Abstract
Rift Valley fever (RVF) virus (RVFV) can cause severe human disease characterized by either acute-onset hepatitis, delayed-onset encephalitis, retinitis and blindness, or a hemorrhagic syndrome. The existing nonhuman primate (NHP) model for RVF utilizes an intravenous (i.v.) exposure route in rhesus macaques (Macaca mulatta). Severe disease in these animals is infrequent, and large cohorts are needed to observe significant morbidity and mortality. To overcome these drawbacks, we evaluated the infectivity and pathogenicity of RVFV in the common marmoset (Callithrix jacchus) by i.v., subcutaneous (s.c.), and intranasal exposure routes to more closely mimic natural exposure. Marmosets were more susceptible to RVFV than rhesus macaques and experienced higher rates of morbidity, mortality, and viremia and marked aberrations in hematological and chemistry values. An overwhelming infection of hepatocytes was a major consequence of infection of marmosets by the i.v. and s.c. exposure routes. Additionally, these animals displayed signs of hemorrhagic manifestations and neurological impairment. Based on our results, the common marmoset model more closely resembles severe human RVF disease and is therefore an ideal model for the evaluation of potential vaccines and therapeutics.
INTRODUCTION
Rift Valley fever (RVF) virus (RVFV) is a negative-sense, single-stranded RNA virus of the genus Phlebovirus (family Bunyaviridae). The virus was first isolated in 1930 in East Africa (14) and has since caused severe epidemics and epizootics throughout Africa and the Arabian peninsula (11, 30). Severe outbreaks have involved tens of thousands of both human and livestock cases for which no effective, commercially available human vaccines or antiviral drugs are available. Due to concerns regarding its use as a potential biological weapon, RVFV has been identified as a category A, high-priority select agent by the National Institute for Allergy and Infectious Diseases (NIAID), the Centers for Disease Control and Prevention (CDC), and the U.S. Department of Agriculture (USDA).
RVFV is an arthropod-borne virus (arbovirus) that causes epizootics associated with abortion and high rates of mortality in livestock, during which humans become infected (30). Human infections result from the bite of an infected mosquito (Culex and Anopheles mosquitoes appear to be the principal vectors for humans) or by contact with tissues, blood, or fluids from infected animals. After an incubation period of 2 to 6 days, an abrupt onset of fever, chills, and general malaise ensues. In most cases, human disease is mild, and recovery occurs without major consequences. Severe cases, which affect around 1 to 2% of infected individuals, are characterized by acute-onset liver disease, delayed-onset encephalitis, retinitis, blindness, or a hemorrhagic syndrome, with a case fatality rate of 10 to 20% for hospitalized individuals (25, 27, 29). Human cases have been reported in much of Africa, Saudi Arabia, and Yemen, with recent outbreaks in Kenya during 2006 to 2007 (11) and South Africa in 2008 to 2011 (34).
The development of an effective vaccine or therapeutic to treat RVF in humans remains an important area of research. The U.S. Food and Drug Administration's animal rule allows for the demonstration of drug or vaccine efficacy using animal studies instead of human clinical trials (44). This rule recommends the testing of potential vaccines and therapeutics with well-described animal models. Ideally, this would involve an animal model using a nonrodent species such as a nonhuman primate (NHP). Several animal models of RVFV infection have been described. Mice are highly susceptible to infection with RVFV by subcutaneous (s.c.) or intraperitoneal (i.p.) injection, leading to fulminant hepatitis and late-developing encephalitis (19, 21, 32, 37, 42). Similar to mice, hamsters and rats are also susceptible to infection (18, 19, 21, 37). Both hepatitis and encephalitis have been described for rats; however, their susceptibility to RVFV can vary significantly depending on the strain of rat used, and usually, only one pathological feature (i.e., hepatitis or encephalitis) is observed for a particular strain (4, 21, 37, 40). The hamster model has relied mainly on experimental infection with the related bunyavirus Punta Toro virus (2, 20), where only hepatitis (and not encephalitis) is the dominant pathological feature. In addition, gerbils infected with RVFV reportedly develop uniformly fatal encephalitis in the absence of significant extraneural lesions (3).
