Abstract
Background: We recently discovered that infants randomly assigned to a formula high in free amino acids (extensive protein hydrolysate formula; ePHF) during infancy consumed less formula to satiation and gained less weight than did infants fed an isocaloric formula low in free amino acids (cow milk formula; CMF).
Objective: Because ePHF and CMF differ markedly in concentrations of free glutamate, we tested the hypothesis that the higher glutamate concentrations in ePHF promote satiation and satiety.
Design: In this counterbalanced, within-subject study, infants <4 mo of age (n = 30) visited our laboratory for 3 sets of 2 consecutive infant-led formula meals over 3 test days. Infants were fed 1 of 3 isocaloric formulas during each first meal: CMF, ePHF, or CMF with added free glutamate to approximate concentrations in ePHF (CMF+glu). When infants signaled hunger again, they were fed a second meal of CMF. From these data, we calculated satiety ratios for each of the 3 formulas by dividing the intermeal interval by the amount of formula consumed during that particular first meal.
Results: Infants consumed significantly less CMF+glu (P < 0.02) and ePHF (P < 0.04) than CMF during the first meals. They also showed greater levels of satiety after consuming CMF+glu or ePHF: satiety ratios for CMF+glu (P < 0.03) and ePHF (P < 0.05) were significantly higher than for CMF.
Conclusion: These findings suggest a role of free glutamate in infant intake regulation and call into question the claim that formula feeding impairs infants’ abilities to self regulate energy intake. This trial was registered at clinicaltrials.gov as NCT00957892.
INTRODUCTION
Several recent studies have shown that accelerated weight gain during the first year of life increases the risk of later obesity (1), metabolic syndrome (2), and mortality from cardiovascular disease (3), which leads some to argue that early life should be the focus for both preventive intervention and further scientific inquiry (4). Previous research has shown that infants fed formula (the vast majority feed CMF5; 5) weigh more by the end of the first year (6, 7) and have greater risk of later obesity than do breastfed infants (8). Some have hypothesized that the higher protein content of formula relative to breast milk is responsible for the greater weight gain of formula-fed infants during later infancy and early childhood (6). Indeed, a recent clinical trial illustrated that infants randomly assigned to feed CMF and follow-on formula, both of which were high in protein, had significantly higher weight-for-age z scores by 2 y of age than did infants randomly assigned to feed a lower-protein CMF and a lower-protein follow-on formula, even when energy intake was controlled for (9).
Emerging research suggests that the form of protein may be as important as the amount of protein in infant formula. In clinical studies of infants randomly assigned to consume either CMF (which is low in FAAs and small peptides; 10, 11) or an isocaloric ePHF (a formula abundant in FAAs and small peptides; 10, 12), weight gain in the CMF-fed infants was accelerated, whereas that of ePHF-fed infants was normative to that of breastfed infants (13, 14). Furthermore, ePHF-fed infants satiated on smaller volumes of formula than did CMF-fed infants during monthly laboratory-based, infant-led feeding sessions (13, 15, 16–18). Thus, formula-fed infants are not a homogeneous group (13).
The mechanisms underlying how infant formulas of different composition affect growth are unclear. Whereas CMF and ePHF have similar percentages of energy from fat and vitamin and mineral contents, ePHF contains slightly more protein than does CMF (11.0% compared with 8.5%); the protein form is a major differentiator (intact compared with extensively hydrolyzed protein) (11, 12). The percentage of energy from carbohydrate also differs (41.0% and 43.5%, respectively) (11, 12). The largest difference between CMF and ePHF is in the FAA profiles, which may affect feeding behaviors and growth. Concentrations of FAAs in ePHF are 120-fold higher and more diversified than those in CMF. Only a few FAAs are present at detectable concentrations in CMF, with taurine being the most abundant (∼7 mg/100 mL) (10). In contrast, almost all types of FAAs can be detected in ePHF, with the most abundant being leucine (∼156 mg/100 mL), glutamate (∼107 mg/100 mL), lysine (∼121 mg/100 mL), and valine (∼82 mg/100 mL) (10).
The objective of this study was to examine whether differences in the concentrations of one FAA—glutamate—are sufficient to produce the intake differences observed when infants consume CMF compared with ePHF in the short term (13, 15, 16). Specifically, we tested the hypothesis that infants will feed lower amounts of formula to satiation and will show greater levels of satiety when fed formulas with higher concentrations of free glutamate than when fed an isocaloric CMF. We investigated this amino acid first because of evidence that free glutamate serves as a key signal for satiation in adults and animal studies (19–21) and because it is of particular relevance for infant feeding, given that it is the most abundant FAA in human breast milk (22).
SUBJECTS AND METHODS
Subjects
Thirty primary caregivers (29 mothers, 1 father) with healthy infants who were younger than 4 mo were recruited through ads in local newspapers, websites, and Philadelphia WIC offices. Only infants who had never been exposed to ePHF but who had previously consumed CMF were considered for the study. At the time of testing, most of the infants (60%) were currently being fed Nestlé GoodStart, 10% Enfamil (Mead Johnson Nutrition), and the remaining infants Similac (Abbott) or Nestle GoodStart Soy.
Infants who were born preterm or had medical conditions that interfered with feeding were excluded. Thirteen additional dyads were recruited but excluded because they did not complete all 3 testing days or did not comply with study procedures (eg, the parent wanted to feed the infant before he or she exhibited hunger cues). All study procedures were approved by the committee on studies involving human subjects at the University of Pennsylvania, and informed consent was obtained from each parent at study entry.
