Abstract
Our knowledge regarding the contribution of the innate immune system in recognizing and subsequently initiating a host response to an invasion of RNA virus has been rapidly growing over the last decade. Descriptions of the receptors involved and the molecular mechanisms they employ to sense viral pathogen-associated molecular patterns have emerged in great detail. This review presents an overview of our current knowledge regarding the receptors used to detect RNA virus invasion, the molecular structures these receptors sense, and the involved downstream signaling pathways.
INTRODUCTION
Recognition of evolutionarily conserved microbial structures, known as pathogen-associated molecular patterns (PAMPs), is an essential function of the innate immune system. These PAMPs are molecular structures, such as glycoproteins, lipopolysaccharides, proteoglycans, and nucleic acid motifs, that are broadly shared by different microorganisms and essential to the survival or infectivity of the microbe. Germ line-encoded pattern recognition receptors (PRRs) are the proteins, expressed by a variety of cells, which are responsible for sensing the presence of microbial invasion. The individual members of these receptor families can be distinguished by ligand specificity, cellular localization, and activation of unique, but converging, downstream signaling pathways. The strategy of employing multiple families of PRRs affords the host a high degree of functional redundancy and thus provides multiple mechanisms to sense and immediately respond to a diverse range of pathogens (3). Furthermore, activation of the innate immune system directs the specific part of the adaptive immune system to be activated upon different threats, thereby launching the most appropriate response to any microbial invasion (2).
Sensing of PAMPs by PRRs markedly upregulates the transcription of genes involved in inflammatory responses. These genes encode proinflammatory cytokines/chemokines, type I interferons (IFNs), and antimicrobial proteins. The expression patterns of the inducible genes differ among activated PRRs (119). Production of type I IFNs (primarily alpha IFN [IFN-α] and IFN-β) plays a central role in the induction of antiviral responses, as these trigger the transcription of many IFN-inducible genes which influence protein synthesis, growth regulation, and apoptosis. Type I IFNs also enhance maturation of dendritic cells (DCs), cytotoxicity of natural killer (NK) cells, and the differentiation of virus-specific cytotoxic T lymphocytes, thus providing an important link between innate and adaptive immune responses (43).
This review presents an overview of the PRRs currently known to be activated by RNA virus invasion. Toll-like receptors (TLRs) are the most extensively studied family of PRRs so far, and they are of substantial importance in the initiation of an antiviral response upon infection. Comprising a gene family of only 10 members in humans (12 in mice), these receptors cover an impressive range of PAMPs involved in the recognition of parasites, fungi, bacteria, and viruses (3). Following early reports of TLR-independent mechanism of viral sensing, the retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) were described as cytosolic sensors of viral RNA species. These were rapidly shown to be of importance in early antiviral responses to several viruses (136).
Besides the TLRs and the RLRs, different other receptors have been described to sense RNA virus invasion; these include the nucleotide-binding oligomerization domain-containing (NOD)-like receptors (NLRs). Some of the important modulators of the innate antiviral response will also be mentioned. These we do not consider sensors, as they do not initiate gene transcription upon specific recognition of viral patterns, but they have been included in this review on the basis of their capacity to influence the final response.
TOLL-LIKE RECEPTORS
Toll-like receptors (TLRs) belong to a conserved family of innate immune recognition receptors acting as the primary sensors of specific PAMPs expressed by numerous pathogens. TLRs are transmembrane glycoprotein receptors with an N-terminal extracellular PAMP-binding region and a C-terminal intracellular signaling region. The N-terminal region contains multiple leucine-rich repeats (LRRs) which form a horseshoe structure. The C-terminal region shows similarity to the intracellular domain of the interleukin-1 receptor (IL-1R), and this region is referred to as the Toll/IL-1R homology (TIR) domain, which mediates downstream signaling events upon activation of the receptor (1, 12). Upon extracellular ligand recognition, TLR dimerization is thought to be induced, bringing together the cytoplasmic TIR domains and subsequently recruiting adaptor molecules to initiate the signaling process (3, 89). The human TLR multigene family comprises 10 members, of which TLR2, -3, -4, -7, and -8 are thought to be of importance in the recognition of structural components of RNA viruses, including viral double-stranded RNA (dsRNA), single-stranded RNA (ssRNA), and surface glycoproteins. Table 1 contains a detailed list of RNA viruses known to be targets of TLR recognition. Among these receptors, TLR3, -7, and -8 recognize nucleic acid motifs and are preferentially confined to intracellular compartments, such as the endoplasmic reticulum (ER), endosomes, lysosomes, and endolysosomes, rather than being expressed at the cell surface, which is the case for the majority of the TLR family members (3).
Table 1.
Individual TLRs and the RNA viruses recognized by thema
| Receptor | Virus | Ligand | Reference(s) |
|---|---|---|---|
| TLR7 | Influenza A virus | ssRNA | 21 |
| Vesicular stomatitis virus | ssRNA | 76 | |
| Human immunodeficiency virus | ssRNA | 9, 36 | |
| Dengue virus | ssRNA | 128 | |
| Sendai virus | ssRNA | 72 | |
| Lactate dehydrogenase-elevating virus | ssRNA | 6 | |
| Mouse mammary tumor virus | ssRNA | 55 | |
| Murine leukemia virus | ssRNA | 55 | |
| TLR8 | Human immunodeficiency virus | ssRNA | 21, 54 |
| TLR3 | Reoviridae | dsRNA | 62, 129 |
| Respiratory syncytial virus | dsRNA | 62, 129 | |
| West Nile virus | dsRNA | 17, 62, 129 | |
| Coxsackievirus B3 | dsRNA | 88 | |
| Poliovirus | dsRNA | 91 | |
| Influenza A virus | dsRNA | 34 | |
| Punta Toro virus | dsRNA | 33 | |
| TLR2 | Measles virus | HA | 11 |
| Lymphocytic choriomeningitis virus | ? | 141, 142 | |
| Hepatitis C virus | Core protein/NS3 | 22 | |
| TLR4 | Respiratory syncytial virus | Fusion protein | 70 |
| Coxsackievirus B4 | ? | 121 | |
| Mouse mammary tumor virus | Envelope protein | 97 | |
| Murine leukemia virus | Envelope protein | 97 |
?, ligand unknown.
TLR7.
Toll-like receptor 7 (TLR7) senses ssRNA oligonucleotides containing guanosine- and uridine-rich sequences from RNA viruses. This takes place in the endosomes of plasmacytoid dendritic cells (pDCs) and B cells (3, 21, 39, 61, 143). TLR7 has been shown also to sense short interfering RNAs (siRNAs) (48).
pDCs comprise a subset of dendritic cells with plasmacytoid morphology, known to secrete large amounts of type I IFN immediately upon viral recognition. The basis for this potent IFN response is a constitutive high expression of IRF7 in pDCs (see below) and a unique attribute of pDCs—retention of the TLR-bound ligands in the endosomal compartment for extended periods of time (42). This increased retention of stimulating ligands allows for a more robust downstream signaling response from the activated TLR before the endosome fuses with lysosome and the ligand is degraded.
In unstimulated cells, TLR7 is localized to the ER, and upon activation, it rapidly traffics to the endosome in a process that depends on UNC93B1 and gp96 (65, 66, 117, 133).