In contrast to the majority of rodent models, infection of NHPs with RVFV does not seem to produce a uniformly fatal infection. The first study describing an infection of NHPs with RVFV was published in 1931 (19), which reported that infection of rhesus macaques induced febrile responses and leukopenia but did not result in a fatal infection. A later study addressed the effects of different exposure routes, such as the i.p., intracerebral (i.c.), s.c., or intranasal (i.n.) inoculation of rhesus macaques using blood or tissues from infected animals as the inoculums. The NHPs became viremic and developed a fever, and leukocytosis was evident, followed by leukopenia, but no signs of disease resulted, except for a decrease in activity during the peak of fever (18). Rhesus macaque (16, 31) and cynomolgus macaque (16) NHPs appeared to be slightly more susceptible to infection by the aerosol route than by peripheral exposure routes (16). The susceptibilities of other species of NHPs were evaluated previously, and four South American NHP species, two species of brown capuchin monkeys (Cebus fatuellus and Cebus chrysopus) and two species of common marmosets (Callithrix jacchus and Callithrix penicillata), were found to be more susceptible to infection than three African species, the green guenon (Cercopithecus callitrichus), the sooty mangabey (Cercocebus fuliginosus), and the Patas guenon (Erythrocebus patas) (17). Spider monkeys (Ateles ater) appeared to be refractory (16), while baboons (Papio anubis) appeared to be comparable to rhesus macaques (15). However, it is important to note that those earlier studies utilized small numbers of NHPs. Therefore, a further evaluation of these species with larger cohorts of animals was warranted.
To date, rhesus macaques infected with RVFV strain ZH501 appear to provide the most realistic model of human infection (37). In previous studies, rhesus macaques were usually infected intravenously (i.v.) with 5 log10 PFU of RVFV. The outcome of these infections can be broadly divided into three groups based on observations of large cohorts of animals (usually 15 to 20 rhesus macaques): (i) fatally infected rhesus macaques that developed severe clinical disease and succumbed (∼18%), (ii) clinically ill rhesus macaques that survived (∼41%), and (iii) rhesus macaques that developed very mild or no apparent clinical illness and survived (∼41%). Rhesus macaques with severe clinical disease developed signs of illness 2 to 4 days after infection, characterized by anorexia, depression, vomiting, and weakness. Coagulopathy was manifested by petechial and purpuric skin lesions on the face, ears, abdomen, and medial thigh. Animals that developed moderate clinical illness and survived were characterized by a reduced appetite, cutaneous petechial hemorrhages on the abdomen and medial aspect of the thigh, and occasional vomiting (33). Pathological changes in the liver were shown to occur in the characteristic midzonal pattern, which is observed for humans (46) and other animals infected with RVFV (16–18, 35). Severe disease in rhesus macaques was accompanied by extensive liver necrosis, evidence of disseminated intravascular coagulation (DIC), and microangiopathic hemolytic anemia (13, 36, 38).
Human RVF presents as a spectrum of disease manifestations ranging from mild febrile episodes to death. The rhesus macaque model faithfully represents this spectrum, because the clinical disease syndromes are similar to those observed for human cases of RVF. However, the use of a moderately susceptible infection model is not ideal to demonstrate the efficacy of newly developed vaccines and therapeutics. Fewer than 20% of rhesus macaques infected with RVFV develop severe disease, and no ocular disease has been reported for this model. Additionally, i.v. infection does not represent a natural exposure route, since RVFV-infected mosquitoes transmit virus primarily extravascularly (45). Therefore, the development of a more susceptible NHP model using a more natural exposure route would be advantageous. In this study, we evaluated the susceptibility of the common marmoset (Callithrix jacchus) to RVFV. This New World primate species was chosen because it has been successfully used to study a number of other viral diseases caused by arenaviruses, herpesviruses, the coronavirus causing severe acute respiratory syndrome, and an alphavirus (1, 5, 12, 22, 23, 28). Here we describe the establishment of a new NHP model for RVF using the common marmoset, which overcomes some of the major limitations of existing primate models. Marmosets were more susceptible to RVFV than rhesus macaques and experienced higher rates of morbidity, mortality, and viremia and marked aberrations in hematological and chemistry values. These animals exhibited acute-onset hepatitis, delayed-onset encephalitis, and hemorrhagic disease, which are dominant features of severe human RVF.
