Abstract
Corneal keratocyte migration can impact both corneal clarity and refractive outcome following injury or refractive surgery. In this study, we investigated how culture conditions, ECM properties, and Rho kinase activity regulate the mechanics of keratocyte migration, using a nested collagen matrix model. Time-lapse imaging demonstrated that both serum and PDGF stimulate keratocyte migration into the outer matrix. Although the velocity of cell migration was similar, cells in serum were bipolar and induced significant matrix deformation during migration, whereas PDGF induced extension of branching dendritic processes with smaller, more localized force generation. These differences in cell-induced matrix reorganization were verified with a global matrix contraction assay and confocal reflection imaging, using both bovine and rat tail collagen. When constructs were detached from the substrate to lower the effective stiffness, migration was significantly reduced in serum; but was unchanged in PDGF. These differences in migration mechanics were mediated, in part, by Rho kinase. Overall, corneal keratocytes can effectively migrate through collagen matrices using varying degrees of cellular force generation. Low-contractility migration may facilitate keratocyte repopulation of the stroma following surgery or injury, without altering the structural and mechanical properties that are critical to maintaining corneal transparency.
Keywords: Cell Mechanics, Corneal Keratocytes, Cell Migration, Rho kinase
INTRODUCTION
The cornea is an optically clear tissue that forms the front surface of the eye, and accounts for nearly two-thirds of its refractive power. The corneal stroma, which makes up 90% of corneal thickness, is a highly ordered structure consisting of approximately 200 collagen lamellae [1]. Each lamella consists of aligned type I collagen fibrils, with highly ordered packing and spacing that is critical to maintenance of corneal transparency. Corneal stromal cells (keratocytes) reside between the collagen lamellae, and are responsible for secreting ECM components required to maintain normal corneal structure and function. From a mechanical standpoint, resting keratocytes are considered quiescent; they do not express stress fibers or generate substantial contractile forces [2, 3].
Because it is directly exposed to environmental conditions, the cornea is susceptible to physical and chemical injuries. During wound healing, quiescent corneal keratocytes surrounding the area of injury generally become activated, and transform into a fibroblastic repair phenotype [4, 5]. These activated fibroblasts proliferate, migrate into the provisional matrix, and generate the forces required for wound closure. In certain wound types, fibroblasts further differentiate into myofibroblasts, which generate even stronger forces and are associated with scar formation [6, 7].
Because of its accessibility and optical power, the cornea is also the target for numerous refractive surgical procedures. Modern laser vision correction procedures, such as photorefractive keratectomy (PRK) and laser-assisted in situ keratomileusis (LASIK), reshape the corneal stroma using photoablation in order to achieve a desired change in refractive power. Both of these procedures induce keratocyte death in stromal tissue surrounding the area of photoablation [8]. While repopulation of these regions is desirable, the normal wound healing response can lead to fibrosis and scarring along the visual axis in a subset of patients, especially following PRK [9]. This can cause a permanent reduction in corneal clarity and a can also decrease the refractive effect of the surgery [9, 10].
In most physiological processes including corneal wound healing, cells migrate through 3-D tissue matrices. We recently developed a model for directly investigating cell-matrix mechanical interactions during migration in which cell-seeded compressed collagen matrices are nested within acellular uncompressed matrices [11, 12]. Compressed matrices can be generated rapidly, and have a geometry and mechanical properties similar to in vivo corneal tissue [13, 14]. In this study we use this model to investigate the mechanics of 3-D keratocyte migration following culture in serum or PDGF. Exogenous serum is commonly used to induce transformation of quiescent corneal keratocytes to a fibroblast phenotype. PDGF is endogenously expressed in corneal tear fluid, and has been shown to increase Rac activity and induce cell spreading and migration in both dermal and corneal fibroblasts [15, 16].
We demonstrate that both PDGF and 10% FBS stimulate increased keratocyte migration into the outer matrix as compared to basal media. However, whereas cells in 10% FBS assumed a bipolar morphology and induced significant matrix deformation during migration, cells in PDGF extended long dendritic processes and produced much smaller matrix deformations. To evaluate the effect of matrix mechanical properties on these two migratory phenotypes, we also studied migration in free floating (FF) nested matrices, which have a lower effective stiffness than matrices attached to the substrate (ATT) [17]. Migration of keratocytes into the outer matrix was significantly reduced in FF matrices as compared to ATT matrices in 10% FBS. In contrast, migration of keratocytes in PDGF was unaffected by changes in the matrix constraints. Rho kinase inhibition induced striking changes both in the mechanics of keratocyte migration in 10% FBS and its dependency on matrix constraints, but had little impact on the mechanical behavior of keratocytes in PDGF. Overall, the low contractility migration stimulated by PDGF may allow keratocytes to repopulate the corneal stroma without disrupting its unique and functional architecture.
MATERIALS AND METHODS
Cell Culture
Corneal keratocytes were isolated from rabbit eyes obtained from Pel Freez (Rogers, AR, USA) as previously described [3]. Cells were cultured in tissue culture flasks with basal medium consisting of DMEM (Invitrogen, Carlsbad, CA), supplemented with 1% RPMI vitamin mix (Sigma-Aldrich, St. Louis, MO), 100μM nonessential amino acids (Invitrogen, Carlsbad, CA), 100μg/ml ascorbic acid, and 1% penicillin/streptomycin amphotericin B (Fungizone; BioWhittaker, Inc., Walkersville, MD) to maintain the keratocyte phenotype [18].