Test formulas
Three isocaloric (68 kcal/100 mL) formulas were used: CMF (Enfamil; Mead Johnson Nutrition), ePHF (Nutramigen; Mead Johnson Nutrition), and CMF with added l-glutamic acid (monosodium salt, monohydrate, MSG; 105 mg/100 mL; USBioAnalyzed), hereafter referred to as CMF+glu. This concentration of MSG increased free glutamate in CMF to 84 mg/100 mL, which was slightly lower than that found in ePHF (107 mg/100 mL) but allowed for similar concentrations (18–32 mg/100 mL) and molarities (8–14 mmol/L) of sodium in CMF, CMF+glu, and ePHF. We note that because breastfed infants ingest ∼23 mg free glutamate within a given meal of 150 mL breast milk (23, 24), the amount added to CMF would be far below what a breastfed infant would consume over the course of a typical day of breastfeeding. The nutritional composition of the test formulas is shown in Table 1.
TABLE 1.
Nutritional composition of the test formulas1
| CMF(Enfamil2) | CMF+glu(Enfamil2 + MSG) | ePHF(Nutramigen2) | |
| Energy (kcal/100 mL) | 67.7 | 67.7 | 67.7 |
| Fat (g/100 mL) | 3.6 | 3.6 | 3.6 |
| Carbohydrate (g/100 mL) | 7.4 | 7.0 | 7.0 |
| Protein (g/100 mL) | 1.4 | 1.4 | 1.9 |
| Free glutamate (mg/100 mL) | 1.83 | 84.14 | 106.53 |
| Sodium (mg/100 mL) | 18.3 | 31.14 | 31.8 |
CMF, cow milk formula; CMF+glu, cow milk formula with added free glutamate; ePHF, extensive protein hydrolysate formula; MSG, monosodium glutamate.
Macronutrient and sodium data obtained from the manufacturer's website: Enfamil (http://www.mjn.com/app/iwp/hcp2/content2.do?dm=mj&id=HCP_Home2/ProductInformation/hcpProducts/hcpInfants/hcpEnfamilLIPIL&iwpst=MJN&ls=0&csred=1&r=3477321385) and Nutramigen (http://www.mjn.com/app/iwp/hcp2/content2.do?dm=mj&id=/HCP_Home2/ProductInformation/hcpProducts/hcpInfants/hcpNutramigen&iwpst=MJN&ls=0&csred=1&r=3477321341) (cited 26 July 2011).
Free glutamate content determined by HPLC analysis (10).
From the addition of 104.7 mg/100 mL of l-glutamic acid, monosodium salt, and monohydrate, which contains 84.1 mg glutamic acid/100 mL and 12.9 mg Na/100 mL.
Before the infants were tested, a trained sensory panel of 10 adults (9 women, 1 man) aged 22–36 y (mean: 28 ± 5 y) evaluated the 3 formulas using the gLMS (25, 26). These adult panelists were recruited through ads in local newspapers and websites, and none were parents of the infants tested in the study. The gLMS is a psychophysical tool that allows subjects to rate perceived intensity along a vertical axis lined with adjectives that are spaced semilogarithmically, based on experimentally determined intervals, to yield data that parallel magnitude estimations. Panelists rated each formula for the basic taste dimensions of bitterness, saltiness, savoriness, sourness, sweetness, and pleasantness.
Procedures
Our study design enabled us to measure the effect of formula type on both satiation (amount consumed within the first formula meal) and satiety (prolonged effect of the first formula meal on a subsequent meal). The methods used were developed and validated at the Monell Center and controlled for many factors to allow evaluation of infants’ hedonic and behavioral responses independent of the parent and experimenter (15, 27, 28).
We accustomed infants to various aspects of the study protocol before testing. Parents were sent bibs, bottles, and masks to use while feeding their infants at home during the 3 d that preceded the first testing session. They were asked to refrain from introducing additional foods or liquids to their infants before and during the experimental period. To encourage compliance, parents kept a daily record of what they fed their infants. Each of the 3 testing sessions occurred at the same time of day to control for circadian rhythmicity.
Each parent-infant dyad came to the Monell Center on 3 separate days for ∼6 h each day. The testing days were separated by, on average, 2.4 ± 0.4 d. On arrival to the Monell Center, parents were asked to change their infants into a light-weight cotton bodysuit to control for clothing thickness. Each infant was then weighed and measured for length, after which a Mini Motionlogger Actigraph (Ambulatory Monitoring Ltd) was placed on the infant's left ankle. Motility levels were sampled in the zero-crossing mode at a constant rate of 10 Hz. In this mode, an activity count was scored each time the infant's leg movement exceeded the sensitivity threshold of the unit. The number of zero crossings was stored in memory in 1-min epochs and later analyzed by a computer program previously validated with behavioral observational state taxonomy (29, 30).
During each of the testing days, we videotaped infants feeding 2 formula meals (hereafter referred to as the first and second formula meals); videotapes were not obtained for the second formula meal for one infant on one test day and for another infant on 2 test days. As shown in Table 2, the testing days differed by the type of formula fed to the infants during the first formula meal (CMF, CMF+glu, ePHF) but not during the the second formula meal, which was always CMF. The type of formula offered at the first formula meal was randomized and counterbalanced across the 3 d of testing. Parents were blinded to the type of formula offered and were unaware of the hypothesis being tested.