MATERIALS AND METHODS
Viral strain, animals, and study design.
Recombinant viral strain ZH501 was rescued as previously described (9), and the exact complete genome sequence was confirmed by techniques described previously by Bird et al. (10). Strain ZH501 was originally isolated from a fatal human case during the 1977 epidemic in Egypt.
Four healthy adult rhesus macaques (Macaca mulatta), 3 to 4 years old and ranging in body weight from 4.8 to 5.7 kg, were obtained from World Wide Primates and Shared Enterprises. Twenty healthy adult marmosets (Callithrix jacchus), 2 to 11 years old and ranging in weight from 279 to 547 g, were obtained from either the Institute of Chemical Defense (ICD), in Aberdeen Proving Ground, MD (the original vendor was World Wide Primates), or GlaxoSmithKline (GSK) (the original vendor was Harlan UK Hillcrest). None of these primates was exposed to any infectious pathogens in previous studies, and all primates were determined to be RVFV naïve by a plaque reduction neutralization test (PRNT) (see methods described below) before the initiation of the study.
For the study design, four animals per exposure route received 7 log10 PFU/ml RVFV in a 1-ml volume i.v., s.c. (one cohort of four animals received 5 log10 PFU/ml), or i.n. Two animals were included as uninfected negative controls: one animal to serve as a negative control for injectable routes of exposure (i.v. and s.c.) and one animal to serve as a negative control for the i.n. exposure route. After RVFV exposure, all animals were monitored for temperature changes, weight loss, and survival, and blood samples were collected on days −3 and 0 to 7 and once a week thereafter (until days 28 to 36 postinfection [p.i.]) for virological, hematological, immunological, and chemical analyses. Additionally, blood was collected from animals exposed i.v. (day 0) within 5 min of virus inoculation to ensure that i.v. exposure occurred. When the animals succumbed to infection or were euthanized, either when moribund or at the study endpoint (ranging from days 28 to 36 p.i.), tissues were collected for viral titer determinations and histopathology.
Research was performed under an Institutional Animal Care and Use Committee-approved protocol in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals (34a). The facility where the research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Hematology, blood chemistries, and virological assays.
Whole blood was added to an EDTA tube (Sarstedt, Numbrecht, Germany) for complete blood count (CBC) determinations using a Hemavet instrument (Drew Scientific, Dallas, TX) according to the manufacturer's instructions. Clinical chemistry analyses were performed by the addition of whole blood to a lithium heparin tube (Sarstedt) using a comprehensive diagnostic panel analyzed with a Vetscan instrument (Abaxis, Union City, CA) according to the manufacturer's instructions. Normal ranges in the chemistry and hematology results of healthy rhesus macaques (26) and marmosets (47) were used as reference values. The plasma was then collected for viral titer determinations by a quantitative RT-PCR as previously described (8). At the time of necropsy, the following tissues were collected for viral titer determinations: liver, cerebrum, spleen, kidney, lung, heart, adrenal gland, inguinal lymph node, axillary lymph node, mesenteric lymph node, duodenum, jejunum, ileum, ovary/testis, skeletal muscle, bone marrow, and retina. Tissues were collected, weighed, and homogenized in Eagle's minimal essential medium (EMEM) containing 5% fetal bovine serum and gentamicin. Tissues were homogenized by using a Qiagen Mixer Mill 300 instrument (Retsch, Newtown, PA) and then centrifuged at 9,000 × g for 10 min, and the supernatant was stored at −70°C until further evaluation. Tissues collected from moribund animals were titrated by a standard plaque assay as previously described (42). Tissues collected at the study endpoint were homogenized according to the methods described above, and a 1:10 dilution of the supernatant was added to a 24-well plate of Vero cells in duplicate in a volume of 100 μl for each well. Plates were incubated for 1 h at 37°C with rocking every 15 min. After incubation, 0.5 ml of EMEM was added to each well, and the mixture was incubated for 4 days to monitor cytopathic effects (CPE).
Histopathology.
