Abstract
The cutaneous beta human papillomavirus (beta HPV) types appear to be involved in skin carcinogenesis. However, only a few beta HPVs have been investigated so far. Here, we compared the properties of E6 and E7 oncoproteins from six uncharacterized beta HPVs (14, 22, 23, 24, 36, 49). Only HPV49 E6 and E7 immortalized primary human keratinocytes and efficiently deregulated the p53 and pRb pathways. Furthermore, HPV49 E6, similarly to E6 from the oncogenic HPV16, promoted p53 degradation.
TEXT
The cutaneous human papillomavirus (HPV) types that belong to the beta genus are suspected to play a role in skin carcinogenesis together with UV irradiation (4, 17). They were originally isolated from patients suffering from a rare autosomal recessive genetic disorder called epidermodysplasia verruciformis (EV), who have an increased susceptibility to beta HPV infection and who develop nonmelanoma skin cancer (NMSC) (17). Epidemiological studies have also provided evidence for the role of beta HPV types in NMSC in immunocompromised individuals, such as organ transplant recipients, as well as in the normal population (2, 3, 5, 7, 8, 12–14, 18). However, due to the high frequency with which cutaneous HPV DNA is found in the skin of healthy individuals, the role of these viruses in carcinogenesis is still unclear.
Studies on the cervical cancer-associated HPV types (for example, see references 16 and 18) showed that their oncogenic potential lies in the transforming activities of two proteins, E6 and E7 (11). By interacting with several host proteins, E6 and E7 of high-risk (HR) HPV types are able to interfere with key cellular processes, such as cell cycle, senescence, differentiation, apoptosis, and telomere shortening.
Only a limited number of studies have addressed the transforming ability of the beta HPV types in primary keratinocytes (6, 9, 15). In the present work, we compared the transforming potential of E6 and E7 proteins from several HPV types, which belong to different subgroups within the beta genus, i.e., HPV14, -24, and -36 (β1), HPV-22, -23, and -38 (β2), and HPV49 (β3).
Beta HPV49 E6 and E7 efficiently immortalize primary human keratinocytes.
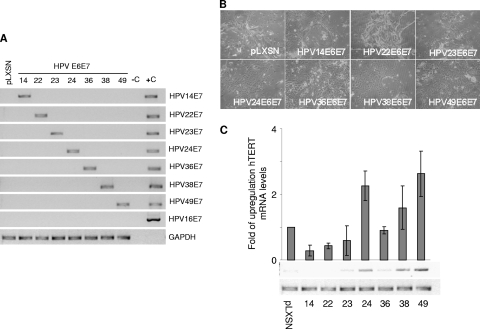
We first compared the ability of E6 and E7 from different beta HPV types to immortalize primary cells. Primary human foreskin keratinocytes (HFK) were transduced with E6/E7 recombinant retroviruses, and viral gene expression was determined by reverse transcription-PCR (RT-PCR) using specific primers for the different E7 genes (10) (Fig. 1A). Twenty days after retroviral transduction, HPV14 and -22 E6/E7-transduced HFK displayed features of senescence, such as irregular shape and intercellular bridges, similar to the HFK transduced with the empty retroviral vector (pLXSN) (mock-transduced HFK) (Fig. 1B). Consistently, all three HFK cultures showed high positivity for the senescent marker β-galactosidase (data not shown) and died after a few population doublings (PD) (Table 1). HPV23, -24, -36, and -38 E6/E7 HFK continued to grow for a few PDs after transduction, then stopped proliferating, enlarged, and remained growth arrested for 3 to 4 weeks. Islands of small proliferating cells appeared in the HPV38, -24, and -36 E6/E7 HFK cultures, whereas HPV23 E6/E7 HFK did not recover from the crisis and died approximately at PD 9. HPV36 E6/E7 HFK, although they overcame the apparent crisis, died immediately afterwards. Long-term culture revealed that HPV24 E6/E7 HFK also failed to become immortalized and underwent senescence. As expected, HPV38 E6/E7 HFK continued to grow without further interruption. Interestingly, HFK expressing E6 and E7 from HPV49 grew at a constant and very high rate without any apparent crisis, similar to what has been observed for HPV16 E6/E7 HFK (11). Table 1 summarizes the findings obtained in three independent experiments using primary keratinocytes from three different donors. The ability of the viral oncoproteins to increase keratinocyte life span correlated with their ability to activate hTERT expression (Fig. 1C). In conclusion, E6 and E7 from HPV38 and HPV49 were the only viral oncoproteins that consistently led to the immortalization of primary HFK. Most importantly, HPV49 E6 and E7 appeared to be more efficient than HPV38 E6 and E7.
