Abstract
Humoral immune responses are thought to play a major role in dengue virus-induced immunopathology; however, little is known about the plasmablasts producing these antibodies during an ongoing infection. Herein we present an analysis of plasmablast responses in patients with acute dengue virus infection. We found very potent plasmablast responses that often increased more than 1,000-fold over the baseline levels in healthy volunteers. In many patients, these responses made up as much 30% of the peripheral lymphocyte population. These responses were largely dengue virus specific and almost entirely made up of IgG-secreting cells, and plasmablasts reached very high numbers at a time after fever onset that generally coincided with the window where the most serious dengue virus-induced pathology is observed. The presence of these large, rapid, and virus-specific plasmablast responses raises the question as to whether these cells might have a role in dengue immunopathology during the ongoing infection. These findings clearly illustrate the need for a detailed understanding of the repertoire and specificity of the antibodies that these plasmablasts produce.
INTRODUCTION
Dengue virus causes an infection with symptoms ranging from a mild fever to severe hemorrhagic fever with vascular leakage that ranges in severity from minor subcutaneous bleeding to severe gastrointestinal bleeding (5, 28, 34). A striking epidemiological and immunological characteristic of dengue fever (DF) is that the severe immunopathology is more likely to occur in individuals who have previously been infected with a heterologous dengue virus serotype (8, 29, 32). While the exact mechanism of this phenomenon remains to be fully elucidated, several hypotheses have been developed over the last few decades to explain the reason for the exacerbated pathology observed in these patients. One of the main hypotheses revolves around a mechanism referred to as antibody-dependent enhancement (ADE) (14). This hypothesis suggests that during a secondary infection, cross-reactive yet poorly cross-neutralizing antibodies produced against a previously encountered serotype will mediate an increased infectivity, in addition to altering the host range of target cells. This mechanism has been extensively studied in vitro (6, 17, 20), and its importance in vivo is beginning to be elucidated (2, 10, 27). Another proposed hypothesis (22, 23) suggests that an enhanced infection together with a potent T cell-mediated recall response produces massive amounts of effector mediators (4, 11–13, 15, 16, 25), a so-called cytokine storm, that is responsible for the observed immunopathology. These two mechanisms are not mutually exclusive and may in fact work in concert to cause the immunopathology of dengue disease.
While human T cell responses during acute dengue virus infection have been studied in some detail, much less is known about the B cell responses. Early studies in dengue patients showed that increases in immunoglobulin-containing cells could be observed during infection and that these cells reached maximal numbers near the time of subsidence of fever (7). It has also been shown that total CD19+ B cells increase during dengue virus infection and that these increases correlate with the presence of so-called atypical lymphocytes (19). In addition, a more recent study of the global gene expression patterns in peripheral blood mononuclear cells (PBMCs) isolated from dengue hemorrhagic fever (DHF) patients showed an enrichment of plasmablast signatures that was accompanied by an increase of plasmablasts by flow cytometric analysis (30).
Here, we have analyzed the magnitude, kinetics, antigen specificity, and isotype usage of the plasmablast responses induced in pediatric and adult patients with acute dengue virus infection. We found during the acute phase of the infection a very potent and rapid induction of virus-specific plasmablasts, which in some cases made up as much as 30% of total lymphocytes. The rapid expansion of plasmablasts was observed in the infected patients at a time point that generally coincides with the subsidence of fever and the most serious symptoms. These findings suggest that these cells and the antibodies that they produce might be involved in dengue immunopathology. However, while suggestive, these findings also clearly illustrate the need for more detailed analyses of the plasmablasts and the antibodies that they produce during the acute phase of dengue virus infection to clearly define their potential role in dengue immunopathology.
MATERIALS AND METHODS
Dengue patient cohort.
Patients enrolled in this study were clinically diagnosed with dengue virus infection upon admission to Siriraj Hospital in Bangkok, Thailand. The dengue virus infection was confirmed by a serotype-specific reverse transcription-PCR (RT-PCR) as well as several other diagnostic tests (NS1 test, dengue-specific IgG and IgM test [enzyme-linked immunosorbent assay {ELISA}, or dipstick tests]). Routine laboratory measurements (complete blood count [CBC], urine and blood chemistry) and clinical manifestations of dengue virus infection were recorded. A final diagnosis and severity classification were done at the conclusion of the trial with a full review of all the clinical and laboratory data. Information about the patient cohort is detailed in Table 1 and in Table S1 in the supplemental material. All studies were preapproved by the Faculty of Medicine at Siriraj Hospital and the Emory institutional review boards.
Table 1.