Preparation of Nested Collagen Matrices
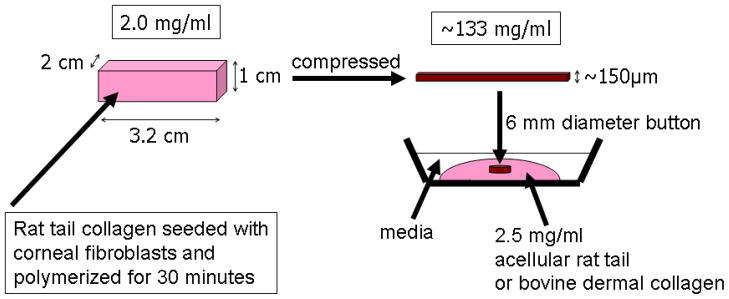
Nested collagen matrices were prepared as outline in Figure 1 [12]. Compressed collagen matrices were first prepared as described previously by Brown and coworkers [13, 14]. Briefly, 10mg/ml of Type I rat tail collagen (BD Biosciences, San Jose, CA) was diluted using 0.02N acetic acid to final concentration of 2mg/ml. After drop-wise neutralization with 1M sodium hydroxide, a suspension of 8×106 keratocytes in 0.6ml basal media was added to the collagen mixture. The solution containing cells and the collagen was poured into a 3x2x1cm stainless steel mould and allowed to set for 30 minutes at 37°C. In order to compact the matrices, a layer of nylon mesh (~50μm mesh size) was placed on a double layer of filter paper. The matrices were placed on the nylon mesh, covered with a pane of glass and loaded with a 130g stainless steel block for 5 minutes at room temperature. This process squeezes media out of the matrix and results in the formation of a flat, cell/collagen sheet with high mechanical stiffness.
Figure 1.
Procedure used for constructing nested matrix model. Reproduced from [12].
To produce the nested matrix, a 6 mm “button” was then punched from the compressed matrix and placed within acellular uncompressed outer collagen matrices [12]. Two different types of collagen were used as outer matrices; pepsinized bovine dermal collagen (Purecol; Inamed Corp., Fremont, CA) and non-pepsinized rat tail collagen (BD Biosciences, San Jose, CA). In both cases, hydrated acellular collagen matrices were prepared by mixing collagen with 10X DMEM to achieve a final collagen concentration of 2.5mg/ml. After adjusting the pH, 100μL of the collagen solution was poured on Bioptechs culture dishes (Bioptechs, Inc., Butler, PA). The compressed matrix button was then pushed into the middle of the solution, and the constructs were placed in a humidified incubator for 60 minutes to allow polymerization of the outer matrix. Constructs were then overlaid with 1.5ml of serum-free media. After 24 hours, media was replaced with either 10% FBS, PDGF BB (50 ng/ml, Millipore) or no growth factor (control) in basal media. In some experiments, media was also supplemented with 2mM thymidine (Sigma) in order to inhibit cell proliferation and/or Y-27632 (10 μM, EMD Chemicals) to inhibit Rho kinase. To investigate how mechanical signals regulate cell migration, nested matrices either remained attached to the bottom of the dish (ATT, attached) or were gently released from the bottom of the dish using a spatula (FF, free floating) following media replacement.
Time-lapse Digital Imaging
In order to assess the dynamics of cell migration, live-cell imaging of constructs was performed using a previously described Nikon TE200 inverted microscope equipped for time-lapse DIC imaging [19]. An environmental chamber (In Vivo Scientific, Valley Park, MO) was used to control temperature, humidity and CO2 during imaging. In each experiment, the dish was transferred to the microscopic stage after changing media to basal media (control), PDGF or 10% FBS. Migration activity was imaged for up to 48 hours using a 20X objective (non-immersion, 0.7 NA, 590μm free working distance). Images were acquired at 20–30 minute intervals. At each interval, a z-series of 20X DIC images was collected from the bottom to the top of the construct, at the edge of the inner matrices. This produced a 4-D dataset which captured the cell migration into the outer matrix. Experiments were performed three times for each condition.
In order to track cell migration, time-lapse sequences from single planes (one z-position) were extracted from the 4-D DIC datasets. In some experiments, there was a shift in the position of the edge of the inner matrix over the course of the experiment. The ‘Align Stack’ tool in MetaMorph software was used to correct for this shift prior to quantitative analysis. The nuclei of cells migrating from the inner to outer matrices were manually tracked using the ‘track points’ module of MetaMorph, and the X–Y coordinates were logged into an Excel file. A custom written “C” program then generated tracks of cell movement over time. To quantify the movement of cells, directional persistence and velocity were used. For directional persistence during migration, T and D were used, where T is the total distance cell traveled and D is the linear distance from starting to end point. D/T is equal to 1 for cells that move along a straight line without changing direction. A value less than 1 indicates that the cell path was more random. The velocity of migrating cells was assessed by calculating the total distance a cell traveled divided by the total time.
Laser Confocal Microscopy
In order to quantify the number of cells in the outer matrix and assess cell-induced matrix remodeling, confocal fluorescence and reflection microscopy were used. Following 4 days of culture, nested constructs were fixed using 3% paraformaldehyde in phosphate buffer for 10 min and permeabilized with 0.5% Triton X-100 in phosphate buffer for 3min. Cells were labeled with Alexa Fluor 546 Phalloidin (1:50; Molecular Probes, Eugene, OR) for 1 hour and then washed in phosphate buffer saline (PBS; 3 times for 5 minutes). TOTO-3 iodide (1:200; Molecular Probes, Eugene, OR) was then added to each construct to stain the cell nuclei. Constructs were then incubated for 15 minutes and washed with PBS (3 times for 5 minutes). All conditions were studied in parallel, and experiments were repeated 3 times.
After labeling with F-actin and nuclei, fluorescent (for f-actin and nuclei) and reflection (for collagen fibrils) images were acquired simultaneously using laser confocal microscopy (Leica SP2, Heidelberg, Germany). A stack of optical sections (z-series) was acquired for each area imaged by changing the position of the focal plane in 10μm steps using a 20X objective (non-immersion, 0.7 NA, 590μm free working distance) or 1μm steps using a 63X objective (water immersion, 1.2 NA, 220μm free working distance).
