Abstract
Mitogen-activated protein kinases (MAPKs) are a family of serine-threonine protein kinases involved in many cellular processes, including cell proliferation, differentiation, inflammation, and cell death. Activation of several MAPKs, including extracellular signal-regulated kinase 1 and 2 (ERK1/2), p38, and c-Jun N-terminal kinase (JNK), results in stimulation of activator protein 1 (AP-1), which promotes gene transcription. Previous studies have demonstrated that varicella-zoster virus (VZV) infection activates ERK1/2, p38, and JNK to promote viral replication, but the underlying mechanism(s) is unclear. To identify viral proteins responsible for the activation of MAPK, we used a proteomic approach to screen viral proteins for AP-1 promoter activation by an AP-1–luciferase reporter assay. We found that VZV ORF12 protein, located in the tegument of virions, enhances AP-1 reporter activity. This effect of ORF12 protein was markedly inhibited by a MAPK/ERK kinase 1 and 2 (MEK1/2) inhibitor (U0126), partially blocked by a p38 inhibitor (SB202190), but not inhibited by a JNK inhibitor (SP600125). Expression of VZV ORF12 protein in cells resulted in phosphorylation of ERK1/2 and p38 but not JNK. Infection of cells with a VZV ORF12 deletion mutant resulted in reduced levels of phosphorylated ERK1/2 (p-ERK1/2) compared to infection with wild-type VZV. Furthermore, deletion of ORF12 rendered VZV-infected cells more susceptible to staurosporine-induced apoptosis. In conclusion, VZV ORF12 protein activates the AP-1 pathway by selectively triggering the phosphorylation of ERK1/2 and p38. Cells infected with a VZV ORF12 deletion mutant have reduced levels of p-ERK1/2 and are more susceptible to apoptosis than cells infected with wild-type VZV.
INTRODUCTION
Mitogen-activated protein kinases (MAPKs) are serine-threonine-specific protein kinases that respond to extracellular stimuli, such as growth factors, cytokines, and stress (e.g., UV irradiation and heat shock). MAPKs regulate various cellular activities, such as gene expression, mitosis, cell differentiation, proliferation, and death (9). The most intensely studied MAPKs are extracellular signal-regulated protein kinase 1 and 2 (ERK1/2), p38 kinase, and c-Jun N-terminal kinase (JNK). ERK1/2 transduces extracellular signals linking the stimulation of membrane-bound receptors to changes in cellular functions (22, 23). After stimulation of cells by growth factors, chemokines, or serum, the GTP-binding protein Ras induces phosphorylation and activation of Raf, which in turn activates MAPK/ERK kinases 1 and 2 (MEK1/2), which results in activation of ERK1/2. Activated ERK phosphorylates numerous substrates in different cellular compartments (30), leading to increased nucleotide synthesis, activation of transcription and translation for protein synthesis, enhanced cell cycle progression and proliferation, and cell survival.
The MAPK pathway is exploited by a number of viruses to manipulate the host cellular environment for optimal virus replication, cell transformation, and prevention of apoptosis. For example, HIV (10), influenza virus (15), human hepatitis viruses (13), rotavirus (8), and vesicular stomatitis virus (19) activate MAPKs to enhance virus replication. Human herpesviruses, such as Epstein-Barr virus (EBV), herpes simplex virus (HSV), or Kaposi's sarcoma-associated herpesvirus, target the MAPK pathway for cell transformation (21), prevention of apoptosis (14), or induction of reactivation (31, 32).
Varicella-zoster virus (VZV) is a neurotropic human alphaherpesvirus. Primary infection causes varicella (chickenpox), which results in a lifelong latent infection in cranial nerve and dorsal root ganglia. VZV can reactivate later in life as a result of waning immunity and result in herpes zoster (shingles). VZV, like other members of the herpesvirus family, activates the MAPK pathway (16–18, 33). Reduction of ERK and p38 activity by chemical inhibitors reduces VZV replication. Expression of VZV ORF61 in cells triggers phosphorylation of JNK, which may be important for VZV replication in specific cell types (16, 33).
We used a proteomic approach to identify individual VZV proteins that may activate AP-1 using an AP-1–luciferase reporter assay. We found that VZV ORF12 protein is able to enhance AP-1 reporter activity through activation of ERK1/2 and p38 and that ORF12 protein inhibits apoptosis.
MATERIALS AND METHODS
Cells and viruses.
Human embryonic kidney (HEK293T) and melanoma (MeWo) cells were grown in Dulbecco's modified Eagle's medium (DMEM) and minimal essential medium (MEM), respectively, containing 10% fetal bovine serum (FBS) supplemented with 1% penicillin-streptomycin at 37°C. VZV was propagated in MeWo cells, and cell-associated virus was titrated in MeWo cells in 2% FBS at 34°C. VZV infections were performed using cell-associated virus.
Plasmids and cosmids.
Individual VZV open reading frames (ORFs) were amplified by PCR of DNA from the Oka vaccine strain of VZV and inserted into the multiple cloning site of pcDNA3.1 (Invitrogen). The following ORFs were cloned: ORFs 0 to 4, 10, 12 and 13, 18, 21, 23 to 26, 29 to 34, 36 to 49, 51 to 56, and 69 and 68. ORF42 and ORF45, which are spliced together to make a single gene, were cloned into individual plasmids. These plasmids express the VZV proteins tagged with a V5 epitope (found in the P and V proteins of the SV5 paramyxovirus) at their carboxyl termini. All plasmid inserts were sequenced, and protein expression was verified by transfection, followed by immunoblotting with anti-V5 antibody. A plasmid expressing HSV-1 UL46 was constructed previously (11), and a plasmid expressing the catalytic domain of MEK kinase 1 (MEKK1) was obtained from Stratagene.
