cell types during VZV disease, including intestinal smooth muscle cells (19) as well as pneumocytes of the lung and liver hepatocytes (17). The potential of VZV to infect several cellular phenotypes makes the inability of the virus to infect pluripotent (“undifferentiated”) hESC even more unique and intriguing.
Insulin-degrading enzyme (IDE) has been proposed as one possible receptor for VZV entry into cells (13), but reverse transcription (RT)-PCR analysis revealed that naïve hESC of both the H9 and HUES9 lines used in this study contain abundant transcripts for this gene (not shown). Therefore, it is apparently not the lack of this molecule that is responsible for lack of VZV infection/replication into pluripotent stem cells in our study.
The apparent inability of VZV to enter and productively infect pluripotent hESC is in striking contrast, however, to our demonstration of the ability of two other alphaherpesviruses, HSV-1 and PrV, to productively infect these cells. We have also obtained preliminary data on the inability of a gammaherpesvirus (KSHV; human herpesvirus 8 [HHV-8]) to infect hESC. Like VZV, KSHV strains do not infect naïve hESC, but readily infect the neighboring mitotically inhibited HFF feeders. Others have found that human cytomegalovirus (HCMV) establishes a latent or latent-like infection in hESC (R. Penker and R. F. Kalejta, presented at the 36th Annual International Herpesvirus Workshop, Gdansk, Poland, 24 to 28 July 2011). Future experiments may reveal what these disparate herpesviruses have in common that prevents their productive infection of naïve hESC.
In addition to VZV's inability to enter hESC, we also found that when viral entry is bypassed by introduction of its genome into hESC by transfection, prevention or shutdown of viral genome expression occurs at a very early stage, prior to IE gene transcription. This suggests that hESC and their immediate progeny have intrinsic restrictive factors or lack cellular factors required for VZV replication. This intrinsic anti-VZV activity is apparently shut off at the stage in our system when hESC neural precursors are detached from their inducing stromal feeders. Recently, promyelocytic leukemia (PML) body entrapment of VZV has been shown to be a cell-intrinsic inhibition of VZV replication (20, 27). Interestingly, naïve hESC have PML bodies with an atypical distribution and composition (3), and it is possible that they have a role in the inability of VZV genomes to express viral genes in hESC, albeit by a different mechanism from that described for fibroblasts. An alternative explanation for the lack of EGFP expression in hESC after transfection with genome-containing BACs is that the virus immediately enters a latent state and does not transcribe even IE genes in the process. Future experiments will evaluate whether the lack of VZV gene expression we observed after transfection of a VZV-containing BAC into hESC is due to a form of latent infection or intrinsic antiviral mechanisms.
HSV-1 and PrV productively infect hESC, so any intrinsic antiviral property of hESC that blocks VZV replication is either insufficient to stop the replication of these viruses or in some way specific for VZV. We do not have an explanation for this disparity in the ability to replicate between HSV-1 and PrV on the one hand and VZV on the other. One can conjecture it is related to the ability of the former viruses to infect in a cell-free manner, while VZV infection is generally performed in a cell-associated manner. The replication of PrV in hESC after transfection of virus-containing BAC DNA suggests that this difference is not only due to the infection process itself. It should be pointed out that others have observed that naïve hESC shut off the cytomegalovirus (CMV) promoter when nonreplicating lentiviral vectors are used for hESC transgenesis (26), consistent with the existence of some mechanism in pluripotent hESC impeding viral gene expression.
Finally, we observed that there is an abrupt transition to permissivity to VZV infection when hESC-derived neurally induced progenitors are placed in suspension. With scaling up of hESC production using bioreactors (24) to produce sufficient numbers of neural precursors, differences in global gene expression in the permissive and nonpermissive states will be assayable, and thus specific information as to mechanisms involved in the inability of VZV to enter and replicate in hESC may be obtained. This experimental model may provide insight into the mechanisms and host factors required for the specificity of productive VZV infection.
ACKNOWLEDGMENTS
This study was supported by Israel Science Foundation grant 158/07 (R.S.G.), NIH grants NS064022 and EY08098, and funds from the Research to Prevent Blindness, Inc., and The Eye & Ear Foundation of Pittsburgh (P.R.K.).
We thank Hua Zhu for VZV-GFP and Lynn Enquist for the PrV BAC. Lina Gamarnik (Ziegler) generated the transgenic EF1α-EGFP-expressing hESC line. Ronit Sarid contributed many helpful discussions and insightful comments on the manuscript, and we thank Inbal Ithak in her lab for communicating her results with KSHV. We also thank Chaya Morgenstern for expert technical and logistic support.
No conflicts of interest are declared.