TABLE 2.
Schedule of testing events1
| Day2 | First formula meal | Second formula meal |
| A | CMF | CMF |
| B | CMF+glu | CMF |
| C | ePHF | CMF |
CMF, cow milk formula; CMF+glu, cow milk formula with added free glutamate; ePHF, extensive protein hydrolysate formula.
Parent-infant dyads participated in 3 d of testing (A, B, and C) that differed by the type of formula fed during the first formula meal.
To ensure that all feedings were infant-led, parents were instructed to 1) feed the infant at his or her customary pace until the infant signaled satiation and 2) wear the mask used at home and refrain from talking to eliminate any potential influence of the facial or verbal responses on infant behaviors. Infants were allowed to feed ad libitum. If the infant finished a bottle, the experimenter immediately gave the parent another bottle so as not to limit infant intake by formula availability. Infant consumption was assessed by weighing the bottles before and after each formula meal on a top-loading balance (model PM 15; Mettler).
The first formula meal began when infants displayed signs of hunger (eg, sucking on hands, rooting, or fussing). At that time, parents were videotaped as they fed their infants CMF, CMF+glu, or ePHF to satiation. To ensure that infants had reached satiation, infants were offered a bottle of CMF after a 5-min rest and allowed to consume this formula ad libitum, if desired. No differences were observed in the amount of CMF infants consumed after being fed CMF, ePHF, or CMF+glu (F2,59 = 1.59, P = 0.21), which indicates that infants were equally satiated by all formulas. Dyads remained at Monell until the infants signaled hunger again, after which infants were fed CMF to satiation for the second formula meal by using the methods described above.
Immediately after each formula meal, parents were asked to rate on a 9-point scale how much they thought the infant enjoyed the formula (1 = extreme dislike), how similar the formula meal was to the infant's typical formula feeding (1 = not at all similar), and how similar the amount of formula consumed was to the infant's typical formula feeding (1 = much less than usual). During the intermeal interval, infants were allowed to rest or sleep in a car seat or crib located in our testing room, and parents completed the Infant Feeding Questionnaire, a 79-item instrument that assesses parental feeding practices and beliefs (31), and the Early Infancy Temperament Questionnaire, an 86-item instrument that assesses parents’ perceptions of their infants’ temperament (32).
Statistical analysis
This study was powered on the basis of the published data on intake differences between CMF and ePHF in infants who were between the ages of 1 and 2 mo (15). Data from this study of 14 infants illustrated formula intake decreased from 141.9 ± 15.6 mL when infants consumed CMF to 116.1 ± 16.3 mL when infants fed consumed ePHF. The within-person mean difference was significant at the P < 0.05 level (paired t [13 df] = 2.31, P = 0.038). The difference in the response of matched pairs is normally distributed with an SD of 41.9 mL. If the true difference in the mean response of matched pairs is 25.8 mL, we needed to study ≥30 parent-infant dyads to be able to reject the null hypothesis that this response difference is zero with a probability (power) of 0.90. The type I error probability associated with this test of this null hypothesis is 0.05.
All analyses were conducted by using SAS version 9.1. All data were assessed for normality. Infant weight and length measurements were normalized by using Epi-Info software version 3.5.2 (http://www.cdc.gov/epiinfo/) to calculate age- and sex-specific z scores and percentiles based on the Centers for Disease Control and Prevention growth references (33), which are based on growth data from predominantly formula-fed infants. Taste panel data were analyzed by separate ANOVAs with Fisher's least-significant-difference post hoc comparisons to determine whether there were differences between formulas for each taste quality and overall pleasantness.
Primary dependent variables for the infant feeding study were 1) intake during each formula meal (mL), 2) duration of each formula meal (min), 3) mean activity count during each formula meal, and 4) intermeal interval, determined by the length of time (min) separating the end of the first formula meal and the beginning of the second formula meal. We also calculated the satiety ratio for each of 3 formulas by dividing the intermeal interval (in min) by the amount of formula (in mL) consumed during the first meal for that particular formula (34).
Repeated-measures ANOVAs were conducted to assess the effects of formula type (CMF, CMF+glu, ePHF) on intake during the first and second formula meals and the remaining primary dependent variables. A priori contrasts were specified to focus on differences between 1) CMF+glu and CMF and 2) ePHF and CMF. We also explored the possible modifying effects of infant temperament and parent feeding practices and beliefs on infant feeding behaviors. The amount of time elapsed since the last feeding (before the infants’ arrival to our laboratory, as reported by parents) and infant age were covaried in the analyses. Preliminary analyses showed that infant sex and weight-for-age z score were not significant covariates for any of the primary dependent variables; thus, they were not included in the statistical models.
RESULTS
Sensory evaluation of formulas
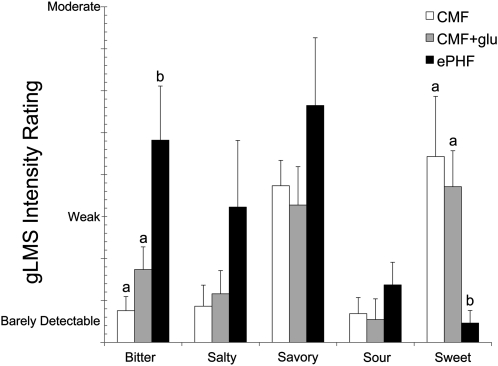
The adult taste panelists perceived ePHF to be significantly more bitter (F2,18 = 10.40, P < 0.001) and less sweet (F2,18 = 8.56, P < 0.003) than CMF and CMF+glu; they reported no differences between CMF and CMF+glu for any of the taste qualities (Figure 1). ePHF was also rated significantly less pleasant (F2,18 = 6.36, P < 0.009) than CMF and CMF+glu, which did not differ from each other (data not shown).