Fig 1.
HPV49 E6 and E7 efficiently immortalize primary HFK. (A) Total RNA was extracted from HFK transduced with the indicated recombinant retroviruses and subjected to reverse transcription. PCR was performed using the different cDNAs as templates with HPV type-specific primers. (B) Morphology of primary HFK transduced with the indicated recombinant retroviruses 20 days postransduction. The same magnification was used (×10) for all photomicrographs. (C) Total RNA was extracted from the indicated cells and subjected to reverse transcription. PCR analysis was performed using specific primers for hTERT and GAPDH. The graph represents the quantification of the results from three independent experiments.
Table 1.
Life span and immortalization of primary keratinocytes transduced with recombinant retroviruses expressing E6 and E7 from different beta HPV typesa
| Retrovirus | No. of population doublings before senescence in cells from donor: |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| None (empty pLXSN vector) | 11 | 11 | 14.2 |
| HPV14 | 8.5 | 6 | 12.7 |
| HPV22 | 6 | 6 | 10.7 |
| HPV23 | 9 | 7 | 12 |
| HPV24 | 27 | 35 | 23 |
| HPV36 | 14 | 19 | 19.5 |
| HPV38 | Immortalized | Immortalized | Immortalized |
| HPV49 | Immortalized | Immortalized | Immortalized |
Primary HFKs from three different donors were transduced with the indicated retroviruses and cultured for several population doublings.
pRb and p53 pathways are altered in HPV49 E6/E7-expressing cells.
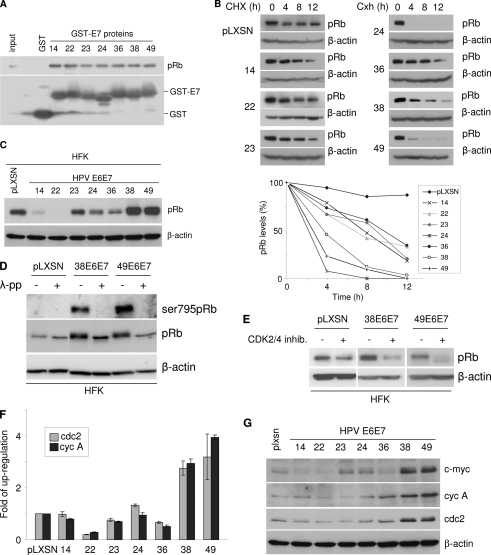
Studies on HR HPV types have shown that inactivation of the two major tumor suppressors, the retinoblastoma protein (pRb) and p53, is an essential event in HPV-mediated keratinocyte immortalization (16). HPV16 E7 associates with and promotes the degradation of pRb (11). All the beta HPV E7 proteins tested contain the typical pRb-binding motif LXCXE (data not shown) and showed similar efficiencies of binding to pRb in a glutathione S-transferase (GST) pulldown assay (Fig. 2A). In addition, pRb half-life was significantly decreased in rodent fibroblasts (NIH 3T3) expressing HPV24, -38, and -49 E6/E7 in comparison to the mock-transduced cells (NIH 3T3-pLXSN), while the reduction in pRb half-life was severely attenuated in HPV14, -22, -23, and -36 E6/E7 cells (Fig. 2B). HPV14 and -22 E7 efficiently induced pRb degradation in HFK (Fig. 2C), although this activity appeared not to be sufficient to promote cellular immortalization. Surprisingly, HPV38 and -49 E6/E7 expression in HFK did not lead to pRb degradation but instead led to its accumulation (Fig. 2C). This phenomenon may be explained by the fact that HPV oncoproteins promote pRb phosphorylation with higher efficiency in HFK than in NIH 3T3 cells, an event known to inhibit interaction of pRb with E7 (16). Accordingly, immunoblotting with a specific anti-phospho-pRB antibody revealed a strong pRb signal in HPV38 and -49 E6/E7 HFK, which was abolished by pretreatment of the protein extracts with lambda phosphatase (Fig. 2D). In addition, exposure of HPV38 and -49 E6/E7-expressing HFK to a specific CDK2/4 inhibitor (Calbiochem) led to a strong reduction in the levels of total pRb (Fig. 2E). Phosphorylation