VZV cosmids NotI A, NotI B, MstII A, and MstII B are derived from the Oka vaccine strain and encompass the VZV genome (4). To construct an ORF12 deletion mutant, we first digested plasmid pSse19, which contains VZV nucleotides 9,780 to 22,586 (5), with BsaBI and BbvCI (which cut VZV at nucleotides 19,496 and 22,347) to remove a DNA fragment containing an NaeI site at VZV nucleotide 21,447, blunted the ends with the Klenow fragment of DNA polymerase I, and religated the larger fragment to itself to create plasmid pSseBB. pSseBB was then digested with NaeI (which cuts at VZV nucleotide 16,298) and SexAI (which cuts at VZV nucleotide 18,255) to delete a 1,957-bp fragment (which encodes amino acids 28 to 661 of VZV ORF12 and extends 55 bp after the ORF12 stop codon); the ends were blunted with the Klenow fragment of DNA polymerase I, and the larger fragment was religated to generate plasmid pSseBB12D. pSseBB12D was then digested with EcoNI (which cuts at VZV nucleotide 10,902) and BsmI (which cuts at VZV nucleotide 18,590), and the smaller fragment containing the region immediately surrounding ORF12 was isolated and inserted into the corresponding EcoNI and BsmI site of plasmid pSse19 to create plasmid pSse12D. The latter plasmid was cut with SbfI (an isoschizomer of Sse8387I which cuts at VZV nucleotides 9,780 and 22,586), and the larger fragment was inserted into the corresponding SbfI site of cosmid NotI A to yield cosmid NotI A12D. This cosmid has a 1,957-bp deletion in the ORF12 gene corresponding to VZV nucleotides 16,298 to 18,255.
To create a cosmid containing a copy of VZV ORF12 at another site in the viral genome, we first amplified the CMV-ORF12-V5 (where CMV is cytomegalovirus) cassette from pcDNA3.1 expressing ORF12-V5 (described above) by PCR using a primer (CMV F AvrII, 5′-GGCCTAGGGGCGTTTTGCGCTGCTTCGCGATG-3′) flanking the beginning of the CMV promoter and a primer (BGH R AvrII, 5′-GGCCTAGGAGAGCCCACCGCATCCCCAGCATG-3′) flanking the end of bovine growth hormone (BGH) poly(A) sequence (AvrII sites are underlined). The PCR product was cloned into pGEM-T Easy (Promega), and the CMV-ORF12-V5 cassette was removed with AvrII and inserted into the AvrII site of cosmid MstII A. The resulting cosmid, MstII A12DR, expresses ORF12 from the human CMV immediate-early (IE) promoter, has a V5 epitope tag at the carboxyl terminus of ORF12, and has a bovine growth hormone poly(A) signal.
We created another cosmid to express ORF12 by PCR amplification of VZV ORF12 along with its endogenous promoter (335 bp upstream of codon 1 of ORF12) and the predicted poly(A) signal AATAAA between VZV nucleotides 19,391 and 19,396 (39 nucleotides downstream of the ORF13 stop codon) which is shared with VZV ORF13. The forward primer extended from VZV nucleotides 15,879 to 15,902 (5′-GGCCTAGGCAGGCATCACCTATAGAGATGAAC-3′), and the reverse primer extended from VZV nucleotides 18,223 to 18,200 [5′-GGCCTAGGTATCATTTTATTATAACTATTAACCGCTGTAC-3′; AvrII sequences are underlined, and the predicted poly(A) signal is in boldface]. The PCR product was cloned into pGEM-T Easy and cut with AvrII, and the ORF12-poly(A) cassette was inserted into the AvrII site of cosmid MstII to yield cosmid MstII A12Dr.
Luciferase reporter assays.
293T cells were transfected using GeneJuice (Novagen) with a combination of the following: (i) 20 ng of plasmid expressing each of the VZV open reading frames (see the paragraph “Plasmids and cosmids” above); (ii) 20 ng of plasmid with AP-1 (Stratagene), NF-κB, or an interferon-stimulated response element (ISRE) driving firefly luciferase (kindly provided by Katherine Fitzgerald, University of Massachusetts); and (iii) 5 μg of plasmid expressing Renilla luciferase under the control of the HSV thymidine kinase promoter (plasmid pRL-TK) (Promega). At 24 h after transfection, cells in 96-well plates were incubated with 50 μl of 1× passive lysis buffer (Promega), and after 12 min, 30 μl of cell lysates was transferred into a luciferase assay plate (Costar). Thirty microliters of Dual-Glo substrate was added, and firefly and Renilla luciferase activities were assayed with a Promega dual-luciferase assay system as described previously (11). The ratio of firefly to Renilla luciferase activity was calculated, and the reporter activity was expressed as the fold induction of luciferase reporter by normalizing values to green fluorescent protein (GFP) expression in plasmid-transfected cells.
Immunoblotting and Southern blotting.
Infected cell lysates were fractionated on polyacrylamide gels, transferred to nitrocellulose membranes, and incubated with rabbit anti-VZV IE62 antibody (a gift from Paul Kinchington, University of Pittsburgh), rabbit anti-phospho-ERK1/2 (p-ERK1/2), p-p38, p-JNK, ERK1/2, p38, JNK, cleaved poly(ADP-ribose) polymerase (PARP), cleaved caspase-3 antibodies (Cell Signaling Technology), mouse anti-V5 antibody (Invitrogen), or mouse anti-actin antibody (Sigma).
Virion DNA was isolated from VZV-infected MeWo cells as described previously (27), and 1.6 μg of virion DNA was digested with BamHI, separated on a 1% agarose gel, transferred to a nylon membrane, and hybridized with a [32P]dCTP-labeled probe corresponding to VZV ORF12.
Immunofluorescence microscopy and inhibition studies.
MeWo cells on coverslips were infected with cell-associated VZV, and after 20 h, the cells were washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 30 min, washed with PBS, and incubated with 4% FBS and 0.05% saponin overnight at 4°C to permeabilize the cells. The cells were washed, incubated with mouse monoclonal antibody to V5 (Invitrogen), washed, incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse antibody, mounted with solution containing 4′,6-diamidino-2-phenylindole ([DAPI] Fluoromount G; Southern Biotech), and visualized by confocal microscopy.
Transfected cells were incubated for 24 h and then treated (or left untreated) for 6 h with U0126 (EMD Chemicals) to inhibit MEK1/2, SB202190 (Gibco) to inhibit p38, or SP600125 (Gibco) to inhibit JNK1/2. Cell lysates were prepared and assayed for luciferase activity as described above.
mRNA extraction and cDNA synthesis.
