Abstract
Background: Plant sterol (PS) supplementation is increasingly accepted as a dietary strategy to lower plasma cholesterol concentrations. However, information is scarce about the effect of increased PS intake in potentially vulnerable groups, such as phytosterolemia heterozygotes (HET).
Objective: This study assessed the responsiveness of circulating PS and lipid concentrations and cholesterol kinetics (absorption and synthesis) to daily PS supplementation in HET (ABCG8 S107X mutation) compared with a healthy control cohort.
Design: A double-blind, randomized, crossover, placebo-controlled study was conducted in 10 HET and 15 control subjects. The participants had a mean (±SEM) age of 34 ± 2 y and a BMI (in kg/m2) of 29.9 ± 1.1 and consumed ∼1.6 g PS or placebo capsules daily with supper for 4 wk. Cholesterol absorption and synthesis were assessed by using [13C]cholesterol and deuterium oxide, respectively.
Results: Plasma LDL-cholesterol concentrations decreased (P = 0.006) in both groups after PS supplementation (HET: 2.73 ± 0.19 mmol/L; control: 3.11± 0.19 mmol/L) compared with placebo (HET: 3.12 ± 0.20 mmol/L; control: 3.50 ± 0.21 mmol/L), whereas PS concentrations (campesterol+β-sitosterol) increased (P = 0.03) in both groups after PS supplementation (HET: 39.72 ± 6.05 μmol/L; control: 24.03 ± 1.65 μmol/L) compared with placebo (HET: 27.32 ± 3.80 μmol/L; control: 21.12 ± 2.05 μmol/L). Cholesterol absorption efficiency decreased (P = 0.010) by ∼22% and ∼17% and synthesis rates increased (P = 0.040) by ∼20% and ∼24% in the HET and control groups, respectively, in response to PS consumption compared with placebo.
Conclusion: These data suggest that heterozygosity for the ABCG8 S107X mutation does not influence the action of dietary PS on circulating cholesterol concentrations but may affect sterol absorption. This trial was registered at clinicaltrials.gov as NCT01102647.
INTRODUCTION
Elevated total cholesterol and LDL-cholesterol concentrations are significantly associated with increased risks of CVD4 (1). Within the past 10 years, a resurgence has occurred in the use of PS and stanols as functional food ingredients to reduce CVD risk, with studies showing that modest doses of 1 to 2 g PS/d can lower total cholesterol and LDL cholesterol by ∼8–15% in normal and moderately hypercholesterolemic individuals (2). As such, various health organizations (3, 4) and advisory groups (5) currently recommend the use of PS as a therapeutic lifestyle change, in coordination with pharmacotherapy to achieve cholesterol-lowering goals. Currently, various PS-enriched foods and supplements are available in Canada, the United States, and other countries. However, recent concern is that a moderate increase in circulating PS concentrations in the general population and subpopulations may increase CVD risk (6–9). Unfortunately, information about the effect of increased PS intakes in potentially vulnerable groups, including individuals heterozygous for phytosterolemia, is scarce.
Normally, low circulating PS concentrations are maintained by the actions of 2-half ATP transporters, ABCG5 and ABCG8, which limit PS absorption from the intestine and facilitate PS excretion via bile. Phytosterolemia, also known as sitosterolemia, is a rare autosomal-recessively inherited disease resulting from mutations in either the ABCG5 or ABCG8 genes. Individuals who are homozygous for phytosterolemia typically exhibit a >30-fold increase in plasma PS concentrations (10, 11) and deposition of sterols in skin and tendons (xanthomas) and in the walls of the coronary arteries, which results in premature coronary atherosclerosis and a high risk of fatal cardiovascular events (12–15). Conversely, individuals that have only one allele with an ABCG5 or ABCG8 mutation are heterozygous for phytosterolemia and appear clinically normal, with normal to only a slight increase in plasma PS concentrations and moderate hypercholesterolemia (12, 13). Whereas it is clear that consuming PS is contraindicated in individuals homozygous for phytosterolemia, limited information is available on the effect of increasing dietary PS on circulating cholesterol and PS concentrations in those heterozygous for phytosterolemia. One concern is that circulating PS concentrations may increase even more distinctly after consumption of PS supplementation in these individuals, which results in an increased risk of atherosclerosis (6), in part because elevated plasma PS concentrations may be seen as surrogate markers for characterizing individuals with higher intestinal cholesterol absorption (16). Therefore, it is important to assess in individuals heterozygous for phytosterolemia whether PS would be an appropriate therapeutic strategy to reduce LDL-cholesterol concentrations. The objectives of this study were thus to investigate the responses of circulating lipid and PS concentrations to PS consumption and to determine the rates of cholesterol absorption and synthesis, analyzed by using stable-isotope approaches, in individuals heterozygous for phytosterolemia and in control subjects.
SUBJECTS AND METHODS
Subjects
Participants were males and females between 16 and 65 y of age, who were recruited from a Hutterite colony in Manitoba. These volunteers were related to a proband reported by Mymin et al (17, 18) to have the ABCG8 S107X mutation. Thus, genotyping for the ABCG8 S107X mutation was used to identify phytosterolemia heterozygotes (HET; n = 10) and control subjects (control; n = 15). Individuals homozygous for the ABCG8 S107X mutation or with thyroid disease, diabetes mellitus, kidney disease, or liver disease were excluded from the study. Volunteers taking medications known to affect lipid metabolism, high-dose dietary supplements, fish-oil capsules, or PS for ≥3 mo before the start of the study were also excluded from participation. The study protocols were approved by the University of Manitoba Biomedical Research Ethics Board. All subjects were informed of the risks associated with the study and gave their written informed consent to participate.
