Abstract
Sterol regulatory element binding proteins (SREBPs) are essential transcriptional factors that control expression of lipogenic genes and adipocyte differentiation. Human cytomegalovirus (HCMV) infection has been shown to require the induction of lipogenesis. Here we show that the induction of lipogenesis and expression of key lipogenic enzymes in human fibroblasts occurs by 24 h post-HCMV infection. This activation correlates with increased cleavage of the SREBP1 precursors to form the mature active transcription factors that enter the nucleus to transcriptionally activate lipogenic genes. SREBP1 cleavage is normally inhibited by increased sterol levels; however, our data show that this level of control is overridden in infected cells to allow constitutive activation of lipogenesis. This process requires viral protein synthesis, since UV-irradiated HCMV cannot activate SREBP cleavage. The cleavage of SREBP1 requires it to be in complex with SREBP cleavage activation protein (SCAP). Depleting SCAP using short hairpin RNA (shRNA) showed that SREBP1 cleavage and the induction of lipogenic genes and lipid synthesis are all inhibited in HCMV-infected cells. As a result, production of infectious virions is reduced in SCAP-depleted cells. Thus, the SCAP-mediated mechanism for SREBP cleavage is utilized by HCMV during infection. Our studies suggest that HCMV induces adipocyte-like lipogenesis and overrides normal sterol feedback controls in order to maintain high levels of constitutive lipid synthesis during infection.
INTRODUCTION
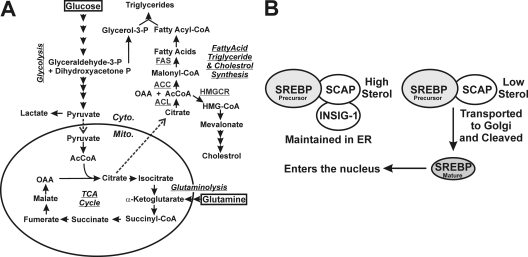
Human cytomegalovirus (HCMV), a betaherpesvirus, is the largest human herpesvirus with a 230-kb double-stranded DNA genome and the potential to encode over 200 proteins (27, 28). HCMV grows slowly in cultured cells and, therefore, must maintain favorable cellular conditions for a long period while manipulating cellular processes to synthesize biomolecules, including proteins, nucleic acids, and lipids, to produce progeny virions. This is supported by increasing uptake of cellular nutrients and significant alteration in intermediary metabolism (6, 25, 26) (reviewed in reference 40). Specifically, glucose uptake is increased in cultured human fibroblasts through the induction of the adipocyte-specific glucose transporter 4 (GLUT4) which replaces GLUT1, the normal fibroblast GLUT, which is a less efficient transporter than GLUT4 (42). Additionally, metabolic flux analysis has shown that glycolysis (Fig. 1A) is greatly increased in infected cells (25); however, glucose carbon is not used for energy production in the tricarboxylic acid (TCA) cycle but is diverted from the TCA cycle at the point of citrate by export from the mitochondrion to the cytoplasm (Fig. 1A) (26). This increase in cytoplasmic citrate is necessary to support cytoplasmic acetyl coenzyme A (AcCoA) production needed for the high levels of fatty acid synthesis induced in HCMV-infected cells (26) (Fig. 1A). This increased fatty acid synthesis has been shown to be critical for the success of the HCMV infection (26, 36), since it is necessary, at least in part, for the formation of membranes that are needed for viral envelopes, enlargement of the infected cells (cytomegalia), and enlargement of the infected cell nucleus and for the many vesicular bodies found in the cytoplasmic viral assembly compartment and throughout the cytoplasm of infected cells.
Fig 1.
(A) The glycolytic, glutaminolytic, fatty acid, triglyceride, and cholesterol biosynthetic pathways discussed in the text. (B) The steps in processing SREBPs to mature transcription factors (see text for details). Cyto., cytoplasm; Mito., mitochondrion; TCA, tricarboxylic acid; OAA, oxyloacetic acid; P, phosphate; ACL, ATP-citrate lyase; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; HMG, 3-hydroxy-3-methylglutaryl; HMGCR, HMG CoA reductase; SREBP, sterol regulatory element binding protein; SCAP, SREBP cleavage-activation protein; INSIG-1, insulin-induced gene 1; ER, endoplasmic reticulum.
The diversion of glucose from energy production to biosynthesis requires the TCA cycle to be supported by other carbon sources. It has been shown that this is accomplished in HCMV-infected cells through the anaplerotic utilization of glutamine where glutaminolysis converts glutamine to α-ketoglutarate which enters the TCA cycle and supports it (6) (reviewed in reference 40). Thus, HCMV infection substantially alters many aspects of intermediary metabolism in order to use glucose biosynthetically for fatty acid production.
Phospholipids and cholesterol are major lipid components of mammalian cell membranes and are required for proper membrane permeability, fluidity, organelle identity, and protein function (13). Cells maintain lipid homeostasis by multiple controls that act through transcriptional and posttranscriptional mechanisms. Sterol regulatory element binding proteins (SREBPs) are the principal regulators to maintain the proper levels of intracellular lipids (15); their biology and means of activation, described below, have been reviewed in references 13 and 32. Briefly, SREBPs belong to the basic helix-loop-helix leucine zipper (bHLH-Zip) family of transcriptional factors that are synthesized as inactive precursors anchored in the membrane of the endoplasmic reticulum (ER) in complex with SREBP cleavage activation protein (SCAP). All SREBP precursors consist of an N-terminal region of approximately 500 amino acids containing the bHLH-Zip region, two hydrophobic transmembrane segments interrupted by a short segment (approximately 30 amino acids), and a C-terminal domain of approximately 590 amino acids (18). In cells with sufficient sterol levels, the SREBP-SCAP complex remains in the ER membrane in association with insulin-induced gene 1 (Insig-1) (Fig. 1B). However, in cells with low levels of sterols, Insig-1 binding is lost, and the SREBP-SCAP complex is loaded into COPII vesicles and transported to the Golgi apparatus where the SREBPs are cleaved to a water-soluble N-terminal domain that is translocated into the nucleus. These mature forms of SREBPs then bind to sterol regulatory elements (SREs) found in the promoters of the genes that encode enzymes needed for lipogenesis, thus upregulating their expression to increase the synthesis of lipids. In return, increased sterols inhibit the cleavage of SREBPs, resulting in reduced synthesis of sterols through a negative-feedback loop (13, 15).