MeWo cells were infected with cell-associated VZV at 0.2 PFU/cell; 24 h after infection the cells were collected, and RNA was isolated using a Qiagen RNeasy minikit. The mRNA was converted to cDNA using SMART Moloney murine leukemia virus (MMLV) reverse transcriptase (Clontech) and oligo(dT)20 (Invitrogen). cDNA was amplified using a primer pair specific for full-length ORF11 (forward, 5′-ATGCAGTCGGGTCATTATAACCGG-3′; reverse, 5′-ATATTTTCGTAGTAAATGCATGGC-3′), full-length ORF12 (forward, 5′-ATGTTTTCTCGGTTTGCGCGTTCC-3′; reverse, 5′-ATGATGACTCTTAGGCGTATTTTTCCT-3′), 370 bp of the N terminus of ORF13 (forward, 5′-ATGGGAGACTTGTCATGTTGGACA-3′; reverse, 5′-ACGTAATAATAAGTCGAAAGTGATG-3′), or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward, 5′-CATCCCTTCTCCCCACACAC-3′; reverse, 5′-AGTCCCAGGGCTTTGATTTG-3′).
Electron microscopy.
MeWo cells were infected with cell-associated VZV at 0.1 PFU/cell, and after 24 h the infected cells were washed with cold PBS and fixed with 4% paraformaldehyde in 0.1 M sodium cacodylate overnight at 4°C. Samples were washed with 0.1 M sodium cacodylate and stained with 1% uranyl acetate prior to being washed with distilled water. The specimens were dehydrated in a graded ethanol series to 95% ethanol, infiltrated and embedded in LR White resin, and cured overnight at 55°C. Sections (120 nm) were cut using an MT-7000 ultramicrotome (Ventana, Tucson, AZ). For immune labeling, the sections were etched for 15 min with 4% metaperiodate, blocked with 1% bovine serum albumin (BSA)–0.1% Tween 20-Tris buffer for 15 min at room temperature, and incubated with mouse anti-V5 antibody diluted 1:100 in blocking buffer for 60 min. The samples were then washed three times for 5 min in blocking buffer and incubated with 10-nm colloidal gold-labeled anti-mouse antibody (BBI International, Cardiff, United Kingdom) for 60 min before a final rinsing step. Sections were stained with 1% uranyl acetate and viewed on a Tecnai BioTWIN transmission electron microscope (FEI, Hillsboro, OR) at 120 kV. Images were acquired with a Hamamatsu XR-100 digital camera system.
Plaque assays and virus replication studies.
MeWo cells in six-well plates were infected with cell-associated VZV in MEM containing 2% fetal bovine serum, and 7 days later the medium was removed, cell monolayers were fixed and stained with crystal violet, and plaque diameters were measured.
For virus replication studies, MeWo cells were infected with cell-associated VZV in MEM containing 0.5% fetal bovine serum at 37°C, and 1 to 4 days after infection, the cells were treated with trypsin. Serial dilutions of cell-associated virus were titrated on MeWo cells in MEM containing 2% fetal bovine serum at 34°C, and plaque numbers were counted 7 days later.
Apoptosis assays.
MeWo cells were infected with VZV at 0.1 PFU/cell in MEM containing 10% serum; 24 h later, cells were treated with staurosporine for 6 h, and cells were collected in 2× sample buffer (Quality Biological). Immunoblotting was performed for detection of cleaved caspase-3 and cleaved PARP. In other experiments, MeWo cells were infected with VZV for 24 h and treated with 1 μM staurosporine for 6 h; cells were then treated with trypsin, collected in MEM containing 10% fetal calf serum (FCS), and washed with PBS. The cells were then stained with annexin V-phycoerythrin (PE; BD Pharmingen) and 7-aminoactinomycin D (7-AAD; BD Pharmingen) for 15 min at room temperature in annexin V binding buffer (BD Pharmingen) and subsequently analyzed by fluorescence-activated cell sorting (FACS).
RESULTS
VZV ORF12 protein activates ERK1/2.
To search for a VZV viral protein(s) that activates MAPKs, we transfected HEK293T cells with (i) individual plasmids encoding VZV open reading frames, (ii) an AP-1 reporter plasmid expressing firefly luciferase under an AP-1-responsive promoter, and (iii) a plasmid expressing Renilla luciferase under the control of HSV-1 thymidine kinase promoter (pRL-TK), which served as a control for transfection efficiency. Of the VZV open reading frames tested, only ORF12 strongly enhanced AP-1 reporter activity, with a level approximately 20-fold higher than that of the plasmid expressing GFP (Fig. 1A). The level of activation of the AP-1 reporter was similar to that observed with the positive-control plasmid expressing the catalytic portion of mitogen-activated protein kinase/extracellular signal-regulated kinase (MEK) kinase 1 (MEKK1). In contrast, expression of HSV-1 UL46, the ortholog of VZV ORF12, did not activate the AP-1 reporter, nor did other VZV-encoded proteins, including ORF13 protein and IE62. To ensure that ORF12 protein activation of the AP-1 reporter was specific for this construct and not due to general effects of ORF12 protein on the luciferase reporter, we tested the ability of ORF12 protein to activate other luciferase reporters such as NF-κB–luciferase and ISRE-luciferase. Transfection of the ORF12-expressing plasmid activated the AP-1 reporter but not the NF-κB or ISRE reporter (Fig. 1B).
Fig 1.