Maximum intensity projection (MIP) images of TOTO-3 image stacks were created using Metamorph and overlaid with reflection images. Photoshop was then used to stitch together these overlapped images, resulting in a 750 μm wide montage image for each quadrant (Supplemental Figure 1). As an index of cell migration, the average number of cells per 750μm wide region that had migrated out of inner compressed matrix was counted. Each field included the border of the inner matrix (detected by reflected light) and the furthest moving cells (detected by nuclei staining with TOTO-3). The distance that cells had traveled was also calculated by drawing a straight line between the interface and the leading edge cells – an average of 10 cells was used for each montage
Standard (Un-Nested) Matrix Contraction Assay
Hydrated collagen matrices were prepared as previously described [3]. Neutralized bovine dermal collagen (Purecol; Inamed, Fremont, CA) was mixed with 10X DMEM to achieve a final collagen concentration of 2.5 mg/ml. A 50 μl of suspension of cells was then mixed with the above collagen solution. The cell/collagen mixture was preincubated at 37°C for 4 min and 30μl aliquots were then spread over a central 12 mm diameter circular region on Bioptechs culture dishes. The dishes were then placed in a humidified incubator for 60 min for polymerization. The matrices were overlaid with 1.5 ml of basal media (serum-free). After 24 hours of incubation the media was changed to basal media or basal media supplemented with 10%FBS or 50ng/ml PDGF. Matrices with both low cell density (2 × 103 cells/matrix) and higher cell density (5 × 104 cells/matrix) were used.
DIC imaging was used to measure global matrix contraction of high cell density matrices. Since the bottom of the matrix remains attached to the dish, cell induced contraction results in a decrease in matrix height [15]. Height was measured by focusing on the top and bottom of each matrix at 6 different locations at 0, 1 and 4 days after plating. Measurements were performed in triplicate for each condition, and repeated 3 times. The percentage decrease in matrix height over time was then calculated. In addition, local cell/matrix interactions were assessed in low density matrices using laser confocal microscopy as described above.
Statistics
All statistical analyses were performed using SigmaStat version 3.11 (Systat Software Inc., Point Richmont, CA). For parametric data, one- and two-way analysis of variance (ANOVA) was used to compare group means. Post-hoc multiple comparisons between groups were performed using the Holm–Sidak method. For non-parametric data, Kruskal-Wallis ANOVA on ranks was used, and post-hoc multiple comparisons were made using Dunn’s Method. Differences were considered significant if P < 0.05.
RESULTS
Effect of Culture Conditions on Migratory Mechanics
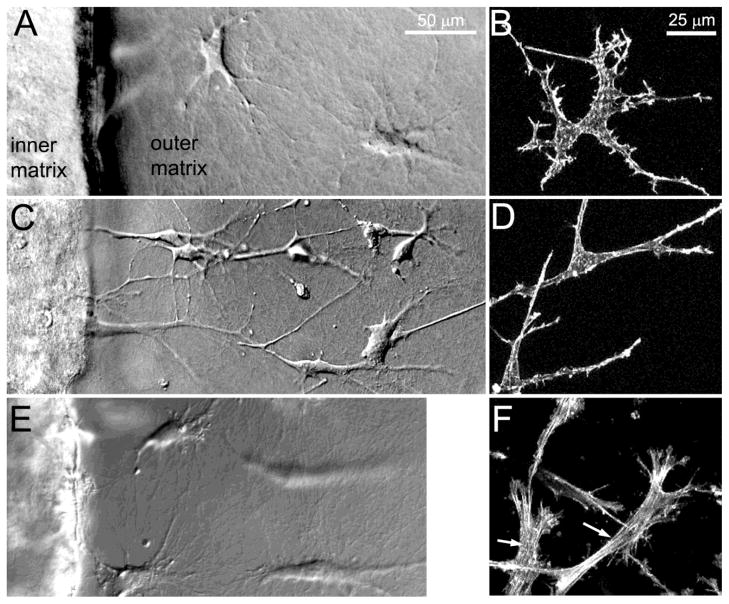
As a model for assessing cell migration, corneal keratocytes were embedded in a compressed inner matrix, which was placed within in an uncompressed acellular outer matrix. Both PDGF and 10% FBS stimulated increased migration into the outer matrix as compared to basal media. However, the morphology and cytoskeletal organization of migrating cells varied substantially depending on the culture conditions used. Corneal keratocytes in basal media assumed a stellate morphology with thin cell processes as they migrated into the outer matrix, consistent with their morphology in normal corneas in vivo (Figure 2A, B). PDGF induced elongation of keratocytes and the extension of numerous dendritic cell processes (Figure 2C, D). In contrast, culture in 10% FBS led to a more polarized, fibroblastic morphology (Figure 2E, F), and the development of intracellular stress fibers (Figure 2F, arrows).
Figure 2.
A, C, E DIC images showing corneal keratocytes migrating from the inner (left) to outer (right) matrix, following 24 hours of culture in basal media (A), PDGF (C) or 10% FBS (E). B, D, F: Maximum intensity projection images of f-actin labeling following culture in basal media (B), PDGF (D) and 10% FBS (F). Corneal keratocytes in basal media assumed a stellate morphology with thin cell processes, consistent with their morphology in normal corneas in vivo. PDGF induced elongation of keratocytes and the extension of numerous dendritic cell processes. In contrast, culture in 10% FBS led to a more polarized, fibroblastic morphology, and the development of intracellular stress fibers.
