Abstract
Background: Epidemiologic evidence shows an increase in obesity concurrent with a reduction in average sleep duration among Americans. Although clinical studies propose that restricted sleep affects hormones related to appetite, neuronal activity in response to food stimuli after restricted and habitual sleep has not been investigated.
Objective: The objective of this study was to determine the effects of partial sleep restriction on neuronal activation in response to food stimuli.
Design: Thirty healthy, normal-weight [BMI (in kg/m2): 22–26] men and women were recruited (26 completed) to participate in a 2-phase inpatient crossover study in which they spent either 4 h/night (restricted sleep) or 9 h/night (habitual sleep) in bed. Each phase lasted 6 d, and functional magnetic resonance imaging was performed in the fasted state on day 6.
Results: Overall neuronal activity in response to food stimuli was greater after restricted sleep than after habitual sleep. In addition, a relative increase in brain activity in areas associated with reward, including the putamen, nucleus accumbens, thalamus, insula, and prefrontal cortex in response to food stimuli, was observed.
Conclusion: The findings of this study link restricted sleep and susceptibility to food stimuli and are consistent with the notion that reduced sleep may lead to greater propensity to overeat. This trial was registered at clinicaltrials.gov as NCT00935402.
INTRODUCTION
The high prevalence of obesity in the United States (1) and the concurrent decrease in the average sleep duration of adult Americans (2) have been well established, and epidemiologic studies have shown a link between the two (3–5). Energy balance is generally tightly regulated by a hormonal system that relays information from the body to brain centers that control energy intake and expenditure (6). This system, which involves insulin, leptin, and ghrelin (7), is thought to provide tight control of energy balance. The development of the obesity epidemic in US adults (1) is attributed to an imbalance between energy intake and expenditure of ∼30 kJ/d (7.2 kcal/d) (8). Restricted sleep can affect the hormonal control of energy balance, and studies have reported higher ghrelin and lower leptin concentrations with short sleep (5, 9). Previous studies have also shown that leptin supplementation alters neuronal responses to food stimuli (10, 11). Together with evidence that direct administration of leptin or insulin to the brain causes a dose-dependent reduction in food intake (7), the data suggest that increased neuronal responsiveness to food under sleep restriction would promote increased food intake.
Integration of signals of hunger and satiety is thought to involve in the hypothalamic arcuate nucleus (7), which allows for the inflow of dietary hormones including insulin and ghrelin from the bloodstream to the brain (12). Hypothalamic cells involved in energy regulation project to many centers of the brain, including the cortex, thalamus, brainstem, limbic structures, and forebrain (13). The hippocampus is believed to contain insulin and leptin receptors and is connected to the hypothalamus and amygdala, which are also thought to play important roles in appetite and motivation (14, 15). The thalamus may also integrate visual signals of food, which leads to an increase in appetite and in the motivation to eat (16). Brain imaging studies have further identified the insula and orbitofrontal cortex (OFC) to be activated in response to food stimuli (17, 18). If short sleep induces alterations in hormonal controls of food intake, then specific brain regions involved in appetite regulation may be differentially affected by food stimuli under conditions of restricted sleep. The use of fMRI to assess neuronal activity in response to food stimuli not only allows us to examine neuroendocrine processes regulating food intake but also the reward-driven, hedonic components of food intake (19). The hypothesis tested in this study is that brain responses to food stimuli after habitual sleep (9 h/night) are more pronounced after restricted sleep (4 h/night) in normal-weight men and women. We expect that limbic regions (OFC, ventral striatum), which are involved in reward evaluation, will be activated to a greater extent by food stimuli in the restricted sleep condition than in the habitual sleep condition.