Full necropsies and histological examination were performed by a board-certified veterinary pathologist. The following tissues were collected during necropsy: axillary, inguinal, submandibular, mesenteric, and tracheobronchial lymph nodes; submandibular salivary gland; haired skin; brachial plexus; sciatic nerve; skeletal muscle; bone marrow (femur); eyes; brain; pituitary gland; spleen; adrenal gland; kidney; liver; stomach; duodenum; pancreas; jejunum; ileum; cecum; colon; testis/ovary; prostate gland/uterus; urinary bladder; tongue; tonsil; trachea; esophagus; thyroid gland; lung; thymus; and heart. All collected tissues were immersion fixed in 10% neutral buffered formalin for at least 21 days. The tissues were trimmed and processed according to standard protocols (39). Histology sections were cut at 5 to 6 μm on a rotary microtome, mounted onto glass slides, and stained with hematoxylin and eosin (HE). For immunohistochemical analysis, serial sections of tissue were cut and stained for RVFV antigen by using a mouse monoclonal antibody (4D4) against the glycoprotein Gn (6, 24) and an immunoperoxidase assay system (EnVision; Dako). Normal hepatic tissue served as the negative control; the positive-control tissue was liver from a known RVFV-positive animal. Normal mouse IgG was used as the negative serum control for the control slides. For the immunohistochemistry study, unstained tissue sections were deparaffinized, rehydrated, subjected to a methanol-hydrogen peroxide block, rinsed, and pretreated with Tris-EDTA buffer at 97°C for 30 min. A serum-free protein block (Dako) plus 5% normal goat serum were applied for 30 min. The primary antibody was then applied onto the tissue at a dilution of 1:100, and the tissue was incubated at room temperature overnight. The tissue sections were rinsed and then exposed to the EnVision horseradish peroxidase-labeled polymer for 30 min at room temperature. All sections were exposed to 3, 3′-diaminobenzidine (DAB) permanent chromogen for about 5 min, rinsed, counterstained with hematoxylin, dehydrated, and applied onto a coverslip with Permount.
Serology.
An anti-RVFV total IgG enzyme-linked immunosorbent assay (ELISA) was performed essentially as described previously (8), with the following modifications necessary for NHP specimens. BHK cell lysate rather than Vero E6 cells and secondary goat anti-monkey IgG horseradish peroxidase-conjugated antibody (catalog number 074-11-021; KPL), which was raised in rhesus macaques and most likely contributed to the low adjusted sum optical density (OD) values, were used. Neutralizing antibodies were assayed in serum from rhesus macaques and in plasma from marmosets with a 50% PRNT as previously described (7).
Statistical analysis.
Repeated-measures analysis of variance (ANOVA) was used to compare chemistries, viremias, percent changes in weight, and percent changes in temperature over time and between groups. Due to decreasing sample sizes, analysis was limited to days 0 through 7. All analyses were conducted by using SAS, version 9.2.
RESULTS
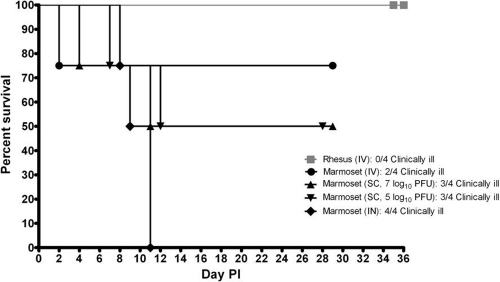
Marmosets were infected with RVFV by three exposure routes, i.v. (7 log10 PFU), s.c. (two doses, 5 and 7 log10 PFU), and i.n. (7 log10 PFU), and rhesus macaques were exposed i.v. to 7 log10 PFU of RVFV for a direct comparison with the current NHP model. The percent survival (Fig. 1), viremia (Fig. 2), weights (Fig. 3), body temperatures (Fig. 4), blood chemistry, and CBC (Fig. 5 and 6 and see Fig. S1 to S3 in the supplemental material) values were determined for all animals throughout the study.
Fig 1.
Percent survival of rhesus macaques exposed i.v. and marmosets exposed i.v., s.c., and i.n. to RVFV (n = 4).
Fig 2.
Viremia in rhesus macaques exposed i.v. (A) and marmosets exposed i.v. (B), s.c. (C), and i.n. (D) to RVFV (n = 4). Asterisks indicate an animal that succumbed or was euthanized. PFUe, plaque-forming unit equivalent.
Fig 3.