Study design and protocol
A double-blind, randomized, crossover, placebo-controlled study was carried out at the Richardson Centre for Functional Foods and Nutraceuticals, University of Manitoba. Participants consumed 2 treatments: ∼1.6 g PS/d or a placebo treatment with the supper meal daily for 4 wk (29 d). A 4-wk washout period separated the 2 treatment periods. Dietary intake was not controlled in this study, and, although many factors in the diet can theoretically modify LDL-cholesterol and PS concentrations, the strengths of this study were that both groups were from the same cohort and were part of the Hutterite lifestyle, whereby meals are eaten communally. Therefore, all participants consumed the same types of foods, although caloric intake varied among members. Throughout the entire study, all participants were instructed to continue to consume their habitual diets at levels consistent with maintenance of a stable body weight. Body weights for all participants were recorded on days 1 and 2 and days 26–29 of each treatment period to determine weight change.
Plant sterol powder (Nutragenius Inc) was packaged into capsules at the Richardson Centre for Functional Foods and Nutraceuticals. Participants were instructed to consume 4 capsules/d (∼1.6 g) of either PS or placebo (cornstarch) with yogurt or milk as part of their evening meal. Adherences to the treatments were based on returned pill counts and study diary. The capsules were analyzed for the PS profile and disintegration time (Table 1).
TABLE 1.
Plant sterol profile and disintegration time of the plant sterol capsules used in the study1
| Analyte | Results |
| Plant sterol profile (%) | |
| β-Sitosterol | 40.94 |
| β-Sitostanol | 1.70 |
| Campesterol | 26.30 |
| Campestanol | 0.85 |
| Stigmasterol | 27.06 |
| Other sterols | 1.39 |
| Total of plant sterols | 98.24 |
| Total of plant stanols | 2.55 |
| Tablet capsule net weight (mg) | 387.9 |
| Disintegration time (min) | 34 |
The capsules were analyzed by Silliker JR Laboratories.
Blood sampling and isotope protocol
Overnight fasting blood samples were collected on days 1 and 2 and on days 26–29 of each treatment period. On day 26 of each treatment period, a fasting baseline blood sample (0 h) was collected before administration of a 75-mg oral dose of [3,4-13C]cholesterol (Cambridge Isotope Laboratories Inc) to measure cholesterol absorption over the following 72 h. The [13C]cholesterol was dissolved in 5 g warmed margarine and spread on a small bun. Additionally, an oral dose of ∼25 mL deuterium oxide (Cambridge Isotope Laboratories Inc) was also given on day 26 as a tracer for measuring cholesterol synthesis. Fasting blood samples were collected on day 27 (24 h), day 28 (48 h), and day 29 (72 h) to monitor enrichment concentrations of both isotopes.
Analyses
Genotyping
In 2003, Mymin et al (17, 18) reported a novel ABCG8:c.320 C→G mutation (NM_022437.2) in the ABCG8 gene that was the cause of phytosterolemia in a Manitoba Hutterite. This mutation results in a premature stop codon replacing serine at codon 107. For genotyping, genomic DNA was isolated from a cheek swab or white blood cells (Gentra Puregene; Qiagen). The ABCG8:c.320C→G mutation was detected by PCR amplifying a 446-bp region containing the mutation from the subjects’ DNA by using forward primer 5′-AGTTGCTGAAGCCCTCTGAA-3 and reverse primer 5′-AGAGGCGCACTCATGACTCT-3′. PCR was performed in a DNA Thermal Cycler (PTC-100; Applied Biosystems) in a final volume of 50 μL contained 32 pmol of each primer, thermopol buffer, dNTPs, water, and 2.5 units of Taq DNA polymerase (New England Biolabs). PCR amplifications were performed with denaturing at 95°C for 2 min, followed by 35 cycles of a denaturing step at 95°C, an annealing step at 58°C, and an extension step at 72°C, each for 30 s. A final extension step at 72°C was performed for 10 min. The PCR products were digested with the SacI restriction endonuclease (New England Biolabs), which recognizes a site that is destroyed in the presence of the c.320C→G mutation. The resulting restriction fragments were separated by electrophoresis on 2% agarose gels and visualized by ethidium bromide followed by ultraviolet illumination. Individuals who did not have the c.320C→G mutation (ie, control) had 3 bands on gel electrophoresis (212, 144, and 90 bp), whereas HET individuals had 4 bands (302, 212, 144, and 90 bp), and affected individuals had 2 bands (302 and 144 bp).
Plasma lipid profile
Plasma and RBCs from fasting blood samples were separated by centrifugation at 3000 × g for 20 min, and the fractions were stored at −80°C. Plasma total cholesterol, HDL cholesterol, and triglyceride concentrations were determined by automated enzymatic methods on a Vitros 350 chemistry analyzer (Ortho-Clinical Diagnostics). LDL-cholesterol concentrations were estimated by using the Friedewald formula (19).