SUBJECTS AND METHODS
Participants
Participants were recruited from New York City and the surrounding area by advertisements on approved media. Eligibility requirements were an age of 30 to 45 y, a BMI (in kg/m2) of 22–26, right handedness, and weight stability for ≥3 mo. Subjects were also required to sleep 7–9 h/night, on average, over a 2-wk period as assessed by actigraphy (ActiGraph LLC) and a sleep diary, with ≤4 nights of sleep <7 h. Exclusion criteria included abnormal scores on the Pittsburgh Quality of Sleep Questionnaire, Epworth Sleepiness Scale, Berlin Questionnaire, Sleep Disorders Inventory Questionnaire, Beck Depression Inquiry, or Composite Scale of Morningness/Eveningness. Additional exclusion criteria included smoking, diabetes, history of drug or alcohol abuse, shift work, travel across time zones within 4 wk of the study, habitual caffeine intake >300 mg/d, regular naps, history of drowsy driving, pregnancy within 1 y of study, contraindications for MRI, or neurologic, sleep, or eating disorders. Operators of heavy machinery and commercial long-distance drivers were also excluded. The study was approved by the Institutional Review Boards of St Luke's–Roosevelt Hospital Center and Columbia University. All subjects provided informed consent before the study.
Design
Subjects were randomly assigned to either 6 d of restricted sleep (4 h/night in bed) or habitual sleep (9 h/night in bed) for phase 1. After a 3-wk washout period, participants returned to complete the second phase of the study, which consisted of the remaining sleep condition. Study phases were specifically designed to start 4 wk apart to ensure that women would be in the same phase of their menstrual cycle during each phase. The restricted sleep duration was chosen to be consistent with previous studies, which showed that 4 h provides sufficient restriction to alter hormonal profiles (20–22). Participants were inpatients for the duration of each sleep phase (Clinilabs), and sleep duration was assessed nightly by using polysomnography (Aurora Recording Systems, Gamma, v4.9; Grass Technologies).
During the first 4 d of each phase, participants were fed a controlled diet determined by the Harris-Benedict equation (23). Food intake was divided into 3 meals and 1 snack provided at 0800, 1300, 1600 (snack), and 1900. Beginning on day 5, the participants were allowed to eat ad libitum. On all days, the subjects were allowed to exercise and use gym facilities ad libitum.
fMRI
On the morning of day 6, after an overnight fast, the subjects were taken to the Columbia University Medical Center Program for Imaging and Cognitive Sciences and were screened for MR safety. Functional images were acquired on a 1.5 T scanner (GE Healthcare). Each scanning session consisted of two 5-min 48-s functional runs. Ten 16-s blocks of 4 food or nonfood images were interspersed with 16-s blocks of baseline. The paradigm used passive viewing as previously described by Hirsch et al (24) and St-Onge et al (25). All images were shown to subjects with Presentation software (Neurobehavioral Systems, http://nbs.neuro-bs.com) viewed through Avotec goggles (http://www.avotecinc.com/eyeTracking.htm). All food and nonfood items were photographed by using the same camera with the same background at our laboratory. Foods included both high- and low-calorie foods: carrots, oatmeal and dried fruit, dried fruit mix, yogurt, fish, doughnuts, pizza, hamburger, potato chips, and candy. Nonfood items were objects that were unrelated to food, such as office supplies (stapler and paper clips), skipping rope, marbles, and stuffed animals.
Functional images were acquired along the anterior commissure–posterior commissure line with a T2*-weighted echo-planar imaging sequence of 27 contiguous axial slices (repetition time = 2000 ms, echo time = 35 ms, flip angle = 90°, field of view = 192 × 192 mm, array size = 64 × 64) of 4.5-mm thickness and 3-mm in-plane resolution. Structural data were acquired with a high-resolution T1-weighted spoiled gradient recalled scan (repetition time = 19 ms, echo time = 5 ms, flip angle = 20°, field of view = 220 × 220 mm), recording 124 slices at a slice thickness of 1.5 mm and an in-plane resolution of 0.86 × 0.86 mm.
All preprocessing and data analyses were performed by using SPM5 (statistical parametric mapping; http://www.fil.ion.ucl.ac.uk/spm/software/spm5). For each participant, the first 4 scans of each functional run were discarded, and the remaining images were slice-time corrected and spatially realigned to the first volume of the first run to correct for motion. Of the 52 scans in this study, all but 2 had motion <2.0 mm; the other 2 scans, both performed in the restricted sleep condition, had motion <4.0 mm. The structural scan was co-registered to a mean image of the realigned functional scans. The co-registered functional scans were then normalized to the Montreal Neurological Institute template brain (resampled voxel size = 2 mm3) and spatially smoothed with a Gaussian kernel of 8 mm3. Vectors of stimulus onsets were created for each of the food and nonfood conditions and convolved with the canonical hemodynamic response function. A 128-s temporal high-pass filter was applied to the data to remove low-frequency artifacts. Contrasts for food > nonfood in both habitual sleep and restricted sleep conditions were created for each subject. Thresholds for active brain regions were set at a cluster extent of ≥10 voxels and a voxel-level of P < 0.01. After individual analyses, a paired t test for group analysis was performed by using the same statistical parameters to compare regional brain activity with food > nonfood for restricted sleep > habitual sleep. Note that the threshold levels (P < 0.01, k > 10) reported in this article are liberal relative to assumptions of Gaussian random field theory. Statistical analyses were performed by using SPM5. Only the coordinates from the largest cluster for each brain region are presented in the main tables for regions with multiple locations. Readers can refer to supplemental material for a complete and comprehensive report of the brain regions that were activated in each contrast.