Summary of dengue patients enrolled in the studya
| Characteristic | DF | DHFb |
|---|---|---|
| Total no. of patients | 28 | 18 |
| No. of males/no. of females | 20/8 | 10/8 |
| Age (yr)c | 17 (1–50) | 20 (7–40) |
| Fever day of sampled | 6 (2–8) | 6 (3–8) |
| No. of patients infected with dengue virus serotypee: | ||
| 1 | 8 | 7 |
| 2 | 10 | 4 |
| 3 | 7 | 6 |
| 4 | 0 | 0 |
Patients were diagnosed on the basis of serology (dengue virus-specific RT-PCR, NS1 test, dengue virus-specific IgG/IgM test, clinical measurements [CBC, urine and blood chemistry]) and clinical manifestations of dengue virus infection.
One of the 18 DHF cases was classified as DSS (DHF class III).
Median age is shown with the range in parentheses.
Median day after fever onset on which the blood sample was obtained. Ranges are in parentheses.
Serotype determination by RT-PCR. Four patients were negative by this assay but classified as having dengue on the basis of other clinical measurements, determined as described in footnote a.
PBMC and plasma isolation.
PBMCs and plasma were isolated essentially as previously described (35). Briefly, blood samples were collected in Vacutainer CPT tubes (Becton Dickinson [BD]). Plasma samples were isolated from the CPT tubes and preserved at −80°C. The PBMCs were collected, washed extensively, and resuspended in phosphate-buffered saline (PBS) with 2% fetal calf serum (FCS) for immediate use or frozen in liquid nitrogen in FCS with 10% dimethyl sulfoxide for subsequent analysis.
Serological determination of primary or secondary dengue virus infection.
Dengue IgM and IgG antibodies were detected by a modified ELISA as described by Innis et al. (18). Briefly, microtiter plates (Nunc) were coated with 100 μl of goat antihuman IgM or IgG and stored at 4°C. Serum specimens were diluted at 1:100 (0.05% PBS–Tween [PBS-T], 3% skim milk), added to the plates, and incubated for 1 h at 37°C. After washing, 50 μl of pooled dengue virus antigens diluted at 1:3 (0.05% PBS-T, 2% lipid-extracted normal human serum, 3% skim milk) was put into each tested well, and the plate was incubated for 1 h at 37°C and washed before adding 50 μl of anti-dengue virus complex (2H2) monoclonal antibody diluted at 1:10,000. After 1 h at 37°C, plates were washed before adding 50 μl of the goat antimouse conjugate. Plates were read at 492 nm using an ELISA reader (Powerwave 340; BioTek), and samples were considered positive at a 0.500 optical density (OD) absorbance.
Identification of dengue virus serotype.
Dengue virus RNA was extracted from patient plasma using a QIAamp viral RNA extraction kit (Qiagen, Hilden, Germany) according to the manufacturer's instruction. Dengue virus serotype was determined by a multiplex nested PCR method as previously described (36) with some modification. Briefly, RNA was subjected to reverse transcription (RT) with primer DEUR (5′-GCTGTGTCACCCAGAATGGCCAT-3′), and a multiplex nested PCR with four primer pairs specific for the E region of each dengue virus serotype was performed. Four pairs of serotype-specific primers were used, including D1L (5′-GGGGCTTCAACATCCCAAGAG-3′) and D1R (5′-GCTTAGTTTCAAAGCTTTTTCAC-3′) for dengue virus serotype 1 (DENV-1), D2L (5′-ATCCAGATGTCATCAGGAAAC-3′) and D2R (5′-CCGGCTCTACTCCTATGATG-3′) for DENV-2, D3L (5′-CAATGTGCTTGAATACCTTTGT-3′) and D3R (5′-GGACAGGCTCCTCCTTCTTG-3′) for DENV-3, and D4L (5′-GGACAACAGTGGTGAAAGTCA-3′) and D4R (5′-GGTTACACTGTTGGTATTCTCA-3′) for DENV-4. The expected sizes of the nested PCR products for DENV-1, DENV-2, DENV-3, and DENV-4 were 504 bp, 346 bp, 196 bp, and 143 bp, respectively.
Viral antigen.
Purified DENV-2 particles were purchased from Microbix Biosystems Inc. (catalog no. EL-22-02; Canada). According to the manufacturer, this preparation contains virus particles (DENV-2 strain 16681) concentrated from tissue culture supernatants by precipitation and ultracentrifugation. The antigen is then purified by sucrose density gradient centrifugation. Virus particles are separated from the sucrose-containing buffer by ultracentrifugation, and the antigen is resuspended in medium 199, followed by inactivation at room temperature with formaldehyde. For the final product formulation, the formaldehyde is neutralized by the addition of sodium bisulfite.
Analytical flow cytometry.
Staining for analytical flow cytometry of plasmablasts was performed on whole blood as previously described (35). Briefly, cells were stained with the appropriately titrated antibodies, followed by lysis of erythrocytes (catalog no. 349202; BD FACS lysing solution; Pharmingen) and fixation in 2% phosphonoformic acid. All antibodies were purchased from Pharmingen (CD19-fluorescein isothiocyanate [555412], CD38-phycoerythrin [555460], CD3-peridinin chlorophyll protein [PerCP; 340663], CD20-PerCP [347674]), except anti-CD27-allophycocyanin (17-0279-73), which was purchased from eBiosciences. Plasmablasts were defined herein as CD19+ CD3− CD20−/low CD27high CD38high cells gated on an extended lymphocyte gate to include blasting cells. Flow cytometry data were analyzed using FlowJo software. Absolute cell numbers per volume blood were calculated using the BD Trucount Tubes bead system (catalog no. 340334BD; eBiosciences) according to the manufacturer's protocol.