Plasma plant sterol concentrations
PS were measured by using a gas-liquid chromatograph equipped with a flame ionization detector and an auto sampler system (Varian 430-GC; Agilent Technologies). An internal standard, 5-α-cholestane, was added to plasma samples that were then saponified with 4 mL methanolic potassium hydroxide. Sterols were extracted twice from the mixture with 4 mL petroleum ether, resuspended in hexane and injected (1 μL) onto a 30-m capillary column (SAC-5; Supelco). The column temperature was 280°C. Isothermal running conditions were maintained for 30 min. The injector and detector were set at 295°C and 300°C, respectively. The carrier gas (helium) flow rate was 1 mL/min with the inlet splitter set at 40:1. Individual PS were identified by using authentic standards (Sigma-Aldrich Canada Ltd). Internal standards were used to calculate detector response factors.
Cholesterol absorption
Cholesterol absorption was assessed by using the stable-isotope single-tracer method (20). Free cholesterol extracted from RBCs (21) was used to determine [3,4-13C]cholesterol enrichments by using online gas chromatography/combustion/isotope mass spectrometry. Lipid extracts were dissolved in hexane and injected into a gas chromatograph (Agilent 6890N) interfaced with a Finnigan Delta V Pulse isotope ratio mass spectrometer (Bremen) through a Finnigan combustion interface (Combustion Interface III; Bremen). Isotope abundances, expressed in delta (δ) per mil (‰), were calculated by using carbon dioxide as a reference gas and further corrected against the international reference standard, Pee Dee Belemnite limestone. Cholesterol absorption was measured by using the [3,4-13C]cholesterol RBC enrichment curve over 72 h after [13C]cholesterol ingestion. Specifically, cholesterol absorption was calculated as area under the [13C]enrichment curve (AUC0–72h) by using the trapezoidal rule and values corrected for baseline values.
Cholesterol synthesis
Cholesterol synthesis rates were assessed based on the rate of deuterium incorporation from body water into newly synthesized RBC membrane free cholesterol over a defined period (22). The measurement of free-cholesterol deuterium enrichment was performed by using online gas chromatography–pyrolysis-isotope ratio mass spectrometry. Isotope abundances, expressed in δ‰, were calculated by using H2 as a reference gas. Deuterium enrichment was measured in both RBC free cholesterol and plasma water. Lipids were extracted from RBCs (21). Enrichments were expressed relative to standard mean ocean water and a series of standards of known enrichment. Cholesterol FSRs are considered to represent RBC free cholesterol deuterium enrichment values relative to the corresponding mean plasma water sample enrichment after correction for the free cholesterol pool. The FSR represents the fraction of the cholesterol pool that is synthesized in 24 h, as shown in the following equation:
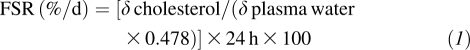 |
where δ is the deuterium enrichment of cholesterol or plasma water above baseline in 24 h. The factor 0.478 reflects the fraction of hydrogen atoms per cholesterol molecule that may become enriched by deuterium during in vivo biosynthesis (23).
The ASR of rapidly exchanging free cholesterol was calculated as follows (23):
where M1 represents the size of the rapidly exchanging free cholesterol pool, as calculated according to the model of Goodman et al (24), based on the following formula:
where TGGP is a variable that is equal to 1, 2, or 3 depending on the serum triglyceride concentration: <2.267, 2.267–3.401, or >3.401 mmol/L, respectively. The factor 0.33 was included to account for the proportion of free cholesterol in the overall plasma total cholesterol pool.
Statistical analyses
Data are expressed as means ± SEMs. Data were analyzed by using SPSS statistical software (version 17; SPSS Inc). Baseline variables were analyzed by using 1-factor ANOVA with an accepted level of significance of P < 0.05. The effects of treatment (PS compared with placebo) and group (HET compared with control subjects) on endpoint variables (PS and lipid profiles, cholesterol metabolism) were assessed by repeated-measures 2-factor ANOVA. Endpoint values for lipid were taken as averages of days 28–29.
RESULTS
Subject characteristics
Baseline characteristics of the study participants at the time of screening are provided in Table 2. No difference was found in the average age between groups. Overall, the HET group had a relatively healthier profile than did the control group. Although no significant difference in body weight was found between groups, based on BMI classification, the HET group was overweight (BMI: 25–29.9), whereas the control group was obese (BMI ≥30). There were no significant weight changes throughout the study or across any of the treatment periods (data not shown). All subjects were mildly hypercholesterolemic, as indicated by total cholesterol concentrations >5.2 mmol/L. The control group was hypertriglycemic, with plasma triglyceride concentrations >1.7 mmol/L. Plasma β-sitosterol and campesterol concentrations were ∼46% and ∼58% higher (P < 0.05), respectively, in the HET group than in the control group. However, even with ∼28% higher plasma lathosterol concentrations in the control group, no significant difference (P > 0.05) was found between groups. All participants completed the study and tolerated the PS treatment well with no reported side effects.
TABLE 2.
