Abstract
The development of small animal models for the study of HIV transmission is important for evaluation of HIV prophylaxis and disease pathogenesis. In humanized bone marrow-liver-thymus (BLT) mice, hematopoiesis is reconstituted by implantation of human fetal liver and thymus tissue (Thy/Liv) plus intravenous injection of autologous liver-derived hematopoietic stem progenitor cells (HSPC). This results in reconstitution of human leukocytes in the mouse peripheral blood, lymphoid organs, and mucosal sites. NOD-scid interleukin-2 receptor-negative (IL-2Rγ−/−) (NSG)-BLT mice were inoculated intravaginally with HIV and were monitored for plasma viremia by a branched DNA assay 4 weeks later. T-cell activation was determined by expression of CD38 and HLA-DR on human CD4+ and CD8+ T cells in mouse peripheral blood at the time of inoculation and 4 weeks later. Additional BLT mice were treated with human alpha interferon 2b (IFN-α2b) (intron A) and assessed for T-cell activation. Productive HIV infection in BLT mice was associated with T-cell activation (increases in CD38 mean fluorescence intensity and both the frequency and absolute number of CD38+ HLA-DR+ T cells) that correlated strongly with plasma viral load and was most pronounced in the CD8+ T-cell compartment. This T-cell activation phenotype was recapitulated in NSG-BLT mice treated with intron A. HIV susceptibility correlated with the number of HSPC injected, yet a number of mice receiving the Thy/Liv implant alone, with no HSPC injection, were also susceptible to intravaginal HIV. These results are consistent with studies linking T-cell activation to progressive disease in humans and lend support for the use of NSG-BLT mice in studies of HIV pathogenesis.
INTRODUCTION
Generalized T-cell activation is one of the hallmarks of progressive human immunodeficiency virus (HIV) disease leading to the onset of AIDS (21). Though many phenotypic markers have been reported to reflect an activated status, the most commonly used are CD38 and HLA-DR. The level of CD38 expression on CD8+ T cells is an important prognosticator of viral replication, eventual CD4+ T-cell depletion, and diminished immune function (7, 14–16). Elevated levels of CD38 expression by CD8+ T cells are a valuable marker for HIV disease progression that may have better predictive value than either CD4+ T-cell count or viral load measurements (16, 21). The dynamics of the immune response to the virus impact the level of viral replication that occurs during the early stages of HIV infection and have a strong impact on subsequent disease progression. For example, in a prospective study of individuals with early HIV infection, viral replication was positively associated with CD38 expression levels on CD8+ T cells, which in turn were associated with the rate of CD4+ T-cell loss carried forward into the chronic stage of infection (7).
Persistent T-cell activation in response to chronic viral infection may occur as an evolutionary remnant of a once-beneficial immunological response. It has been suggested that low-level activation of T cells in response to chronic infections such as HIV leads to functional anergy, in turn leading to reduced numbers of fully activated target cells capable of productively replicating virus (1). Similarly, chronic HIV infection is associated with T-cell exhaustion, during which HIV-specific T cells display reduced function associated with the expression of PD-1 (6). Despite significant levels of HIV-specific CD8+ T cells, high-level expression of PD-1 is associated with the maintenance of elevated viral loads. Blockade of the PD-1 pathway by interfering with PD-1 ligand (PD-1L) interactions restores T-cell function and results in a reduction in viral load, indicating that immune dysfunction in HIV disease may be reversible. The causes of T-cell activation in HIV-infected subjects have been postulated to include the presence of replicating HIV, the translocation of bacterial lipopolysaccharide (LPS) across disrupted gut lumen (3), and the release of type 1 interferons (IFNs) by innate effector cells (4). Though the presence of either replicating virus or LPS may serve to partially explain persistent T-cell activation in HIV-infected individuals, neither is essential for this activation. Murine models have demonstrated generalized T-cell activation and CD4+ T-cell decline in the absence of infection, resulting from chronic CD27 stimulation through transgenic expression of its ligand, CD70, on B cells (35). Persistent T-cell activation, regardless of the cause, can result in immunodeficiency.
Elucidation of the mechanisms of T-cell activation and the subsequent impact on disease progression has been hampered by a lack of experimental models. Samples from human subjects are generally not uniform with respect to duration and stage of infection, and aggressive experimental interventions and tissue excisions pose obvious ethical dilemmas. Much has been learned from the study of simian immunodeficiency virus (SIV) infection of nonhuman primates; however, limitations exist with respect to differences between SIV and HIV and the largely prohibitive costs of this model. Consequently, much effort has gone into the development of a small animal model of HIV infection (30). Efforts to develop humanized mice have focused on the adoptive transfer of human immune system tissues, cells, or hematopoietic progenitors into immunodeficient recipient mice to render them susceptible to infection with HIV. One such model is the bone marrow-liver-thymus (BLT) mouse, in which fragments of human fetal liver and thymus (Thy/Liv) are implanted together under the kidney capsule in recipient mice, followed by the injection of CD34+ hematopoietic stem progenitor cells (HSPC) isolated from the autologous fetal liver (2, 20, 25). The Thy/Liv implant allows for positive and negative selection of human T cells to occur in autologous human thymus tissue, while the injected HSPC seed the mouse bone marrow to reconstitute human hematopoiesis. This approach arguably leads to the most comprehensive reconstitution of the human immune system in a mouse model yet reported, with high levels of multilineage human cell engraftment.
In this study, we examined T-cell activation parameters on human lymphocytes in BLT mice infected intravaginally with HIV. Intravaginal HIV inoculation leads to high levels of viremia in many of these mice, and viremia is strongly correlated with increased expression of CD38 and HLA-DR on both CD4+ and CD8+ T cells. This effect was recapitulated in BLT mice treated with recombinant IFN-α2b (intron A), suggesting that T-cell activation in HIV-infected BLT mice and, by extension, that in HIV-infected human subjects may be mediated by IFN-α.