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Arvin AM, et al. 2010. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr. Top. Microbiol. Immunol. 342:189–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brokhman I, et al. 2009. Genetic modification of human embryonic stem cells with adenoviral vectors: differences of infectability between lines and correlation of infectability with expression of the coxsackie and adenovirus receptor. Stem Cells Dev. 18:447–456 [DOI] [PubMed] [Google Scholar]
- 3. Butler JT, Hall LL, Smith KP, Lawrence JB. 2009. Changing nuclear landscape and unique PML structures during early epigenetic transitions of human embryonic stem cells. J. Cell. Biochem. 107:609–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cowan CA, et al. 2004. Derivation of embryonic stem-cell lines from human blastocysts. N. Engl. J. Med. 350:1353–1356 [DOI] [PubMed] [Google Scholar]
- 5. Decman V, Kinchington PR, Harvey SAK, Hendricks RL. 2005. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 79:10339–10347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eisfeld AJ, Yee MB, Erazo A, Abendroth A, Kinchington PR. 2007. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J. Virol. 81:9034–9049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erazo A, Yee MB, Osterrieder N, Kinchington PR. 2008. Varicella-zoster virus open reading frame 66 protein kinase is required for efficient viral growth in primary human corneal stromal fibroblast cells. J. Virol. 82:7653–7665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gershon MD, Gershon AA. 2010. VZV infection of keratinocytes: production of cell-free infectious virions in vivo. Curr. Top. Microbiol. Immunol. 342:173–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilden D, Mahalingam R, Nagel MA, Pugazhenthi S, Cohrs RJ. 2011. Review: the neurobiology of varicella zoster virus infection. Neuropathol. Appl. Neurobiol. 37:441–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Itsykson P, et al. 2005. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol. Cell Neurosci. 30:24–36 [DOI] [PubMed] [Google Scholar]
- 11. Kawasaki H, et al. 2000. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived activity. Neuron 28:31–40 [DOI] [PubMed] [Google Scholar]
- 12. Koch P, Opitz T, Steinbeck JA, Ladewig J, Brüstle O. 2009. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc. Natl. Acad. Sci. U. S. A. 106:3225–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Q, Ali MA, Cohen JI. 2006. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell 127:305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Markus A, et al. 2011. Varicella-zoster virus (VZV) infection of neurons derived from human embryonic stem cells: direct demonstration of axonal infection, transport of VZV, and productive neuronal infection. J. Virol. 85:6220–6233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moriuchi M, Moriuchi H, Straus SE, Cohen JI. 1994. Varicella-zoster virus (VZV) virion-associated transactivator open reading frame 62 protein enhances the infectivity of VZV DNA. Virology 200:297–300 [DOI] [PubMed] [Google Scholar]
- 16. Nagel MA, et al. 2011. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology 77:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nikkels AF, et al. 1996. Distribution of varicella zoster virus and herpes simplex virus in disseminated fatal infections. J. Clin. Pathol. 49:243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pomp O, et al. 2008. PA6-induced human embryonic stem cell-derived neurospheres: a new source of human peripheral sensory neurons and neural crest cells. Brain Res. 1230:50–60 [DOI] [PubMed] [Google Scholar]
- 19. Pui JC, Furth EE, Minda J, Montone KT. 2001. Demonstration of varicella-zoster virus infection in the muscularis propria and myenteric plexi of the colon in an HIV-positive patient with herpes zoster and small bowel pseudo-obstruction (Ogilvie's syndrome). Am. J. Gastroenterol. 96:1627–1630 [DOI] [PubMed] [Google Scholar]
- 20. Reichelt M, et al. 2011. Entrapment of viral capsids in nuclear PML cages is an intrinsic antiviral host defense against varicella-zoster virus. PLoS Pathog. 7:e1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scassa ME, et al. 2011. Human embryonic stem cells and derived contractile embryoid bodies are susceptible to Coxsakievirus B infection and respond to interferon Iβ treatment. Stem Cell Res. 6:13–22 [DOI] [PubMed] [Google Scholar]
- 22. Reference deleted.
- 23. Smith GA, Enquist LW. 1999. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. J. Virol. 73:6405–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steiner D, et al. 2010. Derivation, propagation and controlled differentiation of human embryonic stem cells in suspension. Nat. Biotechnol. 28:361–364 [DOI] [PubMed] [Google Scholar]
- 25. Tischer BK, von Einem J, Kaufer B, Osterrieder N. 2006. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques 40:191–197 [DOI] [PubMed] [Google Scholar]
- 26. Xia X, Zhang Y, Zieth CR, Zhang S-C. 2007. Transgenes delivered by lentiviral vector are suppressed in human embryonic stem cells in a promoter-dependent manner. Stem Cells Dev. 16:167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zerboni L, Reichelt M, Arvin A. 2010. Varicella-zoster virus neurotropism in SCID mouse-human dorsal root ganglia xenografts. Curr. Top. Microbiol. Immunol. 342:255–276 [DOI] [PubMed] [Google Scholar]
- 28. Zerboni L, Arvin AM. 2008. The pathogenesis of varicella-zoster virus neurotropism and infection, p 225–250 In Reiss CS. (ed), Neurotropic viral infections. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 29. Zhang Z, Huang Y, Zhu H. 2008. A highly efficient protocol of generating and analyzing VZV ORF deletion mutants based on a newly developed luciferase VZV BAC system. J. Virol. Methods 148:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ziegler L, et al. 2010. A human neuron injury model for molecular studies of axonal regeneration. Exp. Neurol. 223:119–127 [DOI] [PubMed] [Google Scholar]