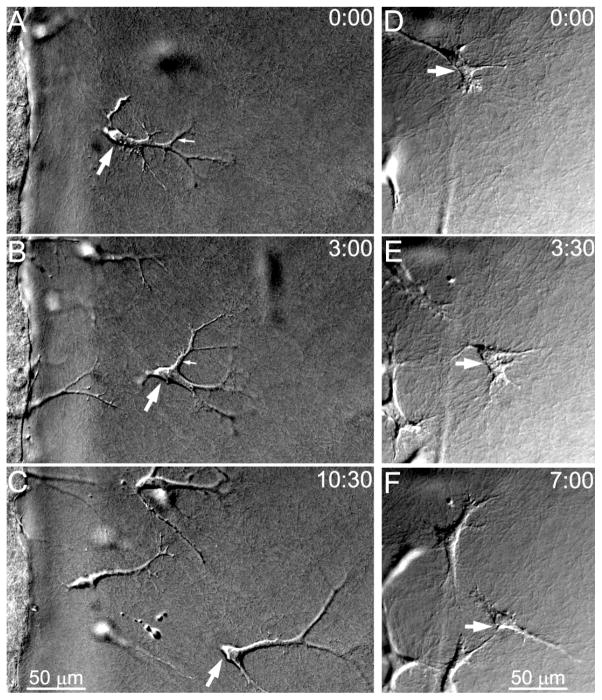
In addition to these morphological changes, time-lapse imaging revealed that the mechanics of cell migration also varied substantially depending on the culture conditions used. Migrating cells in PDGF repeatedly extended and retracted long, thin dendritic extensions while moving through the outer matrix (Figure 3A–C). Small tractional forces were generated at the tips of these branching processes as indicated by inward movement of collagen fibrils (Supplemental Video 1). Dendritic processes generally formed in front of the cell body (Figure 3A, small arrow), progressively elongated (Figure 3B, small arrow), and retracted as the cell body slid past them (Figure 3C). In contrast, migrating cells in 10% FBS developed a more bipolar, fibroblastic morphology with broader pseudopodial processes (Figure 3D–F). These cells generated larger, more sustained forces which deformed and pulled in the surrounding ECM (Supplemental Video 2). Pseudopodial processes were often present at both the front and rear of the cells, and cells made more dramatic changes in direction during migration than those observed in PDGF.
Figure 3.
Time-lapse DIC images of cell migration into bovine collagen outer matrices during culture in PDGF (A–C) or 10% FBS (D–F). A–C. PDGF induced repeated extension and retraction of long dendritic processes as cells migrated. Cells appeared to glide through the ECM without producing large deformations (See Supp Video 1). D–F. In 10% FBS, cells had thicker processes and were less elongated, and much stronger cell-matrix interactions were observed (See Supp Video 2). Large arrow points to same cell in each frame. Times in upper right corner show hours:minutes after starting time lapse imaging.
Figure 4 shows migration paths which were generated by tracking individual cells over 48 hours of time-lapse imaging. Cells in 10% FBS appeared to migrate in more convoluted paths than cells in PDGF (Figure 4A&B). To quantitatively analyze the movement of cells based on the tracking results, velocity and directional persistence (D/T) were measured. PDGF and 10% FBS both stimulated more rapid cell migration through the outer matrix as compared to basal media (Figure 4C). Although the velocity of cell migration was similar, PDGF induced more directional persistence during migration as compared to 10% FBS (Figure 4D). Cells in 10% FBS appeared to interact during cell migration; that is, the matrix compaction and deformation produced by one cell induced directional changes in neighboring cells (Supplemtnal Video 2). These mechanical interactions may have contributed to the more convoluted pattern of migration.
Figure 4.

Quantitative analysis of cell movement. A, B. Migration tracks from one experiment for cells cultured in PDGF (A) or 10% FBS (B). C. Velocity of cell migration. PDGF and 10% FBS both stimulated more rapid cell migration into the outer matrix as compared to basal media (n = 3 experiments per condition). D. PDGF induced more directional persistence (D/T) during migration as compared to 10% FBS (n= 3 experiments per condition).
To quantify the apparent differences in cellular force generation under different culture conditions, a global matrix contraction assay was used. Cell-induced matrix contraction was significantly greater in 10% FBS as compared to PDGF or basal media, after both 24 hours and 4 days of culture (Figure 5A). In order to assess the local pattern of cell-matrix interactions, confocal microscopy was performed. Consistent with previous observations, keratocytes in basal media developed a stellate morphology in 3-D matrices (Figure 5B), with numerous cell processes extending in all directions from the cell body [3]. Keratocytes had a cortical, membrane associated f-actin organization, with more concentrated labeling near the ends of cell processes. In contrast, cells cultured in 10% FBS generally developed a more polarized morphology with thicker pseudopodial processes (Figure 5C). Following culture in PDGF, cells developed long dendritic processes, and stress fibers were not observed (Figure 5D). In general, no significant compaction or realignment of collagen fibrils was observed surrounding cells cultured in basal media or PDGF. In contrast, collagen at the ends of pseudopodial processes in 10% FBS appeared to be compacted and aligned parallel to the ends of pseudopodial processes. These results confirm that keratocytes in 10% FBS generate much larger forces on the matrix than cells in basal media or PDGF.
Figure 5.
