Abstract
We aimed to conduct a placebo-controlled, double-blind, parallel-group design intervention study to evaluate the therapeutic efficacy of hormone therapy (HT) in postmenopausal women with mild to moderate Alzheimer’s disease (AD). The trial was designed to evaluate the dose-dependent effects of transdermal 17-β estradiol, unopposed and opposed with medroxyprogesterone (MPA, Provera©), for 12 months in 43 postmenopausal women with AD. Participants were assessed using cognitive measures at baseline, months 1, 3, 6, and 12 of treatment and eight weeks post treatment (month 15). The dropout rate was 49% across 12 months. As a result of theWomen’s Health Initiative (WHI) and anticipated increased attrition, the protocolwas modified to examine data only at time points where attrition was less than 30%. The results of sensitivity analyses indicated robust and reliable data collected in the first three months of the trial. Data collected in the first three months of the trial for forty-three participants were analyzed. HT had favorable cognitive effects across multiple cognitive domains, including visual memory (p-values < 0.030) and semantic memory (p-values < 0.037) in postmenopausal women with AD. Moreover, treatment-related changes in plasma estradiol were positively correlated with improvements in visual memory. Short-term HT that includes the use of estradiol has favorable effects on cognition in women with AD.
Keywords: Alzheimer’s disease, clinical trial, cognition, estradiol, estrogen, hormone therapy, medroxyprogesterone, memory
INTRODUCTION
Findings from basic science [1], observational [2, 3], and clinical studies [4, 5] suggest that hormone therapy (HT) administered during the menopausal transition could potentially reduce the risk of developing Alzheimer’s disease (AD) [6–8]. After diagnosis, use of estrogen as an alternative therapy for AD is controversial [4, 5, 9]. TheWomen’s Health Initiative [10] (WHI) and the WHI Memory Study [11] (WHIMS), characterized the cognitive efficacy and adverse effects profile of conjugated equine estrogen (CEE) with and without medroxyprogesterone acetate (MPA) in older postmenopausal women [12]. WHIMS found an increased risk for dementia in postmenopausal women aged 65 and older, treated with CEE and MPA. In contrast, other studies have demonstrated HT-related improvements in cognition including reduced risk of dementia [6, 13, 14].
Evidence that fails to support a cognitive benefit of HT in AD emanates from controlled and uncontrolled clinical studies [15–17] that rely on global cognitive measures limited in sensitivity, and have predominately employed CEE instead of estradiol. CEE, the most widely used form of estrogen replacement therapy among postmenopausal women in the United States, is comprised of estrone sulfate and at least ten other steroid hormones with unknown neurobiological effects [4]. Estradiol forms of HT, an alternative to CEE, are comprised of 17-β estradiol, the most potent and natural human form of estrogen [9]. Transdermal estradiol formulations likely confer additional benefits, because oral formulations have been linked to increased thrombotic risk and cognitive deficits due to microthrombi [18–20].
To date, ten placebo-controlled randomized clinical trials have examined the cognitive effects of HT in postmenopausal women with AD [4, 5, 17, 21–27]. A meta-analysis of seven of these trials concluded that estradiol but not estrone forms of HT may confer short term cognitive benefits in women with AD [28]. The limited number of randomized trials conducted thus far, and methodological discrepancies including differences in HT formulation and the cognitive tests employed likely account, at least in part, for conflicting results regarding the cognition-enhancing effects of HT for postmenopausal women.
Our previous randomized clinical trials have shown that treatment with 17β-estradiol for eight weeks resulted in significant improvements in attention, verbal memory, visual memory, and semantic memory in postmenopausal women with AD. The improvements in cognition were correlated with plasma estradiol concentrations and were observed at doses commonly used in clinical practice [4, 29]. Our findings are consistent with the results of studies surveyed in a meta analysis [30] and with the findings of other uncontrolled estradiol studies [6, 13, 31], lending additional support for a potential beneficial effect of estradiol on cognition in postmenopausal women with AD [32, 33].
The current randomized, placebo-controlled, double-blind, parallel-group design intervention study, aimed to evaluate the potential dose-dependent, cognition-enhancing efficacy of opposed and unopposed transdermal estradiol administration for postmenopausal women with AD.
METHODS
Participants
Participants included 43 outpatient, postmenopausal women (ages 55–85 years) with mild to moderate AD, recruited through AD research programs at the University of Washington and Veterans Affairs Puget Sound Health Care System in Seattle, and at the University of Wisconsin-Madison.
Alzheimer’s dementia was diagnosed by consensus through Memory Disorders Clinics using information gathered via medical records, clinical interview, and cognitive testing (Mini-Mental Status Examination, MMSE; Blessed Memory and Information Concentration Test, BMICT). Inclusion criteria included normal gynecological and breast examination within 3 months, normal mammogram and papanicolaou test (pap smear) within the last year, baseline endometrial thickness, as measured by transvaginal ultrasound of less than 5 mm, education level of 9 years or equivalent, Hatchinski score of 4 or less, and a Hamilton Depression Scale score of 14 or below. Both hysterectomized and non-hysterectomized women were recruited.
Exclusion criteria included medical disorders and conditions that contraindicate use of estrogen. Participants were free of any medical or neurological illness apart from AD, and underwent a detailed history, screening blood tests and medical, neurological, and gynecologic examination. HT was discontinued, when applicable, at least 8 weeks before study enrollment.
Study procedures
We conducted a randomized, placebo-controlled, double-blind, parallel-group design intervention study to evaluate the dose-dependent effects of unopposed and opposed (with medroxyprogesterone, MPA) transdermal 17β-estradiol on cognition in postmenopausal women with AD. Participants were assessed on the cognitive outcome measures at baseline, and at months 1, 3, 6, and 12 in the treatment phase of the study. Treatment was discontinued after 12 months and outcome measures were reassessed at month 15 to characterize the effects of HT withdrawal.
Women were assigned to 1 of 5 treatment arms: 1) Low dose unopposed HT: 50µg transdermal 17β-estradiol and a placebo tablet daily, 2) Low dose opposed HT: 50µg transdermal 17β-estradiol and 2.5 mg of MPA daily, 3) High dose unopposed HT: 100µg transdermal 17β-estradiol and a placebo tablet, 4) High dose opposed HT: 100µg transdermal 17β-estradiol and 2.5 mg of MPA daily, or 5) Placebo skin patch and placebo tablet daily.