of pRb by the CDK 1, -2, -4, and -6 leads to the release of E2F, which in turn activates the transcription of several cellular genes, including cyclin A, cdc2, and c-myc. Quantitative PCR performed with Mesa green quantitative PCR master mix (Eurogentec) showed that mRNA levels of the E2F-regulated genes for cyclin A and Cdc2 were higher in HPV38 and -49 E6/E7 HFK than in the other transduced cells (Fig. 2F). Consistently, immunoblotting showed increased protein levels of Cdc2, cyclin A and c-Myc in the same cells (Fig. 2G). Although HPV14 and -22 E7 efficiently induced pRb degradation, it did not significantly activate the expression of cyclin A and Cdc2, suggesting that additional events are required to deregulate cellular gene expression.
Fig 2.
HPV49 E7 alters the pRB-regulated pathway. (A) HaCaT cell extracts were incubated with different GST fusion proteins, and the bound proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The amount of pRb associated with the different GST E7 proteins was determined by immunoblotting, using a specific anti-pRb (554136; BD Pharmingen) antibody. One-tenth of the total cellular extract (60 μg) used in the GST pulldown assay was loaded on the SDS-PAGE gel as a control (input). (B) NIH 3T3 cells were transduced with the indicated recombinant retroviruses. After completion of the drug selection, cells were cultured in the presence of cycloheximide (CHX) for the indicated times. At each time point, cells were collected. Protein extracts were prepared and analyzed by immunoblotting with the indicated antibodies (top). The levels of pRb were quantified by Quantity one (Bio-Rad) and normalized to the levels of β-actin (bottom). (C) Protein extracts of HFK transduced with empty retrovirus vector (pLXSN HFK) or expressing the different beta HPV E6 and E7 genes were analyzed by immunoblotting using anti-pRb (554136; BD Pharmingen) and β-actin (clone C4; MP Biomedicals) antibodies. β-Actin staining was included as a loading control. (D) Protein extracts from HFK transduced with empty (pLXSN) or beta HPV38 or 49 E6/E7 retrovirus were incubated in the presence and absence of lambda phosphatase (λ-pp) and analyzed by immunoblotting using antibodies that recognize pRb phosphorylated at serine 795 (9301S; Cellsignalling), all forms of pRb (554136; BD Pharmingen), or β-actin (clone c4; MP-Biomedicals). (E) HFK transduced with the indicated retroviruses were treated with a CDK inhibitor (219476; Calbiochem) at 520 nM for 48 h. pRb and β-actin levels were determined by SDS-PAGE and immunoblotting. (F) Total RNA was extracted from HFK transduced with the indicated recombinant retroviruses and subjected to reverse transcription. Quantitative PCR analysis was then performed with specific primers for the Cdc2, cyclin A, and GAPDH genes. The data are means from three independent experiments performed in duplicate. (G) Protein extracts from HFK transduced with empty vector (pLXSN) or the indicated beta HPV E6/E7 retrovirus were analyzed by immunoblotting using the following antibodies: anti-c-Myc (1667149; Roche), anti-cyclin A (H-432; Santa Cruz), anti-Cdc2 (sc-54; Santa Cruz), and anti-β-actin (clone C4; MP-Biomedicals).