Mammalian cells have two genes coding for three SREBP isoforms: SREBP1a and SREBP1c are derived from SREBF-1, and SREBP2 is derived from SREBF-2 (reviewed in reference 12). The SREBP1a and -1c transcripts are produced through the use of alternative transcription start sites and differ only in their first exon; the other exons are common to both isoforms. SREBP1a is a more potent transcriptional activator than SREBP1c due to its longer amino-terminal transactivation domain. However, SREBP1c is the predominant isoform expressed in most of the tissues of mice and humans. SREBP1a is highly expressed in cultured cell lines and certain tissues, including the spleen and intestine (35). Transgenic and knockout mouse studies suggest that SREBP1a regulates gene expression for both fatty acid and cholesterol synthesis, SREBP1c activates only genes involved in fatty acid synthesis, and SREBP2 is preferentially involved in cholesterol homeostasis (18). These three proteins regulate the expression of more than 30 genes involved in cholesterol, fatty acid, triglyceride, and phospholipid metabolism.
Recent studies have shown that during HCMV infection the cleavage and activation of SREBP2 is substantially increased (36). Thus, in the following studies, we analyzed the activation of SREBP1 (SREBP1a and SREBP1c collectively, since they cannot be differentiated immunologically). We show that HCMV infection induces lipogenesis by increasing the expression of key lipogenic enzymes as a result of increased cleavage of SREBP1 precursors to form the mature active transcription factors. The activation of cleavage is constitutive, since the normally sterol-mediated feedback control of cleavage is overridden in infected cells. This process requires viral protein synthesis, since UV-irradiated HCMV cannot activate SREBP cleavage. In normal cells, the cleavage of SREBP1 requires that it be in complex with SCAP. Our data show that SCAP is also required in HCMV-infected cells. Depletion of SCAP in infected cells, using short hairpin RNA (shRNA), resulted in inhibition of SREBP1 cleavage and induction of lipogenic genes, lipid synthesis, and viral growth. Our studies suggest that HCMV induces adipocyte-like lipogenesis and overrides normal sterol feedback controls in order to maintain constitutive lipid synthesis during infection.
MATERIALS AND METHODS
Cell culture, viruses, and reagents.
Life-extended human foreskin fibroblasts (HFs) (3) were propagated and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM GlutaMAX (all reagents were obtained from Invitrogen, Carlsbad, CA). HCMV (Towne strain) stocks were prepared and purified as previously described (21). Titers were determined using the 50% tissue culture infective dose (TCID50) method.
Serum-starved HFs were infected with HCMV (multiplicity of infection [MOI] of 3) using the Towne strain modified to express green fluorescent protein (GFP) under the control of the simian virus 40 (SV40) early promoter. For UV-inactivated HCMV (UV-HCMV) infection, the virus was exposed to 254-nm UV light prior to infection at 240 mJ/cm2 in a UV Stratalinker 1800. For fluorescence microscopy studies, the wild-type Towne strain of HCMV without the cassette containing the gene that encodes green fluorescent protein (GFP) was used. The following biochemicals were used at the concentrations discussed below in Results: cholesterol (C3045; Sigma) and 25-hydroxycholesterol (H1015; Sigma).
Immunofluorescence.
After 24 h of serum starvation, confluent HFs on coverslips were either mock infected or infected with HCMV in serum-free DMEM. At 48 hours postinfection (hpi), the cells were washed in phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde at room temperature. After permeabilization with 0.1% Triton X-100 in PBS for 10 min, the cells on coverslips were blocked in Tris-buffered saline (TBS) containing 10% human serum and 0.5% Tween 20 for 1 h, followed by incubation with 10 μg/ml of anti-SREBP1 antibody (IgG-2A4; BD Biosciences) and anti-ex2/3 serum (1:2,000) in blocking buffer for 2 h at room temperature. The coverslips were washed three times with PBS and then incubated with Alexa Fluor 594-labeled donkey anti-rabbit secondary antibody (Invitrogen) and fluorescein isothiocyanate (FITC)-labeled donkey anti-mouse antibody (Santa Cruz Biotech) for 2 h at room temperature. The coverslips were finally washed with PBS and mounted using VectaShield (Vector Laboratories) containing 4′,6′-diamidino-2-phenylindole (DAPI). Fluorescent images were captured using a Nikon Eclipse E600 microscope.
Lipid droplet staining.
Lipid droplets were stained with BODIPY 558/568 C12 [4,4′-difloro-5-(2-thienyl)-4-bora-3a,4a-diaza-s-indacene-3-dodecanoic acid], which stains neutral lipids (Molecular Probes; Invitrogen). For live cell staining (37), the cells were incubated with 20 μg/ml BODIPY 558/568 C12 for 45 min at 37°C in serum-free DMEM, rinsed, and refed with fresh DMEM for a further incubation of 45 min at 37°C. The cells were fixed with 4% paraformaldehyde for 30 min at room temperature, then washed with PBS, and mounted using Vectashield containing DAPI. The images were captured at the same microscopy exposure setting.
Lipid synthesis assay.