Delivery of ssRNA to TLR7 depends on endocytosis of extracellular viral particles and on the cellular process of autophagy, which transports cytosolic viral replication intermediates into the lysosome (72). Notably, paramyxoviruses, which infect cells by direct membrane fusion, effectively circumvent detection by TLR7 in the endosomes of pDCs. Consequently, the type I IFN response to these viruses has been shown to be independent of TLR7 and endosome acidification (50). The IFN response was instead dependent on effective replication in the cytoplasm, suggesting RLR-mediated recognition.
TLR7 is known to recognize influenza A virus (IAV), vesicular stomatitis virus (VSV), Dengue virus, Sendai virus, and human immunodeficiency virus (HIV), and many unknown targets are likely to exist in the group of RNA viruses (21, 55, 72, 76, 128). Interestingly, Kane et al. recently found that in mice from two retrovirus-resistant strains, TLR7 recognition and the subsequent inducement of a virus-specific humoral immune response critically depend upon the viral entry step. This TLR7-mediated humoral immune response is sufficient to impart virus clearance in these mouse strains (55).
TLR8.
Toll-like receptor 8 (TLR8) is phylogenetically and functionally closely related to TLR7 and recognizes ssRNA. TLR8 is known to recognize HIV and likely many other RNA viruses as well. TLR8 is preferentially expressed in myeloid DCs and monocytes (21, 38, 54).
Downstream signaling from TLR7 and TLR8.
A common feature of all TLR recognition is the activation of three major signaling pathways: mitogen-activated protein kinases (MAPKs), one or more interferon regulatory factors (IRFs), and nuclear factor kappa–light-chain-enhancer of activated B cells (NF-κB). Depending on the circumstances, IRF3 and/or IRF7 are essential for induction of type I IFNs, while the MAPKs activates activator protein 1 (AP-1, a heterodimer of activating transcription factor 2 with c-JUN), which together with NF-κB induce the expression of genes required for inflammation and adaptive immune activation, including IL-1β, IL-6, IL-18, and tumor necrosis factor (TNF) (1).
Specifically, upon stimulation, TLR7 and TLR8 recruit a TIR-containing adaptor named myeloid differentiation primary response gene 88 (MyD88) to the cytoplasmic TIR domain of the receptor (82) (Fig. 1). MyD88 consists of a TLR-binding TIR domain in the C-terminal portion and a death domain in the N-terminal part, and through the latter it forms a complex with two interleukin-1 receptor-associated kinases (IRAKs), IRAK-4 and IRAK-1. Upon activation, IRAK-4 phosphorylates IRAK-1; activated IRAK-1 then binds the C-terminal domain of TNF receptor-associated factor 6 (TRAF6), and this IRAK-1/TRAF6 complex then dissociates from the TLR. Upon activation, TRAF6 performs K63-linked polyubiquitination of tumor growth factor beta (TGF-β) activated kinase 1 (TAK1) in addition to IκB kinase gamma (IKKγ), also known as NEMO (NF-κB essential modulator) (126). IKKγ subsequently associates with IKKα and IKKβ. IKKβ is phosphorylated by the activated TAK1 associated with the TAK1 binding protein 1 (TAB1), TAB2, and TAB3. This leads to the IKK-mediated phosphorylation and subsequent degradation of IκB, which in the unphosphorylated state is coupled to NF-κB. NF-κB, formerly sequestered in the cytosol, is now free to enter the nucleus to induce gene expression. TAK1 in association with TAB1, TAB2, and TAB3 also triggers a MAPK pathway leading to the formation of AP-1. Similar to NF-κB, AP-1 enters the nucleus, and together, NF-κB and AP-1 induce the expression of proinflammatory genes (1).
Fig 1.
Signaling cascade following TLR2/TLR4, TLR7/TLR8, and TLR3 activation with envelope glycoprotein, ssRNA, and dsRNA, respectively. MyD88 and TRIF are the adaptor proteins that initiate the different pathways activating IRF3, IRF7 (pDCs only), NF-κB, and AP-1 in order to induce the antiviral response. NF-κB and AP-1 combine to induce the transcription of inflammatory cytokines. IRF3/IRF7 in association with both NF-κB and AP-1 form the enhanceosome for the transcription of IFN-β/IFN-α, respectively. In addition, IRF7 also has some IFN-β-stimulatory effect (not shown in the figure). (…), pathway outlined elsewhere in the illustration.
IRF5 and IRF7 also interact with the complex of IRAKs and TRAF6. This leads to IRAK1-dependent phosphorylation and subsequent nuclear translocation of both molecules (118, 122). While IRF5 is involved primarily in regulating the induction of proinflammatory cytokines (e.g., IL-6 and IL-12p40) (118), this mediator is also essential for in vivo resistance to infection with VSV and herpes simplex virus (HSV). Thus, while infected mouse embryonic fibroblasts from IRF5-deficient mice produce normal amounts of type I IFN, virus-challenged mice exhibit increased mortality and reduced serum levels of IL-6 and type I IFN (IFN-α and IFN-β) (132). Unlike IRF5, IRF7 is a key mediator in TLR7/TLR8-dependent type I IFN production (43, 45), and mice deficient in the expression of IRF7 in hematopoietic cells fail to produce significant amounts of IFN-α systemically and are often more susceptible to systemic viral infection (71, 114). The constitutively high expression of IRF7, required for complex formation, is a unique feature of the pDCs and is not seen for other cells (44, 45, 63); however, in conventional DCs (cDCs), it is possible that IRF1 may take over as the mediator of type I IFN production (107).
TLR3.
Toll-like receptor 3 (TLR3) recognizes dsRNA, which constitutes the genome of some RNA viruses, e.g., rotavirus (a reovirus), and is a viral replication intermediate of ssRNA viruses (4, 10, 16, 130). TLR3 is localized to the intracellular compartment in macrophages, B lymphocytes, and cDCs and is found both intracellularly and on the surfaces of NK cells, epithelial cells, and fibroblasts (49, 62, 80, 92).
RNA virus invasion upregulates type I IFN expression via TLR7 and TLR8 and via constitutively expressed TLR3 (see below). This enhanced IFN expression induces TLR3 gene transcription in many cell types (84). TLR3 function depends on UNC93B (117), but this dependence is not related to the trafficking of TLR3 from the ER to the endosome as is seen for TLR7 and TLR9 (a dsDNA sensor) (65).
Downstream signaling from TLR3 occurs in much the same way as TLR7 and -8 signaling. Upon ligand binding to the TLR3 ectodomain, TLR3 dimerizes (3, 74, 89). This brings together the TIR domains of TLR3s and causes the recruitment of TIR domain-containing adaptor protein inducing IFN-β (TRIF), representing a specific adaptor shared only with TLR4 among the TLRs (131).
As outlined in Fig. 1, TRIF recruits TRAF6 for the activation and translocation of NF-κB and AP-1 by following the same path of activation via TAK1 as that seen for TLR7 and TLR8 signaling described above. In addition to this pathway, a TAK1-independent pathway of NF-κB activation is also triggered. This pathway is initiated when receptor-interacting protein 1 (RIP1) binds to the non-TIR region of TRIF and subsequently converges on IKKβ, which is also used by the TAK1-dependent route (83).