FIGURE 1.
Mean (±SEM) ratings during the sensory evaluation of the formulas used during infant testing. A trained panel of 10 adults rated the sweetness, savoriness, saltiness, sourness, and bitterness of CMF (white bars), CMF+glu (gray bars), and ePHF (black bars) using the gLMS, a psychophysical tool that allows subjects to rate the perceived intensity of sensations that are arranged semilogarithmically and range from “no sensation” to “strongest imaginable” (eg, 0 = no sensation, 1 = barely detectable, 6 = weak, and 16 = moderate). Bars with different lowercase letters are significantly different, P < 0.05 (ANOVA with Fisher's least-significant-difference post hoc comparisons). CMF, cow milk formula; CMF+glu, cow milk formula with added free glutamate; ePHF, extensive protein hydrolysate formula; gLMS, general labeled magnitude scale.
Subject characteristics
The demographic information for the 30 parent-infant dyads included in the data analysis is shown in Table 3. Infants were between the ages of 1.0 and 3.7 mo (8.5 ± 0.5 wk) of age at study entry, and a nearly equal number of boys (n = 14) and girls (n = 16) were tested.
TABLE 3.
Subject characteristics1
| Value | |
| Infant characteristics | |
| Age at study entry (wk) | 8.5 ± 0.52 |
| Sex [% girls (n/N)] | 53.3 (16/30) |
| Weight-for-length percentile at birth | 32.3 ± 6.1 |
| Weight-for-length percentile at study entry | 63.9 ± 4.9 |
| Parent characteristics | |
| Age (y) | 28.3 ± 1.0 |
| BMI (kg/m2) | 28.9 ± 1.1 |
| Education level [% (n/N)] | |
| High school | 46.7 (14/30) |
| Some college or vocational degree | 20.0 (6/30) |
| Bachelors or graduate degree | 33.3 (10/30) |
| Family income level [% (n/N)]3 | |
| <$15,000/y | 27.6 (8/29) |
| $15,000 to <$35,000/y | 20.7 (6/29) |
| $35,000 to <$75,000/y | 34.5 (10/29) |
| ≥$75,000/y | 17.2 (5/29) |
| Race-ethnicity [% (n/N)] | |
| Non-Hispanic white | 23.3 (7/30) |
| Non-Hispanic black | 56.7 (17/30) |
| Asian | 3.3 (1/30) |
| Hispanic white | 16.7 (5/30) |
n = 30.
Mean ± SEM (all such values).
n = 29; one parent chose not to answer this question.
Formula meals
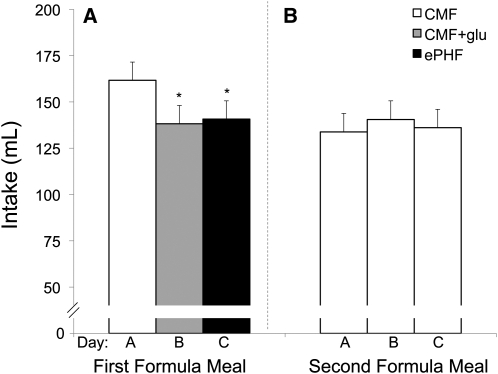
The type of formula the infants were fed affected how much they consumed to satiation during the first formula meal. Infants consumed significantly less CMF+glu (t56 = −2.49, P < 0.02) and ePHF (t58 = −2.21, P < 0.04) than CMF during the first formula meal (Figure 2). Intake was significantly correlated with the duration of the feeding (r89 = 0.72; P < 0.001). There was a tendency for infants to feed for longer periods of time when feeding CMF when compared with CMF+glu (t56 = 1.90, P = 0.06) but not ePHF (t57 = 1.11, P = 0.27) (Table 4). No differences in the level of activity were found when the infants were fed CMF+glu (t58 = 0.01, P = 0.99) or ePHF (t58 = 0.46, P = 0.65) compared with CMF (Table 4).
FIGURE 2.
Amount of formula (mL; mean ± SEM) that the infants consumed while feeding CMF (white bars), CMF+glu (gray bars), and ePHF (black bars) during the first formula meal (A) and the amount of CMF (mL) that the infants consumed during the second formula meal (B). Note that whereas the first formula meal differed across the 3 testing days, the second formula meal was always CMF. Repeated-measures ANOVA with a priori planned comparisons between CMF+glu and CMF and between ePHF and CMF indicated that infants consumed significantly less CMF+glu (P < 0.02) and ePHF (P < 0.04) than CMF during the first formula meal. Infants’ intakes during the second formula meal did not differ across the 3 testing days. *Significantly different from CMF, P < 0.05. CMF, cow milk formula; CMF+glu, cow milk formula with added free glutamate; ePHF, extensive protein hydrolysate formula.
TABLE 4.