VZV ORF12 protein activates ERK1/2 and p38. (A) AP-1 firefly luciferase reporter plasmid, Renilla luciferase plasmid, and plasmids expressing VZV or HSV-1 proteins, MEKK1, or GFP were transfected into HEK293T cells, and AP-1 reporter activity was plotted as luciferase units normalized for transfection efficiency. (B) AP-1 firefly luciferase reporter plasmid, NF-κB firefly luciferase reporter plasmid, or ISRE firefly luciferase reporter plasmid and plasmids expressing VZV ORF12 protein or GFP were transfected into 293T cells, and reporter activity was measured as described for panel A. (C) HEK293T cells were transfected with an AP-1 reporter plasmid, a control pRL-TK plasmid, and a VZV ORF12 expression plasmid for 24 h; cells were then treated with a MEK1/2 inhibitor (U0126), p38 inhibitor (SB202190), or JNK inhibitor (SP600125) at the indicated concentrations, and luciferase activity was measured as described as above. (D) HEK293T cells were transfected with a plasmid expressing ORF12 protein with a V5 epitope tag, a plasmid expressing MEKK1 (positive control), or pcDNA3.1 (negative control), and after 24 h, cells lysates were immunoblotted with antibody to ERK1/2, p-ERK1/2, p38, p-p38, JNK1/2, p-JNK1/2, actin, or V5.
The activation of AP-1 transcription factor is controlled by upstream MAPKs including ERK1/2, p38, and JNK. To determine which MAPK(s) is activated by ORF12 protein, HEK293T cells were transfected with the ORF12-expressing plasmid, AP-1 reporter plasmid, and pRL-TK plasmid, and 20 h later cells were treated with an MEK1/2 inhibitor (U0126), a p38 inhibitor (SB202190), or a JNK inhibitor (SP600125). Measurement of luciferase activity 6 h after treatment showed that ORF12 protein activation of the AP-1 reporter was markedly reduced by the MEK1/2 inhibitor and, to a lesser extent, by the p38 inhibitor but not by the JNK inhibitor (Fig. 1C).
To ensure that the enhanced AP-1 reporter activity was due to the ORF12 protein activation of MAPKs, we measured levels of phosphorylated ERK1/2, p38, and JNK in HEK293T cells transfected with the plasmid expressing ORF12 protein tagged with the V5 epitope. ORF12 protein markedly increased the levels of phosphorylated ERK1/2 and p38 but not JNK, while the total level of ERK1/2, p38, or JNK was unchanged (Fig. 1D). Expression of MEKK1 increased the level of phosphorylated ERK1/2, p38, and JNK as expected. The epitope-tagged ORF12 protein was expressed, and the levels of actin, which served as a loading control, were similar for all samples. ORF12 protein also increased the levels of phosphorylated ERK1/2 in human foreskin fibroblasts (data not shown).
VZV with a deletion of ORF12 is impaired for phosphorylation of ERK1/2.
Since VZV ORF12 protein alone activated an AP-1 reporter and induced ERK1/2 phosphorylation, we constructed a VZV mutant with a deletion of ORF12 to determine if the viral protein is directly responsible for activation of ERK1/2 in VZV-infected cells. We constructed cosmid NotI A12D, which has a deletion of all of ORF12 except the first 27 amino acids (Fig. 2A). Transfection of MeWo cells with VZV cosmids NotI A, NotI B, MstII A, and MstII B yielded VZV ROka with cytopathic effects (CPE) 10 days after transfection. Transfection of the cells with NotI A12D, NotI B, MstII A, and MstII B yielded VZV ROka12D with CPE at day 13. The deletion in ROka12D was confirmed by PCR of virion DNA using primers that flank ORF12, which showed a band of 2.3 kb compared with a band of around 0.3 kb in ROka12D (Fig. 2B). BamHI restriction endonuclease digestion of virion DNA showed an 8.1-kb band in ROka-infected cells (Fig. 2C, star) but not in cells infected with ROka12D; in contrast, an additional 6.1-kb band was present in ROka12D-infected cells (Fig. 2C, closed circle) due to the 1,957 kb deletion of ORF12 in ROka12D. Southern blotting of the same BamHI-digested ROka and ROka12D virion DNA, followed by hybridization with a full-length ORF12 probe, showed an 8.1-kb DNA band in ROka virion DNA but not in ROka12D virion DNA (Fig. 2D). A 6.1-kb band was seen with ROka12D virion DNA with a long exposure (data not shown).
Fig 2.
Map of cosmids used for construction of the ORF12 deletion mutant and confirmation of the genome structure. (A) The prototype VZV genome is 124,884 nucleotides in length (line 1) with unique short (US), unique long (UL), and internal and terminal repeats (IR and TR, respectively) (line 2). Transfection of cells with NotI (line 3) and MstII (line 4) cosmids results in infectious VZV. Cosmid NotI A12D (line 5) has a deletion of all but the first 27 amino acids of ORF12. Cosmid MstII A12DR contains a cassette in which ORF12 with a V5 epitope tag is driven by the CMV promoter. Cosmid MstII A12Dr contains the ORF12 gene driven by its endogenous promoter. Nucleotide numbers are based on the sequence of the Dumas strain of VZV, GenBank number X04370. (B) PCR of VZV ROka or cosmid NotI A using primers (forward primer corresponding to VZV nucleotides 16,214 to 16,237, 5′-ATGTTTTCTCGGTTTGCGCGTTCC-3′; reverse primer corresponding to VZV nucleotides 18,464 to 18,441, 5′-TGTCCAACATGACAAGTCTCCCTA-3′) that span the deletion in ORF12 results in a 2.3-kb band, while PCR of ROka12D or cosmid NotI A12D yields a 0.3-kb band. (C) VZV ROka and ROka12D virion DNAs were digested with BamHI and fractionated on a 1% agarose gel. The star indicates an 8.1-kb band in ROka-infected but not in ROka12D-infected cells, and the circle indicates a 6.1-kb band in ROka12D-infected cells. (D) Southern blot of BamHI-digested virion DNA fractionated and hybridized with a radiolabeled probe corresponding to VZV ORF12 shows a band corresponding to ORF12 in ROka-infected but not ROka12D-infected cells. (E) MeWo cells were infected with VZV ROka, ROka12D, or ROka12DR at 0.1 PFU/cell for 24 h; total mRNA was extracted from infected cells, reverse transcription was performed, and primers specific for ORF11, ORF12, or ORF13 or GAPDH were used to amplify cDNA. mRNA was used as a control in the GAPDH PCR to assess DNA contamination of mRNA.