TRIF also recruits TRAF3 for association with TRAF family member-associated NF-κB activator (TANK)-binding kinase 1 (TBK1), and IKKε. TBK1/IKKε subsequently phosphorylates IRF3 (24, 112); upon phosphorylation, IRF3 dimerizes and translocates to the nucleus to initiate transcription of type I IFNs (IFN-β and IFN-α4). In a positive feedback system, these type I IFNs, among many other effects, upregulate the level of IRF7 expression in responding cells. IRF7, when upregulated, is phosphorylated by TBK1/IKKε, as is IRF3. Dimerized IRF7 then stimulates further type I IFN release (entire range of IFN-α species) (61). Furthermore, as type I IFN release stimulates the expression of TLR3 in cells that were initially TLR3 negative, this adds to the positive feedback loop, enhancing the capacity to provide the antiviral response. In addition to the TLR3-mediated expression of type I IFNs and inflammatory cytokines, TLR3 activation also provides a link between the innate immune system and the adaptive immune system. TLR3 activation in CD8α+ DCs enhances the efficiency of cross-presentation of peptide fragments from apoptotic bodies, derived from virally infected cells. These are taken up by the CD8α+ DCs and presented to CD8+ T lymphocytes (108).
Interestingly, a recent study by Negishi et al. suggests a novel target of TLR3-TRIF signaling, in which TLR3 signaling via TRIF directly induces a type II IFN (i.e., IFN-γ) response. This IFN-γ response was shown to be critical in establishing protection against coxsackievirus B3 (CVB3) infection (88).
In addition to CVB3, the known RNA viruses targeted by TLR3 include reoviruses (dsRNA viruses), respiratory syncytial virus (RSV; ssRNA virus), West Nile virus (WNV; ssRNA virus), poliovirus (PV; ssRNA virus), IAV, and a phlebovirus (Punta Toro virus [PTV]; ssRNA virus) (17, 33, 34, 62, 91). Notably, in the case of WNV, RSV, IAV, and PTV, some studies suggest that the cytokine expression induced by TLR3 (particularly IL-6) may have a detrimental rather than a protective effect on the host (33, 73, 101, 129).
TLR2.
Toll-like receptor 2 (TLR2) differs from the majority of PRRs recognizing viral invasion, as it does not sense nucleic acid motifs; instead, TLR2 recognizes viral glycoproteins. TLR2 is expressed in particular on the cell surface of most immune cells (85, 119). It is believed that TLR2 forms a heterodimer with coreceptors TLR1 or TLR6 and recognizes virion components, such as peptide sequences of envelope glycoproteins (106). Thus, TLR2 has been shown to recognize the hemagglutinin (HA) protein of measles virus (11) and to initiate a response to infection with lymphocytic choriomeningitis virus (LCMV; a murine arenavirus) (141, 142). TLR2 has also been associated with hepatitis C virus (HCV)-induced inflammation via detection of HCV core and nonstructural 3 (NS3) proteins (15, 22). However, a recent study has questioned the in vivo impact of this association, as Hoffmann et al. concluded that only monomeric HCV core protein but not core protein as part of intact nucleocapsid and subviral HCV particles is sensed by TLR2 (41). Therefore, TLR2 expressed at the plasma membrane will be able to bind its relevant ligand only when monomeric core protein is released from disintegrated infected cells containing degraded nucleocapsids. However, as some studies indicate that TLR2 is also expressed in the intracellular compartment, this could support a role for TLR2 in the sensing of HCV core protein (67, 124).
TLR2 signaling involves the recruitment to the TIR domains of the TLR2/TLR1 or TLR2/TLR6 complex of an adaptor named TIR domain-containing adaptor protein/MyD88-adaptor-like (TIRAP/Mal) (Fig. 1) (25, 46). TIRAP/Mal binds MyD88 to initiate the same signaling pathway as that outlined previously for TLR7 and TLR8, culminating in a proinflammatory cytokines response through NF-κB (127), but TLR2 activation may also exert some effect on the expression of type I IFNs (8).
TLR4.
Toll-like receptor 4 (TLR4) has been found to participate in antiviral defense to RNA viruses in human cells by recognizing the fusion protein of respiratory syncytial virus (RSV) (70), and the induction of cytokines in response to coxsackievirus B4 (CVB4) depends on TLR4 (121). In addition, TLR4 is known to recognize the envelope (Env) proteins of both mouse mammary tumor virus (MMTV; a murine retrovirus) and murine leukemia virus (MuLV; a murine retrovirus), and the subsequent triggering of DC maturation and proinflammatory cytokine expression depends on TLR4 functionality (13, 97). Upon activation and dimerization of TLR4, two adaptors are known to be recruited to the TIR domains (as shown in Fig. 1). TIRAP, which was described above, is one, while the other, TRIF-related adaptor molecule (TRAM), is unique to the TLR4 signaling pathway and recruits TRIF, also described previously. Consequently, TLR4 ligation leads to signaling through both the MyD88-dependent pathway as utilized by TLR7, TLR8, and TLR2 and the MyD88-independent pathway shared only with TLR3 (120).
RIG-I-LIKE RECEPTORS AND OTHER CYTOSOLIC NUCLEOTIDE RECEPTORS
The RIG-I-like receptors (RLRs) are cytosolic proteins recognizing viral RNA species. Expressed by most cells of the human organism, though most thoroughly studied for fibroblasts and cDCs, RLRs belong to the family of aspartate-glutamate-any amino acid-aspartate/histidine (DExD/H)-box helicases and include three members of relevance: the retinoic acid-inducible gene I product (RIG-I), melanoma differentiation-associated antigen 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2) (57, 134–136). Studies of gene-deficient mice indicate that RLRs are critical sensors of viral infection in most cell types except pDCs, which preferentially employ TLRs for detection of RNA virus infection (59). However, as mentioned above, in response to viruses that circumvent the endosomal TLRs of the pDCs by direct membrane fusion (e.g., paramyxoviruses), cytosolic recognition by RLRs is assumed to be of great importance also in pDCs. RLRs are expressed at low concentrations in the resting cell, and these concentrations are greatly increased upon stimulation (56, 135, 136).
RIG-I.
Retinoic acid-inducible gene I (RIG-I) contains an ssRNA/dsRNA (ss/dsRNA)-binding C-terminal domain (CTD) which, when unbound, functions as a repressor domain (RD) (103). Upon binding to viral RNA structures produced during viral replication, two repeats of a cysteine-aspartic protease (caspase)-recruiting domain (CARD)-like region at the N terminus are exposed. These are then able to interact with other CARD-containing proteins to trigger downstream signaling events. The middle portion of the RIG-I protein contains the DExD/H helicase domain with an ATP-binding motif. In the inactive state, RIG-I adopts a closed structure with unexposed CARD. The RD specifically recognizes virus-associated RNA species, including dsRNA and 5′-triphosphate ssRNA (60), which differ from self-ssRNA, such as mRNA and tRNA, by having an exposed 5′-triphosphate. Unlike some viral RNA, self-mRNA is inevitably capped posttranscriptionally with a 5′-7-methylguanosine cap, which is subject to 2′-O methylation. While the N-7-methylation is important for stability and translation of the RNA, the 2′-O methylation is suggested to be introduced solely to distinguish self- from nonself (e.g., viral)-5′-capped RNA (18). Self-tRNA undergoes 5′ cleavage and a series of nucleotide base modifications. Association with the ribosome also protects tRNA from being recognized by RIG-I (47). Recent studies suggest a requirement for virus-associated polyuridine-rich sequences in the ssRNA to be recognized by RIG-I in addition to the unmasked 5′-triphosphate (104). Research also suggests that a 3′-phosphoryl group can replace the 5′-triphosphate for recognition by RIG-I (see below) (78).