Infants’ feeding responses and parental perceptions when infants were fed CMF (Day A), CMF+glu (Day B), and ePHF (Day C) during the first formula meal and CMF during the second formula meal for each of these testing days (n = 30)1
| Day A(CMF first meal;CMF second meal) | Day B(CMF+glu first meal;CMF second meal) | Day C(ePHF first meal;CMF second meal) | |
| First formula meal | |||
| Duration (min) | 9.3 ± 0.52 | 8.2 ± 0.5 | 8.7 ± 0.5 |
| Mean activity during feeding | 368.2 ± 18.1 | 368.6 ± 18.1 | 378.8 ± 18.4 |
| Parents’ perceptions | |||
| How much did your infant like the formula?3 | 7.2 ± 0.3 | 7.2 ± 0.3 | 6.3 ± 0.3* |
| How much did your infant eat compared with usual?4 | 5.5 ± 0.3 | 5.6 ± 0.3 | 5.2 ± 0.3 |
| How similar was this feed to your infant's typical formula feeding?5 | 7.6 ± 0.4 | 7.3 ± 0.4 | 7.1 ± 0.4 |
| Intermeal interval (min)6 | 170.5 ± 11.0 | 170.6 ± 11.1 | 163.7 ± 11.2 |
| Satiety ratio7 | 32.2 ± 3.3 | 40.5 ± 3.3* | 38.6 ± 3.3* |
| Second formula meal | |||
| Duration (min) | 8.7 ± 0.6 | 10.1 ± 0.6* | 8.7 ± 0.6 |
| Mean activity during feeding | 213.2 ± 9.2 | 205.6 ± 9.2 | 192.7 ± 9.3 |
| Parents’ perceptions | |||
| How much did your infant like the formula?3 | 6.7 ± 0.4 | 7.0 ± 0.4 | 7.2 ± 0.4 |
| How much did your infant eat compared with usual?4 | 5.5 ± 0.3 | 5.6 ± 0.3 | 5.2 ± 0.3 |
| How similar was this feed to your infant's typical formula feeding?5 | 6.5 ± 0.4 | 6.8 ± 0.4 | 6.8 ± 0.4 |
*Significantly different from CMF, P < 0.05 (repeated-measures ANOVA with a priori planned comparisons between CMF+glu and CMF and between ePHF and CMF). CMF, cow milk formula; CMF+glu, cow milk formula with added free glutamate; ePHF, extensive protein hydrolysate formula.
Mean ± SEM (all such values).
Responses range from 1 (extreme dislike) to 5 (neutral) to 9 (extreme like).
Responses range from 1 (much less than usual) to 5 (about the same) to 9 (much more than usual).
Responses range from 1 (not at all similar) to 9 (very similar).
Defined as time between first and second formula meals.
Defined as intermeal interval (min)/first formula meal intake (mL).
Infants signaled hunger ∼3 h after the first formula meal ended (Table 4). This intermeal interval (ie, time between the first and second formula meals) did not differ when infants were fed CMF+glu (t57 = 0.01, P = 0.99) or ePHF (t58 = −0.61, P = 0.54) compared with when they were fed CMF. Infants showed greater levels of satiety after consuming CMF+glu and ePHF than after consuming CMF, as evidenced by the satiety ratios (intermeal interval in min/amount consumed during the first formula meal in mL), which were significantly higher for CMF+glu (t55 = 2.33, P < 0.03) and ePHF (t57 = 1.78, P = 0.05) than for CMF (Table 4).
The amount of CMF consumed during the second formula meal did not differ as a function of the formula that infants were fed during the first formula meal [CMF+glu (t58 = 0.80, P = 0.43) or ePHF (t60 = 0.27, P = 0.79) compared with CMF] (Figure 2). Thus, when infants consumed less formula during the CMF+glu and ePHF meals, they did not compensate at the subsequent meal, which provides further evidence that, despite ingesting less, they were satiated when they fed these formulas that were higher in free glutamate.
Parent perceptions
As illustrated in Table 4, parents were unaware of any differences in their infants’ behaviors when fed CMF+glu compared with CMF. Parents reported that infants enjoyed CMF+glu as much as CMF but that infants liked the ePHF less than CMF (t59 = −2.18, P < 0.04). Parental perceptions of how much the infant consumed and how similar the feedings were to the infant's typical formula feeding at home did not differ between the 3 formula conditions (Table 4). Finally, parents’ ratings of infant temperament, feeding styles, or feeding attitudes and beliefs were not significant predictors of infant intake during the test meal (data not shown). Thus, the effect of formula type on infant satiation was not modified by infant temperament or parental perceptions, characteristics, and attitudes.
DISCUSSION
Infants consumed less of formulas higher in free glutamate than of an isocaloric formula lower in free glutamate, yet showed equivalent levels of satiation and greater levels of satiety. To our knowledge, this is the first study to examine how infants’ abilities to regulate intake are influenced by variation in the amount of a single FAA in formula. Our findings provide evidence that unbound glutamate, which is abundant in human breast milk (22–24) and in infant formulas with hydrolyzed proteins (10), promotes infant satiation and satiety during formula meals.
The combination of several behaviors showed that infants achieved satiation after feeding less of formulas higher in free glutamate (ePHF and CMF+glu). First, infants consumed significantly less formula on days on which they were given ePHF or CMF+glu during their first formula meal compared with CMF. Second, infants consumed little to no formula when offered CMF 5 min after the feeding ended, regardless of the formula offered during the first formula meal. Third, infants showed no evidence of compensation for lower intakes at the next formula meal: they showed no differences in the average time between the end of the first meal and signaling hunger for the second meal (intermeal interval) or in the amount consumed at the second formula meal. Finally, infants exhibited higher levels of satiety when consuming formulas high in free glutamate, because the satiety ratio was higher for ePHF and CMF+glu than for CMF. Important areas for future research include examining whether expressed breast milk or formula containing free glutamate at concentrations found in breast milk (∼20 mg/100 mL) (23, 24), which are less than that used in the current study, would produce similar effects in infant satiation. Assessment of markers of satiation (35) and gastric emptying (36) and a more fine-grained analysis of infant satiety behaviors would provide more insight into the effects of formula composition on infant feeding.