Keratocyte mechanical behavior in un-nested collagen matrices. A. Global matrix contraction assay. Cell-induced matrix contraction (decrease in matrix height) was significantly greater in 10% FBS as compared to PDGF or basal media, after both 24 hours and 4 days of culture. B–D. Day four overlays of phallodin (green) and collagen (red) obtained using confocal fluorescence and reflection imaging, respectively. Corneal keratocytes developed a stellate morphology in 3-D matrices (B), with a cortical, membrane associated f-actin organization. In contrast, cells cultured in 10% FBS generally developed a more polarized morphology with thicker pseudopodial processes (C). Following culture in PDGF, cells developed long dendritic processes (D). In general, minimal compaction and realignment of collagen fibrils was observed surrounding cells cultured in basal media or PDGF. In contrast, collagen at the ends of cells in 10% FBS appeared to be compacted and aligned parallel to the ends of pseudopodial processes. (* - Significantly greater than all other conditions)
Effect of Mechanical Constraints on 3-D Cell Migration
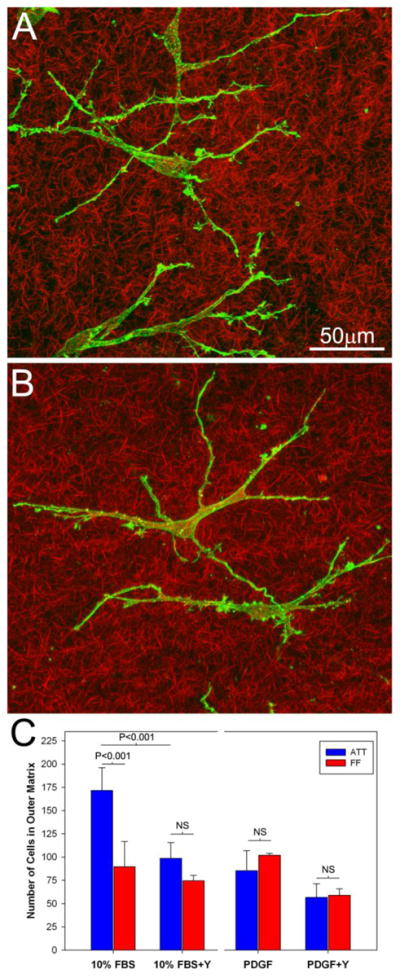
To assess how mechanical signals regulate cell migration, some constructs were released from the bottom of the dish after polymerization. This reduces the effective mechanical stiffness of the outer matrix, by eliminating the fixed constraint which resists displacement [17]. Since time-lapse imaging cannot be performed on floating matrices, constructs were fixed and labeled for static imaging using confocal microscopy. Consistent with the results from time-lapse imaging, PDGF and 10% FBS stimulated more migration into the outer matrix than basal media in both attached (ATT) and free-floating (FF) conditions, as indicated by both the numbers of cells and the distance of the leading edge of the migratory front (Figure 6A&B). Interestingly, reducing the effective stiffness of the outer matrix resulted in a significant decrease in cell numbers and migration distance in 10% FBS, but had no apparent impact on cells in PDGF. Similar to in vivo wound healing, cell counts in the outer matrix likely reflect a combination of both migration and proliferation, particularly in 10% FBS. However, altering matrix constraints produced a similar pattern of changes even when proliferation was inhibited by using a thymidine block (Fig. 6C).
Figure 6.
Comparison of cell migration in attached (ATT) and free-floating (FF) nested matrices. A. Maximum intensity projection images of collagen (red) and TOTO (green) collected after 4 days of culture in basal media, PDGF and 10% FBS under ATT and FF conditions (bovine dermal collagen was used for outer matrices). Green: TOTO-3. Red: collagen. B. Effect of matrix constraints on the number of cells in the outer matrix (left) and the distance from the interface of inner-outer matrix to the cells at the leading edge of the migratory front (right), after 4 days of migration. Changing matrix constraints impacts cell migration in 10% FBS, but not PDGF. C. The effect of changing matrix constraints produced a similar reduction in 10% FBS when proliferation was inhibited using a thymidine block. D. Comparison of cell migration in attached (ATT) and free-floating (FF) nested matrices after 4 days of culture, with rat tail collagen used in the outer matrix. Results were similar to that observed in bovine collagen (compare to A).
We also compared the local cell-induced matrix reorganization produced during migration in ATT and FF conditions (Figure 7). In basal media, collagen fibrils appeared to be randomly oriented without significant cell-induced compaction or alignment in both ATT and FF conditions (Figure 7A and B). When cultured in PDGF, slight compaction of collagen fibrils both around and between cells was sometimes observed (Figure 7C and D). Following culture in 10% FBS, significant cell-induced collagen reorganization was observed in both ATT and FF conditions. Collagen fibrils were generally compacted and aligned parallel to the long axis of pseudopodia (Figure 7E and F). Under FF conditions, cells and collagen both became aligned parallel with the outer edge of the inner matrix (Figure 7F, white arrows). This alignment was even more pronounced after 7 days in 10% FBS (Supplemental Figure 2).
Figure 7.
F-actin (green) and collagen (red) overlays of single optical sections taken at the interface of nested collagen matrices using confocal microscopy after 4 days of culture. In basal media, collagen fibrils appeared to be randomly oriented without significant cell-induced compaction or alignment in both ATT (A) and FF (B) conditions. When cultured in PDGF, compaction of collagen fibrils both around and between cells was rarely observed in either ATT (C) and FF (D) conditions. Following culture in 10% FBS, significant cell-induced collagen reorganization was observed in both ATT (E) and FF (F) conditions. Under FF conditions, cells and collagen both became aligned parallel with the outer edge of the inner matrix (F, white arrows). Because processes in basal media and PDGF extend 3-dimensionally, only small portions of the cells are seen in these single optical sections. Cells in 10% FBS were aligned more parallel with the x–y plane, thus entire cells are observed.
Effect of Matrix Composition on Cell Migration
Consistent with previous studies by others [20], confocal reflection imaging in our model demonstrated that non-pepsinized rat tail collagen forms shorter fibrils, and has smaller pores and a higher fiber density as compared to pepsin-extracted bovine collagen (Supplemental Figure 3). However, when rat tail collagen was used as an outer matrix, the pattern and amount of cell migration for the different conditions studied was nearly identical to that measured in bovine collagen (Figure 6D). Furthermore, time-lapse imaging showed that the mechanics of cell migration was unchanged, with 10%FBS inducing a fibroblastic migration phenotype and PDGF inducing a dendritic, non-contractile migration mechanism (Supplemental Video 3). As in bovine collagen, confocal imaging confirmed that there was significant reorganization of rat tail collagen observed in response to 10% FBS, but not in PDGF or basal media (Supplemental Figure 4). These differences are best appreciated in movies showing maximum intensity projections over a range of projection angles (Supplemental Videos 4 and 5).