Ligand binding combined with ATP binding by the helicase domain unmask the RIG-I CARD, allowing it to interact with the CARD of beta interferon promoter stimulator 1 (IPS-1). IPS-1 is located at the cytosolic face of the outer mitochondrial membrane, and this mitochondrial association is necessary for initiating further signaling events (64, 110). RIG-I is important in recognizing and initiating cytokine production in response to a wide range of viruses from many different families, including Flaviviridae (27, 60, 75, 103, 115), Paramyxoviridae (35, 59, 75, 94, 135), Rhabdoviridae (59, 135), Orthomyxoviridae (60, 75), Arenaviridae (35, 140), and a bunyavirus (35) and in the recognition of Ebola virus (a filovirus). The latter recognition is antagonized by the Ebola virus V35 protein, as this secreted protein binds the dsRNA ligand, preventing activation of RIG-I-mediated signaling (14, 109). Table 2 contains a detailed list of known viral targets of RIG-I. Many unknown targets are likely to exist.
Table 2.
Individual RLRs and the RNA viruses recognized by them
| Receptor | Virus | Ligand | Reference |
|---|---|---|---|
| RIG-I | Sendai virus | ss/dsRNA | 59, 135 |
| Newcastle disease virus | ss/dsRNA | 59 | |
| Respiratory syncytial virus | ss/dsRNA | 75 | |
| Measles virus | ss/dsRNA | 94 | |
| Nipah virus | ss/dsRNA | 35 | |
| Vesicular stomatitis virus | ss/dsRNA | 59, 135 | |
| Rabies virus | ss/dsRNA | 23 | |
| Influenza A virus | ss/dsRNA | 60 | |
| Influenza B virus | ss/dsRNA | 75 | |
| Ebola virus | ss/dsRNA | 14, 35 | |
| Lassa virus | ss/dsRNA | 35 | |
| Lymphocytic choriomeningitis virus | ss/dsRNA | 140 | |
| Rift Valley fever virus | ss/dsRNA | 35 | |
| Japanese encephalitis virus | ss/dsRNA | 60 | |
| Hepatitis C virus | ss/dsRNA | 103, 115 | |
| West Nile virus | ss/dsRNA | 27 | |
| Dengue virus | ss/dsRNA | 75 | |
| Rotavirus | ss/dsRNA | 109 | |
| MDA5 | Encephalomyocarditis virus | dsRNA | 31, 60 |
| Theiler's virus | dsRNA | 60 | |
| Mengo virus | dsRNA | 60 | |
| Rabies virus | dsRNA | 23 | |
| West Nile virus | dsRNA | 27 | |
| Sendai virus | dsRNA | 32 | |
| Dengue virus | dsRNA | 75 | |
| Rotavirus | dsRNA | 109 | |
| Murine hepatitis virus | dsRNA | 99 | |
| Murine norovirus 1 | dsRNA | 81 |
MDA5.
Melanoma differentiation-associated antigen 5 (MDA5) is very homologous to RIG-I, and they exhibit the same overall domains. Upon binding of long dsRNA fragments (at least 2 kbp), MDA5 exposes a CARD and initiates cytokine and type I IFN production via IPS-1 similarly to RIG-I (see below) (57). In uninfected cells, long dsRNA is not normally present and therefore represent an effective PAMP signifying viral invasion. MDA5 is crucial for triggering a cytokine response to an invasion with picornaviruses, such as encephalomyocarditis virus (EMCV), Theiler's virus, and Mengo virus (31, 60). MDA5 has also been shown to be of importance in the antiviral response to Sendai virus (32) and in cooperation with RIG-I as well as WNV, rabies virus, Dengue virus, and rotavirus (23, 27, 75, 109). MDA5 is also responsible for the recognition of murine hepatitis virus (a coronavirus) and murine norovirus 1 (a calicivirus) (81, 99). Table 2 contains a list of known viral targets of MDA5.
LGP2.
Laboratory of genetics and physiology 2 (LGP2) is the third member of the RLRs and is less well-characterized. The LGP2 gene lacks the region encoding CARD in RIG-I and MDA5. Since this region is responsible for the association with IPS-1 and therefore further signaling events, LGP2 is thought to be a negative regulator of RLR signaling via interaction between the RD of LGP2 and that of RIG-I (100, 135). Studies with mice show increased responses to VSV in LGP2-deficient cells (125). VSV is known to be specifically recognized by RIG-I rather than by MDA5. In contrast, some evidence suggests that LGP2 deficiency reduces the response to infection with EMCV, which is known to be recognized preferentially by MDA5 (125). This correlates with a positive regulatory effect on the MDA5 response. Thus, both positive and negative effects have been observed, depending on the virus studied and the RLR involved in recognizing this virus (125). LGP2 is therefore assumed to be a modulator of the innate immune response to a viral infection and not a sensor of PAMPs in that LGP2 does not initiate antiviral gene expression. A study by Pippig et al. (93) showed that LGP2 binds dsRNA independent of 5′-triphosphates. The authors suggest that LGP2 mediates the modulator role by inhibiting RIG-I signaling via competitive interaction with viral dsRNA species in one scenario, while in another, enhancing the ability of MDA5 to sense long dsRNA structures by complexing with MDA5 (93).
RNase L.
RNase L is an endoribonuclease that has been associated with antiviral defense (77). It is known that RNase L-deficient mice are compromised in their response to infection with EMCV, WNV, and CVB4 (26, 105, 139). Furthermore, an increase in prostate infections with xenotropic murine leukemia virus-related virus (XMRV, a retrovirus) is seen in humans homozygotic for a variant of RNase L with reduced activity (123). Viral dsRNA activates 2′-5′-oligoadenylate synthetase (OAS) to increase the concentration of 2′,5′-linked oligoadenylate (2-5A) from cytosolic ATP, and this activates RNase L (113). Malathi et al. propose a model for the antiviral effect of RNase L, in which RNase L, upon activation, cleaves single-stranded regions of RNA to form small pieces of 5′-hydroxylated, 3′-phosphorylated RNAs (78). Substrates for the RNase cleavage process include, in addition to viral RNAs, cellular self-RNA (77). RNase L produces small RNAs, often in the form of duplexes, which are shown to activate RIG-I and MDA5, and via IPS-1 result in an IRF3-mediated IFN-β response (see below). The authors show that the 3′-phosphoryl groups of the RNA products are important to the downstream antiviral activity.
According to this model, by recruiting and cleaving host RNA, RNase L via OAS amplifies the antiviral response to virally derived RNA by increasing the amount of ligand involved in RIG-I and MDA5 recognition. OAS is induced by type I IFNs, which upon binding to type I IFN receptors on surrounding cells triggers an associated JAK kinase and via STAT induces the expression of IFN-inducible antiviral gene products, such as protein kinase R (PKR) and OAS (119).
Downstream signaling from RIG-I and MDA5.