Percent change in baseline of weights of rhesus macaques exposed i.v. (A) and marmosets exposed i.v. (B), s.c. (C), and i.n. (D) to RVFV (n = 4). Asterisks indicate an animal that succumbed or was euthanized.
Fig 4.
Percent change in baseline of the temperature of rhesus macaques exposed i.v. (A) and marmosets exposed i.v. (B), s.c. (C), and i.n. (D) to RVFV (n = 4). Asterisks indicate an animal that succumbed or was euthanized.
Fig 5.
Percent change in baseline of the alanine aminotransferase (ALT) levels in blood of rhesus macaques exposed i.v. (A) and marmosets exposed i.v. (B), s.c. (C), and i.n. (D) to RVFV (n = 4). Asterisks indicate an animal that succumbed or was euthanized. The boxes represent the normal ALT reference range variability in healthy animals.
Fig 6.
Percent change in baseline of the alkaline phosphatase (ALP) levels in blood of rhesus macaques exposed i.v. (A) and marmosets exposed i.v. (B), s.c. (C), and i.n. (D) to RVFV (n = 4). Asterisks indicate an animal that succumbed or was euthanized. The boxes represent the normal ALP reference range variability in healthy animals.
Survival.
None of the rhesus macaques (n = 4) succumbed or presented with clinical illness (Fig. 1). In contrast, the group of marmosets exposed i.v. (n = 4) had one animal that succumbed on day 2 postinfection (p.i.), whereas two others presented with clinical illness, which was characterized by anorexia, decreased activity, and ruffled fur/hunched posture. Half of the marmosets that were exposed s.c. (n = 4) succumbed or were euthanized, and three out of four of the animals presented with a clinical illness similar to that described above for marmosets exposed i.v. For marmosets that received 7 log10 PFU of RVFV, one animal succumbed on day 4 p.i., and another succumbed on day 11 p.i. Of marmosets that received 5 log10 PFU of RVFV, one animal was euthanized on day 7 p.i., and another was euthanized on day 12 p.i. The marmoset that was euthanized on day 12 p.i. had signs of neurological impairment. This animal appeared disoriented and was shaking and falling over in its cage. When marmosets were exposed to RVFV i.n., 100% mortality resulted, and the marmosets succumbed or were euthanized on days 8, 9, and 11 p.i.; all of these animals presented with signs of neurological impairment, as described above.
Viremia and clinical observations. (i) Rhesus macaques and marmosets exposed i.v.
All rhesus macaques did develop viremia, which peaked on day 2 p.i. (Fig. 2A). All of the marmosets exposed i.v. developed viremia, except for the animal that succumbed on day 2 p.i. (Fig. 2B), and the presentation of clinical illness correlated with viremia. Viremia in marmosets exposed i.v. peaked on day 1 p.i., and compared to the peak day of viremia of rhesus macaques (day 2 p.i.), marmosets had levels of viremia that were on average 1 log10 PFU higher (P = 0.27). Over the course of the study, there were no significant changes in the weights of the rhesus macaques (Fig. 3A) and the marmosets exposed i.v. (P = 0.35) (Fig. 3B). The temperatures of rhesus macaques (Fig. 4A) and marmosets (Fig. 4B) exposed i.v. did not differ significantly throughout the study (P = 0.57). One rhesus macaque had a slight increase in temperature, which peaked on day 6 p.i., and one marmoset had a slight decrease in temperature on day 4 p.i., but the general trend remained similar. The level of the liver enzyme alanine aminotransferase (ALT) peaked on days 2 to 3 p.i. in both rhesus macaques and marmosets exposed i.v. (Fig. 5A and B). However, the increase was more significant in marmosets overall than in rhesus macaques (P = 0.03). The white blood cell (WBC) levels varied in rhesus macaques and marmosets exposed i.v. (see Fig. S1A and S1B in the supplemental material). The blood urea nitrogen (BUN) and creatinine (CRE) values for rhesus macaques and marmosets exposed i.v. did not change significantly (P = 0.39 and P = 0.70, respectively) (see Fig. S2A, S2B, S3A, and S3B in the supplemental material).
(ii) Marmosets exposed s.c.