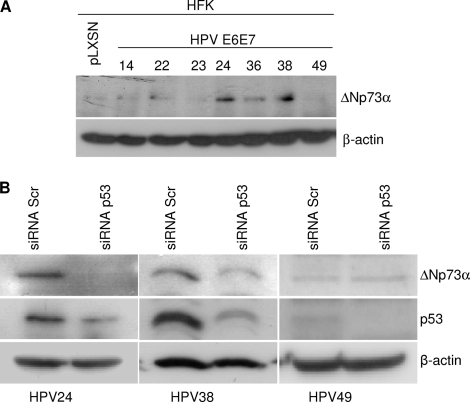
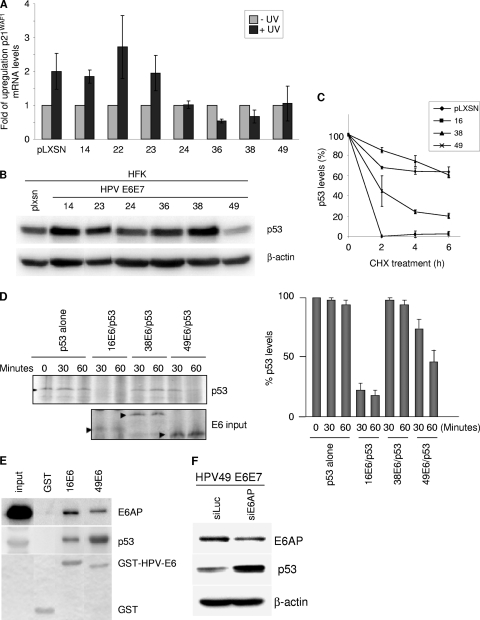
Next we characterized the impact of the viral oncoproteins on the p53 pathway. We showed previously that HPV38 E6/E7 expression leads to the accumulation in the nucleus of a p53 form that is phosphorylated at serines 15 and 392 and has high affinity for the promoter of ΔNp73α, a dominant negative inhibitor of the p53/p73 pathways (1). We observed that HPV24, HPV36, and HPV38 E6/E7 cells contained elevated levels of ΔNp73α (Fig. 3A). This event correlated with p53 phosphorylation at serines 15 and 392 and nuclear localization (data not shown). In addition, downregulation of p53 expression by small interfering RNA (siRNA) decreased ΔNp73α levels in HPV24 and 38 E6/E7 HFK, indicating that in these cells, ΔNp73 transcription is regulated by p53 (Fig. 3B). UV-mediated DNA damage activates p53, which in turn induces cell cycle arrest via transcriptional activation of the CDK inhibitor p21WAF1. UVB irradiation of mock-transduced or HPV14, -22, and -23 E6/E7 HFK led to p21WAF1 accumulation, while the p53 pathway was inhibited in HFK expressing E6 and E7 from HPV24, -36, -38, and -49 (Fig. 4A). As shown above, ΔNp73α was found to be upregulated in HPV24, -36, and -38 E6/E7 HFK and could be partly responsible for the inhibition of p53 functions. In contrast, HPV49 E6 and E7 appeared to inhibit the UV-induced activation of p53 by a ΔNp73α-independent mechanism. Immunoblot analysis revealed that p53 levels were strongly decreased in HPV49 E6/E7 HFK in comparison to those in mock-transduced cells (Fig. 4B). As previously observed, HPV38 E6/E7 had elevated p53 levels, while the HFK expressing the other beta HPV oncogenes showed minor variations in p53 levels (Fig. 4B). Determination of p53 half-life by culturing HPV HFK in the presence of cycloheximide (10 μg/ml) at different times revealed that HPV49 E6 promoted p53 destabilization, although with a lower efficiency than HPV16 E6 (Fig. 4C). Similar results were obtained in an in vitro degradation assay, in which p53 and E6 were cotranslated and coincubated at the indicated times (Fig. 4D). It is known that HPV16 E6/E6AP interaction is required for p53 degradation (16). A GST-HPV49 E6 fusion protein was found to associate with both p53 and E6AP (Fig. 4E). In addition, downregulation of E6AP expression in HPV49 E6/E7 HFK by siRNA, which resulted in increased p53 levels, indicates that E6AP may be involved in HPV49 E6-induced p53 degradation (Fig. 4F). The differences in efficiency of the p53 degradation induced by HPV16 and HPV49 E6s suggest, however, that the mechanisms of degradation and/or interaction with p53 may not be precisely the same between these HPV types.