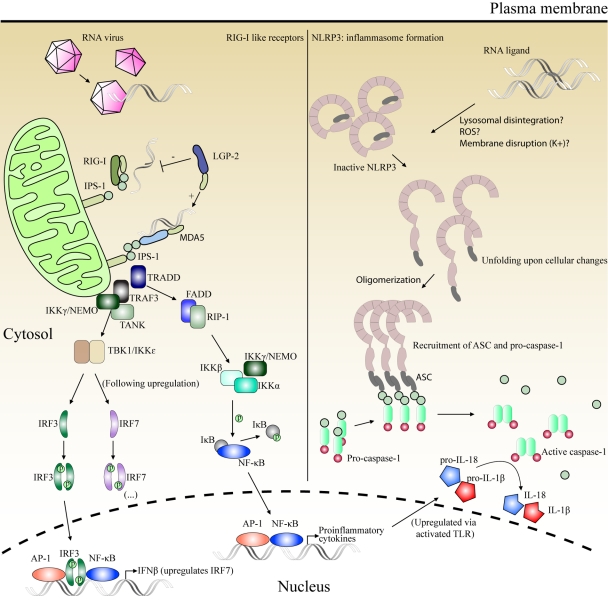
Both RIG-I and MDA5 interact with the adaptor IPS-1 through CARD repeats, and IPS-1-deficient mice are impaired in the production of proinflammatory cytokines and type I IFN in response to all RNA viruses recognized by RIG-I and MDA5 (69, 116), indicating a key role of IPS-1 in downstream signaling from RIG-I and MDA5. IPS-1 itself is probably not directly involved in the signaling process but serves as a platform to orchestrate the molecular interactions which subsequently lead to the activation of IRF3 and NF-κB (136). Recently, another adaptor, stimulator of IFN genes (STING, also called MITA) was described (52, 138). STING is also found in the mitochondrial membrane but resides predominantly in the endoplasmic reticulum. Considering that STING interacts with RIG-I and IPS-1 in the mitochondrial membrane, this potentially opens the possibility for cross talk between the two organelles in viral sensing (7). This is of considerable interest in view of the fact that many viruses replicate in the membranous web connecting these organelles (98). However, the precise importance of such interactions is not clear at this moment.
As shown in Fig. 2, IPS-1 coordinates the activation of two of the same pathways as those activated by TRIF during downstream signaling from TLR3 (cf. Fig. 1), namely, the activation of RIP1 leading to NF-κB nuclear translocation and the activation of IKKs, mediating phosphorylation of IRF3 (40). Central in the initiation of both pathways is the TNF receptor-associated death domain (TRADD), which is recruited to IPS-1 and coordinates interactions with relevant downstream molecules. Thus, the IPS-1/TRADD complex recruits TRAF3, which together with TANK and IKKγ/NEMO initiates the activation of IKKs. Similarly, this complex recruits RIP1 (in a complex with the Fas-associated death domain [FADD]) for the initiation of the NF-κB pathway. Following IRF3-mediated type I IFN release, IRF7 gene expression may be induced in cell types not constitutively expressing the IRF7 gene (i.e., all cell types except pDCs), leading to enhancement of the antiviral IFN response.
Fig 2.
Left half, signaling via IPS-1 following dsRNA/ssRNA recognition by RIG-I and MDA5. IPS-1 serves as a platform to coordinate the activation of two of the signaling pathways also utilized by the TLRs (cf. Fig. 1), that is, the activation of RIP1 for NF-κB nuclear translocation and the activation of IKKs for IRF3 phosphorylation and translocation, leading to the expression of proinflammatory cytokines and IFN-β, respectively. Right half, formation of inflammasomes by NLRP3. Inactive NLRP3 oligomerizes upon stimulation by an unknown mechanism. ASC becomes associated with the complex. This leads to the recruitment of procaspase-1, which dimerizes and autoactivates by proteolytic cleavage, generating active caspase-1. Caspase-1 mediates the activation of IL-18 and IL-1β from inactive pro-IL-18 and pro-IL-1β, respectively. (…), the continuation of this pathway is outlined in Fig. 1.
As RLR signaling converges on pathways also utilized by the TLRs, the induced gene expression is also similar, leading to synthesis and release of type I IFNs and proinflammatory cytokines in order to launch an antiviral inflammatory response (61, 64).
RLR signaling is modified by ubiquitination, direct protein interactions, and caspase activity in an elaborate network of both positive and negative regulation. Numerous ubiquitinating agents have been discovered, of which the RING finger protein, Riplet, and the E3 ubiquitin ligase, tripartite motif 25 (TRIM25), are notably in activating RIG-I through K63 ubiquitin ligation. The modifications imparted by TRIM25 are crucial to the interaction between RIG-I and IPS-1 (29, 30, 90). Zinc finger antiviral protein shorter isoform (ZAPS) has recently been suggested to be a key regulator of RIG-I function by direct protein interaction with the receptor to promote oligomerization and ATPase activity (37). Negative regulation by direct protein interaction is found both at the level of IPS-1, e.g., the proteasomal degradation of IPS-1 following binding of PSMA7(α4), and also of individual receptors, e.g., LGP2 inhibition of RIG-I (see above) and DAK inhibition of MDA5 (20, 37, 53). Caspase-8 has recently been shown to negatively regulate RLR signaling by associating with the assembled IPS-1 signalosome and cleaving RIP1 (96).
In addition to the signaling pathway described above, research also suggests that RIG-I activation can trigger inflammasome formation and cysteine-aspartic protease 1 (caspase-1) activity, leading to the maturation of proinflammatory cytokines such as interleukin-1β (IL-1β) (95). This IPS-1-independent pathway is also used by NLRP3 as described next.
NOD-LIKE RECEPTORS AND INFLAMMASOME FORMATION
Nucleotide-binding oligomerization domain-containing (NOD)-like receptors (NLR)—or, according to newer nomenclature, nucleotide-binding domain, leucine-rich repeat-containing (NLR) proteins—are cytosolic proteins regulating inflammatory and apoptotic responses, and recent studies point to the importance of these receptors in the antiviral defense. Table 3 contains a list of known viral targets. As the new nomenclature implies, these proteins contain an LRR motif located at the C terminus. The LRR domain is considered to be the sensor region of the NLRs. Centrally located is a NACHT (NAIP, CIITA, HET-E, TP-1) domain that mediates oligomerization and activation. In the N terminus, an effector-binding domain, most often a CARD or a pyrin domain (PYD), signals downstream following induced proximity upon activation and oligomerization of the NLRs (28, 68).
Table 3.
Individual NLRs and the RNA viruses recognized by thema
| Receptor | Virus | Ligand | Reference |
|---|---|---|---|
| NLRP3 | Influenza A virus | Virus→cell stress? | 58 |
| Sendai virus | Virus→cell stress? | 58 | |
| NLRC2 | Respiratory syncytial virus | ssRNA | 102 |
| Influenza A virus | ssRNA | 102 | |
| Parainfluenza virus | ssRNA | 102 |
Virus→cell stress?, virus-induced cell stress.
NLRP3.
NOD-like receptor family, pyrin domain-containing 3 (NLRP3) contains a pyrin domain (PYD) which can interact with the N-terminal PYD of apoptosis-associated speck-like protein containing a CARD (ASC). As the name implies, ASC contains a CARD domain in the C terminus (19, 58, 79, 86).
NLRP3 oligomerizes upon activation and recruits ASC and procaspase-1 to form an inflammasome complex. This activates caspase-1, which subsequently mediates the conversion of pro-IL-1β and pro-IL-18 to fully functional IL-1β and IL-18 (Fig. 2) (137). Activation is observed to occur upon infection with adenovirus (dsDNA virus), Sendai virus (ssRNA virus), and IAV (ssRNA virus) (5, 51, 58, 86). The details concerning the specific stimuli initiating aggregation of the NLRP3 inflammasome are still unresolved. Furthermore, the response depends on lysosomal maturation and generation of reactive oxygen species (ROS). This is the basis for the hypothesis that NLRP3 is activated through common intracellular changes, such as lysosomal disintegration, membrane disruption, or generation of ROS, caused either by viral PAMPs or endogenous danger-associated molecular patterns (DAMPs), rather than through a direct interaction of NLRP3 with these ligands. NLRP3 is therefore most likely an indirect sensor of viral invasion (5).