RESULTS
Thirty participants (15 men) were recruited. Of these, one man and one woman were excluded on discovery of exclusionary criteria during the first phase of the study, one woman was unable to complete the fMRI portion of the protocol because of hair extensions that interfered with image acquisition, and another woman withdrew voluntarily after completing the first phase of the study. A total of 26 participants (14 men) completed the fMRI protocol during both study phases (Table 1). The average nightly sleep duration over each study phase was 226.2 ± 38.2 min (∼3 h and 46 min) during restricted sleep and was 457.7 ± 38.2 min (∼7 h and 38 min) during habitual sleep (P < 0.0001).
TABLE 1.
Characteristics of participants1
| Characteristic | Mean (n = 12 F, 14 M) |
| Age (y) | 35.1 ± 5.1 |
| Height (cm) | 172.3 ± 9.5 |
| Weight (kg) | 70.7 ± 10.3 |
| BMI (kg/m2) | 23.6 ± 1.3 |
All values are means ± SDs; n = 26.
Restricted sleep condition: food gt nonfood
Under the restricted sleep condition, greater neuronal activation was observed in response to food > nonfood images in the putamen (thalamus), pulvinar (lentiform nucleus), middle, medial, and superior frontal gyri, which are components of the orbitofrontal cortex (Table 2; also see Supplemental Table 1 under “Supplemental data” in the online issue). Increased activation in the cingulate gyrus, precuneus, pyramis, and inferior parietal lobule was observed.
TABLE 2.
Comparison of regional brain activation in response to food > nonfood stimuli after a period of restricted sleep1
| Montreal Neurological Institute coordinates |
||||||
| Anatomic region | Brodmann area | Clusters (voxels) | z Score | xa | yb | zc |
| Cuneus | 18 | 10 | 2.52 | −26 | −72 | 12 |
| Inferior frontal gyrus | 47 | 82 | 2.86 | −34 | 20 | −2 |
| Superior temporal gyrus | 47 | — | 2.58 | −48 | 14 | −4 |
| Inferior parietal lobule2 | 39/40 | 2004 | 3.75 | 40 | −68 | 42 |
| Precuneus2 | 39 | — | 3.59 | 30 | −62 | 42 |
| Inferior parietal lobule | 40 | 12 | 2.51 | −58 | −52 | 40 |
| Inferior temporal gyrus | 20/21 | 23 | 2.56 | −48 | 0 | −38 |
| Insula2 | 13 | 158 | 3.06 | −42 | −10 | 10 |
| Lateral ventricle | — | 58 | 2.80 | 24 | −34 | −2 |
| Lentiform nucleus/putamen | — | 39 | 2.71 | −20 | 4 | 4 |
| Lingual gyrus2 | 18 | 5943 | 4.44 | 16 | −82 | −12 |
| Middle occipital gyrus2 | 18 | — | 3.92 | 14 | −96 | 10 |
| Middle frontal gyrus2 | 8/10 | 10,389 | 4.48 | −50 | 10 | 46 |
| Cingulate gyrus2 | 23 | — | 4.40 | 4 | −36 | 28 |
| Middle frontal gyrus2 | 10 | — | 4.06 | −34 | 54 | 4 |
| Middle temporal gyrus2 | 21 | 143 | 3.09 | 64 | −34 | −12 |
| Paracentral lobule | 6 | 17 | 2.50 | 6 | −36 | 62 |
| Parahippocampal gyrus | — | 31 | 2.79 | 32 | −10 | −14 |
| Postcentral gyrus | 40 | 45 | 2.83 | −64 | −20 | 14 |
| Precentral gyrus2 | 44/45 | 96 | 3.00 | −58 | 12 | 10 |
| Inferior frontal gyrus | 44 | — | 2.47 | −60 | 6 | 20 |
| Precuneus | 7 | 74 | 2.69 | 6 | −64 | 38 |
| Pyramis2 | — | 266 | 3.80 | 8 | −68 | −32 |
| Nodule | — | — | 2.88 | −4 | −62 | −32 |
| Culmen | — | — | 2.88 | 2 | −48 | −26 |
| Superior frontal gyrus2 | 9/10 | 231 | 3.39 | −22 | 46 | 42 |