ELISPOT assay.
Direct enzyme-linked immunosorbent spot (ELISPOT) assay to enumerate either dengue virus-specific or total IgG-, IgM-, and IgA-secreting plasmablasts present in the PBMC samples was performed as previously described (9, 35). Briefly, 96-well ELISPOT assay filter plates (MAHA N4510; Millipore) were coated overnight either with the optimized amounts of purified dengue virions as described above (10 μg/ml) or with polyvalent goat anti-human immunoglobulins (10 μg/ml; catalog no. 109-005-064; Jackson ImmunoResearch) in PBS. Plates were washed and blocked by incubation with RPMI containing 10% FCS at 37°C for 2 h. Purified and extensively washed PBMCs were added to the plates in dilution series starting at 10 × 105, diluted 3- to 5-fold down the plate, and incubated overnight. Plates were washed with PBS, followed by PBS containing 0.05% Tween, and then incubated with either a biotinylated anti-human IgG antibody (catalog no. H10015; Invitrogen), anti-human IgA (catalog no. H14015; Invitrogen), or anti-human IgM (catalog no. H15015; Invitrogen) for 1.5 h at room temperature. After washing, the plates were incubated with an avidin D-horseradish peroxidase conjugate (catalog no. A-2004; Vector Laboratories) and finally developed using 3-amino-9-ethyl-carbazole (AEC) substrate (Sigma). Developed plates were scanned and analyzed using an automated ELISPOT counter (CTL, Cellular Technologies Ltd.).
ELISA.
Direct ELISA was performed by coating ELISA plates overnight with either anti-human Ig (catalog no. 109-005-064; Jackson ImmunoResearch) or dengue virus antigen (as described above) at 10 mg/ml. Plates were washed with PBS with 0.5% Tween and blocked in PBS with 10% FCS and 0.2% Tween. Serially diluted serum samples were then added to the plates, followed by incubation with a peroxidase-conjugated anti-human IgG antibody (catalog no. H10015; Invitrogen), and were finally developed using o-phenylenediamine substrate. Serum dilution was plotted versus the OD value at 492 nm, and the midpoint dilution values were determined.
Statistical analysis.
Data were collected and graphed using MS Excel and GraphPad Prism software. Data for each individual subject are presented as a circle dot with a bar that represents the median of each group. An unpaired, two-tailed t test was used to determine the statistical significance of the difference observed between groups. Spearman's correlation coefficient test was used to analyze the correlation observed between different parameters.
RESULTS
The patient cohort in this study was comprised of individuals diagnosed with dengue virus infection at Siriraj Hospital, Bangkok, Thailand, from 2009 to 2011. Single blood samples were obtained 2 to 8 days after symptom onset and for some of the donors also at a convalescent-phase time point 1 month or later after discharge. Of 56 patients diagnosed clinically, 46 were confirmed to be infected with dengue virus (Table 1; see Table S1 in the supplemental material), while 10 were found to have some other, nondengue, febrile illness. Those testing positive for dengue were infected with dengue virus serotype 1, 2, or 3, while none were infected with a dengue virus serotype 4 strain. This result is in concordance with previous studies demonstrating the low prevalence of DENV-4 in the area (21). Of the 46 confirmed dengue cases, 28 were classified as having DF, 17 were classified as having DHF class I and II, and 1 patient had dengue shock syndrome (DSS; DHF class III), according to the WHO criteria (34). Three additional patients were also enrolled in 2011, and we obtained two consecutive blood samples during hospitalization. Serological analyses showed that of the 46 dengue patients, only 4 were mounting primary responses, based on an IgM/IgG ratio lower than 1.7 (18) (see Table S1 in the supplemental material). Thus, the majority of the patients enrolled in the study had had previous exposure to dengue virus and were mounting secondary immune responses.
Potent and rapid plasmablast responses induced by dengue virus infection.
Antibodies are thought to play a central role in the development of dengue immunopathology (2, 6, 10, 20). However, relatively little is known about the magnitude or kinetics of the B cell responses induced during the acute phase of dengue virus infection. Figure 1A shows representative examples of the flow cytometric analysis of plasmablasts during the acute infection. These patients demonstrated a massive induction of peripheral plasmablasts, in some cases corresponding to as much as 30% of peripheral lymphocytes. Analysis of the overall set of patients showed that the majority had very high numbers of plasmablasts (Fig. 1B), averaging 47% of the peripheral B cells. At convalescent phase, 1 month after discharge, the numbers of peripheral plasmablasts had returned to baseline levels, similar to those found in healthy adults.