Fig 3.
ΔNp73α is upregulated in HPV24 and 36 E6/E7 HFK but not in HPV49 E6/E7 HFK. (A) Protein extracts of primary keratinocytes transduced with empty retrovirus vector (pLXSN HFK) or the indicated recombinant retroviruses were analyzed by immunoblotting using anti-p73α (OP108; Calbiochem) and anti-β-actin (clone C4; MP-Biomedicals) antibodies. (B) HPV24, -38, or -49 E6/E7 HFK were transiently transfected either with scrambled (Scr) small interfering RNA (siRNA) or with siRNA directed against p53. After preparation of total protein extracts, ΔNp73α, p53, and β-actin protein levels were determined by immunoblotting using specific antibodies.
Fig 4.
HPV49 E6 deregulates the p53 pathway, promoting its degradation. (A) HFK transduced with the indicated retroviruses were UV irradiated (0.08 J/cm2). After 8 h, total RNA was extracted and subjected to reverse transcription. Then qPCR analysis was performed using specific primers for the p21WAF1 and GAPDH genes. The data are means from three independent experiments performed in duplicate. (B) HFK transduced with the indicated retroviruses were cultured, and protein extracts were prepared and analyzed by immunoblotting with the indicated antibodies. (C) HFK transduced with the indicated retroviruses were cultured in the presence of cycloheximide (CHX) for the indicated times. At each time point, cells were collected. Protein extracts were prepared and analyzed by immunoblotting with the indicated antibodies. The levels of p53 were quantified by Quantity one (Bio-Rad) and normalized to the levels of β-actin. The data are means from three independent experiments. (D) In vitro-translated p53 was incubated at 30°C for 0, 30, or 60 min, either alone or with in vitro-translated HPV16, -38, or -49 E6. The remaining p53 was visualized by SDS-PAGE and autoradiography (left). p53 levels were measured after normalization to E6 levels in three independent experiments (right). (E) C33A cell extracts were incubated with GST fusion proteins, and bound proteins were analyzed by SDS-PAGE. The amounts of p53 and E6AP associated with the different GST E6 proteins were determined by immunoblotting, using specific anti-E6AP (clone E6AP-330; Sigma) or anti-p53 antibodies (DO-7; Dako). One-tenth of the total cellular extract (60 μg) used in the GST pulldown assay (input) was loaded on the SDS-PAGE gel as a control. (F) HPV49 E6/E7 HFK were transiently transfected with siRNAs specifically directed against either luciferase (Luc) or E6AP. After preparation of total protein extracts, levels of p53, E6AP, and β-actin were determined by immunoblotting using specific antibodies.
In summary, in this study we characterized the impact of several beta HPV types on the life span and immortalization of primary HFK, and we showed that the ability of the viral oncoproteins to promote these events correlated with their efficiency in targeting the telomerase, p53, and pRb pathways. Our study also led to the identification of HPV49 as an additional beta HPV type that displays transforming activities in primary HFK. Most importantly, we report for the first time that an E6 from a beta HPV type shares with HPV16 E6 the ability to promote p53 degradation through an E6AP-dependent mechanism. In conclusion, this study highlights functional differences of beta HPV types in several in vitro assays, suggesting that they may have different impacts on skin carcinogenesis.