Several hypotheses may explain why infants consume less ePHF or CMF+glu than CMF. First, infants may have rejected the higher-glutamate formulas because of their flavor characteristics. However, whereas the flavor of ePHF differs substantially from that of CMF (37–39), the addition of glutamate did not alter CMF's flavor, as indicated by adult sensory evaluation of the formulas. Additionally, previous research has shown that infants younger than 4 mo of age, the age of the infants in the current study, readily accept ePHF and consume it to satiation (15, 27, 28). Thus, we believe that it is very unlikely that infants rejected the ePHF or CMF+glu before satiation for reasons having to do with the flavors of the formula.
Second, it is possible that parents may have influenced the amount of formula consumed. It is hypothesized that formula feeding is a parent-led process in that parents may feed based on visual cues afforded by bottle-feeding rather than cues produced and displayed by infants (40, 41). This could lead to habitual overfeeding and the loss of infants’ abilities to self regulate intake. However, the experimental design of the current study minimized parental influence on infant intake during formula feeding. Parents were blinded to the type of formula that they fed their infants. All feedings were infant-led, meaning that they occurred in response to infant hunger and satiation cues, not parental perceptions. Parents did not notice a difference in their infants’ behavior across the 3 testing days, and we observed no effect of parental perceptions, feeding attitudes, or practices on infant feeding. Also, because this was a within-subject study, any confounding effects would be similar across all 3 conditions. Thus, it is unlikely that parents moderated the effect of formula type on infant feeding behavior.
Third, the differential intakes may have been due to postingestive effects of free glutamate on infant eating behaviors. We favor this hypothesis for several reasons. Emerging evidence suggests that FAAs (42, 43), particularly free glutamate (19), help regulate energy intake. Free glutamate is sensed by receptors in both the oral cavity (44, 45) and intestinal and gastric walls (20). Glutamate may act as a signal for ingestion of protein and amino acids (46), which is supported by findings that adding MSG to a mixed macronutrient meal induces anticipatory reflexes that prepare the digestive system to metabolize and absorb protein (19). Glutamate may also serve as a satiation signal during feeding: the presence of MSG in the stomach, duodenum, and portal veins increases afferent activity in vagal nerves (21), the principal transmitters of gastrointestinal satiation messages to the central nervous system. Thus, we hypothesize that the detection of glutamate in the gut after feeding formulas higher in free glutamate serves as a satiation signal, stimulating earlier meal termination compared with when infants are fed CMF.
Differences in satiation and satiety between CMF and CMF+glu were most likely due to the addition of glutamate to CMF, but differences between CMF and ePHF could be caused by differences in the many other FAAs between these 2 formula types (10). Receptor mechanisms for several FAAs and small peptides have been discovered in the gut (47), and other FAAs (eg, tryptophan, phenylalanine, arginine, leucine, and alanine) that are present in greater concentrations in ePHF than in CMF (10) also stimulate the vagus nerve (48) and decrease food intake when infused into the intestine (49, 50). Further research should examine whether other components present in ePHF and lacking in CMF also influence infant feeding behaviors. Nonetheless, ePHF and CMF+glu had similar effects on infant satiation and satiety, which supports the conclusion that the glutamate content of these formulas was the primary influence on infant feeding behaviors.
In conclusion, it has been hypothesized that a key difference between breastfeeding and formula feeding is that breastfeeding is an infant-led process, consequently fostering infants’ developing abilities to self regulate intake, whereas formula feeding is a parent-led process that may lead to habitual overfeeding and infants losing their abilities to regulate intake (40, 41). However, data from the current study illustrate that, regardless of feeding history, formula-fed infants can regulate formula intake when given the opportunity to do so through infant-led feeding practices. The finding that infants in the current study consumed more when the bottle contained CMF than when it contained ePHF or CMF+glu indicates that concentrations of free glutamate in formula affect intake, which suggests that what an infant is fed may be as important as how it is fed.
Acknowledgments
We thank Mariya Keselman, Sehris Khawaja, Jillian Fink, Shawna Comalli, and Nicole Halfin, whose positions were created by supplement 3R01HD037119-10S1 received under the ARRA, for technical assistance. We also thank Linda Kilby and the staff at the Philadelphia WIC Program for their assistance with subject recruitment.
The authors’ responsibilities were as follows—JAM: study conception; JAM and AKV: study design, data analyses, and draft of the manuscript; AKV: data collection; and AKV, JAM, and GKB: critical review and editing of the manuscript. None of the authors reported a conflict of interest. The funding agencies had no role in the design or conduct of the study; in the collection, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Abbreviations used: CMF, cow milk formula; CMF+glu, cow milk formula with added free glutamate; ePHF, extensive protein hydrolysate formula; FAA, free amino acid; gLMS, general labeled magnitude scale; MSG, monosodium glutamate; WIC, Special Supplemental Nutrition Program for Women, Infants, and Children.