MATERIALS AND METHODS
BLT mice.
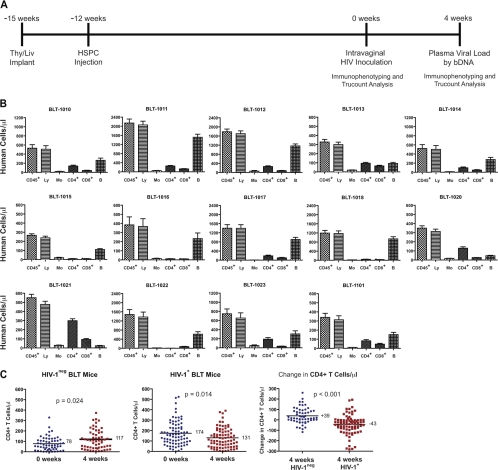
BLT mice were produced as described previously (20, 25), by implanting 1-mm3 pieces of human fetal liver and thymus under the kidney capsule in 6- to 8-week-old female NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; Jackson Laboratories). Each cohort was produced with tissues from a single donor. CD34+ HSPC were purified from fetal liver, isolated by magnetic bead selection for CD34+ cells (Miltenyi), phenotyped cytometrically (34), and cryopreserved until injection into mice 3 weeks after Thy/Liv implantation (Fig. 1A and Table 1).
Fig 1.
Experimental design, HIV inoculation, and loss of CD4+ T cells in HIV-infected BLT mice. (A) Fourteen cohorts of BLT mice (Table 1) were inoculated intravaginally with HIV JR-CSF at 0 weeks (12 weeks after HSPC injection). Peripheral blood of BLT mice was evaluated for human cell reconstitution by Trucount analysis 0 weeks and 4 weeks after inoculation. bDNA, branched DNA assay. (B) Total numbers of peripheral blood leukocytes (CD45+), lymphocytes (Ly), monocytes (Mo), CD4+ T cells (CD4+), CD8+ T cells (CD8+), and B cells (B) were determined for each cohort by Trucount analysis at the time of inoculation. (C) Significant differences were observed in the number of CD4+ T cells and the change in CD4+ T-cell number between HIV-negative (n = 58) and HIV-positive (n = 79) mice at the time of inoculation and 4 weeks after inoculation. The Mann-Whitney U test was used to test the differences between the means.
Table 1.
Numbers of injected cells, levels of human leukocyte reconstitution, and proportions of viremic mice in 14 NSG-BLT mouse cohorts inoculated intravaginally with HIV JR-CSF
| Cohort | Total no. of cells injected per mouse | No. of CD34+ cells injected per mouse | No. of HSPC injected per mouse | Mean no. of human CD45+ cells/μl | Mean no. of human CD4+ cells/μl | No. of TCID50 inoculateda (JR-CSF) | No. of viremic mice/no. of mice in cohort (%) | No. of viremic mice with no HSPC injected/no. of mice in cohort (%) |
|---|---|---|---|---|---|---|---|---|
| BLT-1010 | 6.5 × 105 | 4.7 × 105 | 4,900 | 532 | 138 | 1.4 × 106 | 6/8 (75) | 0/5 (0) |
| BLT-1011 | 5.0 × 105 | 4.3 × 105 | 8,700 | 2,142 | 257 | 0.8 × 106 | 14/15 (93) | 3/5 (60) |
| BLT-1012b | 3.3 × 105 | 2.7 × 105 | 2,500 | 1,785 | 261 | 1.1 × 106 | 14/16 (88) | 2/5 (40) |
| BLT-1013 | 4.4 × 105 | 2.7 × 105 | 350 | 324 | 94 | 1.1 × 106 | 7/15 (47) | 0/5 (0) |
| BLT-1014 | 2.0 × 105 | 1.6 × 105 | 1,400 | 520 | 100 | 1.0 × 106 | 5/7 (71) | 1/5 (20) |
| BLT-1015 | 3.4 × 105 | 1.5 × 105 | 1,100 | 266 | 9 | 0.02 × 106 | 2/10 (20) | 0/2 (0) |
| BLT-1016 | 1.5 × 105 | 1.3 × 105 | 620 | 308 | 10 | 0.4 × 106 | 3/8 (38) | 0/2 (0) |
| BLT-1017 | 3.7 × 105 | 2.6 × 105 | 910 | 1,141 | 148 | 0.03 × 106 | 4/8 (50) | NDc |
| BLT-1018 | 7.5 × 105 | 6.0 × 105 | 760 | 1,195 | 43 | 0.02 × 106 | 2/9 (22) | NDc |
| BLT-1020 | 4.4 × 105 | 2.3 × 105 | 150 | 349 | 129 | 3.7 × 106 | 1/8 (13) | 0/5 (0) |
| BLT-1021 | 1.8 × 105 | 1.6 × 105 | 1,900 | 577 | 317 | 4.5 × 106 | 13/14 (93) | 5/5 (100) |
| BLT-1022 | 11.0 × 105 | 8.8 × 105 | 5,800 | 1,479 | 140 | 3.0 × 106 | 5/9 (56) | 0/5 (0) |
| BLT-1023 | 2.9 × 105 | 2.4 × 105 | 820 | 747 | 188 | 0.5 × 106 | 3/5 (60) | 0/5 (0) |
| BLT-1101 | 3.0 × 105 | 2.2 × 105 | 1,100 | 339 | 82 | 0.5 × 106 | 0/5 (0) | 0/5 (0) |
Assessed by limiting dilution assay in phytohemagglutinin-activated peripheral blood mononuclear cells with supernatant p24 detection 7 days after inoculation.