Since the deletion in ORF12 might affect transcription of the neighboring ORF11 or ORF13 gene, we isolated RNA from cells infected with VZV ROka and ROka12D and performed reverse transcription with oligo(dT), and the resulting cDNA was subjected to PCR using primers specific for VZV ORF11, ORF12, and ORF13. While VZV ORF12 cDNA was detected in ROka-infected but not ROka12D-infected cells with primers to ORF12, levels of ORF11 and ORF13 cDNA were similar in cells infected with ROka and ROka12D (Fig. 2E). GAPDH served as a positive control for PCR, and PCR performed with mRNA verified that DNA was absent from the mRNA preparations. Therefore, the deletion in ORF12 did not affect the level of ORF11 or ORF13 RNA in infected cells.
Two ORF12 rescue viruses were constructed. First, we inserted a cassette containing VZV ORF12 with a V5 epitope driven by the human CMV IE promoter into cosmid MstII A to generate cosmid MstII A12DR (Fig. 2A). Second, we inserted ORF12 driven by its own promoter into cosmid MstII A to yield cosmid MstII A12Dr (Fig. 2A). Transfection of MeWo cells with VZV cosmids NotI A, NotI B, MstII A, and MstII B resulted in CPE 7 days after transfection. Transfection of cells with cosmids NotI A12D, NotI B, MstII A12DR, and MstII B also resulted in CPE 7 days after transfection, and the resulting virus was termed ROka12DR. Transfection of cells with cosmids NotI A12D, NotI B, MstII A12Dr, and MstII B yielded CPE at day 7, and the virus was termed ROka12Dr. ROka12DR expressed ORF12 mRNA while ROka12Dr failed to express detectable levels of ORF12 mRNA in cells and therefore was not evaluated further (Fig. 2E). The lack of detection of an ORF12 transcript in cells infected with ROka12Dr may have been due the failure to insert the full promoter sequence of ORF12 or a functional poly(A) sequence into the ORF12 cassette.
To determine if VZV ORF12 protein is responsible for phosphorylation of ERK1/2 in the context of virus infection, MeWo cells were infected with cell-associated VZV ROka-, ROka12D-, or ROka12DR-infected cells at 0.1 PFU/uninfected cell; cells were harvested at different times after infection, and the level of p-ERK1/2 was determined (Fig. 3A). p-ERK1/2 was detected 3 h after infection of cells with VZV ROka, even in the absence of detectable IE62. p-ERK1/2 was detected until 36 h after infection, after which time the levels declined to those observed in uninfected cells. In contrast, ROka12D-infected cells had markedly reduced levels of p-ERK1/2 compared with ROka-infected cells during first 36 h after infection. Cells infected with the ORF12 rescued virus, ROka12DR, had levels of p-ERK1/2 that were comparable to those seen with ROka (Fig. 3B). These results indicate that ORF12 protein is primarily responsible for phosphorylation of ERK1/2 induced by VZV infection although other viral protein(s) may also contribute to ERK activation, as indicated by the low levels of p-ERK1/2 in ROka12D-infected cells (Fig. 3A).
Fig 3.
Deletion of ORF12 from VZV reduces phosphorylation of ERK1/2 in virus-infected cells. Immunoblotting was performed of MeWo cells infected with 0.1 PFU/cell of ROka or ROka12D (A) or with ROka, ROka12D, or ROka12DR (B) and probed with antibody to p-ERK1/2, total ERK1/2, IE62 protein, or actin.
VZV ORF12 is a tegument protein that localizes in the cytoplasm of virus-infected cells.
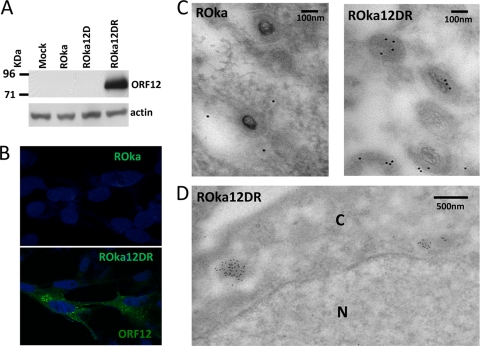
Immunoblotting of ROka12DR-infected MeWo cells showed an approximately 85-kDa band corresponding to the V5 epitope-tagged ORF12 protein (Fig. 4A). Immunofluorescent staining of ROka12DR-infected cells with antibody that recognizes the V5 epitope tag showed that the protein localizes to the perinuclear region of virus-infected cells (Fig. 4B), similar to its ortholog HSV UL46, which is located throughout the cytoplasm but at higher concentrations in the perinuclear region of the cell (29).
Fig 4.
VZV ORF12 protein is localized in the cytoplasm of infected cells and in the tegument of virions. (A) Immunoblotting was performed of MeWo cells infected with 0.1 PFU/cell of ROka, ROka12D, or ROka12DR for 24 h and probed with antibody to V5. (B) Immunofluorescent staining of ORF12 protein in MeWo cells infected with ROka12DR using mouse antibody to V5 followed by FITC-labeled anti-mouse antibody and DAPI. (C) Immunoelectron microscopy in which VZV ROka- or ROka12DR-infected cells were incubated with mouse antibody to V5 followed by colloidal gold-labeled anti-mouse antibody. (D) Lower-power immunoelectron microscopy showing VZV particles labeled with antibody to V5 and colloidal gold. N, nucleus; C, cytoplasm.
Electron microscopy of melanoma cells infected with ROka12DR using mouse anti-V5 antibody and colloidal gold-labeled anti-mouse antibody showed that ORF12 protein is located in the tegument of virion particles (Fig. 4C). In contrast, cells infected with VZV ROka, which does not contain a V5 tag, did not show tegument staining. VZV particles with tegument containing ORF12 protein were localized to the cytoplasm (Fig. 4D).
Deletion of ORF12 from VZV has a modest effect on plaque size.
Since prior studies showed that inhibition of ERK1/2 activation with a chemical inhibitor strongly reduced VZV replication (17), we postulated that the reduced ERK1/2 activity in ROka12D-infected cells compared with ROka-infected cells might correlate with impaired virus growth in cell culture. MeWo cells infected with ROka12D formed slightly smaller plaques than cells infected with ROka or ROka12DR (P < 0.05) (Fig. 5A).
Fig 5.