REFERENCES
- 1.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 2005;331:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekelund U, Ong KK, Linne Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rossner S. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab 2007;92:98–103 [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl 2004;93:26–33 [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Hanson MA. Developmental and epigenetic pathways to obesity: an evolutionary-developmental perspective. Int J Obes (Lond) 2008;32(suppl 7):S62–71 [DOI] [PubMed] [Google Scholar]
- 5.Martinez JA, Ballew MP. Infant formulas. Pediatr Rev 2011;32:179–89 [DOI] [PubMed] [Google Scholar]
- 6.Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B, Dewey KG. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: the DARLING Study. Am J Clin Nutr 1993;58:152–61 [DOI] [PubMed] [Google Scholar]
- 7.Dewey KG, Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B. Breast-fed infants are leaner than formula-fed infants at 1 y of age: the DARLING study. Am J Clin Nutr 1993;57:140–5 [DOI] [PubMed] [Google Scholar]
- 8.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics 2005;115:1367–77 [DOI] [PubMed] [Google Scholar]
- 9.Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, Beyer J, Demmelmair H, Gruszfeld D, Dobrzanska A, et al. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr 2009;89:1836–45 [DOI] [PubMed] [Google Scholar]
- 10.Ventura AK, San Gabriel A, Hirota M, Mennella JA. Determination of free amino acid content in infant formulas. Nutr Food Sci (in press) [Google Scholar]
- 11.Nutritionals MJ. Health Care professional resource center: Enfamil product information. Version current 26 July 2011. Available from: http://www.mjn.com/app/iwp/hcp2/content2.do?dm=mj&id=/HCP_Home2/ProductInformation/hcpProducts/hcpInfants/hcpEnfamilLIPIL&iwpst=MJN&ls=0&csred=1&r=3449135119 (cited 26 July 2011)
- 12.Nutritionals MJ. Health Care Professional Resource Center: Nutramigen product information. Version current 26 July 2011. Available from: http://www.mjn.com/app/iwp/hcp2/content2.do?dm=mj&id=/HCP_Home2/ProductInformation/hcpProducts/hcpInfants/hcpNutramigen&iwpst=MJN&ls=0&csred=1&r=3449135060 (cited 26 July 2011)
- 13.Mennella JA, Ventura AK, Beauchamp GK. Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics 2011;127:110–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rzehak P, Sausenthaler S, Koletzko S, Reinhardt D, von Berg A, Kramer U, Berdel D, Bollrath C, Grubl A, Bauer CP, et al. Short- and long-term effects of feeding hydrolyzed protein infant formulas on growth at < or = 6 y of age: results from the German Infant Nutritional Intervention Study. Am J Clin Nutr 2009;89:1846–56 [DOI] [PubMed] [Google Scholar]
- 15.Mennella JA, Beauchamp GK. Developmental changes in the acceptance of protein hydrolysate formula. J Dev Behav Pediatr 1996;17:386–91 [DOI] [PubMed] [Google Scholar]
- 16.Mennella JA, Beauchamp GK. Development and bad taste. Pediatr Allergy Asthma Immunol 1998;12:161–3 [Google Scholar]
- 17.Hyams JS, Treem WR, Etienne NL, Weinerman H, MacGilpin D, Hine P, Choy K, Burke G. Effect of infant formula on stool characteristics of young infants. Pediatrics 1995;95:50–4 [PubMed] [Google Scholar]
- 18.Hauser B, Keymolen K, Blecker U, Suys B, Bougatef A, Loeb H, Vandenplas Y. A comparative evaluation of whey hydrolysate and whey-predominant formulas. How well do infants accept and tolerate them? Clin Pediatr (Phila) 1993;32:433–7 [DOI] [PubMed] [Google Scholar]
- 19.Viarouge C, Caulliez R, Nicolaidis S. Umami taste of monosodium glutamate enhances the thermic effect of food and affects the respiratory quotient in the rat. Physiol Behav 1992;52:879–84 [DOI] [PubMed] [Google Scholar]
- 20.San Gabriel AM, Maekawa T, Uneyama H, Yoshie S, Torii K. mGluR1 in the fundic glands of rat stomach. FEBS Lett 2007;581:1119–23 [DOI] [PubMed] [Google Scholar]
- 21.Niijima A. Reflex effects of oral, gastrointestinal and hepatoportal glutamate sensors on vagal nerve activity. J Nutr 2000;130:971S–3S [DOI] [PubMed] [Google Scholar]
- 22.Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Lewis DS, Lee DR, Reeds PJ. Amino acid composition of human milk is not unique. J Nutr 1994;124:1126–32 [DOI] [PubMed] [Google Scholar]
- 23.Agostoni C, Carratu B, Boniglia C, Riva E, Sanzini E. Free amino acid content in standard infant formulas: comparison with human milk. J Am Coll Nutr 2000;19:434–8 [DOI] [PubMed] [Google Scholar]
- 24.Agostoni C, Carratu B, Boniglia C, Lammardo AM, Riva E, Sanzini E. Free glutamine and glutamic acid increase in human milk through a three-month lactation period. J Pediatr Gastroenterol Nutr 2000;31:508–12 [DOI] [PubMed] [Google Scholar]
- 25.Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav 2004;82:109–14 [DOI] [PubMed] [Google Scholar]