Fig 1.
Potent plasmablast responses induced during acute dengue virus infection. Plasmablast responses in peripheral blood of healthy controls or patients diagnosed with dengue fever (retrospectively confirmed to be dengue) were measured by flow cytometry. A subset of the patients came back for testing of a follow-up blood sample 1 month after discharge. (A) Representative flow cytometric analysis of the plasmablast frequency in four patients during ongoing dengue virus infection and 1 month after resolution. Plasmablasts are defined herein as CD19+ CD3− CD20−/low CD38hi CD27hi cells. All plots shown are gated on CD3− CD20−/low lymphocytes, including blasting cells. The numbers in the plots represent the percentage of that gate, while numbers in parentheses are the percentage of all lymphocytes. (B) Summary of the frequency of plasmablasts, as a fraction of total CD19+ B cells, in samples obtained from healthy adults, patients with ongoing dengue virus infection, and dengue patients returning after at least 1 month after clearance of infection. Statistical analysis was performed using an unpaired, two-tailed t test. (C) Magnitude of the plasmablast response as absolute numbers in patients infected with different dengue viral serotypes. Statistical analysis was done using an unpaired, two-tailed t test. ND, not detected; ns, not significant. (D) Comparison of the magnitude of the dengue virus infection-induced plasmablast responses to that observed after vaccination with either the inactivated influenza virus vaccine (which induces a recall response that peaks at 7 days after vaccination [35]) or the live attenuated yellow fever virus (YFV) vaccine (which induces a primary response that peaks at day 11/14 after vaccination). Statistical analysis was done using an unpaired, two-tailed t test.
The magnitude of this response is striking, particularly compared with the immune responses to influenza booster vaccination (peak, day 7; historical data from reference 35) or primary infection (peak, days 11 to 14) with the live attenuated yellow fever virus vaccine. While these results are derived from clinical studies performed in the United States (1, 24, 35) and therefore in different cohorts and settings, it is clear that the already sizeable plasmablast responses observed in both these systems (Fig. 1D) are significantly smaller in magnitude than the responses observed during dengue virus infection.
In terms of absolute numbers, several dengue patients carried more than 1 × 106 plasmablasts per ml of blood (median, 3.7 × 105; Fig. 1C and 2A), which is a 1,000-fold increase over what is normally observed in healthy individuals. Not surprisingly, these responses are of a similar magnitude regardless of what serotype of dengue virus the patients were infected with (Fig. 1C). Although this trial was not designed to be a detailed kinetic analysis, the timing of collection of the samples that we analyzed provided interesting information about the kinetics of this response. Samples obtained on day 2 or 3 generally displayed relatively low numbers of plasmablasts, often indistinguishable from the numbers in samples taken from healthy adults. However, as shown in Fig. 2A, the samples obtained from patients later after symptom onset showed a steady and dramatic increase of plasmablasts, reaching peak numbers by day 6 or 7. This finding is substantiated by the analysis of a small number of additional donors in a separate trial, where we found similar increases in consecutive blood samples taken from the same donor. Thus, Fig. 2B shows an analysis of three donors from whom consecutive blood samples were obtained 2 days apart, where we saw a significant increase of plasmablasts when comparing the earlier time point to the later one. The clinical diagnosis of DF or DHF did not correlate with the magnitude or kinetics of the plasmablast response (Fig. 2A), although this might have been confounded by the fact that all patients were unwell enough to require hospital admission. Finally, the responses observed in the four donors who mounted primary responses (based on serological analyses) were similar to those observed in the secondary responders (see Fig. S1 in the supplemental material).
Fig 2.
Rapid expansion of plasmablast responses during acute dengue virus infection. (A) Absolute plasmablast numbers per ml blood (calculated using a bead-based [TruCount; BD] system) for all the donors analyzed as a function of number of days after fever onset. Spearman's correlation coefficient test was used to analyze the correlation observed between different parameters for all the data points. Similar results were obtained when each subgroup was analyzed individually. (B) Plasmablast responses in peripheral blood in three patients diagnosed with and confirmed to have dengue fever infection were measured by flow cytometry at two consecutive time points with 2 days between sample collections. Plots show cells gated for CD3− CD20−/low cells with the CD27hi CD38hi plasmablasts marked by a box. The frequency of these plasmablasts is shown next to the box, and the frequency of total lymphocytes is shown in parentheses.
We conclude from these analyses that dengue virus infection induces a massive plasmablast response, often leading to increases of more than 1,000-fold over the number observed in healthy donors. This increase occurs rapidly over several days after fever onset, with very high numbers reached at about day 6 or 7, in some cases generating a plasmablast pool that makes up as much as a third of all peripheral blood lymphocytes.
The majority of the plasmablast response during the acute phase of dengue virus infection is dengue virus specific.