Deletion of ORF12 from VZV has a modest effect on plaque size. (A) MeWo cells were infected with ROka, ROka12D, or ROka12DR in MEM containing 2% serum; 6 days later medium was removed, and cell monolayers were fixed and stained with crystal violet. More than 20 plaque diameters were measured for each virus. Horizontal bars show mean numbers, and vertical bars show standard error. (B) MeWo cells were infected with cell-associated VZV ROka, ROka12D, or ROka12DR in MEM containing 0.5% fetal bovine serum. At 1 to 4 days after infection, the cells were treated with trypsin, and cell-associated virus was titrated in MeWo cells. The experiment was repeated five times with similar results with fetal bovine serum concentrations ranging from 0.5% to 10%.
ROka12D was not impaired for growth compared with ROka and ROka12DR, with peak titers of ROka12D comparable to those of ROka12DR and only slightly lower than those of ROka (Fig. 5B). These results imply that ORF12 protein activation of ERK1/2 is not essential for virus replication or that the low level of ERK1/2 activity in ROka12D-infected cells (Fig. 3A and B) is sufficient for virus replication. The previous inhibition of VZV replication observed with the ERK1/2 inhibitor (17) may have been due to inhibition of baseline levels of ERK1/2 activity in uninfected cells or to off-target effects of the inhibitor on infected cells.
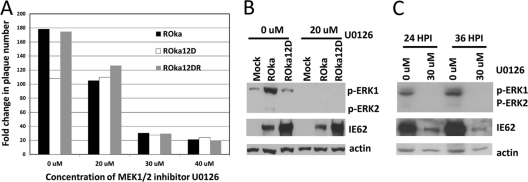
Treatment of VZV-infected cells with an MEK inhibitor reduces plaque numbers and IE62 expression.
We postulated that inhibition of ERK activation would reduce the difference in virus replication between ROka and ROka12D. In the absence of an ERK inhibitor, we observed a 40% reduction in plaque numbers in cells infected with ROka12D compared with cells infected with ROka or ROka12DR (Fig. 6A). However, while treatment of ROka-or ROka12DR-infected cells with 20 μM MEK inhibitor U0126 inhibited the number of plaques in cells infected with ROka or ROka12DR, there was not a similar reduction in plaque numbers for cells infected with ROka12D. With higher concentrations of U0126, similar reductions in plaque numbers were observed for all three viruses. Thus, the modest reduction in plaque size observed with ROka12D may be due to its reduced ERK1/2 activity.
Fig 6.
Chemical inhibition of MEK reduces VZV replication and expression of IE62. (A) MeWo cells were infected with ROka, ROka12D, or ROka12DR virus at similar titers for 24 h in MEM with 10% fetal bovine serum and treated with U0126 at the indicated concentrations for an additional 24 h; cells were treated with trypsin, and virus was titrated on MeWo cells. The fold change in plaque number was determined by dividing the number of plaques in cells infected with virus obtained from wells treated with various concentrations of U0126 (or untreated wells) by the number of plaques in the original inoculum. (B) MeWo cells were infected with VZV ROka or ROka12D at 0.1 PFU/cell and treated with U0126 at 20 μM for 6 h after infection, and cell lysates were prepared and immunoblotted with antibodies to p-ERK1/2, IE62, and actin. (C) MeWo cells were infected with VZV ROka at 0.1 PFU/cell and treated with U0126 at 30 μM for the number of hours indicated after infection, and cell lysates were prepared and immunoblotted.
The MEK1/2 inhibitor U0126 effectively suppressed VZV phosphorylation of ERK1/2 at 20 μM (Fig. 6B) and at 30 μM (Fig. 6C). While no reduction in VZV IE62 was seen at 20 μM U0126, at 30 μM the level of VZV IE62 was reduced, indicating that at higher concentrations inhibition of ERK1/2 may affect viral immediate-early gene expression or that the inhibitor may have off-target effects.
VZV ORF12 protein inhibits apoptosis.
Since VZV ORF12 protein activates ERK and since the MAPK pathway regulates apoptosis (30), we postulated that ORF12 protein may regulate apoptosis in VZV-infected cells. MeWo cells were infected with ROka or ROka12D, and 24 h after infection, cells were treated with staurosporine, an inducer of apoptosis. MeWo cells infected with ROka did not show cleavage of caspase-3 at low concentrations of staurosporine (<0.25 μM) (Fig. 7); however, ROka12D-infected cells showed caspase-3 cleavage with 0.1 μM staurosporine, which increased with higher concentrations of the drug. At each concentration of staurosporine, ROka12D-infected cells had higher levels of cleaved caspase-3 than ROka-infected cells. PARP cleavage was also increased in ROka12D-infected cells compared with levels in ROka-infected cells. VZV IE62 protein was detected in ROka- and ROka12D-infected cells, and its expression was minimally affected by staurosporine treatment. Increased caspase-3 and PARP cleavage was also observed in ROka12D-infected cells compared with ROka-infected cells treated with suberoylanilide hydroxamic acid (SAHA), a pan-histone deacetylase inhibitor which also induces apoptosis (data not shown).
Fig 7.

VZV ORF12 protects VZV-infected cells from staurosporine-induced caspase-3 cleavage. MeWo cells were infected with ROka or ROka12D at 0.1 PFU/cell in MEM containing 10% fetal bovine serum, and 24 h later the cells were treated with staurosporine for 6 h at the indicated concentrations. Cell lysates were immunoblotted with antibodies to cleaved caspase-3, cleaved PARP, VZV IE62 protein, and actin.
To verify that ORF12 protects VZV-infected cells from apoptosis, MeWo cells infected with ROka, ROka12D, or ROka12DR were treated with staurosporine for 6 h and stained with annexin V and 7-AAD. Annexin V-positive, 7-AAD-negative cells are considered apoptotic. Increased numbers of cells infected with ROka and ROka12DR were apoptotic compared with mock-infected cells, while more ROka12D-infected cells underwent apoptosis than ROka- or ROka12DR-infected cells (Fig. 8A). When the experiment was repeated several times, the percentage of VZV ROka12D-infected cells undergoing apoptosis was significantly higher than in cells infected with ROka or ROka12DR (Fig. 8B). Taken together, these results indicate that VZV ORF12 protein protects VZV-infected cells from apoptosis.