CCR5 Δ32+/− cohort susceptible to HIV JR-CSF infection.
ND, not determined.
Mice were conditioned by 225 cGy gamma irradiation from a cesium-137 source (Gammacell 3000; Best Theratronics) 30 h before HSPC injection into the tail vein. A portion of the thawed cells were immunophenotyped by flow cytometry to determine the frequency of CD34+ cells that were CD45+ Lin-1−, and 130,000 to 880,000 CD34+ cells (150,000 to 1,110,000 total viable cells) were injected into each mouse. The CD34+ cells were further evaluated for expression of CD38, c-kit, CD90 (Thy-1), and CD45RA. An estimate of injected fetal liver cells that were HSPC, defined as CD34+ Lin-1− cells that were negative or low for CD38 and c-kit+ CD90+ (Thy-1+) CD45RA−, was determined for each cohort (22).
Animal protocols were approved by the UCSF Institutional Animal Care and Use Committee.
CCR5 genotyping.
Genomic DNA was extracted from tissue by use of an Allprep DNA/RNA miniprep kit (Qiagen). Detection of CCR5-Δ32 by restriction fragment length polymorphism was performed using primers 5′-CCTGGCTGTCGTCCATGCTG-3′ and 5′-CTGATCTAGAGCCATGTGCACAACTCT-3′. The PCR product was digested with EcoRI, giving two bands (332 and 403 bp) for homozygous CCR5/CCR5, two bands (332 and 371 bp) for homozygous CCR5-Δ32/CCR5-Δ32, and three bands (332, 371, and 403 bp) for heterozygous CCR5/CCR5-Δ32 (Δ32+/−).
Intravaginal HIV inoculation.
Twelve weeks after HSPC injection, peripheral blood was collected from the retro-orbital sinus for assessment of human leukocyte reconstitution, and mice were inoculated intravaginally once with 20,000 to 4.5 × 106 50% tissue culture infective doses (TCID50) of the R5 HIV molecular clone JR-CSF in 20 μl, with no abrasion, trauma, or preconditioning with progesterone (Fig. 1A and Table 1). HIV inocula were generated by Lipofectamine transfection of 293T cells with plasmid DNA and were concentrated >100-fold by ultracentrifugation over a 30% sucrose cushion for 2 h at 30,000 × g. Blood was collected 4 weeks after HIV inoculation for quantification of the viral load in plasma by a branched DNA assay (Versant HIV RNA 3.0; Siemens Healthcare) according to the manufacturer's instructions.
Flow cytometry.
Human leukocyte reconstitution was assessed by flow cytometry using Trucount tube (BD Biosciences) enumeration to calculate the absolute numbers of human B cells, CD4+ and CD8+ T cells, monocytes, NK cells, and neutrophils per μl of blood. Anti-human CD45–Alexa 700 (Caltag) and anti-mouse CD45–allophycocyanin (APC) (BD Biosciences) antibodies were used to differentiate mouse from human leukocytes. Human CD45+ cells were typed using antibodies specific for human antigens, including CD3-ECD (Beckman Coulter), CD8-Qdot605 (Invitrogen), CD4-Pacific Blue, CD19-APC-Cy7 (to exclude B cells), HLA-DR–fluorescein isothiocyanate (FITC), and CD38-phycoerythrin (PE) (Biolegend). Data analysis was performed using FlowJo software, version 9.2 (TreeStar).
RESULTS
Experimental design and BLT mouse reconstitution.
A total of 137 cell-injected BLT mice in 14 separate cohorts were inoculated intravaginally with HIV and evaluated for HIV plasma viremia 4 weeks after inoculation (Table 1). Of the 137 inoculated mice, 79 had detectable viral loads (>1.9 log10 RNA copies/100 μl plasma), while 58 mice had undetectable viral loads. Viremic mice included those from cohort BLT-1012, which was produced with tissues from a heterozygous CCR5 Δ32+/− donor. In this group, 14 of 16 HIV-inoculated mice (88%) had detectable viremia (mean of 4.3 log10 copies/100 μl), indicating that CCR5 Δ32+/− mice are just as susceptible to infection with R5 HIV JR-CSF as mice reconstituted with wild-type CCR5 donor tissue. Target cells for HIV infection in this cohort would be expected to express some full-length CCR5 from the wild-type allele, and since these mice displayed no resistance to intravaginal HIV transmission, there must be sufficient expression of the coreceptor to permit viral entry and productive infection of CCR5-positive target cells.
Peripheral blood was stained for polychromatic flow cytometry in Trucount tubes to allow for accurate enumeration of the indicated populations of human leukocytes per μl of whole blood (Fig. 1B). At the time of inoculation (0 weeks), the BLT mice had a mean of 952 human CD45+ cells, 134 CD4+ T cells, 86 CD8+ T cells, and 541 CD19+ B cells per μl of blood. By way of comparison, human peripheral blood control samples (n = 30) had 6,056 CD45+ cells, 808 CD4+ T cells, 527 CD8+ T cells, and 145 CD19+ B cells per μl. In agreement with previous reports on HIV-infected humanized mice (2, 5, 8, 9, 26, 27), we observed a decrease in the number of CD4+ T cells 4 weeks after virus inoculation. The mean number of CD4+ T cells/μl increased from 78 to 117 in the 58 aviremic mice and decreased from 174 to 131 in the 79 mice with detectable viral RNA at 4 weeks. Hence, the aviremic mice had a net gain of 39 CD4+ T cells/μl, whereas HIV-infected mice had a net loss of 43 cells/ml (P < 0.001) (Fig. 1C).