Baseline characteristics of the study participants at the time of screening
| Variable | All subjects (n = 25) | Heterozygotes (n = 10) | Control subjects (n = 15) | P value1 |
| Age (y) | 33.6 ± 2.22 | 33.3 ± 4.0 | 33.8 ± 2.6 | 0.914 |
| Sex | ||||
| Male | 12 | 4 | 8 | |
| Female | 13 | 6 | 7 | |
| Body weight (kg) | 83.4 ± 4.1 | 79.0 ± 4.7 | 86.3 ± 6.1 | 0.395 |
| BMI (kg/m2) | 29.9 ± 1.1 | 29.1 ± 1.1 | 30.4 ± 1.7 | 0.578 |
| Total cholesterol (mmol/L) | 5.49 ± 0.15 | 5.32 ± 0.27 | 5.61 ± 0.16 | 0.336 |
| LDL cholesterol (mmol/L) | 3.30 ± 0.11 | 3.28 ± 0.22 | 3.31 ± 0.12 | 0.886 |
| HDL cholesterol (mmol/L) | 1.23 ± 0.06 | 1.35 ± 0.10 | 1.15 ± 0.06 | 0.102 |
| Triglycerides (mmol/L) | 2.26 ± 0.30 | 1.53 ± 0.22 | 2.74 ± 0.45 | 0.048 |
| β-Sitosterol (μmol/L) | 9.42 ± 0.86 | 11.63 ± 1.71 | 7.95 ± 0.70 | 0.033 |
| Campesterol (μmol/L) | 14.16 ± 1.20 | 18.18 ± 1.99 | 11.49 ± 1.06 | 0.004 |
| Lathosterol (μmol/L) | 10.42 ± 0.83 | 8.88 ± 0.85 | 11.40 ± 1.15 | 0.123 |
Differences between groups (heterozygotes and control subjects) were assessed by 1-factor ANOVA; P < 0.05 indicates significance.
Mean ± SEM (all such values).
Plasma lipid and plant sterol responses to treatment
Plasma lipid concentrations in response to PS and placebo are presented in Table 3. The plasma total cholesterol concentration decreased (P < 0.001) overall by an average of 5.8 ± 1.4% in all subjects after 4 wk of PS, with a change of −8.8 ± 2.4% observed in the HET group and of −3.8 ± 1.6% in the control group. The LDL-cholesterol concentrations decreased (P = 0.001) overall by 9.6 ± 3.2% in all subjects, with a similar magnitude of reduction in both groups (HET group: −10.7 ± 6.6%; control group: −9.0 ± 3.1%) after supplementation with PS. No significant differences in HDL-cholesterol concentrations were found between groups or between treatments within groups. Baseline triglyceride concentrations were found to be higher (P < 0.05) in the control group (Table 2) and remained consistently higher in this group throughout the study (Table 3); thus, there was a significant treatment-group interaction.
TABLE 3.
Serum lipid profile at baseline and after 4 wk of treatment1
| Placebo |
Plant sterols |
P value2 |
|||||||
| Lipid variables and group | Day 1 | Day 29 | Change | Day 1 | Day 29 | Change | Treatment | Group | Interaction |
| Total cholesterol (mmol/L) | |||||||||
| HET | 5.03 ± 0.27 | 5.09 ± 0.23 | 0.06 ± 0.23 | 5.23 ± 0.17 | 4.76 ± 0.19 | −0.46 ± 0.13 | 0.003 | 0.187 | 0.746 |
| Control | 5.63 ± 0.23 | 5.84 ± 0.26 | 0.21 ± 0.13 | 5.85 ± 0.22 | 5.63 ± 0.24 | −0.22 ± 0.10 | |||
| LDL cholesterol (mmol/L) | |||||||||
| HET | 3.13 ± 0.23 | 3.12 ± 0.20 | −0.01 ± 0.16 | 3.09 ± 0.16 | 2.73 ± 0.19 | −0.36 ± 0.18 | 0.006 | 0.073 | 0.403 |
| Control | 3.20 ± 0.17 | 3.50 ± 0.21 | 0.31 ± 0.10 | 3.43 ± 0.19 | 3.11 ± 0.19 | −0.32 ± 0.10 | |||
| HDL cholesterol (mmol/L) | |||||||||
| HET | 1.37 ± 0.13 | 1.27 ± 0.10 | −0.10 ± 0.06 | 1.48 ± 0.14 | 1.36 ± 0.11 | −0.12 ± 0.07 | 0.232 | 0.057 | 0.438 |
| Control | 1.14 ± 0.06 | 1.21 ± 0.06 | 0.07 ± 0.05 | 1.25 ± 0.08 | 1.19 ± 0.06 | −0.06 ± 0.06 | |||
| Triglycerides (mmol/L) | |||||||||
| HET | 1.31 ± 0.11 | 1.55 ± 0.29 | 0.24 ± 0.19 | 1.62 ± 0.23 | 1.52 ± 0.25 | −0.10 ± 0.14 | 0.210 | 0.597 | 0.001 |
| Control | 2.91 ± 0.45 | 2.51 ± 0.37 | −0.40 ± 0.16 | 2.63 ± 0.44 | 2.97 ± 0.48 | 0.34 ± 0.15 | |||
All values are means ± SEMs; n = 10 (HET) and 15 (Control). Day 1 represents baseline, and Day 29 represents the endpoint of each 4-wk treatment period. HET, phytosterolemia heterozygote.
Differences between groups and treatments were assessed by repeated-measures 2-factor ANOVA; P < 0.05 is considered significant.
Plasma PS concentrations in response to PS and placebo treatments are presented in Table 4. Plasma β-sitosterol concentrations in both group were mildly affected (P > 0.05) by PS supplementation (increased by ∼9% in the HET group and by ∼6% in the control group). However, plasma campesterol concentrations increased (P < 0.05) in both groups (HET group: ∼40%; control group: ∼28%). Overall, plasma PS concentrations (campesterol+β-sitosterol) increased (P < 0.05) by ∼28% and ∼20% in the HET and control groups, respectively, after PS supplementation. Plasma lathosterol concentration, a cholesterol precursor sterol, was not significantly different between groups or treatments.
TABLE 4.