The lipid synthesis assay was performed as described previously (9). Briefly, confluent HFs in 12-well plates were serum starved for 24 h. After serum starvation, HFs in triplicate were either mock infected or infected with HCMV at an MOI of 3. At 2 hpi, cells were washed and refed with fresh serum-free DMEM. At 48 hpi, mock- and HCMV-infected HFs were refed with 0.4 ml of fresh medium containing 2 μCi/ml [1,2-14C]acetate and incubated at 37°C for 4 h. Labeled cells were washed three times in 1 ml ice-cold PBS and lysed in 0.3 ml of Triton X-100 (0.5% in H2O). Lipids were extracted by sequentially adding 0.75 ml of methanol, 0.75 ml of chloroform, 0.75 ml of chloroform, and 0.75 ml of H2O, with vortexing. Samples were centrifuged at 4,000 rpm for 15 min; the organic phase was recovered and counted in a scintillation counter. For background subtraction, mock-infected HFs were incubated with labeling medium for 1 s, and then the cells were washed, lysed, and extracted.
Quantitative RT-PCR.
Quantitative reverse transcription-PCR (RT-PCR) was performed as previously described (42). All gene expression data were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA level. The following primer sets (Applied Biosystems) were used: acetyl coenzyme A (acetyl-CoA) carboxylase 1 (ACC1) (assay identification [ID] Hs01046047_m1), ATP-citrate lyase (ACL) (assay ID Hs00153764_m1), fatty acid synthase (FAS) (assay ID Hs00188012_m1), GAPDH (assay ID Hs99999905_m1), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) (assay ID Hs00168352_m1), SCAP (assay ID: Hs00378725_m1), and stearoyl-CoA desaturase 1 (SCD1) (assay ID Hs01682761_m1).
Western analysis.
Western blotting was performed by the procedures described previously (41). The following antibodies were used in this study: anti-ACC1 (4190; Cell Signaling Technology), anti-ACL (15421-1-AP; ProteinTech Group), antiactin (MAB1501; Chemicon), anti-ex2/3 (17), anti-FAS (610962; BD Biosciences), anti-perilipin A (ab3526; Abcam), anti-SCD1 (ab19862; Abcam), anti-SREBP1 (557036; BD Biosciences), anti-pp28 (sc-56975; Santa Cruz), anti-pp52 (sc-69744; Santa Cruz), and anti-pp65 (sc-52401; Santa Cruz).
Utilization of shRNAs.
Confluent HFs in 60-mm dishes were infected with shRNA-expressing lentiviral vectors in the presence of 10 μg/ml Polybrene for 4 h, followed by the addition of fresh medium. After 3 days, the cells were serum starved for 24 h and then infected with HCMV (MOI of 3) in serum-free DMEM. After 2 hours at 37°C, the cells were washed once with serum-free DMEM and then refed with serum-free DMEM. Viruses were harvested at 72 or 96 hpi. The experiments were set up in duplicate. One set of dishes were used to harvest viruses and determine viral titers as described above; the parallel set was used to make whole-cell extracts to determine expression of proteins.
RESULTS
HCMV infection increases cellular lipid biosynthesis and expression of lipogenic genes.
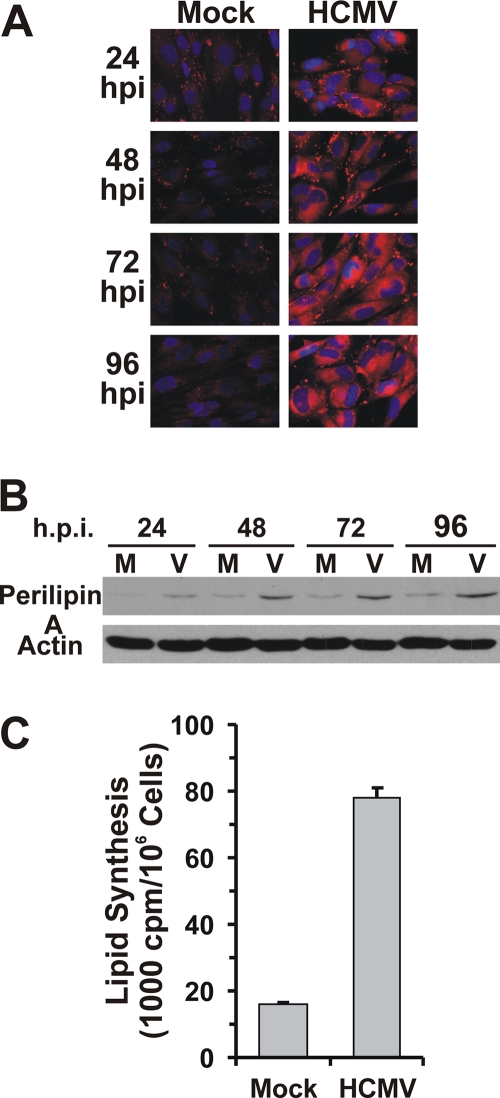
Previous studies have suggested that HCMV-infected cells show increased fatty acid synthesis (24, 26, 33). The increase in fatty acids and lipids can be detected by examining lipid droplets using BODIPY 558/568 C12 which stains neutral lipids. Figure 2A showed that mock-infected HFs contain a few small lipid droplets which is characteristic of fibroblasts. However, after as little as 24 h of HCMV infection, the number and size of lipid droplets are dramatically increased and enlarged, similar to the lipid droplets seen in adipocytes. The lipid droplets continue to increase as seen in the 48, 72, and 96 hpi samples. Lipid droplets are intracellular storage sites for neutral lipids, which are surrounded by a monolayer of phospholipids with embedded proteins (14). Perilipin is the major protein associated with lipid droplet phospholipid membranes (4). Consistent with increased staining of lipid droplets, HCMV-infected cells also had increased protein levels of perilipin A (Fig. 2B). The increase in lipid synthesis in HCMV-infected cells was further confirmed by a total lipid synthesis assay using 14C-labeled acetate. At 48 hpi, lipid synthesis in HCMV-infected cells was almost 5 times higher than that in mock-infected cells (Fig. 1C); similar results have been reported (36).
Fig 2.
HCMV infection increases lipid production in infected HF cells. (A) HF cells on coverslips were stained with lipophilic dye BODIPY 558/568 C12 after mock or HCMV infection for the indicated times. (B) Whole-cell extracts from mock-infected (M) and HCMV-infected (V) HFs at 24, 48, 72, and 96 hpi were used for Western blot analysis to determine the levels of perilipin A. (C) Total lipid synthesis in HFs. HFs were either mock or HCMV infected for 48 h and then labeled with [14C]acetate for 4 h; total lipids were extracted and counted as described in Materials and Methods.