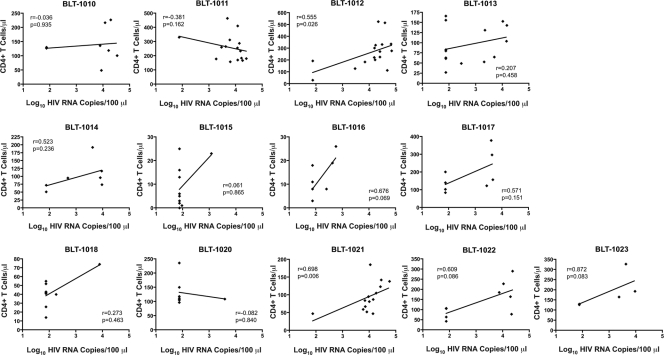
There was wide variation in the numbers of both human CD45+ leukocytes and CD4+ T cells in the peripheral blood across cohorts at the time of inoculation (Table 1 and Fig. 1B). We evaluated the number of CD4+ T cells present in peripheral blood at the time of inoculation in conjunction with the HIV load data obtained 4 weeks later (see Fig. 3). In agreement with our previous results (34), there was only a weak or, for most cohorts, no correlation between viral loads 4 weeks after inoculation and the numbers of human CD4+ T cells or CD45+ human leukocytes in peripheral blood at the time of inoculation (Fig. 2).
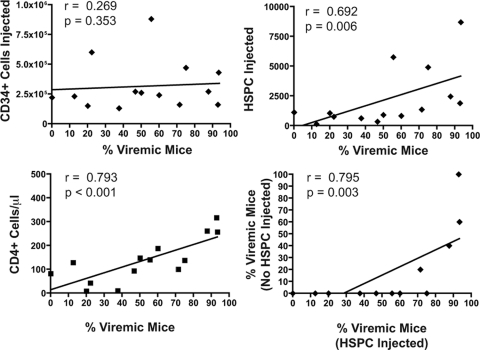
Fig 3.
Numbers of fetal liver-derived CD34+ cells (top left) and HSPC (Lin-1− CD38− CD45RA− CD45+ CD34+ c-kit+ CD90+) (top right) injected into each mouse (−12 weeks) (see Fig. 1A), with the frequency of HIV-viremic mice 4 weeks after inoculation, for the 14 cohorts of BLT mice. The mean number of CD4+ T cells/μl in each cohort at the time of inoculation (0 weeks) correlated with the frequency with which BLT mice became viremic (bottom left). The frequency with which BLT mice that did not receive injected HSPC became infected with HIV following intravaginal inoculation (bottom right) correlated with the frequency with which HSPC-injected BLT mice became infected. Trend lines from linear regression analysis are shown, and the two-tailed Spearman correlation coefficient (r) was calculated, with resulting P values at 95% confidence.
Fig 2.
Overall lack of correlation within cohorts between human CD4+ T-cell reconstitution at the time of inoculation (0 weeks) and the plasma HIV load obtained 4 weeks later. Trend lines from linear regression analysis are shown, and the two-tailed Spearman correlation coefficient (r) was calculated, with resulting P values at 95% confidence.
The autologous fetal liver that was used to isolate CD34+ cells by magnetic bead selection and the resulting CD34+ population were phenotyped extensively by flow cytometry to enumerate the number of potential HSPC injected into each BLT mouse. The CD34+ fraction of cells was analyzed for the expression of lineage markers (Lin-1), CD45, CD38, c-kit, CD90 (Thy-1), and CD45RA, with the cells that were Lin-1− CD45+ CD38− c-kit+ CD90+ (Thy-1+) CD45RA− assumed to be the most enriched for HSPC, as described previously (11, 22). We evaluated both the number of CD34+ cells and the number of HSPC injected per mouse for each cohort and related them to the frequency with which HIV-inoculated mice became viremic (Fig. 3 and Table 1). While the total number of CD34+ cells injected per mouse appeared to have little bearing on intravaginal HIV susceptibility (Spearman correlation coefficient [r] = 0.269; P = 0.353), the number of injected HSPC correlated strongly with the percentage of mice with detectable viremia (r = 0.692; P = 0.006). Similarly, the mean number of CD4+ T cells/μl in each cohort also correlated with the percentage of mice with detectable viremia (r = 0.793; P < 0.001).
Injection of HSPC is not required for HIV infectivity.
For 12 of the 14 cohorts, groups of 2 or 5 Thy/Liv-implanted NSG mice were irradiated and injected with saline rather than autologous HSPC (Table 1). Many of these mice became reconstituted with human T lymphocytes in the peripheral blood and were inoculated intravaginally at the same time as the HSPC-injected mice. In 4 cohorts, these mice had detectable HIV viremia at 4 weeks, despite having not been injected with any HSPC (Table 1). Furthermore, the percentage of the mice that became viremic correlated, on a per-cohort basis, with the percentage of HSPC-injected BLT mice that became viremic (Spearman correlation r = 0.795; P = 0.003) (Fig. 3). In the mice that had no CD34+ HSPC injected, all peripheral human cells were necessarily derived from the Thy/Liv implant. Human peripheral blood leukocytes in these mice consisted nearly entirely of CD3+ T cells and were largely devoid of CD19+ B cells and CD14+ monocytes (data not shown). Furthermore, cohorts of BLT mice that had high levels of human cell reconstitution also had high levels of human T cells in the absence of HSPC injection. For example, cohorts BLT-1011 and BLT-1021 had robust numbers of human CD45+ cells (2,142 cells/μl and 561 cells/μl, respectively) and CD4+ T cells (257 cells/μl and 308 cells/μl, respectively) in HSPC-injected mice, resulting in a high level of HIV infectivity (93% viremic for both cohorts) (Table 1). Similarly, 5 mice from each cohort did not receive HSPC yet still had high levels of peripheral human leukocytes (163 CD45+ cells/μl and 50 CD4+ T cells/μl for BLT-1011 and 292 CD45+ cells/μl and 204 CD4+ T cells/μl for BLT-1021) and high percentages of HIV viremia following intravaginal inoculation (60% and 100%, respectively). Consequently, high-level immune reconstitution with CD3+ T cells, allowing for HIV infectivity, is likely to reflect the quality and/or functional properties of the implanted thymus and liver tissue and is not necessarily correlated with the number of injected fetal liver-derived HSPC. To summarize this observation, it appears from Fig. 3 that susceptibility to viremia is correlated with the number of HSPC injected as well as with the level of CD4+ T-cell reconstitution in peripheral blood, but the correlation with injected HSPC cannot hold true for HIV-viremic mice that were not injected with HSPC. The robust human immune reconstitution in some of the saline-injected mice must have resulted from HSPC present in the liver tissue that was originally implanted. We are now studying this phenomenon more closely to determine whether irradiation and HSPC injection are necessary for intravaginal HIV susceptibility in these mice.