During each clinic visit, participants were evaluated by the study physician for adverse effects. Under the supervision of a neuropsychologist, cognitive tests were administered by trained psychometrist. To maintain the blind, the study physician did not share any participant information with either the neuropsychologist or psychometrist, and the psychometrist did not discuss adverse events with the participant.
Two years into the study, the results from the WHI and the WHIMS indicated that CEE formulations of HT might be associated with increased risk of cardiovascular disease and dementia. In response, a protocol modification was implemented to inform all participants of the WHI findings and alter the randomization protocol for participants who elected to reconsent and continue in the study. Before the WHI, women were randomized to the 5 treatment arms equally, regardless of hysterectomy status. Post-WHI, randomization to progesterone was stratified by hysterectomy status such that hysterectomized women were assigned equally to unopposed HT or placebo and non-hysterectomized women received opposed HT or placebo.
Cognitive tests
Cognitive function was evaluated using a comprehensive battery of nine neuropsychological tests assessing change in cognitive domains reportedly affected by HT and AD [4]. Different but comparable versions of the battery were administered at each visit to avoid practice effects. The battery included measures of semantic memory (Boston Naming Test [34]), visual memory (Figural Memory Test [35], Complex Figure Test [36] and Visual Paired Associates [35, 37]), verbal fluency [38], and verbal memory (Paragraph Recall [35, 37], list learning [39]), attention (Trail-Making Test B), and the Stroop Color-Word Interference test [40, 41]), described in detail elsewhere [4, 5]. Similar batteries have been utilized in our previous HT studies, are well tolerated by persons with AD, and are used to evaluate aspects of cognition selectively impaired in early AD [4].
Profile of mood states questionnaire
The effects of HT on mood were measured using the Profile of Mood States (POMS) [42]. The POMS queries mood changes over the preceding week on six categories (anger, anxiety, confusion, depression, fatigue, and vigor) comprised of 65 adjectives (such as friendly or listless) using a 5-point Likert scale. The POMS has been extensively validated to evaluate mood effects of estrogen treatment and is commonly used in AD populations [43–46].
Laboratory tests
Participants underwent an extensive diagnostic work-up including laboratory blood tests, a urinalysis, and an electrocardiogram to assess overall health and exclude treatable medical disorders with cognitive symptoms similar to AD. If not available within the past 12 months, a mammography and a pap smear were conducted during the gynecologic examination. In addition, a trans-vaginal ultrasound (TVUS) measurement of endometrial thickness was performed at baseline, 6 months and 12 months. Participants with an endometrial thickness of 5mm or more at baseline were excluded.
Adherence and correlational analyses were performed on estradiol, estrone, testosterone (T), and luteinizing hormone (LH) levels in blood collected at baseline and at month 3 using an enzyme immunoassay (EIA kit, Alpco Diagnostics, Salem, NH, USA).
Estrogen (estradiol and placebo skin patches) was provided by Berlex Pharmaceuticals (now Bayer HealthCare Pharmaceuticals). Provera© and matching placebo tablets were provided by the Pharmaceutical Research Centers at the University of Washington and the University of Wisconsin-Madison, per FDA regulations.
Analyses
Primary outcomes were selected in light of our studies [5, 29] and earlier reports [47–50], and included scores on tests of semantic and visual memory. The remaining cognitive variables were examined as secondary outcomes given prior reports of HT-related improvements in these measures. Covariates included baseline age, education, and MMSE scores. As a result of the WHI and WHIMS, we anticipated high attrition rates and modified our protocols accordingly [51].
To minimize the potential impact on power or sample selection, we conducted analyses for time points when attrition in treatment arms was less than 30%. Sensitivity analyses evaluate potential bias caused by missing values in follow-up measures to ensure peak sensitivity at all time points [52, 53] and reliability at time points where attrition is less than 30%. Sensitivity analyses are typically conducted to assess the effect of dropouts on inferences about the target parameters, and are particularly important when the treatment arms are unequal [53]. Although there are no clear guidelines regarding the acceptable amount of missing data in a clinical trial (or any longitudinal study), values ranging from 60%–80% as minimum acceptable follow-up rates have been proposed [54–56].
To maximize power in light of high attrition rates, we conducted analyses on all participants taking any form of HT (‘any HT’ group) versus placebo, collapsing across dose. Next, for those cognitive tests that showed a significant difference between treatment and placebo, we examined potential differences between opposed versus unopposed treatment arms, once again, collapsing across dose. All analyses were conducted using linear mixed-effects (LME) models. The LME approach has the advantage of incorporating fixed-effects parameters and random effects, are more suitable for unbalanced data, and take full advantage of all available variables [57].
Cognitive outcomes collected at baseline and months 1 and 3 were included as repeated outcome measures. In all models, the parameter estimate of primary interest was the treatment by time interaction. Estimation of regression parameters as well as the variance-covariance matrix of random effects was conducted using restricted maximum likelihood (REML) algorithms and robust standard errors. In all models, we assumed an unstructured covariance structure for the two repeated measures. REML methods are generally more appropriate than standard maximum likelihood, particularly in small samples [58]. All models were estimated using SAS, version 9.2.
For all treatment groups (collapsed across dose), we also examined relationships between cognition and hormone levels when 1) hormone levels changed over 3 months of treatment and 2) cognitive performance was altered by treatment. Correlations were calculated using Spearman’s rank method.