Plasma plant sterol concentrations at baseline and after 4 wk of treatment1
| Placebo |
Plant sterols |
P value2 |
|||||||
| Plant sterol variables and group | Day 1 | Day 29 | Change | Day 1 | Day 29 | Change | Treatment | Group | Interaction |
| β-Sitosterol (μmol/L) | |||||||||
| HET | 10.77 ± 1.60 | 10.79 ± 1.36 | 0.02 ± 0.97 | 12.44 ± 1.40 | 13.57 ± 1.59 | 1.12 ± 1.37 | 0.617 | 0.886 | 0.734 |
| Control | 8.72 ± 1.00 | 9.01 ± 1.17 | 0.29 ± 1.49 | 7.86 ± 0.88 | 8.36 ± 0.82 | 0.50 ± 0.80 | |||
| Campesterol (μmol/L) | |||||||||
| HET | 17.15 ± 2.28 | 16.53 ± 2.47 | −0.62 ± 1.81 | 18.66 ± 2.30 | 26.15 ± 4.75 | 7.49 ± 3.56 | 0.008 | 0.381 | 0.176 |
| Control | 11.57 ± 1.21 | 12.11 ± 1.19 | 0.53 ± 0.80 | 12.29 ± 1.11 | 15.67 ± 1.10 | 3.37 ± 0.89 | |||
| β-Sitosterol+campesterol (μmol/L) | |||||||||
| HET | 27.92 ± 3.49 | 27.32 ± 3.80 | −0.60 ± 2.20 | 31.10 ± 3.31 | 39.72 ± 6.05 | 8.61 ± 4.58 | 0.030 | 0.496 | 0.256 |
| Control | 20.29 ± 2.00 | 21.12 ± 2.05 | 0.83 ± 1.97 | 20.15 ± 1.87 | 24.03 ± 1.65 | 3.88 ± 1.31 | |||
| Lathosterol (μmol/L) | |||||||||
| HET | 8.61 ± 0.82 | 7.30 ± 1.12 | −1.31 ± 1.09 | 8.00 ± 0.71 | 8.34 ± 0.57 | 0.33 ± 0.46 | 0.816 | 0.710 | 0.614 |
| Control | 10.0.42 ± 1.09 | 10.17 ± 1.37 | −0.25 ± 1.23 | 11.48 ± 1.05 | 10.79 ± 0.98 | −0.61 ± 0.95 | |||
All values are means ± SEMs; n = 10 (HET) and 15 (Control). Day 1 represents baseline, and Day 29 represents the endpoint of each 4-wk treatment period. HET, phytosterolemia heterozygote.
Differences between groups and treatments were assessed by repeated-measures 2-factor ANOVA; P < 0.05 is considered significant.
Cholesterol absorption and synthesis responses to treatment
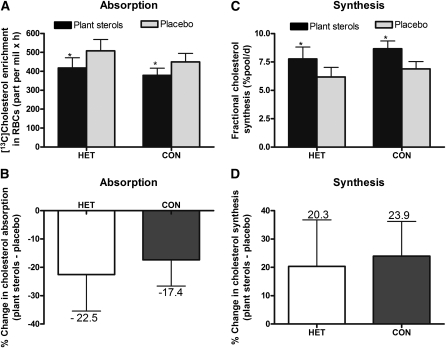
Cholesterol absorption, as measured by the area under the [3,4-13C]cholesterol RBC enrichment curve, decreased (P = 0.010) in both groups in response to PS consumption compared with placebo (Figure 1). No difference (P = 0.445) in the magnitude of cholesterol absorption reduction was observed between groups (Figure 1).
FIGURE 1.
Mean (±SEM) cholesterol absorption as measured by area under the [13C]cholesterol red blood cell enrichment curve over 72 h after ingestion of 75 mg [3,4-13C]cholesterol in response to consumption of plant sterols compared with placebo capsules in phytosterolemia heterozygotes and control cohorts (A), the percentage change in cholesterol absorption between treatments within group (B), the fractional synthesis rate of cholesterol measured at 24 h by deuterium incorporation after deuterium oxide consumption (C), and the percentage change in cholesterol synthesis between treatments within group (D). Differences between groups and treatments were assessed by repeated-measures 2-factor ANOVA; *P < 0.05 indicates a significant difference between treatments within group. A: P-interaction = 0.732. C: P-interaction = 0.904. CON, control group (n = 15); HET, phytosterolemia heterozygote group (n = 10); RBCs, red blood cells.
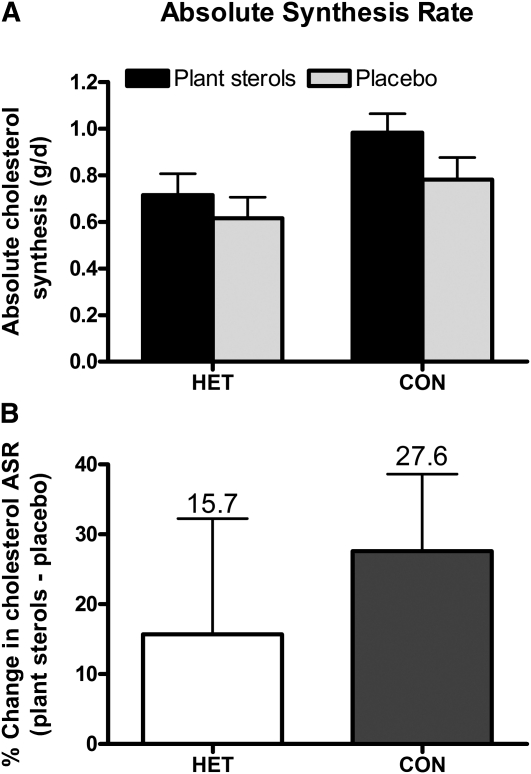
Cholesterol FSR, which represents the proportion of the rapidly turning over pool of cholesterol synthesized per day, increased (P = 0.040) in both groups in responses to PS supplementation (Figure 1). The magnitude of the increase in cholesterol FSR due to PS consumption did not differ between groups (P = 0.330). Similarly, cholesterol ASR, the absolute amount of de novo cholesterol synthesized per day, showed a trend (P = 0.070) toward increased rates in both groups as a result of PS consumption (Figure 2).