NLRC2.
NOD-like receptor family, CARD-containing 2 (NLRC2) has recently been shown to recognize ssRNA species derived from RSV, influenza A virus, and parainfluenza virus, making NLRC2 the only NLR reported to directly sense viral components (102, 111). Upon recognition, NLRC2 associates with IPS-1 through an interaction dependent upon the LRR and nucleotide-binding domains (NBDs) of NLRC2. This initiates the IPS-1-dependent pathway previously described for the RLRs and culminates in type I IFN and proinflammatory cytokine release. In contrast to the RLRs, the NLRC2 interaction with IPS-1 does not involve CARD-CARD binding (102).
NLRC5.
NOD-like receptor family, CARD-containing 5 (NLRC5) participates in the response to RNA viruses but has not been reported as having a direct sensor function (68, 87). NLRC5 is highly expressed in hematopoietic cells and is further induced in many cell types following viral invasion. NLRC5 contains a CARD domain and a NACHT domain but differs from other members of the NLR family by having a longer LRR domain that consequently adopts a different structure from the conventional horseshoe shape, perhaps forming a helical shape as suggested by Neerincx et al. (68, 87).
Kuenzal et al. (68) showed that NLRC5 gene expression is markedly increased upon infection with human cytomegalovirus (HCMV) and that this response depends on the IFN-γ-induced JAK-STAT (Janus kinase-signal transducer and activator of transcription) pathway. Apart from being a target of antiviral signaling, NLRC5 has also been identified as an important activator of antiviral signaling pathways. This suggests a role of NLRC5 as an endogenous amplifier of antiviral responses without sensor function (68, 87).
CONCLUSION
Nucleic acid motifs are the main virus-derived PAMPs to be recognized by the innate immune system. Viral ssRNA is distinguished from the capped mRNA of the host by an exposed 5′-triphosphate moiety in a subset of viral RNA and is sensed by TLR7 and TLR8 in the endosomes of pDCs and myeloid DCs and by RIG-I in the cytosol of many cell types. Viral dsRNA is sensed by RIG-I and MDA5 in the cytosol, by TLR3 in the endosome compartment in innate immune cells, and by certain NLRs. In addition to these first-line sensors, RNase L amplifies the RLR response, and IFN-inducible antiviral proteins such as PKR and OAS are upregulated upon viral recognition to control the infection. Autophagy is utilized to bring cytosolic RNA species to the endosomal compartment. The potential importance of understanding innate recognition of viral invasion cannot be overstated. The role of the innate antiviral response in protecting the host during the early phase of a viral infection has been known for a long time. More recently, it has also become apparent that the innate response greatly influences the formation of the subsequent adaptive response. Consequently, clarifying the underlying processes and the many ways in which viruses have evolved to try and evade innate immunity is extremely important with regard to our general understanding of viral pathogenesis, and is the only way in which we will stand reasonably prepared for the next emerging virus disease.
Footnotes
Published ahead of print 18 January 2012
REFERENCES
- 1. Akira S, Takeda K. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511 [DOI] [PubMed] [Google Scholar]
- 2. Akira S, Takeda K, Kaisho T. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680 [DOI] [PubMed] [Google Scholar]
- 3. Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801 [DOI] [PubMed] [Google Scholar]
- 4. Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. 2001. Recognition of double-stranded RNA and activation of NF-kappa B by Toll-like receptor 3. Nature 413:732–738 [DOI] [PubMed] [Google Scholar]
- 5. Allen IC, et al. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30:556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ammann CG, Messer RJ, Peterson KE, Hasenkrug KJ. 2009. Lactate dehydrogenase-elevating virus induces systemic lymphocyte activation via TLR7-dependent IFNalpha responses by plasmacytoid dendritic cells. PLoS One 4:e6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arnoult D, Carneiro L, Tattoli I, Girardin SE. 2009. The role of mitochondria in cellular defense against microbial infection. Semin. Immunol. 21:223–232 [DOI] [PubMed] [Google Scholar]
- 8. Barbalat R, Lau L, Locksley RM, Barton GM. 2009. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 10:U1200–U1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beignon AS, et al. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via toll-like receptor-viral RNA interactions. J. Clin. Invest. 115:3265–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bell JK, Askins J, Hall PR, Davies DR, Segal DM. 2006. The dsRNA binding site of human toll-like receptor 3. Proc. Natl. Acad. Sci. U. S. A. 103:8792–8797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bieback K, et al. 2002. Hemagglutinin protein of wild-type measles virus activates Toll-like receptor 2 signaling. J. Virol. 76:8729–8736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bowie A, O'Neill LAJ. 2000. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J. Leukoc. Biol. 67:508–514 [DOI] [PubMed] [Google Scholar]
- 13. Burzyn D, et al. 2004. Toll-like receptor 4-dependent activation of dendritic cells by a retrovirus. J. Virol. 78:576–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cardenas WB, et al. 2006. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J. Virol. 80:5168–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang S, Dolganiuc A, Szabo G. 2007. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J. Leukoc. Biol. 82:479–487 [DOI] [PubMed] [Google Scholar]
- 16. Choe J, Kelker MS, Wilson IA. 2005. Crystal structure of human Toll-like receptor 3 (TLR3) ectodomain. Science 309:581–585 [DOI] [PubMed] [Google Scholar]
- 17. Daffis S, Samuel MA, Suthar MS, Gale M, Diamond MS. 2008. Toll-like receptor 3 has a protective role against West Nile virus infection. J. Virol. 82:10349–10358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daffis S, et al. 2010. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature 468:452–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Delaloye J, et al. 2009. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 5:e1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Diao F, et al. 2007. Negative regulation of MDA5- but not RIG-I-mediated innate antiviral signaling by the dihydroxyacetone kinase. Proc. Natl. Acad. Sci. U. S. A. 104:11706–11711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diebold SS, Kaisho T, Hemmi H, Akira S, Sousa CRE. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529–1531 [DOI] [PubMed] [Google Scholar]
- 22. Dolganiuc A, et al. 2004. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology 127:1513–1524 [DOI] [PubMed] [Google Scholar]
- 23. Faul EJ, et al. 2010. Rabies virus infection induces type I interferon production in an IPS-1 dependent manner while dendritic cell activation relies on IFNAR signaling. PLoS Pathog. 6:e1001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fitzgerald KA, et al. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491–496 [DOI] [PubMed] [Google Scholar]
- 25. Fitzgerald KA, et al. 2001. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature 413:78–83 [DOI] [PubMed] [Google Scholar]
- 26. Flodstrom-Tullberg M, et al. 2005. RNase L and double-stranded RNA-dependent protein kinase exert complementary roles in islet cell defense during coxsackievirus infection. J. Immunol. 174:1171–1177 [DOI] [PubMed] [Google Scholar]
- 27. Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M., Jr 2008. Establishment and maintenance of the innate antiviral response to West Nile virus involves both RIG-I and MDA5 signaling through IPS-1. J. Virol. 82:609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. 2006. Nod-like proteins in immunity, inflammation and disease. Nat. Immunol. 7:1250–1257 [DOI] [PubMed] [Google Scholar]
- 29. Gack MU, et al. 2007. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446:U916–U912 [DOI] [PubMed] [Google Scholar]
- 30. Gao D, et al. 2009. REUL is a novel E3 ubiquitin ligase and stimulator of retinoic-acid-inducible gene-I. PLoS One 4:e5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gitlin L, et al. 2006. Essential role of mda-5 in type IIFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. U. S. A. 103:8459–8464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gitlin L, et al. 2010. Melanoma differentiation-associated gene 5 (MDA5) is involved in the innate immune response to Paramyxoviridae infection in vivo. PLoS Pathog. 6:e1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gowen BB, et al. 2006. TLR3 deletion limits mortality and disease severity due to phlebovirus infection. J. Immunol. 177:6301–6307 [DOI] [PubMed] [Google Scholar]