RESULTS
Participants
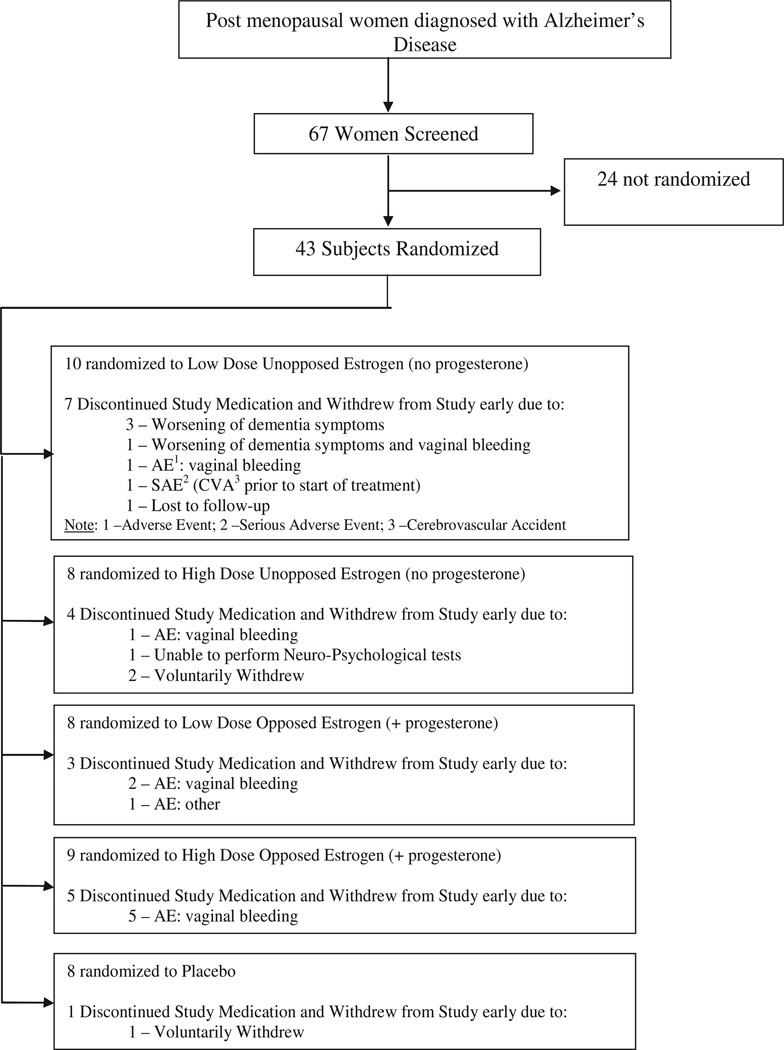
Figure 1 illustrates participant enrollment, randomization, and adverse events by the five treatment arms using a CONSORT-style diagram. A total of 43 subjects were randomized across five treatment groups. Prior to the publication of the WHI results, nine of the 43 women had been randomized. Of this group of nine participants, five withdrew early (self-reported worsening of dementia symptoms, n = 4; vaginal bleeding, n = 1). Post WHI, 34 women were randomized to the study. Sixteen of the 34 (47%) withdrew early. The main reason for discontinuation was vaginal bleeding (n = 8) resulting in unblinding of participants. Unlike many HT studies, including the WHI and WHIMS, no incidence of venous thromboembolism, stroke or cardiac events were observed.
Fig. 1.
Diagram describing study enrollment, randomization, and follow-up protocol.
Table 1 lists attrition rates for the five treatment arms by study visit. Participants who completed the month 12 visit were considered to have completed the study regardless of whether they returned for the month 15 follow-up visit. The overall withdrawal rate was 49%.
Table 1.
Attrition by treatment arm
| Sample Size at Month – Subjects with Any Cognitive Data | ||||||
|---|---|---|---|---|---|---|
| Group | Baseline | 1 | 3 | 6 | 12 | 15 |
| Placebo | 8 | 8 (100%) | 8 (100%) | 8 (100%) | 7 (87.5%) | 7 (87.5%) |
| Low Dose Estrogen Unopposed | 10 | 7 (70%) | 6 (60%) | 5 (50%) | 3 (30%) | 2 (20%) |
| High Dose Estrogen Unopposed | 8 | 7 (87.5%) | 7 (87.5%) | 6 (75%) | 4 (50%) | 4 (50%) |
| Low Dose Estrogen Opposed | 8 | 8 (100%) | 6 (75%) | 5 (62.5%) | 5 (62.5%) | 5 (62.5%) |
| High Dose Estrogen Opposed | 9 | 9 (100%) | 7 (78%) | 4 (44%) | 4 (44%) | 3 (33%) |
| Estrogen Unopposed | 18 | 14 (78%) | 13 (72%) | 11 (61%) | 7 (39%) | 6 (33%) |
| Estrogen Opposed | 17 | 17 (100%) | 13 (76%) | 9 (53%) | 9 (53%) | 8 (47%) |
| Any HT | 35 | 31 (89%) | 26 (74%) | 20 (57%) | 16 (46%) | 14 (40%) |
| Total | 43 | 39 (91%) | 34 (79%) | 28 (65%) | 23 (53%) | 21 (49%) |
Sensitivity
Imputation through month 3 added 18 to 25 more observations to the analyses, depending on the outcome measure, representing a 22–25% increase in available data. Analyses of 3-month imputed values yielded similar results to analyses of the original dataset that included only non-missing values, indicating that the month 3 results are robust. Analyses through month 6 resulted in an additional 44 records (a 35% increase in available data). Parameter estimates and p-values showed high variability between the original and imputed data for the any HT versus placebo comparisons. These findings suggest that modeling results up to, and beyond month 6 are unreliable, and thus were not subjected to analysis in this study.
In light of the results of the sensitivity analyses indicating reliable and robust data in the first 3 months of the trial, we examined the effects of HT treatment on cognitive performance at baseline, month 1 and month 3. While participants had complete data at baseline for certain cognitive tests (e.g., Boston Naming Test), some tests had a higher rate of non-completion due to issues associated with advanced stages of AD (e.g., fine motor tremors and executive function problems). Thus, tests such as Trails B had higher rates of missing data, even at baseline (23% missing). At baseline, there were no differences between any of the treatment groups in age, education, MMSE, GDS, BMICT, or ApoE4 status (Table 2). As expected, there was a significant difference between opposed and unopposed treatment groups by hysterectomy status (p < 0.001, data not shown).
Table 2.