All animals developed viremia (Fig. 2C), with the marmosets that were exposed to 5 log10 PFU of RVFV s.c. having the highest levels (P = 0.04). Viremia persisted until the animals succumbed or were euthanized, and the level of viremia correlated with the presentation of signs of clinical illness as described above for animals exposed i.v. An overall decrease in the weights was observed for marmosets exposed s.c. (P = 0.04) (Fig. 3C). In contrast to marmosets exposed i.v., marmosets that were exposed s.c. and that succumbed or were euthanized had decreases in temperature (Fig. 4C). The ALT (Fig. 5C) and alkaline phosphatase (ALP) (Fig. 6C) levels were highest in the animals exposed s.c. compared to the other exposure routes (P = 0.02 for both). The WBC levels varied in marmosets exposed s.c. (see Fig. S1C in the supplemental material), showing sporadic increases and decreases for most animals. In contrast to the i.v. exposure route, the BUN and CRE levels increased in marmosets exposed s.c. (P < 0.01 and P = 0.79, respectively) (see Fig. S2C and S3C in the supplemental material), suggesting kidney disease in these animals. Among the marmosets with notable increases in levels, the BUN/CRE ratios ranged from 24 to 78, which provides further evidence for the occurrence of kidney disease in these animals. However, one marmoset had BUN/CRE ratios that ranged from 50 to 148, which suggests that dehydration occurred in this animal.
(iii) Marmosets exposed i.n.
All marmosets exposed i.n. developed viremia (Fig. 2D), which peaked on day 2 or 3 p.i. and declined steadily until the animals succumbed or were euthanized. In contrast to marmosets exposed i.v. and s.c., marmosets that were exposed i.n. did show a significant overall decrease in weight after exposure (P < 0.01) (Fig. 3D). Similar to marmosets that were exposed s.c., the marmosets exposed i.n. that succumbed or were euthanized had decreases in temperature (P = 0.66) (Fig. 4D). The ALT (Fig. 5D) and ALP (Fig. 6D) levels were elevated in the animals exposed i.n. but to a lesser extent than that of animals exposed i.v. and s.c. In contrast to the other exposure routes, three out of four marmosets exposed i.n. had a significant increase in WBC on day 1 p.i. (P = 0.02) (see Fig. S1D in the supplemental material). Similar to the marmosets exposed s.c., the BUN and CRE levels increased in marmosets exposed i.n. (P < 0.01 and P = 0.67, respectively) (see Fig. S2D and S3D in the supplemental material), suggesting kidney disease in these animals. However, the BUN/CRE ratios ranged from 55 to 245, which suggests that these animals were dehydrated.
Viral titers in tissues.
Tissues were collected at the time of necropsy, and the viral titers were determined by a standard plaque assay for the animals that succumbed or were euthanized (see Table S1 in the supplemental material). Virus was found in the liver of all marmosets exposed i.v. and s.c. but in only two out of four of the animals exposed i.n. Virus was found in the cerebrum of six out of nine of the marmosets exposed by all three exposure routes. The kidney was the only tissue where virus was detected in all marmosets. It is interesting that virus was detected in the retinas of two of the marmosets exposed s.c. and of all marmosets exposed i.n. Other tissues where virus was detected in marmosets depending on the exposure route included the spleen; lung; heart; adrenal gland; inguinal, axillary, and mesenteric lymph nodes; duodenum; jejunum; ileum; ovary/testis; skeletal muscle; and bone marrow. Tissues were also collected at the time of necropsy from the surviving animals at the study endpoint (days 28 to 35 p.i.) and were analyzed for virus by a cytopathic effect assay, where all tissues were found to be negative for virus (data not shown).
Pathology findings.