| Medial frontal gyrus | 9 | — | 2.58 | −8 | 42 | 28 |
| Superior occipital gyrus | 19 | 12 | 2.46 | −30 | −76 | 24 |
| Thalamus/pulvinar | — | 10 | 2.47 | −16 | −32 | 10 |
Additional clusters and peak coordinates for some brain regions can be found elsewhere (see Supplemental Table 1 under “Supplemental data” in the online issue). Data are peak coordinates of brain regions represented by the largest cluster of activation, as assessed by using SPM5 (cluster-level P < 0.01, cluster ≥ 10). n = 26. aPositive x coordinate indicates right hemisphere. bPositive y coordinate indicates anterior-to-commissure landmark. cPositive z coordinate indicates above anterior-posterior commissure line.
Regions with voxel-level P ≤ 0.001.
Habitual sleep condition: food gt nonfood
Under habitual sleep, responses to food > nonfood stimuli were generally attenuated. The inferior and middle frontal gyri remained significantly activated in response to food > nonfood stimuli (Table 3). The hypothalamus was also activated in response to food > nonfood stimuli.
TABLE 3.
Comparison of regional brain activation in response to food > nonfood stimuli after a period of habitual sleep1
| Montreal Neurological Institute coordinates |
||||||
| Anatomic region | Brodmann area | Clusters (voxels) | z Score | xa | yb | zc |
| Hypothalamus | — | 29 | 3.43 | 6 | 0 | −6 |
| Inferior frontal gyrus2 | 10/46 | 355 | 3.97 | −40 | 36 | 12 |
| Middle frontal gyrus | 46 | — | 2.97 | −50 | 34 | 18 |
| Inferior parietal lobule2 | 40 | 77 | 3.51 | −46 | −60 | 48 |
| Lateral ventricle/corpus callosum | — | 37 | 2.92 | −16 | 32 | 8 |
| Lateral ventricle/corpus callosum | — | 14 | 2.77 | −4 | 4 | 22 |
| Middle frontal gyrus | 11 | 14 | 3.02 | −24 | 36 | −12 |
| Superior frontal gyrus2 | 9 | 29 | 3.09 | 12 | 50 | 34 |
| Superior frontal gyrus | 10 | 40 | 3.16 | 24 | 54 | 12 |
| Superior frontal gyrus | 10 | 79 | 3.12 | −18 | 58 | 14 |
| Medial frontal gyrus | 10 | — | 3.06 | −20 | 50 | 10 |
Data are peak coordinates of activated brain regions, as assessed by using SPM5 (cluster-level P < 0.01, cluster ≥ 10). n = 26. aPositive x coordinate indicates right hemisphere. bPositive y coordinate indicates anterior-to-commissure landmark. cPositive z coordinate indicates above anterior-posterior commissure line.
Regions with voxel-level P ≤ 0.001.
Restricted relative to habitual sleep: food gt nonfood
When brain responses to food > nonfood stimuli were compared after a period of restricted relative to habitual sleep, significantly greater activation was found in the putamen (lentiform nucleus), nucleus accumbens (putamen), thalamus, insula, and inferior, middle, and superior frontal gyri (Brodmann areas 6, 8, and 10) (Table 4 and Figure 1; also see Supplemental Table 2 under “Supplemental data” in the online issue). Other areas that were activated to a greater extent in response to food > nonfood stimuli under restricted sleep conditions relative to habitual sleep included the precentral gyrus, lentiform nucleus, supramarginal gyrus, superior and transverse temporal gyri, inferior parietal lobule, paracentral lobule, middle occipital gyrus, precuneus, and cuneus.