FIGURE 2.
Mean (±SEM) absolute synthesis rates of cholesterol in response to consumption of plant sterol compared with placebo capsules in phytosterolemia heterozygotes and control cohorts (A) and the percentage change in the absolute synthesis rate of cholesterol between treatments within group (B). Differences between groups and treatments were assessed by repeated-measures 2-factor ANOVA; P < 0.05 indicates a significant difference between treatments within group. A: P-interaction = 0.522. ASR, absolute synthesis rate; CON, control group (n = 15); HET, phytosterolemia heterozygote group (n = 10).
DISCUSSION
The current work is the first study, to our knowledge, to use stable-isotope techniques in a population that is heterozygous for phytosterolemia, to show that there were no significant differences in cholesterol absorption efficiency or FSRs between these individuals and a control group consuming PS supplements, despite differences in plasma PS concentrations between the groups. In the current study, consumption of ∼1.6 g PS/d for 4 wk reduced serum total cholesterol and LDL-cholesterol concentrations in both groups, which agrees with other PS consumption studies (13, 15, 25, 26). Previous studies in phytosterolemia heterozygotes have shown that 2–3 g PS/d in margarine for 4 to 16 wk resulted in a reduction in plasma cholesterol concentrations (13, 15). The findings of the current work, which highlights the reductions in circulating LDL-cholesterol concentrations and cholesterol absorption, support the hypothesis that PS produce their LDL-cholesterol-lowering effect in part by inhibiting the intestinal absorption of cholesterol, which is postulated to occur via decreases in the cholesterol contents of intestinal mixed lipid micelles (27). Furthermore, in the current investigation, we observed a reciprocal increase in cholesterol synthesis in tandem with the suppression in cholesterol absorption. Through regulation of enterohepatic sterol homeostasis, cholesterol absorption and synthesis are believed to adopt a reciprocal relation to sustain the body cholesterol pools (28, 29). Overall, our results are in accordance with those of other studies that used stable-isotope techniques to indicate that consumption of PS reduced cholesterol absorption and consequently increased cholesterol synthesis, with net reduction in LDL-cholesterol concentrations (25, 26).
Some researchers have suggested that circulating PS concentrations may reflect PS absorption efficiency (30). The current study indicated that consumption of PS for 4 wk significantly increased plasma PS (campesterol+β-sitosterol) concentrations in both groups, with a slightly greater increase in the HET group than in the control group. The 28% increase in PS concentrations (campesterol+β-sitosterol) due to PS supplementation noted in this study for the HET group was similar to what has been reported in studies examining the general population (26, 31). Furthermore, although baseline plasma campesterol and β-sitosterol concentrations were higher in the HET group, PS concentrations remained within the ranges reported for the general population (32, 33). Plasma concentrations of β-sitosterol and campesterol in the general population are reported to be in the ranges of 3 to 16 μmol/L and 7 to 28 μmol/L, respectively (32, 33). The work of Salen et al (34), using the dual-radioisotope ratio method, suggests that even if there is enhanced PS (β-sitosterol) absorption in phytosterolemia heterozygotes, overall body pools remain small because of rapid elimination associated with adequate cholesterol synthesis in these individuals. Overall, plasma campesterol and β-sitosterol concentrations in the HET group in the current study were similar to those reported by others (13, 15).
Some researchers suggest that an increase in circulating PS concentrations may increase CVD risk (6–9). One proposition is that serum PS concentrations are seen as significant indirect indexes of cholesterol absorption and synthesis, whereby a higher ratio of serum PS concentrations (β-sitosterol and campesterol) was shown to be positively associated with fractional absorption of dietary cholesterol and negatively with cholesterol synthesis in humans (16). Higher serum PS (β-sitosterol+campesterol) concentrations in our HET group did not indicate higher cholesterol absorption and/or a greater reduction in cholesterol synthesis in this group than in the control cohort. Overall, the results indicate that cholesterol absorption, as measured by using [13C]cholesterol, was consistently ∼13% (placebo treatment) to 10% (PS treatment) higher in the HET group than in the control group; however, this finding was not statistically significant. In addition, the FSR, as measured by assessing deuterium incorporation into newly synthesized cholesterol, was consistently ∼10% (both treatments) lower in the HET group than in the control group; again, this was not found to be significant. Moreover, these observed differences in plasma PS did not affect the magnitude of the plasma LDL-cholesterol responses to PS supplementation between the groups. The basal serum ratio of campesterol to cholesterol, a surrogate marker of intestinal cholesterol absorption, has been reported to predict the LDL-cholesterol-lowering response to PS (35). Houweling et al (36) found that baseline plasma PS concentrations did not determine changes in serum lipids and plasma PS in hypercholesterolemia individuals after intake of 2 g/d of PS-enriched food. The findings of the current study, as it relates to LDL-cholesterol concentrations, agree with those from the study by Houweling et al (36). Additionally, an interesting finding from the current study was that, despite the higher plasma PS (β-sitosterol+campesterol) concentrations in the HET group, overall, these individuals had a relatively favorably lipid profile compared with the control cohort. For instance, plasma triglyceride concentrations were significantly higher in the control group throughout the study.