Increased expression of T-cell activation markers in HIV-infected BLT mice.
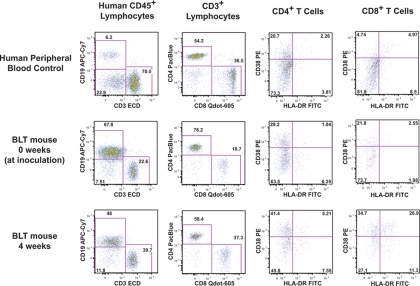
A large body of previous work has examined generalized T-cell activation in progressive HIV disease (7, 14–16, 21). The expression level of CD38, with or without coexpression of HLA-DR, has been used as a measure of the activation status of CD4+ and CD8+ T cells, with higher levels of expression, especially for CD8+ T cells, correlating strongly with a loss of CD4+ cells and with disease progression (7, 14, 15). Peripheral blood leukocytes from 123 HIV-inoculated BLT mice (of the 137 total inoculated mice) were assessed for activation using polychromatic flow cytometry to measure the expression of HLA-DR and CD38 on CD4+ and CD8+ T cells. Matched blood samples at the time of inoculation (0 weeks) and 4 weeks after inoculation were evaluated using the gating scheme shown in Fig. 4.
Fig 4.
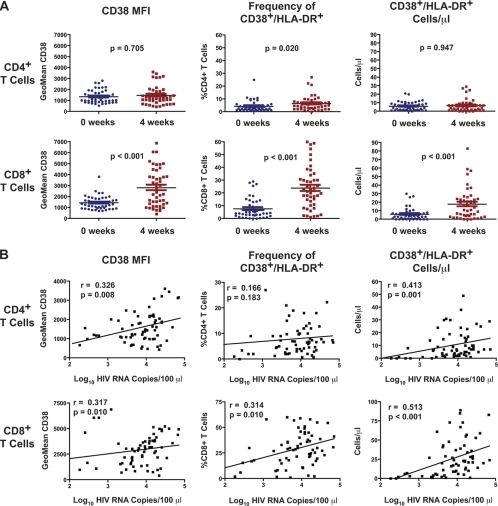
T-cell activation in HIV-infected BLT mice. Peripheral blood was collected from BLT mice at the time of inoculation (0 weeks) (middle row) and 4 weeks later (bottom row). Human peripheral blood was used as a staining and gating control (top row). Blood was immunophenotyped for the presence of human leukocytes and further analyzed for the expression of activation markers. Human CD45+ leukocytes were gated by side scatter into lymphocytes (not shown) and further subdivided into CD3+ (T cells) and CD19+ cells (B cells). CD3+ cells were separated into CD4+ and CD8+ T cells and evaluated for CD38 and HLA-DR expression. Shown in the middle and bottom rows are data for the same BLT mouse at the 0-week and 4-week time points (4.5 log10 copies HIV RNA per 100 μl), where an increase in the frequency of CD38+ HLA-DR+ cells was observed in the CD4+ T-cell and CD8+ T-cell compartments.
Of the 123 mice for which we obtained activation data, 66 (54%) had detectable viremia at 4 weeks. Of these, matched activation data from 0 weeks and 4 weeks were available for 46 mice (Fig. 5A). We observed significant increases in the expression level of CD38 and the frequency and absolute number of CD8+ T cells expressing both CD38 and HLA-DR (CD38+ HLA-DR+) in HIV-viremic mice. This increased expression of activation markers was reflected only weakly in the CD4+ T-cell compartment. The HIV load in the 66 viremic mice was correlated with the mean fluorescence intensity (MFI) of CD38 and both the frequency and absolute number of CD38+ HLA-DR+ cells observed 4 weeks after inoculation (Fig. 5B). The MFI of CD38 correlated strongly with plasma HIV load for both CD4+ (r = 0.326; P = 0.008) and CD8+ (r = 0.317; P = 0.010) T cells. Although the frequency of CD38+ HLA-DR+ CD4+ T cells did not correlate with viral load (r = 0.166; P = 0.183), the absolute number per μl did correlate with viral load (r = 0.413; P = 0.001). Both the frequency (r = 0.314; P = 0.010) and absolute number (r = 0.513; P < 0.001) of CD38+ HLA-DR+ CD8+ T cells correlated strongly with the HIV load. The fact that CD8+ T-cell activation appears to be correlated more tightly with HIV load than with CD4+ T-cell activation is consistent with previous reports linking HIV disease progression with T-cell activation where the effect was most pronounced in the CD8+ T-cell compartment (14, 21, 28).
Fig 5.