TABLE 4.
Comparison of regional brain activation in response to food > nonfood stimuli after a period of restricted sleep > habitual sleep1
| Montreal Neurological Institute coordinates | ||||||
| Anatomic region | Brodmann area | Clusters (voxels) | z Score | xa | yb | zc |
| Caudate/nucleus accumbens | — | 14 | 2.57 | −12 | 14 | −10 |
| Cingulate gyrus | — | 18 | 2.78 | −10 | −10 | 32 |
| Corpus callosum | — | 28 | 2.60 | 6 | −34 | 14 |
| Culmen | — | 19 | 2.72 | −10 | −68 | −8 |
| Inferior frontal gyrus2 | 44 | 394 | 3.42 | 50 | −2 | 22 |
| Middle frontal gyrus | 9 | — | 2.65 | 50 | 24 | 36 |
| Inferior parietal lobule2 | 7/19 | 167 | 3.16 | −36 | −62 | 46 |
| Insula | 13 | 50 | 2.89 | −36 | −8 | 6 |
| Claustrum | 13 | — | 2.69 | −32 | 0 | 6 |
| Lateral ventricle/corpus callosum2 | — | 55 | 3.08 | −12 | −26 | 26 |
| Lentiform nucleus/putamen | — | 46 | 2.92 | 28 | −6 | 10 |
| Lingual gyrus | 18 | 18 | 2.54 | 12 | −74 | −2 |
| Middle frontal gyrus2 | 6 | 231 | 3.34 | −18 | −2 | 64 |
| Superior frontal gyrus | 6 | — | 2.87 | −20 | 12 | 64 |
| Middle frontal gyrus2 | 9 | 122 | 3.07 | 36 | 36 | 42 |
| Middle occipital gyrus2 | 30 | 453 | 3.69 | −28 | −74 | 10 |
| Cuneus2 | 18 | — | 3.12 | −18 | −80 | 14 |
| Nucleus accumbens/putamen | — | 24 | 2.93 | 12 | 14 | −8 |
| Paracentral lobule2 | 9 | 138 | 3.42 | 8 | −36 | 64 |
| Precentral gyrus2 | 6 | 323 | 3.33 | −48 | 0 | 50 |
| Middle frontal gyrus2 | 6 | — | 3.16 | −50 | 10 | 46 |
| Inferior frontal gyrus | 9 | — | 2.90 | −48 | 8 | 38 |
| Precuneus2 | 19 | 160 | 3.79 | −24 | −84 | 42 |
| Subcallosal gyrus | 34 | 13 | 2.86 | −22 | 6 | −16 |
| Superior frontal gyrus | 6 | 224 | 2.94 | 2 | 8 | 54 |
| Superior temporal gyrus2 | 22/42 | 214 | 3.29 | −62 | −28 | 12 |
| Supramarginal gyrus2 | 40 | 592 | 3.31 | 52 | −40 | 36 |
| Thalamus2 | — | 306 | 3.41 | −18 | −36 | 10 |
| Transverse temporal gyrus2 | 41 | 769 | 3.32 | 44 | −22 | 10 |
| Insula2 | 13 | — | 3.30 | 32 | −30 | 20 |
Additional clusters and peak coordinates for some brain regions can be found elsewhere (see Supplemental Table 2 under “Supplemental data” in the online issue). Data are peak coordinates of brain regions represented by the largest cluster of activation, as assessed by using SPM5 (cluster-level P < 0.01, cluster ≥ 10). n = 26. aPositive x coordinate indicates right hemisphere. bPositive y coordinate indicates anterior-to-commissure landmark. cPositive z coordinate indicates above anterior-posterior commissure line.
Regions with voxel-level P ≤ 0.001.
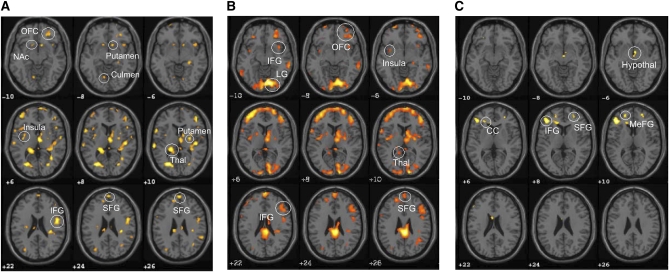
FIGURE 1.