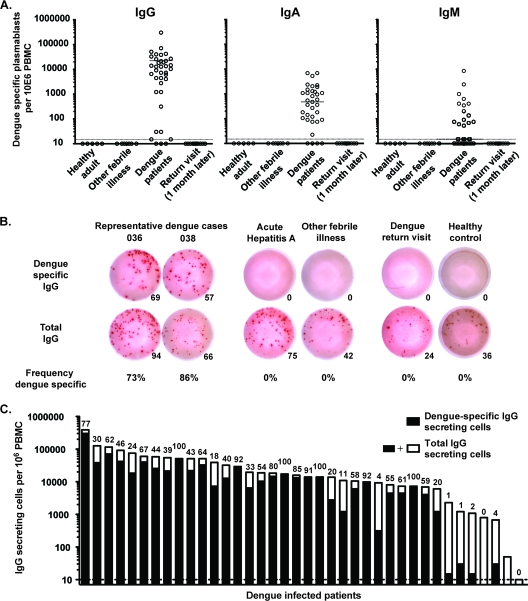
It has been proposed not only that infection can lead to activation of antigen-specific cells but also that the proinflammatory environment could lead to the activation of both B and T cells in a nonspecific manner, i.e., bystander activation (3, 31, 33). To determine the extent to which the dengue virus infection-induced plasmablast response was the result of antigen-specific or nonspecific bystander activation, we examined the specificity and isotype usage of the antibodies that they produced using a dengue virus-specific ELISPOT assay (Fig. 3A). This analysis showed that the response was dominated by dengue virus-specific IgG-secreting cells, with several donors showing more than 105 dengue virus-specific IgG-secreting cells per 106 PBMCs. We also observed dengue virus-specific IgA-secreting cells in most donors but at about a 100-fold lower frequency. Finally, a small number of the donors had detectable IgM-secreting plasmablast responses. Importantly, none of these were IgM only, and only one (donor Den01-066) had an IgM response that was larger than the IgG response in the same donor. It is interesting to note that of the four donors identified to be primary responders, all had detectable IgM responses by ELISPOT assay. Of these, donor Den01-066 mentioned above had a very high serum IgM/IgG ratio (see Table S1 in the supplemental material) and also showed the highest IgM response by ELISPOT assay.
Fig 3.
The majority of the plasmablasts induced by dengue virus infection are virus specific. Plasmablast responses in peripheral blood were measured by ELISPOT analysis during infection and 1 month after discharge. (A) Graphs showing the number of IgG-, IgA-, or IgM-secreting dengue virus-specific plasmablasts per million PBMCs. ELISPOT assay plates were coated with purified 16681 DENV-2 antigen. (B) Representative IgG-specific ELISPOT analysis of two dengue virus-infected patients, two patients with other acute febrile illnesses, a dengue patient returning for a 1-month follow-up, and a healthy control. Upper row, dengue virus-specific IgG spots; lower row, total IgG-secreting cells. From left to right, the numbers of cells plated was 823, 823, 2,489, 7,407, 66,000, and 66,000 PBMCs. The number given below each well is the spot count for that well. Relative frequency (percent) of dengue virus-specific IgG-secreting plasmablasts over total IgG secreting plasmablasts is also shown. (C) Summary of the number of total IgG-secreting cells and dengue virus-specific IgG-secreting cells for all the donors analyzed. The entire bar (black and white together) represents the total number of IgG-secreting cells per million PBMCs, while the numbers of dengue virus-specific IgG-secreting cells are marked in black only. Shown above each bar is the percentage of dengue virus-specific cells over total IgG-secreting cells.
Comparing the number of specific and total IgG-secreting plasmablasts, we found that the majority of the IgG response was indeed dengue virus specific, often with 70% or more of the IgG-secreting cells being specific for the virus (Fig. 3B and C). This frequency is likely an underestimation, as these assays were performed using formalin-inactivated purified DENV-2. A large proportion of these responses are likely directed against proteins or epitopes shared between the dengue virus serotypes, as no significant difference in the frequency of virus-specific cells was seen between patients infected with DENV-2 and those with DENV-1 or DENV-3 infections (see Fig. S2 in the supplemental material). The current study was aimed at understanding the overall immune responses induced by dengue virus infection, but future studies will be aimed at understanding the serotype-specific responses and their functional properties. Figure 3B also shows results for two patients, one with acute hepatitis A virus infection and one with an unidentified febrile illness. Both patients showed substantial plasmablast responses; however, none of the induced cells were dengue virus specific. When samples from patients returning 1 month after hospital discharge were analyzed, plasmablast numbers had returned to baseline. This illustrates that these responses are transient in peripheral blood, likely reflecting substantial cell death as well as migration to tissues capable of long-term maintenance of plasma cells, such as the bone marrow.