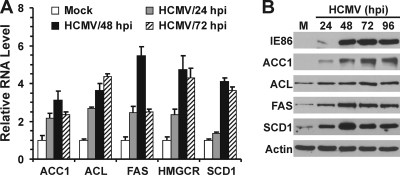
In mammalian cells, ATP-citrate lyase (ACL) is the primary enzyme that produces cytosolic acetyl-CoA from citrate exported from the mitochondrion; it is an essential building block for the synthesis of fatty acids and cholesterol (Fig. 1). Acetyl-CoA carboxylase (ACC), sterol CoA desaturase, and HMG CoA reductase (HMGCR) are rate-limiting enzymes for the synthesis of fatty acids, monounsaturated fatty acids, and cholesterol, respectively (16, 20, 29). The synthesis of fatty acids from acetyl-CoA and malonyl-CoA is carried out by fatty acid synthase (FAS). We determined the mRNA levels of these key lipogenic enzymes by quantitative RT-PCR. As shown in Fig. 3A, the mRNA levels of all of these enzymes increased after HCMV infection; in most cases, the RNA levels peaked at 48 hpi. In each case, the increase in mRNA levels was reflected by a concomitant increase at the protein level. The Western analysis in Fig. 3B showed that the protein levels of ACC1, ACL, FAS, and SCD1 all increased during the course of HCMV infection. Due to the lack of suitable antibody, HMGCR protein data are not presented. The increase in ACC1 during HCMV infection was reported recently (36).
Fig 3.
HCMV-infected cells have increased expression of key lipogenic enzymes. (A) RNA levels of lipogenic genes in mock-infected HFs and HFs infected with HCMV for the indicated times. Total RNA was isolated, and the mRNA levels of lipogenic genes were measured by quantitative RT-PCR and normalized to GAPDH mRNA levels. (B) Protein levels of lipogenic enzymes. Whole-cell extracts were prepared from mock-infected HFs or HFs infected with HCMV for the indicated times. Western blot analysis was used to quantitate the levels of acetyl-CoA carboxylase 1 (ACC1), ATP-citrate lyase (ACL), fatty acid synthase (FAS), sterol CoA desaturase 1 (SCD1), the HCMV 86-kDa immediate-early protein (IE86), and actin.
SREBP1 is cleaved in HCMV-infected cells.
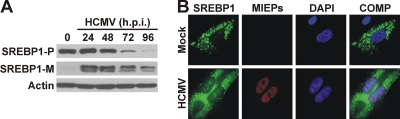
The promoters of the genes for each of the above enzymes contain sterol regulatory elements (SREs) (2). This is also true for the promoter of GLUT4 which we previously showed was unregulated during HCMV infection (42). As discussed in the introduction, SREs are bound by the mature SREBPs which are formed through cleavage of a larger precursor. In resting, mock-infected HF cells (zero time point [Fig. 4A]), the SREBP1 precursor (SREBP1-P) was readily detected but very little mature SREBP1 (SREBP1-M) was detected by Western analysis (Fig. 4A), indicating that SREBP1 processing was inhibited. In contrast, within 24 h of HCMV infection, the mature form of SREBP1 was readily detected, suggesting that HCMV infection activated the SREBP maturation mechanism. At times later than 48 hpi, the levels of the SREBP1 precursor declined significantly; however, the levels of the mature form were more stable, suggesting that the mature forms made at earlier times are retained.
Fig 4.
HCMV infection induces the cleavage of SREBP1. (A) Whole-cell extracts were prepared from HCMV-infected HFs at 0 (mock), 24, 48, 72, and 96 hpi. SREBP1 and actin protein levels were determined by Western blot analysis. The SREBP1 precursor (SREBP1-P) and mature form of SREBP1 (SREBP1-M) are shown. (B) SREBP1 presents in the nuclei of HCMV-infected HFs. Mock- and HCMV-infected (48 hpi) HFs were fixed and stained for the HCMV major immediate-early proteins (MIEPs) and SREBP1 as described in Materials and Methods. The SREBP1, MIEPs, and DAPI images are overlaid into a composite image (COMP).
The mature form of SREBP1 is capable of translocation into the nucleus to function as a transcription factor. To show that SREBP1 was present in the nuclei of infected cells, we examined mock- and HCMV-infected cells using immunofluorescence microscopy with an IgG-2A4 antibody against the N terminus of SREBP1 (34), which can detect both the precursor and mature, nuclear form of SREBP1. Figure 4B showed little to no SREBP1 staining in the nuclei of mock-infected cells. However, after HCMV infection, SREBP1 staining was present in the infected cell nuclei, indicating that mature SREBP1 is transported into the nuclei of HCMV-infected cells.
The cleavage of SREBP1 is independent of cellular sterol level and requires expression of viral proteins.