Comparison of treatment groups according to demographic, mood, ApoE4 status and cognitive variables at baseline
| Variable | Treatment Group | n | Mean | SD | Contrast | KW* p-value |
|---|---|---|---|---|---|---|
| Age (y) | 1. Placebo | 8 | 74.4 | 5.2 | ||
| 2. Estrogen Unopposed | 18 | 78.1 | 8.4 | 2, 1 | 0.131 | |
| 3. Estrogen Opposed | 17 | 76.5 | 7.8 | 3, 1 | 0.431 | |
| 4. Any Estrogen | 35 | 77.3 | 8 | 4, 1 | 0.205 | |
| Blessed Memory | 1. Placebo | 8 | 28 | 6.8 | ||
| 2. Estrogen Unopposed | 14 | 26.7 | 4.8 | 2, 1 | 0.321 | |
| 3. Estrogen Opposed | 16 | 27.3 | 6.3 | 3, 1 | 0.781 | |
| 4. Any Estrogen | 30 | 27 | 5.6 | 4, 1 | 0.495 | |
| Education (y) | 1. Placebo | 8 | 13.9 | 1.5 | ||
| 2. Estrogen Unopposed | 17 | 13.2 | 2.1 | 2, 1 | 0.239 | |
| 3. Estrogen Opposed | 16 | 13.3 | 1.9 | 3, 1 | 0.398 | |
| 4. Any Estrogen | 33 | 13.3 | 1.9 | 4, 1 | 0.255 | |
| GDS | 1. Placebo | 8 | 3.5 | 1.9 | ||
| 2. Estrogen Unopposed | 18 | 2.4 | 2.1 | 2, 1 | 0.114 | |
| 3. Estrogen Opposed | 17 | 2.5 | 2.7 | 3, 1 | 0.121 | |
| 4. Any Estrogen | 35 | 2.5 | 2.4 | 4, 1 | 0.087 | |
| MMSE | 1. Placebo | 8 | 21.8 | 6.4 | ||
| 2. Estrogen Unopposed | 15 | 22.4 | 3.8 | 2, 1 | 1 | |
| 3. Estrogen Opposed | 17 | 24.5 | 3.8 | 3, 1 | 0.365 | |
| 4. Any Estrogen | 32 | 23.5 | 3.9 | 4, 1 | 0.599 | |
| Variable | Treatment Group | n | N | % | Contrast | Chisq |
| ApoE ε4+ | 1. Placebo | 5 | 7 | 71.43 | ||
| 2. Estrogen Unopposed | 7 | 12 | 58.33 | 2, 1 | 0.568 | |
| 3. Estrogen Opposed | 12 | 15 | 80 | 3, 1 | 0.655 | |
| 4. Any Estrogen | 19 | 27 | 70.37 | 4, 1 | 0.956 |
The nonparametric Wilcoxon scores one-way ANOVA approach of the Kruskal Wallis test was used to assess differences between the treatment groups.
HT and cognitive performance
At baseline, there were no group differences (HT versus placebo) for any of the cognitive measures. Table 3 provides a list of the cognitive tests administered and indicates significant treatment effects when appropriate. The black box highlights the primary outcomes of interest in light of our previous findings. Three months of HT had significant favorable effects on semantic memory (Boston Naming Test, p = 0.036), an effect that did not differ across the opposed and unopposed HT groups (p = 0.85). Three months of HT had favorable effects on episodic visual memory (Figural Memory Test, p = 0.015), and this effect was more pronounced for women who received opposed rather than unopposed HT (p = 0.08). A similar pattern of results, though not reaching statistical significance, was also observed on a second test of visual memory, the Complex Figure Test (p = 0.09). No significant difference in mood as measured by the total POMS score was observed between the HT and placebo groups (p = 0.22)
Table 3.
Treatment effects on cognitive performance scores
| Cognitive Tests | Any HT vs. Placebo | Opposed vs. Unopposed p-value |
|||
|---|---|---|---|---|---|
| Estimate | SE | t-ratio | p-value | ||
| Boston Naming (Total) | 1.48 | 0.66 | 2.24 | 0.032 | 0.853 |
| Boston Naming (Spontaneous) | 1.13 | 0.52 | 2.19 | 0.036 | 0.889 |
| Figure Memory-Total | 0.63 | 0.24 | 2.60 | 0.015 | 0.082 |
| CFT-Immediate Recall | 2.08 | 1.19 | 1.75 | 0.090 | 0.029 |
| CFT-Total Recall | 4.66 | 2.57 | 1.81 | 0.081 | 0.279 |
| CFT Delayed Recall | 2.04 | 1.62 | 1.26 | 0.218 | 0.664 |
| VPA: Immediate | −0.89 | 0.88 | −1.01 | 0.319 | |
| VPA: Delayed Recall | 0.43 | 0.30 | 1.42 | 0.166 | |
| VPA: Total | −0.43 | 0.97 | −0.44 | 0.663 | |
| Fluency | 0.87 | 1.65 | 0.53 | 0.602 | |
| Paragraph-Delayed Recall | −0.07 | 1.43 | −0.05 | 0.960 | |
| Paragraph: Immediate Recall | −0.47 | 1.67 | −0.28 | 0.780 | |
| Paragraph: Total Recall (Imm. + Del) | −0.63 | 2.49 | 0.25 | 0.800 | |
| List Learning-Total Recall | −1.78 | 1.49 | −1.19 | 0.244 | |
| List Learning-Delayed Recall | −0.23 | 0.44 | −0.51 | 0.614 | |
| List Learning-Immediate | −1.43 | 1.27 | −1.13 | 0.268 | |
| Trails B (time) | −11.58 | 17.75 | −0.65 | 0.519 | |
| Stroop-Interference (time) | 28.75 | 18.57 | 1.55 | 0.132 | |
All linear mixed effects models controlled for age, education level and Mini-Mental State Examination (MMSE) scores. Models were estimated using restricted maximum likelihood and robust standard errors.
Treatment effects on hormone levels
Table 4 shows change in hormone levels by treatment group over time. As expected, plasma levels of estradiol and estrone increased for the treated groups (p-values < 0.01). Although plasma T levels were not significantly affected by treatment for any of the groups, LH levels decreased for women receiving unopposed or opposed HT (p-values < 0.015). Hormone levels remained stable over time for women in the placebo group.