Increased expression of cellular activation markers on lymphocytes from HIV-infected BLT mice. (A) The geometric MFI of CD38 (left), as well as the frequency (center) and absolute number per μl (right) of CD38+ HLA-DR+ cells, was determined for CD4+ and CD8+ T cells from a subset of HIV-positive (n = 46) BLT mice at 0 weeks (the time of inoculation) and 4 weeks. P values are shown for the two-tailed, nonparametric Mann-Whitney U test. (B) Activation marker data for HIV-infected mice were correlated with plasma HIV load 4 weeks after inoculation. The MFI of CD38 (left) on CD4+ and CD8+ T cells correlated strongly with viral load, as did the frequency (center) and absolute number (right) of CD38+ HLA-DR+ cells. Trend lines from linear regression analysis are shown, and the two-tailed Spearman correlation coefficient (r) was calculated, with resulting P values at 95% confidence. Data are from cohorts BLT-1012, BLT-1013, BLT-1014, BLT-1015, BLT-1016, BLT-1017, BLT-1018, BLT-1020, BLT-1022, and BLT-1023 (described in Table 1).
Treatment with intron A causes T-cell activation in BLT mice.
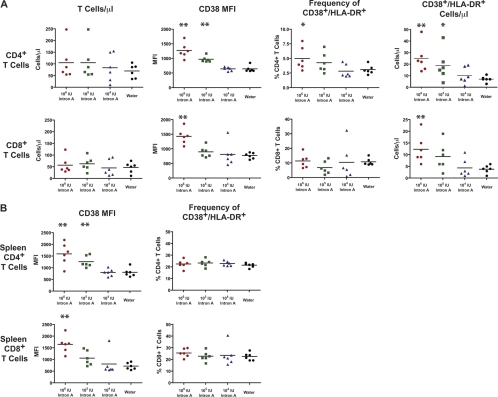
To explore a mechanism by which HIV may increase the activation state of the immune system, we treated groups of BLT mice with recombinant IFN-α2b (intron A). IFN-α is a proinflammatory cytokine released by innate immune effector cells that induces upregulation of a number of IFN-α-stimulated genes (ISGs) and has strong links to HIV pathogenesis (17). The importance of this cytokine is highlighted by studies of SIV in nonhuman primates, where low levels of T-cell activation have been associated with nonpathogenic infection and vice versa. In those studies, SIV-mediated IFN-α release triggered through Toll-like receptor 7 (TLR7) and TLR9 stimulation of plasmacytoid dendritic cells was reduced in nonpathogenic infection of sooty mangabeys and elevated in pathogenic infection of rhesus macaques (23). Further work in our own lab demonstrated an expanded tropism of HIV resulting from IFN-α-mediated upregulation of CCR5 (33). More recently, IFN-α was found to contribute to CD4+ T-cell loss from HIV-infected fetal thymic organ cultures (31). To assess the effect of IFN-α on T-cell activation in our model, BLT mice were treated daily with intron A (106, 105, and 104 IU) for 6 days, and T-cell activation was assessed by flow cytometry on day 7. Intron A treatment resulted in elevated expression of CD38 on both CD4+ and CD8+ T cells, in a dose-dependent manner (Fig. 6A). Similarly, the frequency and absolute number of CD38+ HLA-DR+ cells were elevated in the CD4 compartment, and the number of double-positive cells was increased in the CD8 compartment, all in a dose-dependent manner. The frequency of CD38+ HLA-DR+ CD8+ T cells in the intron A-treated mice was not higher than that for water-treated controls, but both CD38 MFI and the absolute number of CD38+ HLA-DR+ CD8+ T cells in peripheral blood were significantly higher in the mice treated with 106 IU intron A. This highlights the importance of multiple endpoint analyses, especially absolute counts, rather than relying on percentages of populations of cells for assessment of lymphocyte activation. To ascertain whether T-cell activation was anatomically restricted to the peripheral blood or diffuse throughout secondary lymphoid tissue, we measured the levels of CD38 and HLA-DR on splenic T cells (Fig. 6B). MFI measurements of CD38 on splenic CD4+ and CD8+ T cells mirrored those on peripheral blood T cells, though unexpectedly, the frequencies of CD38+ HLA-DR+ cells were not different among the treatment groups. These results show that the activation phenotype observed for viremic HIV-infected BLT mice is reproduced by treatment with intron A, confirming that IFN-α is an important mediator of T-cell activation.
Fig 6.
Treatment with intron A causes increased expression of CD38 and HLA-DR. (A) Groups of 6 BLT mice from the same cohort were treated with 3 different doses of intron A (106, 105, and 104 IU), beginning 12 weeks after HSPC injection. One group was treated with sterile water. Mice were dosed once daily by intraperitoneal injection for 6 days. On day 7, peripheral blood was phenotyped and examined for expression of CD38 and HLA-DR. (Left) The numbers of CD4+ and CD8+ T cells per μl of whole blood were elevated only slightly in the treated groups. The MFI of CD38 and both the frequency and absolute number per μl of CD38+ HLA-DR+ cells (center left, center right, and right panels, respectively) were determined for CD4+ T cells (top row) and CD8+ T cells (bottom row). (B) Spleens were collected from groups of intron A-treated mice as described for panel A and were processed to produce single-cell suspensions for immunophenotyping by flow cytometry. The MFI of CD38 was increased significantly, in a dose-dependent manner, on both splenic CD4+ and CD8+ T cells (left), yet there was no difference in the frequency of CD38+ HLA-DR+ cells (right). Statistical significance compared with the water-treated group was determined by the Mann-Whitney U test. *, P < 0.05; **, P < 0.01.