ACKNOWLEDGMENTS
We are grateful to all the members of our laboratories for their cooperation. The study was partly supported by grants from La Ligue Contre le Cancer (Comités Rhône, Savoie and Drôme) to M.T. and B.S.S., the Association pour la Recherche sur le Cancer to M.T., Association for International Cancer Research to M.T., M.S.C., and L.B., and the Associazione Italiana per la Ricerca sul Cancro to L.B. M.S.C. was supported by Cancer Research UK.
Footnotes
Published ahead of print 14 December 2011
REFERENCES
- 1. Accardi R, et al. 2006. Skin human papillomavirus type 38 alters p53 functions by accumulation of deltaNp73. EMBO Rep. 7:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersson K, et al. 2008. Seroreactivity to cutaneous human papillomaviruses among patients with nonmelanoma skin cancer or benign skin lesions. Cancer Epidemiol. Biomarkers Prev. 17:189–195 [DOI] [PubMed] [Google Scholar]
- 3. Berkhout RJ, Bouwes Bavinck JN, Ter Schegget J. 2000. Persistence of human papillomavirus DNA in benign and (pre)malignant skin lesions from renal transplant recipients. J. Clin. Microbiol. 38:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernard HU, Burk RD, Chen Z, van Doorslaer K, Hzur Hausen de Villiers EM. 2010. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401:70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouwes Bavinck JN, et al. 2010. Multicenter study of the association between betapapillomavirus infection and cutaneous squamous cell carcinoma. Cancer Res. 70:9777–9786 [DOI] [PubMed] [Google Scholar]
- 6. Caldeira S, et al. 2003. The E6 and E7 proteins of cutaneous human papillomavirus type 38 display transforming properties. J. Virol. 77:2195–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casabonne D, et al. 2007. A prospective pilot study of antibodies against human papillomaviruses and cutaneous squamous cell carcinoma nested in the Oxford component of the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 121:1862–1868 [DOI] [PubMed] [Google Scholar]
- 8. de Jong-Tieben LM, et al. 1995. High frequency of detection of epidermodysplasia verruciformis-associated human papillomavirus DNA in biopsies from malignant and premalignant skin lesions from renal transplant recipients. J. Investig. Dermatol. 105:367–371 [DOI] [PubMed] [Google Scholar]
- 9. Gabet AS, et al. 2008. Impairment of the telomere/telomerase system and genomic instability are associated with keratinocyte immortalization induced by the skin human papillomavirus type 38. FASEB J. 22:622–632 [DOI] [PubMed] [Google Scholar]
- 10. Gheit T, et al. 2007. Development of a sensitive and specific multiplex PCR method combined with DNA microarray primer extension to detect Betapapillomavirus types. J. Clin. Microbiol. 45:2537–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghittoni R, et al. 2010. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes. 40:1–13 [DOI] [PubMed] [Google Scholar]
- 12. Harwood CA, et al. 2000. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J. Med. Virol. 61:289–297 [DOI] [PubMed] [Google Scholar]
- 13. Iftner A, et al. 2003. The prevalence of human papillomavirus genotypes in nonmelanoma skin cancers of nonimmunosuppressed individuals identifies high-risk genital types as possible risk factors. Cancer Res. 63:7515–7519 [PubMed] [Google Scholar]
- 14. Karagas MR, et al. 2006. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J. Natl. Cancer Inst. 98:389–395 [DOI] [PubMed] [Google Scholar]
- 15. Muench P, et al. 2009. Binding of PDZ proteins to HPV E6 proteins does neither correlate with epidemiological risk classification nor with the immortalization of foreskin keratinocytes. Virology 387:380–387 [DOI] [PubMed] [Google Scholar]
- 16. Munger K, et al. 2004. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 78:11451–11460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfister H. 2003. Human papillomavirus and skin cancer. J. Natl. Cancer Inst. Monogr. 31:52–56 [DOI] [PubMed] [Google Scholar]
- 18. Waterboer T, et al. 2008. Serological association of beta and gamma human papillomaviruses with squamous cell carcinoma of the skin. Br. J. Dermatol. 159:457–459 [DOI] [PubMed] [Google Scholar]