Table 4.
Hormone levels across time and treatment group
| Estradiol BL pg/mL |
Estradiol M3 pg/mL |
Estrone BL pg/mL |
Estrone M3 pg/mL |
Estradiol Estrone Ratio BL pg/mL |
Estradiol Estrone Ratio M3 pg/mL |
T BL pg/mL |
T M3 pg/mL |
LH BL pg/mL |
LH M3 pg/mL |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unopposed | Mean | 16.56 | 96.33 | 32.61 | 76.83 | 0.59 | 1.23 | 269.94 | 211.25 | 12.33 | 7.69 |
| n | 18 | 12 | 18 | 12 | 18 | 12 | 18 | 12 | 18 | 12 | |
| SD | 7.16 | 98.01 | 17.00 | 56.47 | 0.26 | 0.64 | 320.53 | 139.68 | 4.57 | 3.62 | |
| pvalue | 0.005* | 0.008* | 0.041 | 0.433 | 0.012* | ||||||
| Opposed | Mean | 14.00 | 107.38 | 28.76 | 59.15 | 0.56 | 1.76 | 302.29 | 174.08 | 14.87 | 8.06 |
| n | 17 | 13 | 17 | 13 | 17 | 13 | 17 | 13 | 17 | 13 | |
| SD | 0.00 | 93.29 | 12.08 | 34.58 | 0.21 | 0.92 | 274.46 | 127.96 | 6.35 | 4.46 | |
| pvalue | 0.002* | 0.004* | 0.001* | 0.055 | 0.001* | ||||||
| Any HT | Mean | 15.31 | 102.08 | 30.74 | 67.64 | 0.58 | 1.50 | 285.66 | 191.92 | 13.56 | 7.88 |
| n | 35 | 25 | 35 | 25 | 35 | 25 | 35 | 25 | 35 | 25 | |
| SD | 5.23 | 93.73 | 14.73 | 46.27 | 0.23 | 0.83 | 295.10 | 132.24 | 5.57 | 4.00 | |
| pvalue | 0.000* | 0.000* | 0.000* | 0.045 | 0.000* | ||||||
| Placebo | Mean | 14.00 | 19.75 | 35.75 | 25.00 | 0.54 | 0.81 | 282.50 | 145.13 | 13.12 | 13.75 |
| n | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | |
| SD | 0.00 | 16.26 | 25.18 | 14.31 | 0.25 | 0.28 | 237.67 | 114.40 | 6.82 | 8.77 | |
| pvalue | 0.317 | 0.183 | 0.017 | 0.036 | 0.401 |
P-values reflect the results from pair-wise Wilcoxon rank sum tests.
Change in estradiol and the estradiol-to-estrone ratio were positively correlated with scores obtained on the Boston Naming task (estradiol: r = 0.80, p = 0.002; ratio: r = 0.81, p = 0.001) in the any treatment group. In addition, change in estrone was positively correlated with immediate recall on the Complex Figure Test (r = 0.64, p = 0.048) in the opposed HT group. There were no significant correlations between estrogen levels and cognition for the unopposed HT group.
DISCUSSION
Our findings indicate that three months of HT administration with transdermal 17β-estradiol had significant favorable effects on semantic memory (Boston Naming Test) and visual memory (Figural Memory Test) in postmenopausal women with AD. These findings, consistent with our earlier reports and the reports of others [4, 5, 30], indicate that short-term HT that includes transdermal 17 β-estradiol may augment some cognitive abilities in older postmenopausal women with AD. Given the small sample size and short duration of treatment, the clinical relevance of the present and other similar studies needs to be confirmed in larger clinical trials of HT over extended periods of time.
Presently, drugs designed to treat AD mainly include cholinesterase inhibitors, which work by preventing the synaptic breakdown of acetylcholine in the brain. However, cholinesterase inhibitors attenuate only some AD symptoms and a positive treatment response is seen in a considerably small subset of the affected population. An ideal pharmacologic treatment for AD should be directed towards multiple pathophysiological mechanisms, have the potential to favorably alter disease neurobiology, be associated with minimum toxicity, and result in clinically significant improvements in AD symptoms. Short-term use of HT with estradiol may represent one such alternative treatment to improve cognitive symptoms associated with AD in older postmenopausal women. Unlike cholinergic drugs that primarily enhance cholinergic neurotransmission [59], estrogen exerts multiple salutary effects on the brain that have the potential to both enhance cognition and favorably alter AD pathology (for a comprehensive review, see [60]). Among others, some of these salutary effects include enhanced serotonergic, cholinergic and dopaminergic neurotransmission, anti-inflammatory effects, antioxidative efficacy, an ability to favorably alter amyloid-β protein precursor metabolism, multiple neurotrophic effects (e.g., increased synaptogenesis and dendritic spine density), and an ability to enhance glucose metabolism in areas known to be afflicted by AD pathology and involved in memory (e.g., the hippocampus) [61–63]. Findings from clinical studies suggest that, in addition to improving cognition, HT enhances cerebral blood flow and glucose utilization [64–67].
The results of the current study not only replicate our previous findings, but also are consistent with other reports demonstrating cognitive improvement associated with increased endogenous estradiol levels in younger women and exogenous levels via HT in healthy older women [68, 69]. Cognitive assessment was obtained using a comprehensive battery of well-established tests, across multiple cognitive domains pertinent to HT and AD. Together, these findings provide further support for a favorable effect of estradiol for healthy and pathological aging. This conclusion is not without controversy, as others have failed to show a beneficial effect on cognitive function [17, 22]. The lack of a clinical consensus likely relates to a variety of complex financial, social, psychological, and scientific issues, including several important methodological inconsistencies between studies, such as formulation and route of estrogen administration, hysterectomy status, age at time of exposure, exposure duration, sensitivity of cognitive tests administered, and inter-individual differences in hormone levels achieved following HT.