On the basis of the hypothesis that plasma PS concentrations are good surrogate markers of cholesterol absorption and synthesis, findings from some studies suggest that heterozygosity of a mutation or single nucleotide polymorphisms of the ABCG8 gene may influence PS concentrations, which suggests that the existence of a relation between cholesterol absorption and synthesis may be mediated by ABCG8. Identification of an exon 2 mutation in ABCG8 19H was found to be associated with increased plasma PS (campesterol and β-sitosterol) concentrations and decreased lathosterol concentrations, with the suggestion by the researchers that such a PS profile will enhance cholesterol absorption with a reciprocal decrease in synthesis (37). In the current study, heterozygosity for the ABCG8 S107X mutation was found to be associated with increased plasma PS (campesterol+β-sitosterol) concentrations and lower lathosterol concentrations; however, using stable-isotope techniques we found no significant effects on cholesterol absorption or fractional synthesis in the HET group compared with the control group.
In conclusion, the current work suggests that, although the ABCG8 S107X heterozygous mutation affected plasma PS concentrations, there seems to be a lack of effect of this mutation on cholesterol metabolism. Thus, PS supplementation modulated cholesterol absorption efficiency and cholesterol FSRs to similar degrees in the HET subjects and their control cohort, which produced similar significant reductions in circulating LDL-cholesterol concentrations.
Acknowledgments
We gratefully thank Sam Guo (Nutragenius Inc, Ontario, Canada) for donating the plant sterols; the study participants for their contribution; Candice Cryne, Caitlin McFadden, and Meriam Mohammed for clinical and technical assistance; and Peter Eck, Natalia Yurkova, Haifeng Yang, and Dylan MacKay for technical assistance.
The authors’ responsibilities were as follows—PJHJ, SBM, and DM: designed the research; SBM: conducted the research, analyzed the data, and wrote the manuscript; BT-R: participated in the data analysis; and SBM and PJHJ: had primary responsibility for final content. All authors participated in editing and approving the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: ABC, adenosine triphosphate binding cassette transporter; ASR, absolute synthesis rate; CVD, cardiovascular disease; FSR, fractional synthesis rate; HET, phytosterolemia heterozygotes; PCR, polymerase chain reaction; PS, plant sterols; RBC, red blood cell.
REFERENCES
- 1.Stanner S, ed. British Nutrition Foundation. Cardiovascular disease: diet, nutrition and emerging risk factors 1st ed. Oxford, UK: Blackwell Publishing Ltd, 2005 [Google Scholar]
- 2.Abumweis SS, Barake RI, Jones PJ. Plant sterols/stanols as cholesterol lowering agents: a meta-analysis of randomized controlled trials. Food Nutr Res; 2008:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heart and Stroke Foundation of Canada Living with cholesterol. cholesterol and healthy living. Ottawa, CA: Heart and Stroke Foundation of Canada, 2005 [Google Scholar]
- 4.American Heart Association. Diet and lifestyle recommendations. Dallas, TX: AHA, 2009 [Google Scholar]
- 5.Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227–39 [DOI] [PubMed] [Google Scholar]
- 6. Hansel B, Courie R, Bayet Y, Delestre F, Bruckert E. [Phytosterols and atherosclerosis.] Rev Med Interne 2010;32:124ndash9s. [DOI] [PubMed]
- 7.Glueck CJ, Speirs J, Tracy T, Streicher P, Illig E, Vandegrift J. Relationships of serum plant sterols (phytosterols) and cholesterol in 595 hypercholesterolemic subjects, and familial aggregation of phytosterols, cholesterol, and premature coronary heart disease in hyperphytosterolemic probands and their first-degree relatives. Metabolism 1991;40:842–8 [DOI] [PubMed] [Google Scholar]
- 8.Sudhop T, Gottwald BM, von Bergmann K. Serum plant sterols as a potential risk factor for coronary heart disease. Metabolism 2002;51:1519–21 [DOI] [PubMed] [Google Scholar]
- 9.Assmann G, Cullen P, Erbey J, Ramey DR, Kannenberg F, Schulte H. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the Prospective Cardiovascular Munster (PROCAM) study. Nutr Metab Cardiovasc Dis 2006;16:13–21 [DOI] [PubMed] [Google Scholar]
- 10.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 2000;290:1771–5 [DOI] [PubMed] [Google Scholar]
- 11.Lee MH, Lu K, Patel SB. Genetic basis of sitosterolemia. Curr Opin Lipidol 2001;12:141–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V. Sitosterolemia. J Lipid Res 1992;33:945–55 [PubMed] [Google Scholar]
- 13.Kwiterovich PO, Jr, Chen SC, Virgil DG, Schweitzer A, Arnold DR, Kratz LE. Response of obligate heterozygotes for phytosterolemia to a low-fat diet and to a plant sterol ester dietary challenge. J Lipid Res 2003;44:1143–55 [DOI] [PubMed] [Google Scholar]