- 34. Guillot L, et al. 2005. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280:5571–5580 [DOI] [PubMed] [Google Scholar]
- 35. Habjan M, et al. 2008. Processing of genome 5′ termini as a strategy of negative-strand RNA viruses to avoid RIG-I-dependent interferon induction. PLoS One 3:e2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hardy AW, Graham DR, Shearer GM, Herbeuval J-P. 2007. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc. Natl. Acad. Sci. U. S. A. 104:17453–17458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hayakawa S, et al. 2011. ZAPS is a potent stimulator of signaling mediated by the RNA helicase RIG-I during antiviral responses. Nat. Immunol. 12:U37–U56 [DOI] [PubMed] [Google Scholar]
- 38. Heil F, et al. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526–1529 [DOI] [PubMed] [Google Scholar]
- 39. Hemmi H, et al. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196–200 [DOI] [PubMed] [Google Scholar]
- 40. Hemmi H, et al. 2004. The roles of two I kappa B kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J. Exp. Med. 199:1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoffmann M, et al. 2009. Toll-like receptor 2 senses hepatitis C virus core protein but not infectious viral particles. J. Innate Immun. 1:446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Honda K, et al. 2005. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 434:1035–1040 [DOI] [PubMed] [Google Scholar]
- 43. Honda K, Takaoka A, Taniguchi T. 2006. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity 25:349–360 [DOI] [PubMed] [Google Scholar]
- 44. Honda K, et al. 2004. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc. Natl. Acad. Sci. U. S. A. 101:15416–15421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Honda K, et al. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772–777 [DOI] [PubMed] [Google Scholar]
- 46. Horng T, Barton GM, Medzhitov R. 2001. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2:835–841 [DOI] [PubMed] [Google Scholar]
- 47. Hornung V, et al. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997 [DOI] [PubMed] [Google Scholar]
- 48. Hornung V, et al. 2005. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 11:263–270 [DOI] [PubMed] [Google Scholar]
- 49. Hornung V, et al. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531–4537 [DOI] [PubMed] [Google Scholar]
- 50. Hornung V, et al. 2004. Replication-dependent potent IFN-α induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 173:5935–5943 [DOI] [PubMed] [Google Scholar]
- 51. Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. 2009. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206:79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ishikawa H, Barber GN. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455:674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jia Y, et al. 2009. Negative regulation of MAVS-mediated innate immune response by PSMA7. J. Immunol. 183:4241–4248 [DOI] [PubMed] [Google Scholar]
- 54. Jurk M, et al. 2002. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 3:499. [DOI] [PubMed] [Google Scholar]
- 55. Kane M, et al. 2011. Innate immune sensing of retroviral infection via Toll-like receptor 7 occurs upon viral entry. Immunity 35:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kang DC, et al. 2004. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene 23:1789–1800 [DOI] [PubMed] [Google Scholar]
- 57. Kang DC, et al. 2002. mda-5: an interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. U. S. A. 99:637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kanneganti TD, et al. 2006. Critical role for cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 281:36560–36568 [DOI] [PubMed] [Google Scholar]
- 59. Kato H, et al. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19–28 [DOI] [PubMed] [Google Scholar]
- 60. Kato H, et al. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105 [DOI] [PubMed] [Google Scholar]
- 61. Kawai T, Akira S. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131–137 [DOI] [PubMed] [Google Scholar]
- 62. Kawai T, Akira S. 2008. Toll-like receptor and RIG-1-like receptor signaling. Ann. N. Y. Acad. Sci. 1143:1–20 [DOI] [PubMed] [Google Scholar]
- 63. Kawai T, et al. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061–1068 [DOI] [PubMed] [Google Scholar]
- 64. Kawai T, et al. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981–988 [DOI] [PubMed] [Google Scholar]
- 65. Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. 2008. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452:U234–U280 [DOI] [PubMed] [Google Scholar]
- 66. Kiyokawa T, et al. 2008. A single base mutation in the PRAT4A gene reveals differential interaction of PRAT4A with Toll-like receptors. Int. Immunol. 20:1407–1415 [DOI] [PubMed] [Google Scholar]
- 67. Kobayashi M, et al. 2009. Expression of toll-like receptor 2, NOD2 and dectin-1 and stimulatory effects of their ligands and histamine in normal human keratinocytes. Br. J. Dermatol. 160:297–304 [DOI] [PubMed] [Google Scholar]
- 68. Kuenzel S, et al. 2010. The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J. Immunol. 184:1990–2000 [DOI] [PubMed] [Google Scholar]
- 69. Kumar H, et al. 2006. Essential role of IPS-1 in innate immune responses against RNA viruses. J. Exp. Med. 203:1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kurt-Jones EA, et al. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398–401 [DOI] [PubMed] [Google Scholar]
- 71. Lang PA, et al. 2009. Hematopoietic cell-derived interferon controls viral replication and virus-induced disease. Blood 113:1045–1052 [DOI] [PubMed] [Google Scholar]
- 72. Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. 2007. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 315:1398–1401 [DOI] [PubMed] [Google Scholar]
- 73. Le Goffic R, et al. 2006. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu L, et al. 2008. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 320:379–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Loo Y-M, et al. 2008. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J. Virol. 82:335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lund JM, et al. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 101:5598–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Malathi K, Dong B, Gale M, Jr, Silverman RH. 2007. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448:U816–U819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Malathi K, et al. 2010. RNase L releases a small RNA from HCV RNA that refolds into a potent PAMP. RNA 16:2108–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Masumoto J, et al. 1999. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J. Biol. Chem. 274:33835–33838 [DOI] [PubMed] [Google Scholar]
- 80. Matsumoto M, et al. 2003. Subcellular localization of toll-like receptor 3 in human dendritic cells. J. Immunol. 171:3154–3162 [DOI] [PubMed] [Google Scholar]
- 81. McCartney SA, et al. 2008. MDA-5 recognition of a murine norovirus. PLoS Pathog. 4:e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Medzhitov R, et al. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2:253–258 [DOI] [PubMed] [Google Scholar]
- 83. Meylan E, et al. 2004. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat. Immunol. 5:503–507 [DOI] [PubMed] [Google Scholar]
- 84. Miettinen M, Sareneva T, Julkunen I, Matikainen S. 2001. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2:349–355 [DOI] [PubMed] [Google Scholar]
- 85. Mogensen TH. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22:240–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Muruve DA, et al. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452:103–107 [DOI] [PubMed] [Google Scholar]
- 87. Neerincx A, et al. 2010. A role for the human nucleotide-binding domain, leucine-rich repeat-containing family member NLRC5 in antiviral responses. J. Biol. Chem. 285:26223–26232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Negishi H, et al. 2008. A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proc. Natl. Acad. Sci. U. S. A. 105:20446–20451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. O'Neill LAJ. 2006. How Toll-like receptors signal: what we know and what we don't know. Curr. Opin. Immunol. 18:3–9 [DOI] [PubMed] [Google Scholar]
- 90. Oshiumi H, et al. 2010. The ubiquitin ligase Riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell Host Microbe 8:496–509 [DOI] [PubMed] [Google Scholar]