Our findings provide preliminary evidence to suggest that opposed 17 β-estradiol administration may confer greater benefits for visual memory than unopposed therapy. This finding is surprising, given recent evidence that MPA is neurotoxic and could further worsen cognitive performance [70]. When the current study was designed, MPA was the most commonly used progesterone to oppose estrogen. Unlike natural progesterone, MPA binds to glucocorticoid receptors with a much higher affinity and may have a greater impact on the hypothalamic–pituitary–adrenal axis, basal forebrain and limbic areas such as the amygdala and hippocampus, areas of the brain that are particularly stress-sensitive [71]. It is possible that the MPA-induced androgenic and progestogenic actions may result in short-term improvements on visuospatial ability, which may also explain reports that CEE + MPA was associated with a trend toward beneficial effects on figural memory in the Women’s Health Initiative Study of Cognitive Aging (WHISCA) study [72]. Taken together, these results suggest that the potential negative cognitive effects associated with MPA administration likely take longer than 3 months to manifest.
The primary limitation of the present study relates to attrition. Published risks from the WHI and WHIMS significantly increased attrition of participants in our study relative to attrition rates observed in our previous HT trials and adversely affected the overall recruitment resulting in a smaller than anticipated sample size. In a retrospective analysis of 29,718 new HT users, 54.4% were non-adherent after one year [73]. Moreover in women over 65 years of age, 62% discontinued HT within 12 months compared to 48% of younger women 50 to 55 years [74]. To minimize the potential impact on power or bias caused by such limitations, we performed analyses only on data collected up to month 3 for comparison groups collapsed across HT dose (i.e., any HT, versus placebo; opposed versus unopposed). A second important limitation relates to the mandated IRB modification to the randomization scheme in the wake of the WHI and WHIMS reports. That is, hysterectomized women were assigned equally to unopposed HT or placebo and non-hysterectomized women received opposed HT or placebo. As a result, the potential effects of hysterectomy status on cognitive response to HT could not be evaluated. Type of hysterectomy and duration since surgery is now recognized as an important factor and has been associated with an increased risk of cognitive impairment [75]. Finally, we were not able to address whether variables such as type of hysterectomy, duration and type of HT for prior users, cardiovascular history, and smoking history modulated response to HT as this information was not collected in our study.
In summary, our findings indicate favorable effects of short-term HT that includes estradiol on cognitive function in older postmenopausal women with AD. Future, larger trials to examine the cognitive effects of HT for older women with AD will be essential to further investigate the short-term therapeutic benefit of estradiol HT.
ACKNOWLEDGMENTS
The study utilized the resources of the School of Medicine and Public Health at the University of Wisconsin, UW Department of Medicine Division of Geriatrics and Gerontology, the Geriatric Research Education and Clinical Center (GRECC) of the William S. Middleton Memorial Veterans Hospital, Madison WI. This research was supported by NIH grants AG029624, K07AG021582, K23AG024302, K23AG026752, P50AG033514 and 1UL1RR025011.
GRECC Manuscript # 2009-08
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=862).
REFERENCES
- 1.Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci U S A. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEwen B, Alves S, Bulloch K, Weiland N. Ovarian steroids and the brain: Implications for cognition and aging. Neurology. 1997;48(7):8-S8–8-S15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: Effects on cognitive function and dementia. JAMA. 1998;279:688–695. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 4.Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24:657–677. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 5.Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, Plymate SR. High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology. 2001;57:605–612. doi: 10.1212/wnl.57.4.605. [DOI] [PubMed] [Google Scholar]
- 6.Henderson V, Paganini-Hill A, Emanuel C, Dunn M, Buckwalter J. Estrogen replacement therapy in older women. Comparisons between Alzheimer’s disease cases and nondemented control subjects. Arch Neurol. 1994;51:896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- 7.Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- 8.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson VW. Action of estrogens in the aging brain: Dementia and cognitive aging. Biochim Biophys Acta. 2010;1800:1077–1078. doi: 10.1016/j.bbagen.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 12.Wharton W, Dowling M, Khosropour CM, Carlsson C, Asthana S, Gleason CE. Cognitive benefits of hormone therapy: cardiovascular factors and healthy-user bias. Maturitas. 2009;64:182–187. doi: 10.1016/j.maturitas.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 14.Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 15.Henderson V. Estrogen replacement therapy for the prevention and treatment of Alzheimer’s disease. CNS Drugs. 1997;8:343–351. [Google Scholar]
- 16.Honjo H, Ogino Y, Tanaka K, Urabe M, Kashiwagi T, Ishihara S, Okada H, Araki K, Fushiki S, Nakajima K, Hayashi K, Hayashi M, Sakaki T. An effect of conjugated estrogen to cognitive impairment in women with senile dementia-Alzheimer’s type: A placebo-controlled, double-blind study. J Jpn Menopause Soc. 1993;1:167–171. [Google Scholar]
- 17.Wang PN, Liao SQ, Liu RS, Liu CY, Chao HT, Lu SR, Yu HY, Wang SJ, Liu HC. Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology. 2000;54:2061–2066. doi: 10.1212/wnl.54.11.2061. [DOI] [PubMed] [Google Scholar]