Effect of Rho Kinase on Keratocyte Migration Mechanics
In order to determine the role of Rho kinase in regulating keratocyte migration mechanics, we used the established Rho kinase inhibitor Y-27632. Overall, the mechanics of cell migration in PDGF+Y-27632 appeared to be similar to that observed in PDGF alone. Keratocytes formed dendritic processes during migration, and collagen fibrils appeared to be randomly oriented without significant cell-induced compaction or alignment (Figure 8A). Furthermore, keratocytes continued to repeatedly extend and retract dendritic extensions while moving through the outer matrix while generating only small, localized tractional forces (Supplemental Video 6). Although not statistically significant, the number of cells in the outer matrix after 4 days was somewhat reduced in the presence of Y-27632. However, no difference in the number of cells that invaded the outer matrix was detected between ATT and FF conditions, similar to culture in PDGF alone (Figure 8C).
Figure 8.
Effect of Rho kinase inhibition on 3-D cell migration. A. Maximum intensity projection of f-actin (green) and collagen fibrils (red) following 4 days of migration in media containing PDGF + Y-27632. Keratocytes formed dendritic processes similar to those observed in PDGF alone. Collagen fibrils appeared to be randomly oriented without significant cell-induced compaction or alignment. B. Maximum intensity projection of f-actin (green) and collagen fibrils (red) following 4 days of migration in media containing 10%FBS + Y-27632. Unlike cells in 10% FBS alone, keratocytes formed thin dendritic processes and did not induce substantial matrix compaction and alignment. C. Effect of Rho kinase inhibition on keratocyte migration numbers under ATT and FF conditions.
In contrast to PDGF, Rho kinase inhibition induced striking changes in the mechanics of migration in 10% FBS. Unlike cells in 10% FBS alone, keratocytes formed long, thin processes and did not produce substantial matrix compaction and alignment (Figure 8B). During migration, these dendritic processes generated tractional forces at the leading edge of cells (Supplemental Video 7) that were smaller and more transient than those produced in 10% FBS alone. Furthermore, following Rho kinase inhibition, the difference in cell invasion between ATT and FF conditions was eliminated (Figure 8C).
DISCUSSION
Migration of corneal keratocytes is involved in matrix patterning during developmental morphogenesis and is also required for repopulation of corneal tissue following wounding due to injury or surgery [21, 22]. Following lacerating injury or incisional surgery, contractile force generation is needed to facilitate wound closure and prevent loss of the mechanical integrity of the cornea. However, following refractive surgical procedures such as PRK or LASIK, it is preferable to minimize cellular force generation and fibrosis during stromal repopulation, since these processes can alter corneal shape and transparency. Such non-disruptive stromal repopulation is also needed following UV cross-linking of the cornea, which is increasingly used as a treatment for keratoconus, since this procedure kills corneal keratocytes in the area of treatment. In order to identify the growth factor environment that could stimulate non-disruptive keratocyte migration, a model that allows cells to be studied in their natural quiescent state is required. In this study, we used a model for investigating cell-matrix mechanical interactions during migration in which cell-seeded compressed collagen matrices are nested within acellular uncompressed matrices [11, 12]. An important feature of this model is that quiescent (non-contractile) cells can be used in the inner matrix, thus the effects of specific growth factors and their downstream signaling pathways can be assessed without pre-exposure to serum or other factors which may permanently alter these responses [18].
In our original nested matrix model, there was a delay of 2–3 days before cells invaded the outer matrix, thus cell migration was investigated after 7 days of culture using only static confocal imaging. Using this approach, we demonstrated that corneal keratocytes cultured in PDGF develop an elongated morphology when migrating in 3-D collagen matrices, but did not produce large changes in matrix reorganization [12]. In contrast, keratocytes cultured in 10% FBS became fibroblastic and produced significant compaction and alignment of ECM. In the current study we directly assessed the underlying mechanics of keratocyte migration under these culture conditions for the first time, using modifications to our model that facilitate earlier invasion and allow time-lapse DIC imaging [19]. We also evaluate the effects of matrix composition, effective mechanical stiffness, and Rho kinase activity on the migratory process.
Effects of Serum and PDGF on Keratocyte Migration Mechanics
Both PDGF and 10% FBS stimulated more rapid cell migration into the outer matrix as compared to basal media. Interestingly, although the velocity of cell migration was similar, the mechanics of cell motility were strikingly different. Cells in 10% FBS assumed a fibroblastic morphology and generated significant tractional forces, as indicated by pulling in of the matrix at the leading edge of migrating cells. In contrast, PDGF induced repeated extension and retraction of branching dendritic processes at the leading edge with smaller, more localized matrix deformations. A significant increase in cell-induced matrix reorganization in 10% FBS was confirmed quantitatively using a global matrix contraction assay. In contrast, both global and local ECM reorganization induced by PDGF were comparable to that produced by quiescent keratocytes in basal media.
Overall, the migration pattern observed in 10% FBS is consistent with the migratory phenotype used by other fibroblastic cells, which is generally associated with high levels of cell attachment and cytoskeletal contractility [23]. In contrast, keratocytes in PDGF did not produce strong forces or develop a fibroblastic morphology. Instead, corneal keratocytes developed an elongated morphology during migration with branching dendritic cell processes at the leading edge. These dendritic processes were dynamic; new processes generally formed in front of the cell body, progressively elongated and formed branches, and retracted as the cell body slid past them. Dermal fibroblasts form similar “dendritic” cell processes when contractility is blocked by inhibiting myosin II [24] and corneal fibroblasts develop dendritic processes in response to Rho kinase inhibition [25]. Thus the dendritic morphology is a hallmark of cells in a low tension environment [26].