Brain activation in response to food > nonfood stimuli during a period of restricted sleep > habitual sleep (A), restricted sleep (B), and habitual sleep (C), as assessed by using SPM5 (cluster-level P < 0.01, cluster ≥ 10). n = 26. Corresponding coordinates are provided in Tables 2–4 and in supplementary materials under “Supplemental data” in the online issue. Contrasts for food > nonfood were performed for each subject and averaged. After individual analyses, a paired t test for group analysis was performed by using the same statistical parameters to compare regional brain activity with food > nonfood for restricted sleep > habitual sleep. CC, corpus callosum; Hypothal, hypothalamus; IFG, inferior frontal gyrus; LG, lingual gyrus; MeFG, medial frontal gyrus; NAc, nucleus accumbens; OFC, orbitofrontal cortex; SFG, superior frontal gyrus; SPM, Statistical Parametric Mapping; Thal, thalamus.
DISCUSSION
This study extends our previous work (26) by examining the neuronal pathways involved in the brain responses to food stimuli. We found that food stimuli increased regional brain activity in the OFC, insula, and regions of the basal ganglia and limbic system after restricted sleep. Although food stimuli also elicited increases in regional brain activity in areas of the OFC during habitual sleep, activation was reduced and much less widespread. In fact, direct comparison of the effects of food stimuli on regional brain activity during restricted and habitual sleep highlight the OFC, insula, thalamus, precuneus, cingulate gyrus, and supramarginal gyrus as being activated to a greater extent after restricted sleep. These regions have been associated with motivation and the reward value of food as well as cognitive processing, decision-making, and self-control (27). Our data thus suggest that restricting sleep alters neuronal activity, which predisposes individuals to enhanced susceptibility to food stimuli and may partly explain the relation observed between sleep duration and BMI (4, 28, 29).
Of specific interest was the comparison of restricted to habitual sleep in regional brain responses to food stimuli. Significantly greater activity was observed in the thalamus, insula, and OFC, consistent with our hypothesis that restricted sleep would lead to a state of greater susceptibility to food stimuli via greater motivation to seek food as a reward (30). Activation in the insula, involved in processing interoceptive signals such as hunger, is also consistent with our hypothesis. The lentiform nucleus, putamen, and nucleus accumbens—associated with emotional responses to stimuli and motivation and reward systems (27)—were activated to a greater extent in response to food after restricted compared with habitual sleep. These data suggest that restricted sleep induces a state of greater responsiveness to food stimuli and heightened awareness of the rewarding properties of food. Enhanced activity in the prefrontal cortex may also signify greater recruitment of memory and cognitive branching centers of the brain (27) to process complex behaviors associated with food seeking.
Interestingly, the inferior and middle frontal gyri, lingual gyrus, and culmen—activated to a greater extent after restricted sleep than after habitual sleep—were also found as being activated in response to food stimuli in individuals in a weight-reduced state as compared with higher initial body weight (11). These similarities between studies suggest that restricted sleep may lead to a neuronal state that signals reduced energy stores and may act to promote a positive energy balance. We reported greater food intake (26) and Nedeltcheva et al (31) reported greater intakes from snacks during a period of short sleep than during habitual sleep. Because there was no effect of sleep duration on circulating leptin and ghrelin concentrations, the authors suggested that “prolonged exposure to palatable food and sleep-loss-related changes in reward seeking and motivation may underlie these changes in feeding behavior” (31). Our results support this hypothesis.
Our results suggest separate neuronal activity patterns under restricted and habitual sleep. Similar to Rosenbaum et al's (11) description of a neuronal network involved in energy homeostasis, the hypothalamus, middle frontal gyrus, and inferior parietal lobule were activated in response to food stimuli after habitual sleep. However, after a period of restricted sleep, the neuronal pattern was similar to one that would be in place when the body is at lower body weight and aiming to restore initial body weight. As observed by Rosenbaum et al (11) at low body weight, we also noted that the culmen, parahippocampal gyrus, inferior and middle frontal gyri, and lingual gyrus were responsive to food stimuli after restricted sleep. We additionally observed the nucleus accumbens and putamen as being activated to a greater extent after restricted sleep than after habitual sleep. These regions have been associated with reward, pleasure, reinforcement learning, and drug addiction (32) and suggest enhanced salience of food stimuli during restricted sleep, similar to previous observations of enhanced salience of food stimuli under reduced leptin concentrations (11).