Analyzing the serological responses induced at the same time point as the plasmablast analyses showed that there were high titers of dengue virus-specific IgG present during the acute phase of the infection (Fig. 4A). All the infected patients had very high levels of dengue virus-specific antibody, but there was a wide variability between individuals. Of interest, the two patients with primary infection (based on the IgG/IgM ratios) showed very low overall titers at this time point. Furthermore, as expected, among the healthy adult controls from Thailand, 2/3 had detectable titers, whereas the healthy controls from the United States were seronegative. Finally, comparing the dengue virus-specific serological responses to the plasmablast responses, we found that they correlated well, as shown in Fig. 4B and C.
Fig 4.
Dengue virus-specific serum antibody correlates with the observed plasmablast responses. ELISA analysis of serum samples obtained from the acutely infected dengue patients as well as healthy control samples. This group of samples was from 17 cases of secondary infection, 2 primary cases (based on IgG/IgM ratios, as described in the text), and 3 healthy Thai and 2 healthy U.S. volunteers. Dengue virus-specific IgG was measured by direct ELISA using the same dengue virus antigen used for the ELISPOT analysis of the cellular responses described in the legend to Fig. 3. (A) ELISA measurement of total dengue virus-specific IgG. Midpoint dilution values were determined as the intersection of the dotted line at 50% of the OD value and the ELISA binding curves. (B and C) Correlation of the serological responses (as determined by the midpoint dilution value determined in panel A) and the plasmablast response by absolute plasmablasts in blood (flow cytometry) (B) and dengue virus-specific plasmablasts by ELISPOT assay (C).
Given the size and isotype of the plasmablast response, it might be expected that these patients show evidence of hypergammaglobulinemia. To this end, we also measured the total IgG in these patients and healthy controls. However, no significant differences were observed (data not shown) between the patients and the healthy controls.
Collectively, this study shows that dengue virus infection induced a very potent virus-specific plasmablast response in nearly all of the patients (93%). These responses are dominated by IgG-secreting cells and increased as much as 1,000-fold in magnitude, reaching maximal numbers by day 6 or 7 after fever onset. These findings regarding the magnitude and specificity of the plasmablast responses during the acute phase of dengue virus infection prompt more in-depth analyses of plasmablast responses with regard to the repertoire breadth and fine specificity of the antibodies that they produce, as well as their possible involvement in dengue-induced immunopathology.
DISCUSSION
In an effort to analyze the plasmablast responses induced during the acute phase of dengue virus infection, we have examined a cohort of patients from both pediatric and adult clinics identified at Siriraj Hospital in Bangkok. This patient group contained both patients with mild symptoms (DF) and patients with more severe symptoms (DHF). We found very potent plasmablast responses induced in almost all of the dengue virus-infected donors. In many cases, these responses completely dominated the B cell compartment (often as much as 80% of the CD19+ B cells were plasmablasts), making up as much as 30% of the total peripheral lymphocytes. In contrast, by way of comparison to U.S. volunteers receiving the influenza vaccine (35) or a primary vaccination with the yellow fever vaccine, we find much smaller responses, on the order of 2 to 3% of the total CD19+ B cells. The magnitude of the plasmablast response during dengue virus infection correlated with the time following the onset of febrile symptoms. Thus, most samples taken 2 to 3 days after fever onset showed a barely detectable response, but over time the response increased to the massive responses observed in samples obtained on day 6 or 7. These findings were substantiated by additional analysis of three donors from whom two samples were obtained 2 days apart, which showed a similar increase with time.
Importantly, ELISPOT assay analysis showed that the majority, often 80% or more, of the dengue virus-induced plasmablasts were secreting dengue virus-specific IgG. This suggests that these cells were induced by interaction with their cognate antigen and were not the result of nonspecific, bystander mechanisms. Interestingly, even though we used a DENV-2 clone for detection of antigen-specific cells in the ELISPOT assay, there was little difference in the relative frequency of antigen-specific cells over total IgG-secreting cells in patients infected with DENV-1 or -3 compared to patients infected with DENV-2 (see Fig. S2 in the supplemental material). This indicates that most of the plasmablast cells induced by infection are specific for proteins or epitopes present in multiple serotypes. Future studies, on a monoclonal level, are needed to identify what protein/epitope these responses target, what proportion of these responses are functionally active, and how this activity correlates with serotype specificity.
Patients returning to the hospital for a follow-up visit about 1 month after discharge showed essentially baseline numbers of plasmablasts by flow cytometry with no dengue virus-specific cells by ELISPOT assay. This demonstrates that these cells are present in the peripheral circulation only for a relatively short time, likely representing either contraction through extensive cell death or migration of at least a subset of these cells to tissues where long-term antibody production can be sustained, such as bone marrow or secondary lymphoid tissues. Furthermore, while patients suffering from other febrile illnesses often showed sizeable numbers of plasmablasts in the periphery, these cells were not dengue virus specific by the ELISPOT assay.
The patient cohort studied here almost exclusively represented secondary exposures to dengue virus, based on serological analyses (see Table S1 in the supplemental material) as well as the fact that the virus is endemic in this area. In fact, the vast majority of the population in this region seroconverts very early in life (8). Although the few primary cases identified in the current cohort showed responses similar to those of the overall cohort, they were too few to be able to reach a solid conclusion regarding differences between the responses induced in primary versus secondary responses. Ongoing studies of primary dengue virus infection in areas where the virus is not endemic will determine how these data compare to primary immune responses in terms of magnitude, kinetics, and isotype distribution in the plasmablast response.