Effect of Mechanical Constraints on Migration in Serum and PDGF
It is well established that mechanical stimuli play a key role in regulating growth and function in a variety of cell types. In the cornea, large shifts in the global distribution of ECM tension can be induced by lacerating injury, penetrating keratoplasty, or refractive surgery [10]. In addition, the provisional wound healing matrix is less dense, more disorganized and more compliant than normal corneal tissue. Thus the response of corneal fibroblasts to changes in the ECM composition, stress and stiffness may play an important role in both the acute and long-term clinical responses to injury or surgery. To evaluate the effect of matrix mechanical properties on the keratocyte migratory phenotypes, we also compared migration in attached and free floating nested matrix constructs. During migration, tractional forces result in both cell and matrix translocation (cells move forward as matrix is pulled backward). Previous studies have shown that for dermal fibroblasts, cell translocation predominates in attached nested matrices, where contractile forces can be propagated through the matrix to the underlying restraining surface. In floating nested matrices, where there is no fixed constraint against which to pull, matrix translocation predominates and cell migration is significantly reduced [27]. Consistent with these studies, invasion of keratocytes into the outer matrix in 10% FBS was significantly reduced in floating matrices as compared to attached matrices. In contrast, invasion of keratocytes in PDGF was unaffected by changes in the matrix constraints. When Rho kinase was inhibited in 10% FBS, the dependency of cell invasion on matrix constraints was eliminated, further suggesting that migration of cells with lower contractility is less sensitive to ECM stiffness.
Differences in the pattern of cell alignment were also observed between ATT and FF matrices. Under FF conditions, cells and collagen both became aligned parallel with the outer edge of the inner matrix in 10% FBS, whereas cells were more randomly aligned in ATT conditions. Interestingly, a similar pattern of cell alignment is observed during corneal wound contraction following incisional surgery. In these studies a force-balance analysis predicts that contractile forces pull cells into alignment with the wound edge, due to its increased stiffness as compared to the provisional wound ECM [28, 29]. For fibroblastic cells, focal contacts and stress fibers generally align along the tensile axis under anisotropic conditions [30–32]. Similarly, in 3-D matrices pre-fabricated with directional gradients in collagen density, fibroblasts migrate towards the stiffer region [33], a phenomenum called “durotaxis” [34]. It has recently been suggested that durotaxis may be unique to contractile cells [17]. Consistent with this hypothesis, cells in PDGF did not align parallel to the matrix interface, even under free floating conditions.
Dynamic mechanical feedback between cells and matrices can also occur in 3-D culture, due to time-dependent ECM remodeling. For example, as tumour cells migrate from tissue explants into 3-D matrices, they exert ROCK-dependent tractional forces which progressively compact and align surrounding collagen fibrils, providing increased rigidity and contact guidance for other cells [35]. This type of positive feedback loop between cells and ECM likely plays a role in regulating differentiation and patterning during embryonic development, as well as remodeling during wound healing [36]. In the current study, cells in 10% FBS also appeared to interact mechanically during cell migration; that is, the matrix compaction and deformation produced by one cell induced directional changes in neighboring cells. Perhaps as a result of these mechanical interactions, cells in 10% FBS migrated in more convoluted paths than cells in PDGF.
3-D culture models typically use either bovine dermal collagen (which is pepsinized), or rat tail tendon collagen (which is not pepsinized). Pepsin treatment reductively cleaves cross-link mediating telopeptides from collagen monomers, which alters the structural and mechanical properties of re-assembled collagen matrices [20]. Recent studies have shown that both the mechanics and protease dependency of migration by certain tumor cell lines is impacted by the type of collagen used [20, 37, 38]. Interestingly, the morphological, migratory and mechanical responses of corneal keratocytes were remarkably similar for these two matrix types, with serum inducing a contractile phenotype and PDGF inducing a non-contractile phenotype in both cases. For both matrix types, migration in serum was reduced under free floating conditions, but migration in PDGF was unaffected by changing matrix constraints.
Effect of Rho Kinase Inhibition of Keratocyte Migration Mechanics
For a cell to migrate, at least two distinct types of force must be generated: the protrusive force needed to extend lamellipodia or filopodia at the leading edge, and the tractional force needed to overcome the adhesive interactions between the cell and matrix. We previously demonstrated that while most of the large tractional forces generated by corneal fibroblasts are Rho-kinase/myosin II dependent, smaller forces can be generated at the tips of pseudopodia that are not produced by actomyosin contraction [16]. Thus we speculated that keratocyte migration in 10% FBS would be mediated primarily by Rho-kinase dependent actomyosin contractile force generation; whereas migration in PDGF may be mediated by a Rho kinase-independent mechanism. Consistent with this hypothesis, Rho kinase inhibition induced striking changes in both the mechanics of keratocyte migration in 10% FBS and its dependency on matrix constraints, but had little impact on the mechanical behavior of keratocytes in PDGF.
Although the velocity of cell migration and distance to the migratory front were similar for cells cultured in serum and PDGF, the total number of cells invading the outer matrix was higher for serum. One possible explanation is that increased contractility enhances the ability of cells to pull themselves out of the inner matrix. Our finding that Rho kinase inhibition reduces keratocyte invasion of the outer matrix is consistent with this possibility.
Taken together, the results of this study demonstrate that corneal keratocytes can effectively migrate through 3-D collagen matrices using both a high-contractility mechanism stimulated by serum, and a low-contractility mechanism stimulated by PDGF. Low-contractility migration in PDGF was consistently observed in both pepsinized and non-pepsinized matrices, and was not impacted by altering the mechanical constraints of the constructs. From a clinical standpoint, this may facilitate keratocyte repopulation of the stroma following surgery or injury, without altering the structural and mechanical properties that are critical to maintaining corneal clarity.