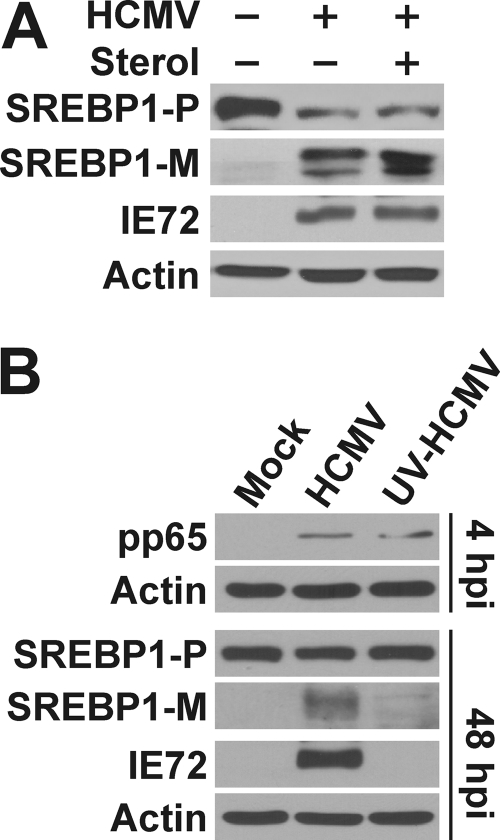
As discussed in the introduction, the cleavage of SREBP1 is regulated by cellular sterol levels; cleavage is enhanced by sterol depletion and inhibited by sterol supplement (1, 19). To find out whether the cleavage of SREBPs is affected by sterol levels in HCMV-infected cells, we serum starved HF cells prior to infection with HCMV at an MOI of 3. At 2 hpi, HCMV-infected cells were either refed with serum-free medium alone or medium supplemented with 10 μg/ml cholesterol and 1 μg/ml 25-hydroxycholesterol. At 72 hpi, protein samples were harvested and tested for the effects of sterols on SREBP1 cleavage. Figure 5A showed the increased levels of mature SREBP1 in HCMV-infected cells under serum-free conditions in agreement with the data in Fig. 4A. This was not decreased by the addition of the sterol to HCMV-infected cells, indicating that the cleavage of SREBPs in HCMV-infected cells is independent of the normal control by cellular sterol levels. A caveat of this experiment is that there is very little cleaved SREBP in uninfected cells even when there is no sterol present. This may be because the cells used are quiescent. However, as discussed above, the results reported in the literature are quite convincing that cleavage can be inhibited by sterols; since we do not see this with HCMV infection, the most straightforward conclusion is that this inhibitory mechanism is bypassed in infected cells.
Fig 5.
The cleavage of SREBP1 is independent of cellular sterol level in HCMV-infected cells and requires viral protein expression. (A) Serum-starved HFs were infected with HCMV (+) (MOI of 3) or mock infected (−). At 2 hpi, the cells were either refed with serum-free medium alone or medium supplemented with 10 μg/ml cholesterol and 1 μg/ml 25-hydroxycholesterol. At 72 hpi, protein samples were harvested and tested for the effects of sterols on SREBP1 cleavage. (B) HFs were mock infected or infected with normal HCMV or UV-inactivated HCMV (UV-HCMV) for 4 or 48 h. Whole-cell extracts were prepared and analyzed by Western analysis for the HCMV tegument protein pp65, the 72-kDa immediate-early protein (IE72), SREBP1, and actin. SREBP1-P, precursor SREBP1; SREBP1-M, mature form of SREBP1.
These experiments used serum-free conditions, however, HCMV infection enhances SREBP cleavage under conditions when serum is present (with or without additional sterol; not shown). We use serum-free conditions because HCMV infection in cultured human fibroblasts can proceed quite well without serum; the virus can activate needed signaling pathways that can also be activated by growth factors in serum (21). Thus, to ensure that the effects we are seeing are arising from a viral mechanism, not serum, we use serum-free conditions and purified virus stocks.
To determine whether de novo viral protein expression is required for the cleavage of SREBP1, HF cells were infected with either HCMV or UV-inactivated HCMV (UV-HCMV). UV irradiation inactivated the viral genome but still allows virus to enter cells to release viral tegument proteins. In Fig. 5B, Western blot analysis is shown for cell extracts taken at 4 hpi and showed that UV-HCMV-infected cells had the same amount of viral tegument protein pp65 as HCMV-infected cells, indicating an equal viral loading between HCMV and UV-HCMV infections. Samples harvested at 48 hpi were tested for SREBP1 cleavage. The lack of detectable, mature SREBP1 in UV-HCMV-infected cells suggests that de novo viral protein synthesis is needed to set into motion the virus-mediated means of activating SREBP1 cleavage. The lack of detectable expression of the 72-kDa major immediate-early protein (IE72) confirmed the success of UV inactivation.
SCAP is required for SREBP1 cleavage in HCMV-infected cells.
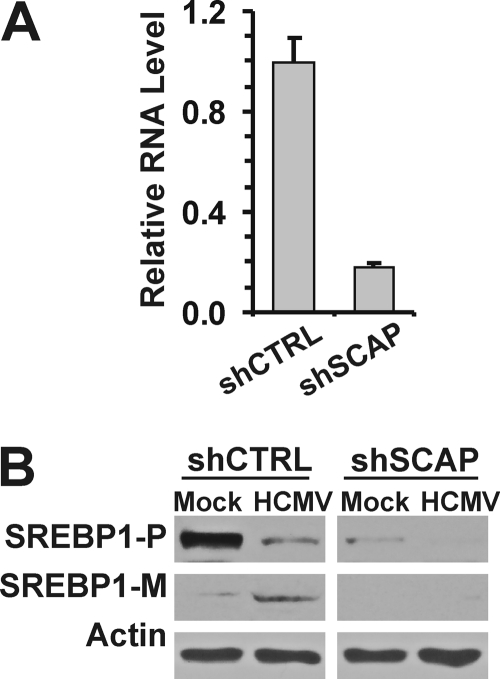
The observation that the cleavage of SREBP1 in infected cells was insensitive to sterol levels led us to ask whether the virus circumvents the normal cleavage process that involves its chaperone SCAP. SCAP forms a complex with SREBP1 in the ER membrane and is required to transport it to the Golgi apparatus for proteolytic cleavage (see the introduction). Mice with conditional SCAP deficiency in liver have a profound reduction in RNA levels and cleavage of all three SREBPs (23). To determine whether or not SCAP was necessary for SREBP cleavage during HCMV infection, we used lentiviral vectors to introduce an shRNA against SCAP (shSCAP) or a control shRNA. Due to the lack of anti-SCAP antibody for Western analysis, we evaluated the depletion efficiency by measuring SCAP mRNA levels using quantitative RT-PCR. The shSCAP suppressed SCAP expression by 80 to 90% compared to a control shRNA, shCTRL (Fig. 6A). Normal levels of precursor and mature SREBP1 were detected in both mock- and HCMV-infected HFs treated with the control shRNA. However, in SCAP-depleted, HCMV-infected cells, there was a severe loss of both the precursor and mature forms of SREBP1. In part, this loss is due to the lowering of the precursor levels as seen in the mock-infected, SCAP-depleted HFs. One argument from this observation is that SCAP may stabilize SREBP. However, the literature suggests that this loss arises because the mature SREBP1 transcription factors transcriptionally activate their own gene (23), an autoregulatory mechanism. In other words, SCAP depletion results in the inability to cleave SREBP1 precursors, thus lowering the levels of mature SREBP transcription factors. Without the mature SREBP transcription factors, expression of the SREBP genes decreases as do the levels of SREBP precursors. Thus, our data suggest that SCAP is essential for the cleavage of SREBP1 in infected cells.