- 18.Modena MG, Sismondi P, Mueck AO, Kuttenn F, Lignieres B, Verhaeghe J, Foidart JM, Caufriez A, Genazzani AR. New evidence regarding hormone replacement therapies is urgently required transdermal postmenopausal hormone therapy differs from oral hormone therapy in risks and benefits. Maturitas. 2005;52:1–10. doi: 10.1016/j.maturitas.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Garza-Meilandt A, Cantu RE, Claiborne BJ. Estradiol’s effects on learning and neuronal morphology vary with route of administration. Behav Neurosci. 2006;120:905–916. doi: 10.1037/0735-7044.120.4.905. [DOI] [PubMed] [Google Scholar]
- 20.Scarabin P, Oger E, Plu-Bureau G group obotEaTEs. Differential association of oral and transdermal oestrogen replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428–432. doi: 10.1016/S0140-6736(03)14066-4. [DOI] [PubMed] [Google Scholar]
- 21.Birge S. The role of estrogen in the treatment of Alzheimer’s disease. Neurology. 1997;48:36-. S36–36-. S41. doi: 10.1212/wnl.48.5_suppl_7.36s. [DOI] [PubMed] [Google Scholar]
- 22.Henderson VW, Paganini-Hill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, McCleary CA, Klein RA, Hake AM, Farlow MR. Estrogen for Alzheimer’s disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- 23.Honjo H, Tanaka K, Kashiwagi T, Urabe M, Okada H, Hayashi M, Hayashi K. Senile dementia-Alzheimer’s type and estrogen. Horm Metab Res. 1995;27:204–207. doi: 10.1055/s-2007-979941. [DOI] [PubMed] [Google Scholar]
- 24.Caldwell B, Watson R. An evaluation of psychologic effects of sex hormone administration in aged women: Results after six months. J Gerontol. 1952;7:228–244. doi: 10.1093/geronj/7.2.228. [DOI] [PubMed] [Google Scholar]
- 25.Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Luo G, Guo ZJ, Cui RY, Wang LQ, Zhou CL. Quantitative evaluation of the intervention al effect of estrogen on Alzheimer’s disease. Chin J Clin Rehab. 2006;10:37–91. [Google Scholar]
- 27.Valen-Sendstad A, Engedal K, Stray-Pedersen B, Strobel C, Barnett L, Meyer N, Nurminemi M. Effects of hormone therapy on depressive symptoms and cognitive functions in women with Alzheimer disease: a 12 month randomized, double-blind, placebo-controlled study of low-dose estradiol and norethisterone. Am J Geriatr Psychiatry. 18:11–20. doi: 10.1097/JGP.0b013e3181beaaf4. [DOI] [PubMed] [Google Scholar]
- 28.Hogervorst E, Yaffe K, Richards M, Huppert FA. Hormone replacement therapy to maintain cognitive function in women with dementia. Cochrane Database Syst Rev. 2009:D003799. doi: 10.1002/14651858.CD003799.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asthana S, Baker L, Craft S, Lofgreen C, Avery E, Raskind M, Cherrier M, Wong M, Veith R, Plymate S. Estrogen-induced enhancement in memory in postmenopausal women with Alzheimer’s disease. Neurobiol Aging. 1998;19:748. Abstract # 748, page S178. [Google Scholar]
- 30.Hogervorst E, Yaffe K, Richards M, Huppert F. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst Rev. 2002:CD003122. doi: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawas C, Resnick S, Morrison A. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: The Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 32.Kantor H, Michael C, Shore H. Estrogen for older women. Am J Obstet Gynecol. 1973;116:115–118. doi: 10.1016/0002-9378(73)90894-6. [DOI] [PubMed] [Google Scholar]
- 33.Caldwell B. An evaluation of psychological effects of sex hormone administration in aged women. J Gerontol. 1954;9:168–174. doi: 10.1093/geronj/9.2.168. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 35.Wechsler D. A standardized memory scale for clinical use. J Psychology. 1945;19:87–95. [Google Scholar]
- 36.Corwin J, Bylsma F. Translations of excerpts from Andre Rey’s Psychological examination of traumatic encephalopathy and P.A. Osterrieth’s The Complex Figure Copy Test. Clin Neuropsychologist. 1993;7:3–15. [Google Scholar]
- 37.Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 38.Spreen O, Strauss E. A compendium of neuropsychological tests. New York: Oxford University Press; 1998. [Google Scholar]
- 39.Buschke H. Selective reminding for analysis of memory and learning. J Verb Learn Verb Behav. 1973;12:543–550. [Google Scholar]
- 40.Dodrill C. A neuropsychological battery for epilepsy. Epilepsia. 1978;19:611–623. doi: 10.1111/j.1528-1157.1978.tb05041.x. [DOI] [PubMed] [Google Scholar]
- 41.Stroop J. Studies of interference in serial verbal reactions. J Exp Psychology. 1935;18:643–662. [Google Scholar]
- 42.McNair DM, Lorr M, Droppleman LF. POMS manual. San Diego, CA: EdITS; 1981. [Google Scholar]
- 43.Sherwin B. Affective changes with estrogen and androgen replacement therapy in surgically menopausal women. J Affective Disord. 1988;14:177–187. doi: 10.1016/0165-0327(88)90061-4. [DOI] [PubMed] [Google Scholar]
- 44.Hosaka T, Sugiyama Y. Structured intervention in family caregivers of the demented elderly and changes in their immune function. Psychiatry Clin Neurosci. 2003;57:147–151. doi: 10.1046/j.1440-1819.2003.01094.x. [DOI] [PubMed] [Google Scholar]
- 45.Studd J, Panay N. Hormones and depression in women. Climacteric. 2004;7:338–346. doi: 10.1080/13697130400012262. [DOI] [PubMed] [Google Scholar]
- 46.Zweifel JE, O’Brien WH. A meta-analysis of the effect of hormone replacement therapy upon depressed mood. Psychoneuroendocrinology. 1997;22:189–212. doi: 10.1016/s0306-4530(96)00034-0. [DOI] [PubMed] [Google Scholar]
- 47.Carlson LE, Sherwin BB. Steroid hormones, memory and mood in a healthy elderly population. Psychoneuroendocrinology. 1998;23:583–603. doi: 10.1016/s0306-4530(98)00025-0. [DOI] [PubMed] [Google Scholar]
- 48.Kampen DL, Sherwin BB. Estradiol is related to visual memory in healthy young men. Behav Neurosci. 1996;110:613–617. doi: 10.1037//0735-7044.110.3.613. [DOI] [PubMed] [Google Scholar]