Acute infection with dengue virus results in symptoms ranging from a mild fever to severe vascular leakage and massive internal bleeding. Interestingly, epidemiological studies suggest that the immunological history might be important for the outcome of infection, such that while a primary exposure to this virus generally leads to a mild disease and lifelong protection against that serotype, a subsequent exposure to a heterologous strain can sometimes lead to the more severe form of dengue, with the accompanying hemorrhagic symptoms described above. Both T and B cell-mediated mechanisms have been implicated in dengue-induced immunopathogenesis, but the importance of either of these mechanisms has not been fully characterized in vivo (23). Recently, two papers have also described dengue virus-specific memory B cells at convalescent-phase time points and characterized the antibodies that the memory B cells produce (2, 10). However, studies describing the antibodies produced by responding plasmablasts during the acute phase of the infection have not been reported. Ongoing studies to clearly define the repertoire breadth of the infection-induced plasmablasts, the epitopes that they recognize, and their ability to cause immunopathology by ADE-like responses at a monoclonal level will provide further insight into the role of these cells.
In addition to their role in binding antigen, antibodies are able to regulate immune responses through interaction with several different Fc receptors, a function that is directly dependent on their Fc-region glycosylation pattern. Recent studies (reviewed in reference 26) have clearly shown that glycosylation patterns regulated on IgG can mediate pro- or anti-inflammatory functions. These findings suggest a mechanism for ensuring that steady-state serum IgG maintains an anti-inflammatory state, while, in contrast, upon antigenic challenge by a pathogen, the antigen-specific IgG antibodies produced can mediate a more proinflammatory state. Given the large antibody-secreting cell responses that we have observed in dengue patients, it is possible that the IgG produced by the plasmablasts is also contributing to pathology by exacerbating the proinflammatory state in these patients. However, while the dengue virus-infected patients showed very high titers of dengue virus-specific antibody that were proportional to the magnitude of the plasmablast response (Fig. 4), no overall increases of total IgG were found in these patients (data not shown). Given the massive numbers of plasmablasts present in these patients during the acute phase of infection, this would indicate that only a smaller number of them are able to survive long term as long-lived plasma cells. It is unclear if this is due to the fact that the bone marrow can sustain only a smaller number of plasma cells or if the majority of the induced plasmablasts are predestined to a short life span.
In conclusion, human dengue virus infection induces a very rapid and potent plasmablast response, often dominating the B cell compartment of these patients. We show that these responses are largely dengue virus specific and almost entirely made up of IgG-secreting cells and that they reach very large numbers at a time after fever onset that generally coincides with the window where the most serious dengue virus-induced pathology is observed. The findings are suggestive of a role for these cells in dengue immunopathogenesis. However, more detailed analyses of the fine specificity and functional properties of the antibodies that they produce would be needed to truly address this important question.
Supplementary Material
ACKNOWLEDGMENTS
We thank Chris Chiu, Carl Davis, and Megan McCausland for critical reading of the manuscript and S. Kongprasit, K. Intalapaporn, A. Maleesatharn, N. Kongstan, C. Utenpitak, and R. Arjwong for excellent technical and clinical support.
This work was funded by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) grant U19-AI057266 and by the Office of the Higher Education Commission and Mahidol University under the Thai National Research Universities Initiative. N.O. is supported by a Chalermphrakiat grant from the Faculty of Medicine, Siriraj Hospital, Mahidol University. K.P. is a Thailand Research Fund Senior Research Scholar.