Fig 6.
Effects of SCAP depletion on the cleavage of SREBP1. (A) Efficiency of shRNA treatment on the levels of SCAP mRNA in HFs. Lentiviral vectors were used to introduce a control shRNA (shCTRL) or an shRNA directed to SCAP RNA (shSCAP). (B) Whole-cell extracts were prepared from HFs that had been treated with lentiviral vectors expressing shCTRL or shSCAP prior to mock infection or HCMV infection for 3 days. SREBP cleavage was determined by Western analysis. SREBP1-P, precursor SREBP1; SREBP1-M, mature form of SREBP1.
The inhibition of SREBP1 cleavage reduces the expression of lipogenic enzymes and lipid synthesis.
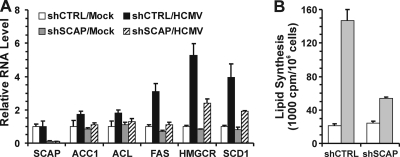
Given the importance of SCAP for SREBP1 cleavage, we predicted that SCAP depletion should reduce the expression of lipogenic genes. To test this idea, we used RT-PCR to determine the mRNA levels of key lipogenic enzymes in SCAP-depleted HFs which were mock infected or infected with HCMV (Fig. 7A). SCAP mRNA levels were also determined to show that shSCAP suppressed SCAP expression. HCMV infection was able to induce expression of ACC1, ACL, FAS, HMGCR, and SCD1 in cells treated with shCTRL; however, this induction was substantially reduced by SCAP depletion.
Fig 7.
Depletion of SCAP inhibits lipogenic gene expression and total lipid synthesis in HCMV-infected HFs. (A) mRNA levels of lipogenic enzymes were determined by RT-PCR using total RNA extracted from mock- and HCMV-infected cells that had been treated with the shSCAP- or shCTRL-expressing lentiviral vectors. (B) Total lipid synthesis was assayed in mock-infected cells (white bars) or HCMV-infected cells (gray bars) as described in Materials and Methods.
To further evaluate the effect of SCAP depletion on lipid synthesis in HCMV infection, total lipid synthesis was assayed. Figure 7B shows that the depletion of SCAP caused no change in the low level of lipid synthesis occurring in the mock-infected cells. However, the large induction of lipid synthesis seen in HCMV-infected cells was substantially reduced (nearly 70%) when SCAP was depleted. Thus, the data show that SCAP and the SCAP-dependent cleavage of SREBP1 are essential for lipogenic gene expression and fatty acid synthesis in HCMV-infected cells.
The inhibition of SREBP1 cleavage impairs HCMV growth.
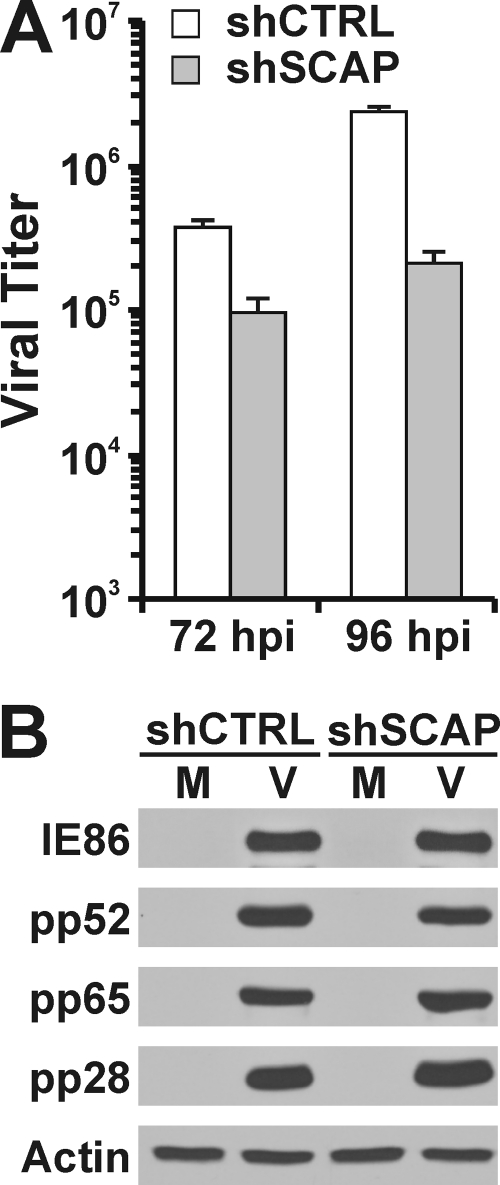
It has been reported that inhibition of fatty acid synthesis by chemical inhibitors greatly inhibits HCMV growth (26). Given this and the above results, it is predicted that SCAP and the cleavage of SREBP1 should be essential for HCMV growth. The growth data in Fig. 8A show that SCAP depletion slows viral growth in HFs. Figure 8B shows that the expression of viral proteins is not affected by SCAP depletion, as the levels of an immediate-early protein (IE86), and early protein (pp52) and two late proteins (pp65 and pp28) were not altered by SCAP depletion. This suggests that the slowdown of HCMV growth is due to the decrease in lipid synthesis by SCAP depletion.
Fig 8.