- 49.Albertazzi P, Natale V, Barbolini C, Teglio L, Di Micco R. The effect of tibolone versus continuous combined norethisterone acetate and oestradiol on memory, libido and mood of postmenopausal women: a pilot study. Maturitas. 2000;36:223–229. doi: 10.1016/s0378-5122(00)00147-x. [DOI] [PubMed] [Google Scholar]
- 50.Smith YR, Giordani B, Lajiness-O’Neill R, Zubieta JK. Long-term estrogen replacement is associated with improved nonverbal memory and attentional measures in postmenopausal women. Fertil Steril. 2001;76:1101–1107. doi: 10.1016/s0015-0282(01)02902-8. [DOI] [PubMed] [Google Scholar]
- 51.Sano M, Jacobs D, Andrews H, Bell K, Graff-Radford N, Lucas J, Rabins P, Bolla K, Tsai WY, Cross P, Andrews K, Costa R, Xiaodong L. A multi-center, randomized, double blind placebo-controlled trial of estrogens to prevent Alzheimer’s disease and loss of memory in women: design and baseline characteristics. Clin Trials. 2008;5:523–533. doi: 10.1177/1740774508096313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathibe LJ. Drop-out rates of cancer patients participating in longitudinal RCTs. Contemp Clin Trials. 2007;28:340–342. doi: 10.1016/j.cct.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Touloumi G, Babiker AG, Pocock SJ, Darbyshire JH. Impact of missing data due to drop-outs on estimators for rates of change in longitudinal studies: a simulation study. Stat Med. 2001;20:3715–3728. doi: 10.1002/sim.1114. [DOI] [PubMed] [Google Scholar]
- 54.Kristman V, Manno M, Cote P. Loss to follow-up in cohort studies: how much is too much? Eur J Epidemiol. 2004;19:751–760. doi: 10.1023/b:ejep.0000036568.02655.f8. [DOI] [PubMed] [Google Scholar]
- 55.Lohr S. Sampling: design and alanysis. In: SL L, editor. Nonresponse. Pacific Grove: Duxbury Press; 1999. pp. 255–287. [Google Scholar]
- 56.Altman DG. Statistics in medical journals: some recent trends. Stat Med. 2000;19:3275–3289. doi: 10.1002/1097-0258(20001215)19:23<3275::aid-sim626>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 57.Littell RMG, Stroup W, Wolfinger R. SAS System for Mixed Models. Cary, NC: 1996. [Google Scholar]
- 58.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 59.Knapp M, Knopman D, Solomon P, Pendlebury W, Davis C, Gracon S. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. JAMA. 1994;271:985–991. [PubMed] [Google Scholar]
- 60.Gleason CE, Cholerton B, CarlssonCM,Johnson SC, Asthana S. Neuroprotective effects of female sex steroids in humans: current controversies and future directions. Cell Mol Life Sci. 2005;62:299–312. doi: 10.1007/s00018-004-4385-z. [DOI] [PubMed] [Google Scholar]
- 61.Hu L, Yue Y, Zuo PP, Jin ZY, Feng F, You H, Li ML, Ge QS. Evaluation of neuroprotective effects of long-term low dose hormone replacement therapy on postmenopausal women brain hippocampus using magnetic resonance scanner. Chin Med Sci J. 2006;21:214–218. [PubMed] [Google Scholar]
- 62.Yue Y, Hu L, Tian QJ, Jiang JM, Dong YL, Jin ZY, Cheng YH, Hong X, Ge QS, Zuo PP. Effects of long-term, low-dose sex hormone replacement therapy on hippocampus and cognition of postmenopausal women of different apoE genotypes. Acta Pharmacol Sin. 2007;28:1129–1135. doi: 10.1111/j.1745-7254.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- 63.Bhavnani BR. Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer’s. J Steroid Biochem Mol Biol. 2003;85:473–482. doi: 10.1016/s0960-0760(03)00220-6. [DOI] [PubMed] [Google Scholar]
- 64.Stirone C, Boroujerdi A, Duckles SP, Krause DN. Estrogen receptor activation of phosphoinositide-3 kinase, akt, and nitric oxide signaling in cerebral blood vessels: rapid and long-term effects. Mol Pharmacol. 2005;67:105–113. doi: 10.1124/mol.104.004465. [DOI] [PubMed] [Google Scholar]
- 65.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- 66.Florian M, Lu Y, Angle M, Magder S. Estrogen induced changes in Akt-dependent activation of endothelial nitric oxide synthase and vasodilation. Steroids. 2004;69:637–645. doi: 10.1016/j.steroids.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe Y, Littleton-Kearney MT, Traystman RJ, Hurn PD. Estrogen restores postischemic pial microvascular dilation. Am J Physiol Heart Circ Physiol. 2001;281:H155–H160. doi: 10.1152/ajpheart.2001.281.1.H155. [DOI] [PubMed] [Google Scholar]
- 68.Grodstein F, Chen J, Pollen DA, Albert MS, Wilson RS, Folstein MF, Evans DA, Stampfer MJ. Postmenopausal hormone therapy and cognitive function in healthy older women. J Am Geriatr Soc. 2000;48:746–752. doi: 10.1111/j.1532-5415.2000.tb04748.x. [DOI] [PubMed] [Google Scholar]
- 69.Yaffe K, Lui L-Y, Grady D. Cognitive decline in women in relation to non-protein bound oestradiol concentrations. Lancet. 2000;356:708–712. doi: 10.1016/S0140-6736(00)02628-3. [DOI] [PubMed] [Google Scholar]
- 70.Bjorn I, Bixo M, Nojd KS, Nyberg S, Backstrom T. Negative mood changes during hormone replacement therapy: a comparison between two progestogens. Am J Obstet Gynecol. 2000;183:1419–1426. doi: 10.1067/mob.2000.107781. [DOI] [PubMed] [Google Scholar]
- 71.Kolber BJ, Muglia LJ. Defining brain region-specific glucocorticoid action during stress by conditional gene disruption in mice. Brain Res. 2009;1293:85–90. doi: 10.1016/j.brainres.2009.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Resnick SM, Espeland MA, AnY, Maki PM, Coker LH, Jackson R, Stefanick ML, Wallace R, Rapp SR. Effects of conjugated equine estrogens on cognition and affect in postmenopausal women with prior hysterectomy. J Clin Endocrinol Metab. 2009;94:4152–4161. doi: 10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faulkner DL, Young C, Hutchins D, McCollam JS. Patient noncompliance with hormone replacement therapy: a nationwide estimate using a large prescription claims database. Menopause. 1998;5:226–229. [PubMed] [Google Scholar]
- 74.Ettinger B, Pressman A, Silver P. Effect of age on reasons for initiation and discontinuation of hormone replacement therapy. Menopause. 1999;6:282–289. doi: 10.1097/00042192-199906040-00003. [DOI] [PubMed] [Google Scholar]
- 75.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]