DISCUSSION
Generalized T-cell activation is an established phenomenon in human HIV infection and is implicated in HIV disease progression (7, 15). HIV-infected BLT mice recapitulate the T-cell activation observed in human HIV-infected subjects and may be a useful model for dissecting the mechanisms by which HIV infection causes T-cell activation. The NSG mouse is a major improvement over the CB17-scid and NOD-scid mouse strains for human immune reconstitution, because it lacks murine NK cells and other innate immune activities that limit the engraftment of human HSPC (30). While NOD-scid mice are superior to CB17-scid mice for human reconstitution, we (34) and others (24) have shown that NSG mice allow even higher levels of engraftment than NOD-scid mice; moreover, we have shown that NSG mice are far more susceptible to intravaginal HIV transmission than NOD-scid mice in side-by-side comparisons (34). Although such side-by-side comparisons have not been reported for BLT mice generated with and without HSPC injection, the cell injection step was envisioned by Melkus et al. (25) to engraft the mouse bone marrow with human hematopoietic progenitors capable of developing into B cells, NK cells, monocytes, and dendritic cells, in addition to the human T cells generated by the Thy/Liv implant. The HSPC injection thus provides a more complete immune reconstitution of the mice, which serves to more closely model events occurring in HIV-infected humans.
The T-cell activation we observed during what can be described as the acute phase of infection in BLT mice highlights the relevance of studying this phenomenon with the aim of establishing an effective prophylaxis. As a study by Deeks et al. (7) highlights, an immune activation set point is established early in HIV infection, is carried forward into the chronic stage, and determines the rate of disease progression. This implies that interventions aimed at reducing T-cell activation early in HIV disease may serve to reduce the CD4+ T-cell loss that occurs during late-stage HIV disease and may impede AIDS-related morbidity and mortality. This was further bolstered by a study of HIV-positive “elite controllers,” defined as a population that is able to maintain clinically undetectable plasma viral loads in the absence of therapy, showing significantly lower levels of CD38+ HLA-DR+ CD8+ T cells (28). It was shown that reduced levels of activation correlate with maintenance of T-cell function, and these subjects rarely progress to AIDS. Our data demonstrate that the T-cell activation observed in HIV-infected human subjects is reproduced in HIV-infected BLT mice, allowing investigators to address preventive measures in a relevant in vivo model. One such preventive measure was recently reported in which HIV-infected subjects treated with atorvastatin for an 8-week period displayed significantly reduced numbers of activated CD8+ T cells (13). Although statins have been shown to exhibit antiviral activity against HIV, no reduction in viral load was detected. Measurements of CD38 and HLA-DR revealed reductions in the numbers of CD8+ T cells that expressed HLA-DR and that coexpressed CD38 and HLA-DR. By making use of BLT mice, agents with the potential to reduce persistent T-cell activation in the setting of HIV infection may be screened more rapidly to expedite their adoption in a clinical setting.
Our description of T-cell activation following HIV infection is in agreement with previous findings on BLT mice described by Brainard and colleagues (2), who described a 4-fold increase in Ki-67-expressing CD4+ and CD8+ T cells in matched samples obtained before and 12 weeks after intraperitoneal HIV inoculation. This increase in Ki-67 coincided with a decrease in CD27 in both the CD4 and CD8 compartments and with increases in CD69, HLA-DR, and perforin expression by CD8+ T cells.
There are few published studies on IFN-α levels in HIV-infected patients. One group reported that when patients were studied during primary HIV infection (2 months after infection, on average), type I IFN was not detectable in the plasma, at least by conventional methods (18, 29), while another group detected IFN-α in the sera of 3 of 9 patients only within the first week of symptomatic primary HIV infection (36). We have not yet assayed specimens from HIV-infected NSG-BLT mice for IFN-α levels but plan to do so in future experiments designed to explore the role of IFN-α in the model in more detail. While we have not formally demonstrated that the release of IFN-α in response to HIV infection is the cause of the elevated T-cell activation observed in the mice, we believe that our data support this possibility. IFN-α induces or enhances the expression of more than 100 genes (10), and a portion of these ISG products may contribute to the lymphocyte activation we observe after intron A treatment of the mice. IFN-α is one of multiple cytokines and chemokines induced during early HIV infection, including rapid and transient elevations of interleukin-15 (IL-15), a large increase in inducible protein 10 (IP-10), rapid and more-sustained increases in tumor necrosis factor alpha (TNF-α) and monocyte chemotactic protein 1, more slowly initiated elevations in IL-6, IL-8, IL-18, and IFN-γ, and a late-peaking (15 days after HIV RNA detection) increase in IL-10 (32).
It is not clear why the increase in CD38 expression was more pronounced on CD8+ T cells than CD4+ T cells, but previous reports showed this to be true for HIV-infected individuals. It may be that CD8+ T cells are more susceptible to the activation signals that cause increased expression of CD38 because of their key role in cytotoxic immune responses. Fewer CD38-expressing CD4+ T cells may have been detected because they were activated and killed by the virus, although the overall loss of CD4+ T cells in the HIV-positive mice 4 weeks after inoculation was modest, with a mean reduction of 43 cells per μl (from 174 to 131 CD4+ T cells per μl) (Fig. 1C).
CD8+ T-cell activation and subsequent dysfunction have been linked causally to IFN-α, as HIV infection induces increased IFN-α production, resulting in increased major histocompatibility complex class I (MHC-I) expression (19). This in turn correlates with an increase in the frequency of CD8low T cells that display increased levels of CD38 and are functionally impaired in response to T-cell receptor (TCR) stimulation (12). As such, a prophylactic treatment that limits the effect of IFN-α during the acute phase of the infection may reduce the T-cell activation set point and ultimately delay disease progression. Our future studies include experiments to determine the effect of neutralizing anti-IFN-α antibody on T-cell activation in HIV-infected BLT mice, with the goal of examining the feasibility of using this treatment to alter the activation set point during acute infection. Ultimately, if applied to the human setting, this may provide a treatment for delaying or preventing HIV disease progression and the onset of AIDS.