Fig 8.

VZV ORF12 protects VZV-infected cells from staurosporine-induced apoptosis. (A) Uninfected MeWo cells or cells infected with ROka, ROka12D, or ROka12DR for 24 h were treated with 1 μM staurosporine for 6 h and stained with annexin V and 7-AAD, and flow cytometry was performed. (B) Numbers of apoptotic cells (annexin V-positive, 7-AAD-negative cells) from wells of MeWo cells infected with VZV ROka, ROka12D, or ROka12DR and treated with staurosporine were normalized to the number of apoptotic cells in uninfected wells treated with staurosporine, and the percentage of apoptotic cells was determined. Each dot represents a separate experiment. P values were determined by one-way analysis of variance with a posthoc Student t test.
DISCUSSION
While previous reports showed that VZV infection activates the ERK pathway, the mechanism for this effect and its consequences for infected cells were not understood. Here, we report that VZV ORF12 protein, a tegument protein, triggers phosphorylation of ERK1/2, enhances AP-1 reporter activity, and protects cells from apoptosis.
Several viruses phosphorylate ERK1/2 using different mechanisms, including EBV, HSV, hepatitis C virus (HCV), and HIV. EBV latent membrane protein 1 (LMP1) or LMP2A activates ERK by a Ras-dependent pathway (21) or through association with ERK (12). The HCV envelope protein E2 interacts with CD81 and the low-density lipoprotein receptor on target cells to trigger activation of ERK (35). The HIV-1 transactivator protein Tat activates the Ras/ERK signaling pathway to regulate cell cycle progression (28). The HSV-2 large subunit of ribonucleotide reductase (ICP10 protein kinase [PK]) binds the GDP/GTP exchange factor SOS (son of sevenless) and phosphorylates Ras-GAP to activate Ras, resulting in ERK activation (26). The protein kinase domain of HSV-2 ICP10 PK is required for ERK activation and inhibition of staurosporine-induced apoptosis (14). In contrast, the large subunit of ribonucleotide reductase from VZV lacks the protein kinase domain present in its HSV-2 ortholog. We have shown that VZV, like other viruses, also activates ERK, through expression of VZV ORF12 protein. In addition, deletion of ORF12 from VZV resulted in impaired phosphorylation of ERK.
We found that treatment of VZV-infected cells with a low concentration of a MEK1/2 inhibitor (20 μM U0126) reduced the growth of VZV ROka but not ROka12D. In contrast, increasing concentrations of the MEK1/2 inhibitor (30 μM U2016) reduced the growth of both wild-type VZV, as has been reported previously with MAPK inhibitors (17), and the VZV ORF12 deletion mutant. However, this concentration of the MEK1/2 inhibitor also reduced VZV IE62 expression, which likely reduced virus growth. Based on these results, MEK inhibitors, which have been used as therapeutic agents for a number of different malignancies (20), might be considered as an additional approach to treat acyclovir-resistant VZV.
VZV activation of ERK by ORF12 protein had a modest effect on plaque size. Similar to prior investigators (34), we did not observe a reproducible effect on peak virus replication using an ORF12 deletion mutant. Prior studies did not describe the effect of an ORF12 deletion mutant on plaque size. In fact, when we compared plaque sizes of one of the previously published ORF12 mutants with its parental virus (34), we found that the ORF12 mutant formed smaller plaques than its parental virus (data not shown). Deletion of ORF12 did not affect the ability of VZV to replicate in tonsillar T cells or skin xenografts implanted into SCID mice (2); however, the phenotype of a VZV ORF12 mutant might be more pronounced when VZV replicates in other cells in the body.
We found that cells infected with VZV with a deletion of ORF12 were more susceptible to staurosporine-induced apoptosis than cells infected with wild-type virus. VZV infection induces apoptosis in fibroblasts (7), Vero cells (24), MeWo cells (1), and T cells (25) but not neurons (7). Two VZV proteins have been reported previously to inhibit apoptosis. ORF66 inhibits VZV apoptosis in T cells (25), while ORF63 inhibits apoptosis in neurons after withdrawal of nerve growth factor (6) but does not affect apoptosis in rodent ganglia in vivo (3). We found that VZV ORF12, which induces ERK, inhibited apoptosis in MeWo cells. Previous data have shown that VZV induction of ERK contributes to the induction of cell survival signals (17). Interestingly, HSV-2 ICP10 PK, which also activates ERK, protects cells from staurosporine-induced apoptosis (14). Activation of MEK/MAPK is required for the antiapoptotic activity of HSV-2.
Apoptosis was reported 24 to 48 h after VZV infection of fibroblasts (7), 48 h after infection of T cells (25), 64 to 72 h after infection in MeWo cells (1), and 72 to 96 h after infection of Vero cells (24). We found that ORF12 protein inhibited apoptosis of VZV-infected MeWo cells early (≤30 h) after infection. Since we have shown that ORF12 is a tegument protein in the virion, ORF12 protein may be released upon entry of the virus into the cell and inhibit apoptosis at an early stage of infection, allowing the virus to initiate replication.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases.
We thank Katherine Press for technical assistance, Paul Kinchington for antibody to VZV IE62, and Hua Zhu for the VZV bacterial artificial chromosome with the ORF12 deletion.