- 91. Oshiumi H, et al. 2011. The TLR3/TICAM-1 pathway is mandatory for innate immune responses to poliovirus infection. J. Immunol. 187:5320–5327 [DOI] [PubMed] [Google Scholar]
- 92. Paludan SR, Bowie AG, Horan KA, Fitzgerald KA. 2011. Recognition of herpesviruses by the innate immune system. Nat. Rev. Immunol. 11:143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pippig DA, et al. 2009. The regulatory domain of the RIG-I family ATPase LGP2 senses double-stranded RNA. Nucleic Acids Res. 37:2014–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Plumet S, et al. 2007. Cytosolic 5′-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS One 2:e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Poeck H, et al. 2010. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1[beta] production. Nat. Immunol. 11:63–69 [DOI] [PubMed] [Google Scholar]
- 96. Rajput A, et al. 2011. RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity 34:340–351 [DOI] [PubMed] [Google Scholar]
- 97. Rassa JC, Meyers JL, Zhang YM, Kudaravalli R, Ross SR. 2002. Murine retroviruses activate B cells via interaction with Toll-like receptor 4. Proc. Natl. Acad. Sci. U. S. A. 99:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rehermann B, Nascimbeni M. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215–229 [DOI] [PubMed] [Google Scholar]
- 99. Roth-Cross JK, Bender SJ, Weiss SR. 2008. Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J. Virol. 82:9829–9838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rothenfusser S, et al. 2005. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J. Immunol. 175:5260–5268 [DOI] [PubMed] [Google Scholar]
- 101. Rudd BD, Burstein E, Duckett CS, Li XX, Lukacs NW. 2005. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J. Virol. 79:3350–3357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sabbah A, et al. 2009. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 10:1073–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Saito T, et al. 2007. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl. Acad. Sci. U. S. A. 104:582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M., Jr 2008. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454:523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Samuel MA, et al. 2006. PKR and RNase L contribute to protection against lethal West Nile virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 80:7009–7019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sandor F, et al. 2003. Importance of extra- and intracellular domains of TLR1 and TLR2 in NF kappa B signaling. J. Cell Biol. 162:1099–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schmitz F, et al. 2007. Interferon-regulatory-factor 1 controls Toll-like receptor 9-mediated IFN-beta production in myeloid dendritic cells. Eur. J. Immunol. 37:315–327 [DOI] [PubMed] [Google Scholar]
- 108. Schulz O, et al. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433:887–892 [DOI] [PubMed] [Google Scholar]
- 109. Sen A, Pruijssers AJ, Dermody TS, Garcia-Sastre A, Greenberg HB. 2011. The early interferon response to rotavirus is regulated by PKR and depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3. J. Virol. 85:3717–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Seth RB, Sun LJ, Ea CK, Chen ZJJ. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappa B and IRF3. Cell 122:669–682 [DOI] [PubMed] [Google Scholar]
- 111. Shapira SD, et al. 2009. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell 139:1255–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sharma S, et al. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148–1151 [DOI] [PubMed] [Google Scholar]
- 113. Silverman RH. 2007. Viral encounters with 2 ′,5′-oligoadenylate synthetase and RNase L during the interferon antiviral response. J. Virol. 81:12720–12729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Steinberg C, et al. 2009. The IFN regulatory factor 7-dependent type I IFN response is not essential for early resistance against murine cytomegalovirus infection. Eur. J. Immunol. 39:1007–1018 [DOI] [PubMed] [Google Scholar]
- 115. Sumpter R, Jr, et al. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79:2689–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Sun Q, et al. 2006. The specific and essential role of MAVS in antiviral innate immune responses. Immunity 24:633–642 [DOI] [PubMed] [Google Scholar]
- 117. Tabeta K, et al. 2006. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7:156–164 [DOI] [PubMed] [Google Scholar]
- 118. Takaoka A, et al. 2005. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434:243–249 [DOI] [PubMed] [Google Scholar]
- 119. Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820 [DOI] [PubMed] [Google Scholar]
- 120. Thompson MR, Kaminski JJ, Kurt-Jones EA, Fitzgerald KA. 2011. Pattern recognition receptors and the innate immune response to viral infection. Viruses 3:920–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Triantafilou K, Triantafilou M. 2004. Coxsackievirus B4-induced cytokine production in pancreatic cells is mediated through Toll-like receptor 4. J. Virol. 78:11313–11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Uematsu S, et al. 2005. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-alpha induction. J. Exp. Med. 201:915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Urisman A, et al. 2006. Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2:e25. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 124. Uronen-Hansson H, et al. 2004. Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: integrity of microtubules is required for interleukin-12 production in response to internalized bacteria. Immunology 111:173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Venkataraman T, et al. 2007. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J. Immunol. 178:6444–6455 [DOI] [PubMed] [Google Scholar]
- 126. Wang C, et al. 2001. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412:346–351 [DOI] [PubMed] [Google Scholar]
- 127. Wang JP, et al. 2005. Varicella-zoster virus activates inflammatory cytokines in human monocytes and macrophages via Toll-like receptor 2. J. Virol. 79:12658–12666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wang JP, et al. 2006. Flavivirus activation of plasmacytoid dendritic cells delineates key elements of TLR7 signaling beyond endosomal recognition. J. Immunol. 177:7114–7121 [DOI] [PubMed] [Google Scholar]
- 129. Wang T, et al. 2004. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 10:1366–1373 [DOI] [PubMed] [Google Scholar]
- 130. Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80:5059–5064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yamamoto M, et al. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640–643 [DOI] [PubMed] [Google Scholar]
- 132. Yanai H, et al. 2007. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc. Natl. Acad. Sci. U. S. A. 104:3402–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yang Y, et al. 2007. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 26:215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Yoneyama M, Fujita T. 2007. Function of RIG-I-like receptors in antiviral innate immunity. J. Biol. Chem. 282:15315–15318 [DOI] [PubMed] [Google Scholar]
- 135. Yoneyama M, et al. 2005. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175:2851–2858 [DOI] [PubMed] [Google Scholar]
- 136. Yoneyama M, et al. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730–737 [DOI] [PubMed] [Google Scholar]
- 137. Yu JW, et al. 2006. Cryopyrin and pyrin activate caspase-1, but not NF-kappa B, via ASC oligomerization. Cell Death Differ. 13:236–249 [DOI] [PubMed] [Google Scholar]
- 138. Zhong B, et al. 2008. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29:538–550 [DOI] [PubMed] [Google Scholar]
- 139. Zhou AM, et al. 1997. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 16:6355–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhou S, et al. 2010. Induction and inhibition of type I interferon responses by distinct components of lymphocytic choriomeningitis virus. J. Virol. 84:9452–9462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhou S, et al. 2008. Lymphocytic choriomeningitis virus (LCMV) infection of CNS glial cells results in TLR2-MyD88/Mal-dependent inflammatory responses. J. Neuroimmunol. 194:70–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhou SH, et al. 2005. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 35:822–830 [DOI] [PubMed] [Google Scholar]
- 143. Zucchini N, et al. 2008. Cutting edge: overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. J. Immunol. 180:5799–5803 [DOI] [PubMed] [Google Scholar]