- 26.Mennella JA, Forestell CA, Morgan LK, Beauchamp GK. Early milk feeding influences taste acceptance and liking during infancy. Am J Clin Nutr 2009;90(suppl):780S–8S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mennella JA, Lukasewycz LD, Castor SM, Beauchamp GK. The timing and duration of a sensitive period in human flavor learning: a randomized trial. Am J Clin Nutr 2011;93:1019–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mennella JA, Griffin CE, Beauchamp GK. Flavor programming during infancy. Pediatrics 2004;113:840–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadeh A, Lavie P, Scher A, Tirosh E, Epstein R. Actigraphic home-monitoring sleep-disturbed and control infants and young children: a new method for pediatric assessment of sleep-wake patterns. Pediatrics 1991;87:494–9 [PubMed] [Google Scholar]
- 30.Sadeh A, Acebo C, Seifer R, Aytur S, Carskadon MA. Activity-based assessment of sleep-wake patterns during the 1st year of life. Infant Behav Dev 1995;18:329–37 [Google Scholar]
- 31.Baughcum AE, Powers SW, Johnson SB, Chamberlin LA, Deeks CM, Jain A, Whitaker RC. Maternal feeding practices and beliefs and their relationships to overweight in early childhood. J Dev Behav Pediatr 2001;22:391–408 [DOI] [PubMed] [Google Scholar]
- 32.Carey WB, McDevitt SC. Revision of the infant temperament questionnaire. Pediatrics 1978;61:735–9 [PubMed] [Google Scholar]
- 33.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data 2000;Jun 8:1–27 [PubMed] [Google Scholar]
- 34.Bellisle F, Dalix AM, Mennen L, Galan P, Hercberg S, de Castro JM, Gausseres N. Contribution of snacks and meals in the diet of French adults: a diet-diary study. Physiol Behav 2003;79:183–9 [DOI] [PubMed] [Google Scholar]
- 35.James RJ, Drewett RF, Cheetham TD. Low cord ghrelin levels in term infants are associated with slow weight gain over the first 3 months of life. J Clin Endocrinol Metab 2004;89:3847–50 [DOI] [PubMed] [Google Scholar]
- 36.Riezzo G, Indrio F, Montagna O, Tripaldi C, Laforgia N, Chiloiro M, Mautone A. Gastric electrical activity and gastric emptying in preterm newborns fed standard and hydrolysate formulas. J Pediatr Gastroenterol Nutr 2001;33:290–5 [DOI] [PubMed] [Google Scholar]
- 37.Cook DA, Sarett HP. Design of infant formulas for meeting normal and special need. Pediatric nutrition: Infant feeding, deficiencies, disease. New York, NY: Marcel Dekker Inc, 1982 [Google Scholar]
- 38.Mennella JA, Beauchamp GK. Understanding the origin of flavor preferences. Chem Senses 2005;30(Suppl 1):i242–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedrosa M, Pascual CY, Larco JI, Esteban MM. Palatability of hydrolysates and other substitution formulas for cow's milk-allergic children: a comparative study of taste, smell, and texture evaluated by healthy volunteers. J Investig Allergol Clin Immunol 2006;16:351–6 [PubMed] [Google Scholar]
- 40.Bernal J, Richards MP. The effects of bottle and breast feeding on infant development. J Psychosom Res 1970;14:247–52 [DOI] [PubMed] [Google Scholar]
- 41.Crow RA, Fawcett JN, Wright P. Maternal behavior during breast- and bottle-feeding. J Behav Med 1980;3:259–77 [DOI] [PubMed] [Google Scholar]
- 42.Diepvens K, Häberer D, Westerterp-Plantenga M. Different proteins and biopeptides differently affect satiety and anorexigenic/orexigenic hormones in healthy humans. Int J Obes (Lond) 2008;32:510–8 [DOI] [PubMed] [Google Scholar]
- 43.Uneyama H, Niijima A, San Gabriel A, Torii K. Luminal amino acid sensing in the rat gastric mucosa. Am J Physiol Gastrointest Liver Physiol 2006;291:G1163–70 [DOI] [PubMed] [Google Scholar]
- 44.Torii K, Cagan RH. Biochemical studies of taste sensation. IX. Enhancement of L-[3H]glutamate binding to bovine taste papillae by 5′-ribonucleotides. Biochim Biophys Acta 1980;627:313–23 [PubMed] [Google Scholar]
- 45.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci 2000;3:113–9 [DOI] [PubMed] [Google Scholar]
- 46.Naim M, Ohara I, Kare MR, Levinson M. Interaction of MSG taste with nutrition: perspectives in consummatory behavior and digestion. Physiol Behav 1991;49:1019–24 [DOI] [PubMed] [Google Scholar]
- 47.Conigrave AD, Brown EM. Taste receptors in the gastrointestinal tract. II. L-amino acid sensing by calcium-sensing receptors: implications for GI physiology. Am J Physiol Gastrointest Liver Physiol 2006;291:G753–61 [DOI] [PubMed] [Google Scholar]
- 48.Jeanningros R. Vagal unitary responses to intestinal amino acid infusions in the anesthetized cat: a putative signal for protein induced satiety. Physiol Behav 1982;28:9–21 [DOI] [PubMed] [Google Scholar]
- 49.Yox DP, Ritter RC. Capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol 1988;255:R569–74 [DOI] [PubMed] [Google Scholar]
- 50.Meyer JH, Hlinka M, Tabrizi Y, DiMaso N, Raybould HE. Chemical specificities and intestinal distributions of nutrient-driven satiety. Am J Physiol 1998;275:R1293–307 [DOI] [PubMed] [Google Scholar]