Footnotes
Published ahead of print 11 January 2012
REFERENCES
- 1. Brazeau E, et al. 2010. Varicella-zoster virus-induced apoptosis in MeWo cells is accompanied by down-regulation of Bcl-2 expression. J. Neurovirol. 16:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Che X, et al. 2008. Functions of the ORF9-to-ORF12 gene cluster in varicella-zoster virus replication and in the pathogenesis of skin infection. J. Virol. 82:5825–5834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen JI, Cox E, Pesnicak L, Srinivas S, Krogmann T. 2004. The varicella-zoster virus open reading frame 63 latency-associated protein is critical for establishment of latency. J. Virol. 78:11833–11840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen JI, Krogmann T, Pesnicak L, Ali MA. 2007. Absence or overexpression of the varicella-zoster virus (VZV) ORF29 latency-associated protein impairs late gene expression and reduces VZV latency in a rodent model. J. Virol. 81:1586–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen JI, Seidel KE. 1993. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro. Proc. Natl. Acad. Sci. U. S. A. 90:7376–7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hood C, et al. 2006. Varicella-zoster virus ORF63 inhibits apoptosis of primary human neurons. J. Virol. 80:1025–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hood C, Cunningham AL, Slobedman B, Boadle RA, Abendroth A. 2003. Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response. J. Virol. 77:12852–12864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jafri M, Donnelly B, McNeal M, Ward R, Tiao G. 2007. MAPK signaling contributes to rotaviral-induced cholangiocyte injury and viral replication. Surgery 142:192–201 [DOI] [PubMed] [Google Scholar]
- 9. Johnson GL, Lapadat R. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–1912 [DOI] [PubMed] [Google Scholar]
- 10. Kumar S, et al. 1996. Activation of the HIV-1 long terminal repeat by cytokines and environmental stress requires an active CSBP/p38 MAP kinase. J. Biol. Chem. 271:30864–30869 [DOI] [PubMed] [Google Scholar]
- 11. Liu X, Fitzgerald K, Kurt-Jones E, Finberg R, Knipe DM. 2008. Herpesvirus tegument protein activates NF-κB signaling through the TRAF6 adaptor protein. Proc. Natl. Acad. Sci. U. S. A. 105:11335–11339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panousis CG, Rowe DT. 1997. Epstein-Barr virus latent membrane protein 2 associates with and is a substrate for mitogen-activated protein kinase. J. Virol. 71:4752–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Panteva M, Korkaya H, Jameel S. 2003. Hepatitis viruses and the MAPK pathway: is this a survival strategy? Virus Res. 92:131–140 [DOI] [PubMed] [Google Scholar]
- 14. Perkins D, Pereira EF, Gober M, Yarowsky PJ, Aurelian L. 2002. The herpes simplex virus type 2 R1 protein kinase (ICP10 PK) blocks apoptosis in hippocampal neurons, involving activation of the MEK/MAPK survival pathway. J. Virol. 76:1435–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pleschka S, et al. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3:301–305 [DOI] [PubMed] [Google Scholar]
- 16. Rahaus M, Desloges N, Wolff MH. 2004. Replication of varicella-zoster virus is influenced by the levels of JNK/SAPK and p38/MAPK activation. J. Gen. Virol. 85:3529–3540 [DOI] [PubMed] [Google Scholar]
- 17. Rahaus M, Desloges N, Wolff MH. 2006. Varicella-zoster virus influences the activities of components and targets of the ERK signalling pathway. J. Gen. Virol. 87:749–758 [DOI] [PubMed] [Google Scholar]
- 18. Rahaus M, Wolff MH. 2003. Reciprocal effects of varicella-zoster virus (VZV) and AP1: activation of jun, fos and ATF-2 after VZV infection and their importance for the regulation of viral genes. Virus Res. 92:9–21 [DOI] [PubMed] [Google Scholar]
- 19. Renukaradhya GJ, Khan MA, Shaji D, Brutkiewicz RR. 2008. Vesicular stomatitis virus matrix protein impairs CD1d-mediated antigen presentation through activation of the p38 MAPK pathway. J. Virol. 82:12535–12542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rinehart J, et al. 2004. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J. Clin. Oncol. 22:4456–4462 [DOI] [PubMed] [Google Scholar]
- 21. Roberts ML, Cooper NR. 1998. Activation of a ras-MAPK-dependent pathway by Epstein-Barr virus latent membrane protein 1 is essential for cellular transformation. Virology 240:93–99 [DOI] [PubMed] [Google Scholar]
- 22. Robinson MJ, Cobb MH. 1997. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9:180–186 [DOI] [PubMed] [Google Scholar]
- 23. Roux PP, Blenis J. 2004. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68:320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sadzot-Delvaux C, Thonard P, Schoonbroodt S, Piette J, Rentier B. 1995. Varicella-zoster virus induces apoptosis in cell culture. J. Gen. Virol. 76:2875–2879 [DOI] [PubMed] [Google Scholar]
- 25. Schaap A, et al. 2005. T-cell tropism and the role of ORF66 protein in pathogenesis of varicella-zoster virus infection. J. Virol. 79:12921–12933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith CC. 2005. The herpes simplex virus type 2 protein ICP10PK: a master of versatility. Front. Biosci. 10:2820–2831 [DOI] [PubMed] [Google Scholar]
- 27. Straus SE, et al. 1981. Structure of varicella-zoster virus DNA. J. Virol. 40:516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toschi E, et al. 2006. HIV-1 Tat regulates endothelial cell cycle progression via activation of the Ras/ERK MAPK signaling pathway. Mol. Biol. Cell 17:1985–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willard M. 2002. Rapid directional translocations in virus replication. J. Virol. 76:5220–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wortzel I, Seger R. 2011. The ERK cascade: distinct functions within various subcellular organelles. Genes Cancer 2:195–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xie J, Ajibade AO, Ye F, Kuhne K, Gao SJ. 2008. Reactivation of Kaposi's sarcoma-associated herpesvirus from latency requires MEK/ERK, JNK and p38 multiple mitogen-activated protein kinase pathways. Virology 371:139–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu F, et al. 2007. Systematic identification of cellular signals reactivating Kaposi sarcoma-associated herpesvirus. PLoS Pathog. 3:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zapata HJ, Nakatsugawa M, Moffat JF. 2007. Varicella-zoster virus infection of human fibroblast cells activates the c-Jun N-terminal kinase pathway. J. Virol. 81:977–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Z, et al. 2010. Genome-wide mutagenesis reveals that ORF7 is a novel VZV skin-tropic factor. PLoS Pathog. 6:e1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao LJ, et al. 2006. Up-regulation of ERK and p38 MAPK signaling pathways by hepatitis C virus E2 envelope protein in human T lymphoma cell line. J. Leukoc. Biol. 80:424–432 [DOI] [PubMed] [Google Scholar]