Conversely, it has been proposed that low activation of brain reward centers in response to salient stimuli could predispose to obesity, because such responses would indicate that more stimuli are necessary to produce pleasure in genetically susceptible individuals (33). The same report acknowledged that the opposite is also true, that greater activation of brain reward centers may predispose to overeating and obesity. Because all of our participants were nonobese and because we previously reported overeating after a period of short sleep relative to habitual sleep in this study (26), we believe that we can safely interpret our results to mean the latter: greater activation of brain reward centers would increase susceptibility to food stimuli.
This study investigated the effect of visual food stimuli on neuronal activity, but did not distinguish between high- and low-calorie foods. High-calorie foods may be preferred by subjects after sleep restriction. This would be consistent with previous findings (17, 18) and would be particularly compelling in light of significant increases in snack food consumption during a period of restricted relative to habitual sleep (31). In this study, food intake on the day before the fMRI measurement was 296 kcal more during the restricted sleep period relative to habitual sleep (26). This would serve to bias our findings toward more conservative measures, which was likely attenuated by conducting the scans in the fasted state. Some (21), but not all (34–36), studies have reported increased cortisol concentrations after a period of restricted sleep. Cortisol release via activation of the hypothalamic-pituitary-adrenal axis could lead to overeating in the presence of highly palatable food (37) and may explain some of the findings. However, general stress is controlled by comparing food and nonfood stimuli. Finally, our participants were all relatively normal weight (BMI <26). Studies have shown that brain responses to satiation (38, 39) and food images (40) differ between lean and obese individuals, and it is unknown whether sleep restriction would accentuate or attenuate this difference. Even though sample size limits rigorous evaluation of factors such as sex and body weight, our observations provide a foundation for future research.
Although we enrolled both men and women, our sample size was too small to conduct separate analyses by sex. Nevertheless, our data showed similar results between men and women for food > nonfood after a period of restricted sleep (data not shown). On the other hand, during habitual sleep, men showed less overall brain activity in response to foods compared with women.
Finally, we obtained fMRI data on 2 separate occasions ∼4 wk apart. Although the time difference between measurements may have affected our results, this was minimized by the randomized design, the control nonfood stimuli condition, and the known high test-retest reliability of fMRI (41).
Our results suggest that the association between sleep restriction and obesity may in part be the result of changes in neuronal activity when exposed to food stimuli. These changes associated with reduced sleep apparently affect brain regions known to be linked to motivation and desire and may indicate an increased propensity to seek food in individuals who are not getting enough sleep. These actions, in a world where food is readily accessible, would promote weight gain. Overall, these findings suggest that changes in neuronal activity in response to food stimuli after insufficient sleep are precursors to energy balance regulation mechanisms in the brain.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—M-PS-O and JH: designed the research; ALR, AM, ZBT, and MS: conducted the research; M-PS-O, ALR, MS, AM, and ZBT: collected the data; AM, ZBT, MS, and M-PS-O: analyzed the data; AM, ZBT, MS, JH, and M-PS-O: interpreted the data; M-PS-O, ZBT, AM, and JH: wrote the manuscript; and M-PS-O: had primary responsibility for the final content. All authors read and approved the final manuscript. The funding agencies played no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. None of the authors had any conflicts of interest to disclose.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–41 [DOI] [PubMed] [Google Scholar]
- 2.Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, Ruden D, Pietrobelli A, Barger JL, Fontaine KR, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 2006;30:1585–94 [DOI] [PubMed] [Google Scholar]
- 3.Cappuccio FP, Taggart FM, Kandala NB, Currie C, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008;31:619–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep 2005;28:1289–96 [DOI] [PubMed] [Google Scholar]
- 5.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 2004;1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes 2003;52:232–8 [DOI] [PubMed] [Google Scholar]