Supplementary Material
Acknowledgments
This study was supported in part by NIH R01 EY 013322, NIH P30 EY020799, and an unrestricted grant and Senior Scientific Investigator Award (WMP) from Research to Prevent Blindness, Inc., NY, NY. The authors wish to thank Dr. Dwight Cavanagh for his helpful comments and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pepose JS, Ubels JL. The Cornea. In: Hart WM, editor. Adler’s Physiology of the Eye. Mosby Year book; St. Louis: 1992. pp. 29–70. [Google Scholar]
- 2.Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: In situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994;35:730–743. [PubMed] [Google Scholar]
- 3.Lakshman N, Kim A, Petroll WM. Characterization of corneal keratocyte morphology and mechanical activity within 3-D collagen matrices. Exp Eye Res. 2010;90:350–359. doi: 10.1016/j.exer.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of the myofibroblast. Prog Retinal Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 5.Stramer BM, Zieske JD, Jung J-C, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- 6.Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999;40:1959–1967. [PubMed] [Google Scholar]
- 7.Blalock TD, Duncan MR, Varela JC, Goldstein MH, Tuli MH, Grotensdorst GR, Schultz GS. Connective tissue growth factor expression and action in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2003;44:1879–1887. doi: 10.1167/iovs.02-0860. [DOI] [PubMed] [Google Scholar]
- 8.Wilson SE. Analysis of the keratocyte apoptosis, keratocyte proliferation, and myofibroblast trasnformation responses after photorefractive keratectomy and laser in situ keratomileusis. Trans Am Ophthalmol Soc. 2002;100:411–433. [PMC free article] [PubMed] [Google Scholar]
- 9.Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: A 1-year confocal microscopic study. Ophthalmology. 2000;107:1235–1245. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 10.Dupps WJ, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006;83:709–720. doi: 10.1016/j.exer.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karamichos D, Lakshman N, Petroll WM. An experimental model for assessing fibroblast migration in 3-D collagen matrices. Cell Motil Cytoskeleton. 2009;66:1–9. doi: 10.1002/cm.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim A, Lakshman N, Karamichos D, Petroll WM. Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Invest Ophthalmol Vis Sci. 2010;51:864–875. doi: 10.1167/iovs.09-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RA, Wiseman M, Chuo C-B, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv Funct Mater. 2005;15:1762–1770. [Google Scholar]
- 14.Neel EAA, Cheema U, Knowles JC, Brown RA, Nazhat SN. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter. 2006;2:986–992. doi: 10.1039/b609784g. [DOI] [PubMed] [Google Scholar]
- 15.Grinnell F. Fibroblast-collagen matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- 16.Petroll WM, Ma L, Kim A, Ly L, Vishwanath M. Dynamic assessment of fibroblast mechanical activity during Rac-induced cell spreading in 3-D culture. J Cell Physiol. 2008;217:162–171. doi: 10.1002/jcp.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010;26:335–361. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- 18.Jester JV, Chang J-H. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Petroll WM. Rho Kinase Regulation of Fibroblast Migratory Mechanics in Fibrillar Collagen Matrices. Cell Mol Bioeng. 2010;3:76–83. doi: 10.1007/s12195-010-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Sem Cell Dev Biol. 2009;20:931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomasek JJ, Hay ED, Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: Distribution of actin, α-actinin and myosin. Dev Biol. 1982;92:107–122. doi: 10.1016/0012-1606(82)90155-5. [DOI] [PubMed] [Google Scholar]
- 22.Netto MV, Mohan RR, Ambrosio R, Hutcheon AEK, Zieske JD, Wilson SE. Wound healing in the cornea: A review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- 23.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2009;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee S, Jiang H, Ho C-H, Grinnell F. Microtubule function in fibroblast spreading is modulated according to the tension state of cell-matrix interactions. Proc Natl Acad Sci. 2007;104:5425–5430. doi: 10.1073/pnas.0608030104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim A, Lakshman N, Petroll WM. Quantitative assessment of local collagen matrix remodeling in 3-D culture: the role of Rho kinase. Exp Cell Res. 2006;312:3683–3692. doi: 10.1016/j.yexcr.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grinnell F, Ho C-H, Tamariz E, Lee DJ, Skuta G. Dendritic fibroblasts in three-dimensional collagen matrices. Mol Biol Cell. 2003;14:384–395. doi: 10.1091/mbc.E02-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grinnell F, Rocha LB, Iucu C, Rhee S, Jiang H. Nested collagen matrices: A new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res. 2006;312:86–94. doi: 10.1016/j.yexcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Petroll WM, Cavanagh HD, Barry-Lane P, Andrews P, Jester JV. Quantitative analysis of stress fiber orientation during corneal wound contraction. J Cell Sci. 1993;104:353–363. doi: 10.1242/jcs.104.2.353. [DOI] [PubMed] [Google Scholar]
- 29.Petroll WM, Cavanagh HD, Jester JV. Assessment of stress fiber orientation during healing of radial keratotomy wounds using confocal microscopy. Scanning. 1998;20:74–82. doi: 10.1002/sca.1998.4950200202. [DOI] [PubMed] [Google Scholar]
- 30.Kolodney MS, Wysolmerski RB. Isometric contraction by fibroblasts and endothelial cells in tissue culture: A quantitative study. J Cell Biol. 1992;117:73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takakuda K, Miyairi H. Tensile behavior of fibroblasts cultured in collagen gel. Biomaterials. 1996;17:1393–1397. doi: 10.1016/0142-9612(96)87280-2. [DOI] [PubMed] [Google Scholar]
- 32.Wakatsuki T, Elson EL. Reciprocal interactions between cells and extracellular matrix during remodeling of tissue constructs. Biophys Chem. 2003;100:593–605. doi: 10.1016/s0301-4622(02)00308-3. [DOI] [PubMed] [Google Scholar]
- 33.Hadjipanayi E, Mudera V, Brown RA. Guding cell migration in 3D: A collagen matrix with graded directional stiffness. Cell Motil Cytoskeleton. 2009;66:121–129. doi: 10.1002/cm.20331. [DOI] [PubMed] [Google Scholar]
- 34.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophysical Journal. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Provenzano PP, InMan DR, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys J. 2008;95:5374–5384. doi: 10.1529/biophysj.108.133116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- 37.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus - independent cancer cell invasion programs: three-dimensional amoeboid movement revisted. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaman MH, Trapani LM, Siemeski A, MacKellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.