- 14.Salen G, Xu G, Tint GS, Batta AK, Shefer S. Hyperabsorption and retention of campestanol in a sitosterolemic homozygote: comparison with her mother and three control subjects. J Lipid Res 2000;41:1883–9 [PubMed] [Google Scholar]
- 15.Stalenhoef AF, Hectors M, Demacker PN. Effect of plant sterol-enriched margarine on plasma lipids and sterols in subjects heterozygous for phytosterolaemia. J Intern Med 2001;249:163–6 [DOI] [PubMed] [Google Scholar]
- 16.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol 1990;131:20–31 [DOI] [PubMed] [Google Scholar]
- 17.Mymin D, Wang J, Frohlich J, Hegele RA. Aortic xanthomatosis with coronary ostial occlusion in a child homozygous for a nonsense mutation in ABCG8. Circulation 2003;107:791. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Joy T, Mymin D, Frohlich J, Hegele RA. Phenotypic heterogeneity of sitosterolemia. J Lipid Res 2004;45:2361–7 [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 20.Wang Y, Vanstone CA, Parsons WD, Jones PJ. Validation of a single-isotope-labeled cholesterol tracer approach for measuring human cholesterol absorption. Lipids 2004;39:87–91 [DOI] [PubMed] [Google Scholar]
- 21.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 22.Jones PJ. Use of deuterated water for measurement of short-term cholesterol synthesis in humans. Can J Physiol Pharmacol 1990;68:955–9 [DOI] [PubMed] [Google Scholar]
- 23.Jones PJH, Ausman LM, Croll DH, Feng JY, Schaefer EA, Lichtenstein AH. Validation of deuterium incorporation against sterol balance for measurement of human cholesterol biosynthesis. J Lipid Res 1998;39:1111–7 [PubMed] [Google Scholar]
- 24.Goodman DS, Smith FR, Seplowitz AH, Ramakrishnan R, Dell RB. Prediction of the parameters of whole body cholesterol metabolism in humans. J Lipid Res 1980;21:699–713 [PubMed] [Google Scholar]
- 25.AbuMweis SS, Vanstone CA, Lichtenstein AH, Jones PJ. Plant sterol consumption frequency affects plasma lipid levels and cholesterol kinetics in humans. Eur J Clin Nutr 2009;63:747–55 [DOI] [PubMed] [Google Scholar]
- 26.Jones PJ, Raeini-Sarjaz M, Ntanios FY, Vanstone CA, Feng J, Parsons WE. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J Lipid Res 2000;41:697–705 [PubMed] [Google Scholar]
- 27.Ikeda I, Tanabe Y, Sugano M. Effects of sitosterol and sitostanol on micellar solubility of cholesterol. J Nutr Sci Vitaminol (Tokyo) 1989;35:361–9 [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM, Ahrens EH, Jr, Davignon J. The interaction of cholesterol absorption and cholesterol synthesis in man. J Lipid Res 1969;10:304–15 [PubMed] [Google Scholar]
- 29.Santosa S, Varady KA, AbuMweis S, Jones PJ. Physiological and therapeutic factors affecting cholesterol metabolism: does a reciprocal relationship between cholesterol absorption and synthesis really exist? Life Sci 2007;80:505–14 [DOI] [PubMed] [Google Scholar]
- 30.Sudhop T, von Bergmann K. Sitosterolemia—a rare disease. Z Kardiol 2004;93:921–8 [DOI] [PubMed] [Google Scholar]
- 31.Maki KC, Davidson MH, Umporowicz DM, Schaefer EJ, Diklin MR, Ingram KA, Chen S, McNamara W Jr, Gebhart B, Ribaya-Mercado JD, et al. Lipid responses to plant sterol-enriched reduced-fat spreads incorporated into a National Cholesterol Education step 1 diet. Am J Clin Nutr 2001;74:33–43 [DOI] [PubMed] [Google Scholar]
- 32.Chan YM, Varady KA, Lin Y, Trautwein E, Mensink RP, Plat J, Jones PJ. Plasma concentrations of plant sterols: physiology and relationship with coronary heart disease. Nutr Rev 2006;64:385–402 [DOI] [PubMed] [Google Scholar]
- 33.Weingärtner O, Böhm M, Laufs U. Controversial role of plant sterol esters in the management of hypercholesterolemia. Eur Heart J 2009;30:404–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salen G, Tint GS, Shefer S, Shore V, Nguyen L. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler Thromb 1992;12:563–8 [DOI] [PubMed] [Google Scholar]
- 35.Fuentes F, Lopez-Miranda J, Garcia A, Perez-Martinez P, Moreno J, Cofan M, Caballero J, Paniagua JA, Ros E, Perez-Jimenez F. Basal plasma concentrations of plant sterols can predict LDL-C response to sitosterol in patients with familial hypercholesterolemia. Eur J Clin Nutr 2008;62:495–501 [DOI] [PubMed] [Google Scholar]
- 36.Houweling AH, Vanstone CA, Trautwein EA, Duchateau GS, Jones PJ. Baseline plasma plant sterol concentrations do not predict changes in serum lipids, C-reactive protein (CRP) and plasma plant sterols following intake of a plant sterol-enriched food. Eur J Clin Nutr 2009;63:543–51 [DOI] [PubMed] [Google Scholar]
- 37.Sehayek E, Yu HJ, von Bergmann K, Lutjohann D, Stoffel M, Duncan EM, Garcia-Naveda L, Salit J, Blundell ML, Friedman JM, et al. Phytosterolemia on the island of Kosrae: founder effect for a novel ABCG8 mutation results in high carrier rate and increased plasma plant sterol levels. J Lipid Res 2004;45:1608–13 [DOI] [PubMed] [Google Scholar]