- 7.Woods SC, Seeley RJ. Adiposity signals and the control of energy homeostasis. Nutrition 2000;16:894–902 [DOI] [PubMed] [Google Scholar]
- 8.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011;378:826–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–50 [DOI] [PubMed] [Google Scholar]
- 10.Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A 2007;104:18276–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 2008;118:2583–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav 2001;74:683–701 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–71 [DOI] [PubMed] [Google Scholar]
- 14.Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: appetite and activity. Behav Brain Res 2001;127:13–23 [DOI] [PubMed] [Google Scholar]
- 15.York DA. Peripheral and central mechanisms regulating food intake and macronutrient selection. Obes Surg 1999;9:471–9 [DOI] [PubMed] [Google Scholar]
- 16.Stellar E. The physiology of motivation. Psychol Rev 1954;61:5–22 [DOI] [PubMed] [Google Scholar]
- 17.Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex 2003;13:1064–71 [DOI] [PubMed] [Google Scholar]
- 18.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage 2003;19:1381–94 [DOI] [PubMed] [Google Scholar]
- 19.Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav 2004;81:781–93 [DOI] [PubMed] [Google Scholar]
- 20.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354:1435–9 [DOI] [PubMed] [Google Scholar]
- 21.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 2004;89:5762–71 [DOI] [PubMed] [Google Scholar]
- 22.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003;26:117–26 [DOI] [PubMed] [Google Scholar]
- 23.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA 1918;4:370–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch J, Ruge MI, Kim KH, Correa DD, Victor JD, Relkin NR, Labar DR, Krol G, Bilsky MH, Souweidane MM, et al. An integrated functional magnetic resonance imaging procedure for preoperative mapping of cortical areas associated with tactile, motor, language, and visual functions. Neurosurgery 2000;47:711–21, discussion 721–2 [DOI] [PubMed] [Google Scholar]
- 25.St-Onge MP, Sy M, Heymsfield SB, Hirsch J. Human cortical specialization for food: a functional magnetic resonance imaging investigation. J Nutr 2005;135:1014–8 [DOI] [PubMed] [Google Scholar]
- 26.St-Onge M-P, Roberts A, Chen J, Kelleman M, O'Keeffe M, Jones P. Short sleep duration increases energy intakes but does not change expenditure expenditure in normal weight individuals. Am J Clin Nutr 2011;94:410–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schloegl H, Percik R, Horstmann A, Villringer A, Stumvoll M. Peptide hormones regulating appetite-focus on neuroimaging studies in humans. Diabetes Metab Res Rev 2011;27:104–12 [DOI] [PubMed] [Google Scholar]
- 28.Seicean A, Redline S, Seicean S, Kirchner HL, Gao Y, Sekine M, Zhu X, Storfer-Isser A. Association between short sleeping hours and overweight in adolescents: results from a US Suburban High School survey. Sleep Breath 2007;11:285–93 [DOI] [PubMed] [Google Scholar]
- 29.Sekine M, Yamagami T, Handa K, Saito T, Nanri S, Kawaminami K, Tokui N, Yoshida K, Kagamimori S. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care Health Dev 2002;28:163–70 [DOI] [PubMed] [Google Scholar]
- 30.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 2005;6:691–702 [DOI] [PubMed] [Google Scholar]
- 31.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89:126–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology 2004;47(suppl 1):3–13 [DOI] [PubMed] [Google Scholar]
- 33.Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage 2010;50:1618–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW, Corssmit EP, Romijn JA. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab 2010;95:2963–8 [DOI] [PubMed] [Google Scholar]
- 35.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 2009;94:3242–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Lehnert H, Born J, Schultes B. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep 2011;34:371–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav 2007;91:449–58 [DOI] [PubMed] [Google Scholar]
- 38.Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, Ravussin E, Reiman EM, Tataranni PA. Differential brain responses to satiation in obese and lean men. Diabetes 2000;49:838–46 [DOI] [PubMed] [Google Scholar]
- 39.Gautier JF, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE, Ravussin E, Reiman EM, Tataranni PA. Effect of satiation on brain activity in obese and lean women. Obes Res 2001;9:676–84 [DOI] [PubMed] [Google Scholar]
- 40.Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an FMRI study. Obesity (Silver Spring) 2011;19:1775–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aron AR, Gluck MA, Poldrack RA. Long-term test-retest reliability of functional MRI in a classification learning task. Neuroimage 2006;29:1000–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.