Footnotes
Published ahead of print 11 January 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Akondy RS, et al. 2009. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J. Immunol. 183:7919–7930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beltramello M, et al. 2010. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8:271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bernasconi NL, Traggiai E, Lanzavecchia A. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199–2202 [DOI] [PubMed] [Google Scholar]
- 4. Bethell DB, et al. 1998. Pathophysiologic and prognostic role of cytokines in dengue hemorrhagic fever. J. Infect. Dis. 177:778–782 [DOI] [PubMed] [Google Scholar]
- 5. Bhamarapravati N, Tuchinda P, Boonyapaknavik V. 1967. Pathology of Thailand haemorrhagic fever: a study of 100 autopsy cases. Ann. Trop. Med. Parasitol. 61:500–510 [DOI] [PubMed] [Google Scholar]
- 6. Blackley S, et al. 2007. Primary human splenic macrophages, but not T or B cells, are the principal target cells for dengue virus infection in vitro. J. Virol. 81:13325–13334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boonpucknavig S, Lohachitranond C, Nimmanitya S. 1979. The pattern and nature of the lymphocyte population response in dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 28:885–889 [PubMed] [Google Scholar]
- 8. Burke DS, Nisalak A, Johnson DE, Scott RM. 1988. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 38:172–180 [DOI] [PubMed] [Google Scholar]
- 9. Crotty S, Aubert RD, Glidewell J, Ahmed R. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286:111–122 [DOI] [PubMed] [Google Scholar]
- 10. Dejnirattisai W, et al. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dung NT, et al. 2010. Timing of CD8+ T cell responses in relation to commencement of capillary leakage in children with dengue. J. Immunol. 184:7281–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Green S, et al. 1999. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J. Infect. Dis. 179:755–762 [DOI] [PubMed] [Google Scholar]
- 13. Green S, et al. 1999. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J. Med. Virol. 59:329–334 [PubMed] [Google Scholar]
- 14. Halstead SB, O'Rourke EJ. 1977. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265:739–741 [DOI] [PubMed] [Google Scholar]
- 15. Hober D, Delannoy AS, Benyoucef S, De Groote D, Wattre P. 1996. High levels of sTNFR p75 and TNF alpha in dengue-infected patients. Microbiol. Immunol. 40:569–573 [DOI] [PubMed] [Google Scholar]
- 16. Hober D, et al. 1993. Serum levels of tumor necrosis factor-alpha (TNF-alpha), interleukin-6 (IL-6), and interleukin-1 beta (IL-1 beta) in dengue-infected patients. Am. J. Trop. Med. Hyg. 48:324–331 [DOI] [PubMed] [Google Scholar]
- 17. Huang KJ, et al. 2006. The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J. Immunol. 176:2825–2832 [DOI] [PubMed] [Google Scholar]
- 18. Innis BL, et al. 1989. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am. J. Trop. Med. Hyg. 40:418–427 [DOI] [PubMed] [Google Scholar]
- 19. Jampangern W, et al. 2007. Characterization of atypical lymphocytes and immunophenotypes of lymphocytes in patients with dengue virus infection. Asian Pac. J. Allergy Immunol. 25:27–36 [PubMed] [Google Scholar]
- 20. Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. 1989. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 40:444–451 [DOI] [PubMed] [Google Scholar]
- 21. Klungthong C, Zhang C, Mammen MP, Jr, Ubol S, Holmes EC. 2004. The molecular epidemiology of dengue virus serotype 4 in Bangkok, Thailand. Virology 329:168–179 [DOI] [PubMed] [Google Scholar]
- 22. Kurane I, et al. 1994. Immunopathologic mechanisms of dengue hemorrhagic fever and dengue shock syndrome. Arch. Virol. Suppl. 9:59–64 [DOI] [PubMed] [Google Scholar]
- 23. Mathew A, Rothman AL. 2008. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol. Rev. 225:300–313 [DOI] [PubMed] [Google Scholar]
- 24. Miller JD, et al. 2008. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 28:710–722 [DOI] [PubMed] [Google Scholar]
- 25. Mustafa AS, Elbishbishi EA, Agarwal R, Chaturvedi UC. 2001. Elevated levels of interleukin-13 and IL-18 in patients with dengue hemorrhagic fever. FEMS Immunol. Med. Microbiol. 30:229–233 [DOI] [PubMed] [Google Scholar]
- 26. Nimmerjahn F, Ravetch JV. 2010. Antibody-mediated modulation of immune responses. Immunol. Rev. 236:265–275 [DOI] [PubMed] [Google Scholar]
- 27. Onlamoon N, et al. 2010. Dengue virus-induced hemorrhage in a nonhuman primate model. Blood 115:1823–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Piyaratn P. 1961. Pathology of Thailand epidemic hemorrhagic fever. Am. J. Trop. Med. Hyg. 10:767–772 [DOI] [PubMed] [Google Scholar]
- 29. Sangkawibha N, et al. 1984. Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand. I. The 1980 outbreak. Am. J. Epidemiol. 120:653–669 [DOI] [PubMed] [Google Scholar]
- 30. Simmons CP, et al. 2007. Patterns of host genome-wide gene transcript abundance in the peripheral blood of patients with acute dengue hemorrhagic fever. J. Infect. Dis. 195:1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suwannasaen D, Romphruk A, Leelayuwat C, Lertmemongkolchai G. 2010. Bystander T cells in human immune responses to dengue antigens. BMC Immunol. 11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thein S, et al. 1997. Risk factors in dengue shock syndrome. Am. J. Trop. Med. Hyg. 56:566–572 [DOI] [PubMed] [Google Scholar]
- 33. Tough DF, Borrow P, Sprent J. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272:1947–1950 [DOI] [PubMed] [Google Scholar]
- 34. World Health Organization 1997. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. World Health Organization, Geneva, Switzerland [Google Scholar]
- 35. Wrammert J, et al. 2008. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 453:667–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yenchitsomanus PT, et al. 1996. Rapid detection and identification of dengue viruses by polymerase chain reaction (PCR). Southeast Asian J. Trop. Med. Public Health 27:228–236 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.