HCMV growth is attenuated in SCAP-depleted HFs. (A) HCMV viral titer. (B) Viral protein expression in SCAP-depleted HFs was evaluated by Western analysis.
DISCUSSION
Lipids are essential molecules for cell growth and proliferation. In quiescent cells, lipid synthesis is diminished, since lipid requirements are low. In contrast, rapidly proliferating cells, like tumor cells, require a high rate of de novo lipid synthesis for cell membrane production and lipid-based posttranslational protein modification (10). HCMV-infected cells alter intermediary metabolism in a manner that is very similar to tumor cells where glucose is used biosynthetically for fatty acid synthesis and the tricarboxylic acid cycle is anaploretically maintained by glutaminolysis (reviewed in reference 40). The activation of fatty acid synthesis results in the accumulation of enlarged lipid droplets in the cytoplasm of cells in the infected cultures.
The shift in metabolism to support fatty acid synthesis requires the activation of many genes associated with lipogenesis. Indeed, HCMV-induced lipogenesis appears to be very similar to that which occurs during adipocyte differentiation where large amounts of lipids are produced and large lipid droplets form. Studies of adipocyte differentiation have suggested the involvement of a number of transcription factors in adipocyte differentiation, notably peroxisome proliferator-activated receptor γ (PPARγ), several members of the CCAAT/enhancer binding protein (C/EBP) and Krüppel-like factor (KLF) families, signal transducer and activator of transcription factor 5 (STAT5), and SREBP1 (reviewed in reference 38). The various SREBP transcription factors have been shown to be able to bind either classic SRE elements (ATCACCCCAC), SRE-like elements (CTCACACGAG), E-boxes, or E-box-like sequences (2). Studies using specific promoter-reporter constructs suggest that there is significant differentiation in the promoters that the various SREBP isoforms can activate (2). For example, cholesterogenic gene promoters, containing classic SREs, were strongly and efficiently activated by both SREBP1a and SREBP2, but not by SREBP1c. Whereas an E-box-containing reporter was activated by SREBP1a and -1c, it was not activated by SREBP2. Promoters of lipogenic enzymes containing SRE-like sequences were strongly activated by SREBP1a and activated only modestly by either SREBP1c or -2. Studies using transgenic and knockout mice agree, in large part, with these analyses showing that SREBP1a regulates gene expression for both fatty acid and cholesterol synthesis, SREBP1c activates only genes in fatty acid synthesis, and SREBP2 is preferentially involved in cholesterol homeostasis (18). A recent study has implicated SREBP2 activation in increased lipogenesis in HCMV-infected cells (36). In this report, we have concentrated on SREBP1, since both SREBP1a and -1c are more directly related to fatty acid synthesis and adipocyte differentiation. Our studies examine SREBP1a and -1c collectively, since they cannot be differentiated by the immunological techniques utilized in these studies.
Our data show that the HCMV-mediated infection can induce lipogenesis through increased cleavage of the SREBP1 precursor to the mature transcription factor. The cleavage process induced by HCMV requires SCAP, the SREBP chaperone that accompanies SREBP to the Golgi apparatus and through cleavage to the mature transcription factor (Fig. 1B). However, the virus-induced cleavage is insensitive to high levels of sterol, which suggests that the virus has circumvented the normal sterol control mechanism. A potential mechanism for this is virus-induced alteration in the Insig interaction with the SREBP-SCAP complex (Fig. 1B), as such a modification could free the SREBP-SCAP complex from sequestration in the ER regardless of the sterol level.
Additional effects of HCMV infection on SREBP cleavage are suggested by our previous observations of mammalian target of rapamycin (mTOR) activation in HCMV-infected cells (8, 21, 22). It has been reported that mTOR complex 1 (mTORC1) stimulates lipogenesis through activation of SREBP1 cleavage (11, 31). In addition, it has been suggested that mTORC1 regulates SREBP by controlling the nuclear entry of lipin 1 to promote nuclear remodeling and nuclear entry of the mature form of SREBP (30). This may also be induced during HCMV infections where there is well-documented remodeling of the nucleus and changes in its permeability (5). Other studies also suggest that AKT kinase can stimulate lipogenesis in an mTORC1-independent mechanism by suppression of Insig gene expression which further activates the SREBP1 cleavage (39). However, this mechanism is unlikely in HCMV-infected cells; while the activation of AKT is important in the very early phases of infection, its activity declines significantly after 24 h (7, 21).
The involvement of SCAP in the HCMV-induced SREBP cleavage mechanism was demonstrated by depletion of SCAP using shRNAs. Since SREBP2 also utilizes SCAP, our experiments show that the specific inhibition of all SREBP protein cleavage, through SCAP depletion, affects induction of lipogenic gene expression and lipid synthesis during HCMV infection. Viewing all of the data available, we note that the induction of SREBP cleavage, the concomitant activation of lipogenic genes, and the accumulation of large lipid droplets in HCMV-infected cells are quite reminiscent of adipocyte differentiation which also includes the induction of glucose transporter 4 which we previously showed to occur during HCMV infection (42). These finding lead us to conclude that HCMV induced adipocyte-like lipogenesis or adipocyte differentiation in infected cells.
ACKNOWLEDGMENTS
We thank all the member of the Alwine laboratory for helpful suggestions and guidance.
This work was supported by NIH grant R01 CA157679 awarded to J.C.A.