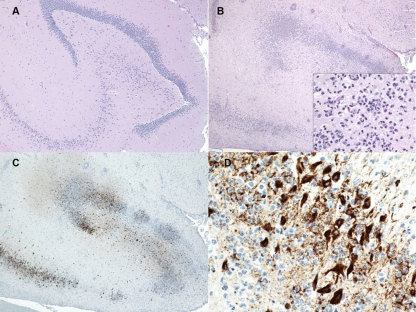
The primary gross pathology finding identified in marmosets that succumbed to infection by the i.v. or s.c. route of exposure was an enlarged (about 2 to 3 times the size of an uninfected control), yellow-orange, friable liver (Fig. 7A and B). The histopathologic findings in the liver were characterized by hepatocellular degeneration and necrosis that affected primarily the centrilobular to midzonal areas (Fig. 7C). Degenerate hepatocytes often contained variably sized, clear, round vacuoles (lipid) in the cytoplasm. Scattered among necrotic hepatocytes were brightly eosinophilic, round bodies interpreted as Councilman-like bodies (remnants of apoptotic hepatocytes). Rarely, eosinophilic intranuclear inclusion bodies were observed in hepatocytes. Immunohistochemically, hepatocytes exhibited intense positive staining for RVFV antigen (Fig. 7D). No directly attributable pathological changes in the liver were noted for the rhesus macaques exposed to RVFV.
Fig 7.
Gross, histologic, and immunohistochemical findings in the liver of RVFV-infected marmosets. (A) Gross image of the liver of an uninfected control marmoset. (B) Gross image of the liver of an RVFV-infected marmoset exposed i.v. on day 2 p.i. (C) Hematoxylin and eosin stain. Hepatocellular degeneration and necrosis in the liver on day 4 p.i. of a marmoset exposed s.c. are shown. (D) Immunohistochemistry demonstrating the amount of RVFV antigen (brown staining) on day 4 p.i. in the liver (primarily degenerate/necrotic hepatocytes) of a marmoset exposed s.c.
An additional histologic finding observed for the marmoset exposed to RVFV by the i.v. route that succumbed on day 2 p.i. was the presence of fibrin deposition and fibrin thrombi in several tissues (kidney, lung, choroid layer of the eye, and brain), suggesting that DIC was present at the time of death (Fig. 8). None of the other animals that succumbed to infection by any other route of exposure exhibited evidence of DIC.
Fig 8.
Histologic findings consistent with DIC in a marmoset exposed to RVFV i.v. that succumbed on day 2 p.i. (A) Hematoxylin and eosin (HE) stain of the kidney showing a fibrin thrombus in a vessel (delineated by asterisks). (B) HE stain of the lung showing a fibrin thrombus in a vessel (delineated by an asterisk). (C) HE stain of the choroid layer of the eye showing fibrin thrombi in the vessels (delineated by arrows). (D) HE stain of the pons region of the brain showing an acute infarct (delineated by arrows) with a fibrin thrombus in an adjacent blood vessel (delineated by an asterisk).
Gross lesions were not observed in the brain of any of the marmosets that succumbed to infection by the multiple exposure routes. However, histopathological changes in the brains of marmosets exposed s.c. that succumbed on days 11 and 12 p.i. and all marmosets exposed i.n. were noted. Representative histopathological changes portrayed widespread brain lesions with moderate numbers of inflammatory cells consisting primarily of lymphocytes and neutrophils affecting the gray matter (encephalitis), changes that were not found in uninfected controls (Fig. 9A and B). Neuronal necrosis, satellitosis (surrounding of neurons by glial cells), gliosis (reactive glial cells), and neuronophagia (phagocytosis of degenerate/necrotic neurons) were also commonly observed. Immunohistochemically, there was intense multifocal positive staining of neurons for RVFV antigen (Fig. 9C and D). For rhesus macaques, one out of four animals displayed minimal to mild multifocal lymphoplasmacytic inflammation of the brain accompanied by gliosis at the study endpoint. Interestingly, one of the marmosets exposed s.c. that succumbed to RVFV on day 11 p.i. exhibited widespread positive staining for RVFV antigen in the retina of the eye without histologic changes (Fig. 10). However, positive antigen staining of the retina was not observed for any of the other marmosets that succumbed to RVFV despite virus being detected by a cytopathic effect assay (see Table S1 in the supplemental material), as discussed above.
Fig 9.
Histologic and immunohistochemical findings in the brain of RVFV-infected marmosets. (A) HE stain of the hippocampus region from an uninfected control marmoset. (B) HE stain demonstrating increased cellularity with perivascular inflammation in the hippocampus of a marmoset exposed to 5 log10 PFU s.c. that was euthanized on day 12 p.i. The inset is a magnified view of the same region, showing necrotic neurons surrounded by numerous neutrophils. (C) Immunohistochemistry demonstrating the amount of RVFV antigen in the hippocampus of the same marmoset exposed s.c. that was euthanized on day 12 p.i. (D) Higher magnification of the immunohistochemistry of the hippocampus demonstrating the intense immunoreactivity of neurons for RVFV antigen.