ACKNOWLEDGMENTS
We thank Pheroze Joshi, Mary Beth Moreno, Jose M. Rivera, Sofiya Galkina, Galina Kosikova, Barbara Sloan, Ekaterina Maidji, and Maudi Killian for expert technical assistance.
This work was supported in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (contract HHSN266200700002C/N01-AI-70002). This work was also supported in part by the AIDS Research Institute at UCSF and the Harvey V. Berneking Living Trust.
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Bentwich Z, Kalinkovich A, Weisman Z, Grossman Z. 1998. Immune activation in the context of HIV infection. Clin. Exp. Immunol. 111:1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brainard DM, et al. 2009. Induction of robust cellular and humoral virus-specific adaptive immune responses in human immunodeficiency virus-infected humanized BLT mice. J. Virol. 83:7305–7321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brenchley JM, et al. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 4. Chang JJ, Altfeld M. 2010. Innate immune activation in primary HIV-1 infection. J. Infect. Dis. 202(Suppl 2):S297–S301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choudhary SK, et al. 2009. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2−/− γc−/− mouse. J. Virol. 83:8254–8258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Day CL, et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354 [DOI] [PubMed] [Google Scholar]
- 7. Deeks SG, et al. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104:942–947 [DOI] [PubMed] [Google Scholar]
- 8. Denton PW, et al. 2008. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 5:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denton PW, et al. 2010. Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS One 5:e8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Der SD, Zhou A, Williams BR, Silverman RH. 1998. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc. Natl. Acad. Sci. U. S. A. 95:15623–15628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dick JE, Bhatia M, Gan O, Kapp U, Wang JC. 1997. Assay of human stem cells by repopulation of NOD/SCID mice. Stem Cells 15(Suppl 1):199–203 [DOI] [PubMed] [Google Scholar]
- 12. Favre D, et al. 2011. HIV disease progression correlates with the generation of dysfunctional naive CD8(low) T cells. Blood 117:2189–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ganesan A, et al. 2011. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J. Infect. Dis. 203:756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giorgi JV, et al. 1994. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA-DR+ CD38− CD8+ cells is associated with subsequent stable CD4+ cell levels. J. Infect. Dis. 170:775–781 [DOI] [PubMed] [Google Scholar]
- 15. Giorgi JV, et al. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179:859–870 [DOI] [PubMed] [Google Scholar]
- 16. Giorgi JV, et al. 2002. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J. Acquir. Immune Defic. Syndr. 29:346–355 [DOI] [PubMed] [Google Scholar]
- 17. Giri MS, Nebozhyn M, Showe L, Montaner LJ. 2006. Microarray data on gene modulation by HIV-1 in immune cells: 2000–2006. J. Leukoc. Biol. 80:1031–1043 [DOI] [PubMed] [Google Scholar]
- 18. Hosmalin A, Lebon P. 2006. Type I interferon production in HIV-infected patients. J. Leukoc. Biol. 80:984–993 [DOI] [PubMed] [Google Scholar]
- 19. Keir ME, Stoddart CA, Linquist-Stepps V, Moreno ME, McCune JM. 2002. IFN-alpha secretion by type 2 predendritic cells up-regulates MHC class I in the HIV-1-infected thymus. J. Immunol. 168:325–331 [DOI] [PubMed] [Google Scholar]
- 20. Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. 2006. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108:487–492 [DOI] [PubMed] [Google Scholar]
- 21. Liu Z, et al. 1997. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:83–92 [DOI] [PubMed] [Google Scholar]
- 22. Majeti R, Park CY, Weissman IL. 2007. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell 1:635–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mandl JN, et al. 2008. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 14:1077–1087 [DOI] [PubMed] [Google Scholar]
- 24. McDermott SP, Eppert K, Lechman ER, Doedens M, Dick JE. 2010. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood 116:193–200 [DOI] [PubMed] [Google Scholar]
- 25. Melkus MW, et al. 2006. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 12:1316–1322 [DOI] [PubMed] [Google Scholar]
- 26. Neff CP, et al. 2011. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice. Sci. Transl. Med. 3:66ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olesen R, Wahl A, Denton PW, Garcia JV. 2011. Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection. J. Reprod. Immunol. 88:195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Owen RE, et al. 2010. HIV+ elite controllers have low HIV-specific T-cell activation yet maintain strong, polyfunctional T-cell responses. AIDS 24:1095–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pacanowski J, et al. 2001. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 98:3016–3021 [DOI] [PubMed] [Google Scholar]
- 30. Shultz LD, Ishikawa F, Greiner DL. 2007. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7:118–130 [DOI] [PubMed] [Google Scholar]
- 31. Sivaraman V, Zhang L, Su L. 2011. Type 1 interferon contributes to CD4+ T cell depletion in HIV-1 induced human thymus. J. Virol. 85:9243–9246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stacey AR, et al. 2009. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 83:3719–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stoddart CA, Keir ME, McCune JM. 2010. IFN-alpha-induced upregulation of CCR5 leads to expanded HIV tropism in vivo. PLoS Pathog. 6:e1000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stoddart CA, et al. 2011. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rgamma(−/−) (NSG) BLT mice. Virology 417:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tesselaar K, et al. 2003. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat. Immunol. 4:49–54 [DOI] [PubMed] [Google Scholar]
- 36. Von Sydow M, Sonnerborg A, Gaines H, Strannegard O. 1991. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res. Hum. Retroviruses 7:375–380 [DOI] [PubMed] [Google Scholar]