Footnotes
Published ahead of print 18 January 2012
REFERENCES
- 1. Adams CM, et al. 2004. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem. 279:52772–52780 [DOI] [PubMed] [Google Scholar]
- 2. Amemiya-Kudo M, et al. 2002. Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J. Lipid Res. 43:1220–1235 [PubMed] [Google Scholar]
- 3. Bresnahan WA, Hultman GE, Shenk T. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816–10818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown DA. 2001. Lipid droplets: proteins floating on a pool of fat. Curr. Biol. 11:R446–R449 [DOI] [PubMed] [Google Scholar]
- 5. Buchkovich NJ, Maguire TG, Alwine JC. 2010. Role of the endoplasmic reticulum chaperone BiP, SUN domain proteins, and dynein in altering nuclear morphology during human cytomegalovirus infection. J. Virol. 84:7005–7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chambers JW, Maguire TG, Alwine JC. 2010. Glutamine metabolism is essential for human cytomegalovirus infection. J. Virol. 84:1867–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chuluunbaatar U, et al. 2010. Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev. 24:2627–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clippinger AJ, Maguire TG, Alwine JC. 2011. The changing role of mTOR kinase in the maintenance of protein synthesis during human cytomegalovirus infection. J. Virol. 85:3930–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deberardinis RJ, Lum JJ, Thompson CB. 2006. Phosphatidylinositol 3-kinase-dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J. Biol. Chem. 281:37372–37380 [DOI] [PubMed] [Google Scholar]
- 10. DeBerardinis RJ, Sayed N, Ditsworth D, Thompson CB. 2008. Brick by brick: metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 18:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Düvel K, et al. 2010. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39:171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eberlé D, Hegarty B, Bossard P, Ferré P, Foufelle F. 2004. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86:839–848 [DOI] [PubMed] [Google Scholar]
- 13. Espenshade PJ, Hughes AL. 2007. Regulation of sterol synthesis in eukaryotes. Annu. Rev. Genet. 41:401–427 [DOI] [PubMed] [Google Scholar]
- 14. Fujimoto T, Ohsaki Y. 2006. Cytoplasmic lipid droplets: rediscovery of an old structure as a unique platform. Ann. N. Y. Acad. Sci. 1086:104–115 [DOI] [PubMed] [Google Scholar]
- 15. Goldstein JL, DeBose-Boyd RA, Brown MS. 2006. Protein sensors for membrane sterols. Cell 124:35–46 [DOI] [PubMed] [Google Scholar]
- 16. Hampton R, Dimster-Denk D, Rine J. 1996. The biology of HMG-CoA reductase: the pros of contra-regulation. Trends Biochem. Sci. 21:140–145 [PubMed] [Google Scholar]
- 17. Harel NY, Alwine JC. 1998. Phosphorylation of the human cytomegalovirus 86-kilodalton immediate early protein IE2. J. Virol. 72:5481–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horton JD, et al. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. U. S. A. 100:12027–12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hua X, Nohturfft A, Goldstein JL, Brown MS. 1996. Sterol resistance in CHO cells traced to point mutation in SREBP cleavage-activating protein. Cell 87:415–426 [DOI] [PubMed] [Google Scholar]
- 20. Iliffe J, Myant NB. 1970. The sensitivity of acetyl-coenzyme A carboxylase to citrate stimulation in a homogenate of rat liver containing subcellular particles. Biochem. J. 117:385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kudchodkar S, Yu Y, Maguire T, Alwine JC. 2004. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J. Virol. 78:11030–11039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kudchodkar SB, Yu Y, Maguire TG, Alwine JC. 2006. Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of raptor- and rictor-containing complexes. Proc. Natl. Acad. Sci. U. S. A. 103:14182–14187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsuda M, et al. 2001. SREBP cleavage-activating protein (SCAP) is required for increased lipid synthesis in liver induced by cholesterol deprivation and insulin elevation. Genes Dev. 15:1206–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McArdle J, Schafer XL, Munger J. 2011. Inhibition of calmodulin-dependent kinase kinase blocks human cytomegalovirus-induced glycolytic activation and severely attenuates production of viral progeny. J. Virol. 85:705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munger J, Bajad SU, Coller HA, Shenk T, Rabinowitz JD. 2006. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2:1165–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Munger J, et al. 2008. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26:1179–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy E, Rigoutsos I, Shibuya T, Shenk TE. 2003. Reevaluation of human cytomegalovirus coding potential. Proc. Natl. Acad. Sci. U. S. A. 100:13585–13590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murphy E, et al. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 25:14976–14981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ntambi JM, Miyazaki M. 2004. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 43:91–104 [DOI] [PubMed] [Google Scholar]
- 30. Peterson TR, et al. 2011. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146:408–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Porstmann T, et al. 2008. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8:224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raghow R, Yellaturu C, Deng X, Park EA, Elam MB. 2008. SREBPs: the crossroads of physiological and pathological lipid homeostasis. Trends Endocrinol. Metab. 19:65–73 [DOI] [PubMed] [Google Scholar]
- 33. Sanchez V, Dong JJ. 2010. Alteration of lipid metabolism in cells infected with human cytomegalovirus. Virology 404:71–77 [DOI] [PubMed] [Google Scholar]
- 34. Sato R, et al. 1994. Assignment of the membrane attachment, DNA binding, and transcriptional activation domains of sterol regulatory element-binding protein-1 (SREBP-1). J. Biol. Chem. 269:17267–17273 [PubMed] [Google Scholar]
- 35. Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. 1997. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J. Clin. Invest. 99:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spencer CM, Schafer XL, Moorman NJ, Munger J. 2011. Human cytomegalovirus induces the activity and expression of acetyl-coenzyme A carboxylase, a fatty acid biosynthetic enzyme whose inhibition attenuates viral replication. J. Virol. 85:5814–5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Targett-Adams P, et al. 2003. Live cell analysis and targeting of the lipid droplet-binding adipocyte differentiation-related protein. J. Biol. Chem. 278:15998–16007 [DOI] [PubMed] [Google Scholar]
- 38. White UA, Stephens JM. 2010. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell. Endocrinol. 318:10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yecies JL, et al. 2011. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 14:21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu Y, Clippinger AJ, Alwine JC. 2011. Viral effects on metabolism: changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol. 19:360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu Y, Kudchodkar SB, Alwine JC. 2005. Effects of simian virus 40 large and small tumor antigens on mammalian target of rapamycin (mTOR) signaling: small tumor antigen mediates hypophosphorylation of eIF4E-binding protein 1 late in infection. J. Virol. 79:6882–6889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu Y, Maguire TG, Alwine JC. 2011. Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection. J. Virol. 85:1573–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]