Fig 10.
Immunohistochemical findings in the retina of a marmoset exposed to 7 log10 PFU s.c. that was euthanized on day 11 p.i. Neurons and associated fibers are shown to be multifocally positive for RVFV antigen.
The histopathology analysis focused on the liver, brain, and retina because these areas have been shown to be important RVFV targets in other animals. However, other gross and/or histological changes were noted for the lymphoid tissues, adrenal gland, kidney, and heart. The lungs were also noted as a target tissue in marmosets exposed i.n. A detailed study is under way to further characterize the pathology of RVFV in these tissues.
Serology.
All rhesus macaques and some marmosets developed neutralizing antibody titers as early as day 7 p.i. (see Table S2 in the supplemental material). By day 14 p.i., all animals sampled developed neutralizing antibody titers except for the uninfected controls. On day 14 p.i., all rhesus macaques and some marmosets developed RVFV IgG titers, which increased steadily until the study endpoint (Fig. 11). The animals exposed s.c. that were euthanized on days 11 and 12 p.i. developed neutralizing antibody titers, but only one animal developed an RVFV IgG titer (Fig. 11C). For marmosets that succumbed to RVFV i.n., all but one developed neutralizing antibody titers either before or on the day of euthanasia (note that some samples were not collected because the animal succumbed to infection before sample collection), but none of the animals developed an RVFV IgG titer (Fig. 11D).
Fig 11.
Results of anti-RVFV total IgG adjusted sum OD ELISA of sera from rhesus macaques exposed i.v. (R-IV) (A) and marmosets exposed i.v. (M-IV) (B), s.c. (M-SC) (C), and i.n. (M-IN) (D) to RVFV (n = 4).
DISCUSSION
The results of this study identified the common marmoset as a useful model of severe human RVF for the evaluation of potential vaccines and therapeutics. The manifestations of hemorrhagic disease, acute-onset hepatitis, and delayed-onset encephalitis observed for these marmosets are similar to the most severe consequences of RVFV infection of humans. Accordingly, the marmoset model could be useful for investigating key processes in the pathogenesis of severe RVF. We identified a number of advantages of using this new primate model, which overcomes limitations of the existing rhesus macaque RVF primate model.
Overall, marmosets were more susceptible to infection with RVFV and had more marked changes in their clinical chemistry and hematology values than rhesus macaques. Viremia was observed for all animals, and clinical illness did correlate with higher levels of viremia in marmosets. The mortality rate for all the marmosets ranged from 25 to 100%, with 50 to 100% showing signs of clinical illness depending on the route of exposure. This is in contrast to the finding of no mortality or signs of clinical illness in any of the rhesus macaques. Previous studies reported that a minority of rhesus macaques exposed to RVFV do succumb to infection and have signs of clinical illness (37), but those studies used large cohorts of animals (15 to 20 rhesus macaques), and we evaluated a smaller cohort of four animals. A major advantage of the marmoset RVF primate model is the manifestation of severe disease in a small cohort of animals. Marmosets therefore become a cost-beneficial model, which is enhanced by their lower purchase price than that of rhesus macaques.
The earliest fatality observed was for the marmosets exposed to RVFV i.v. One marmoset succumbed on day 2 p.i. and had histological evidence of DIC, suggested by thrombocytopenia and fibrin thrombi. These features are also observed for human hemorrhagic RVFV infections (41) and have also been reported to occur in rhesus macaques exposed i.v. (13), but they were not observed in the rhesus macaques used in this study, most likely due to the small cohort of animals. Evidence of DIC was observed only for the marmosets exposed i.v. and not by the other routes of exposure. This finding suggests that marmosets exposed i.v. may serve as a beneficial model for human hemorrhagic RVF disease.
Marmosets exposed to RVFV s.c. developed both hepatitis and encephalitis, and in one instance, viral antigen was detected in the retina by immunohistochemistry. Marmosets euthanized on day 4 p.i. (exposure to 7 log10 PFU) and day 7 p.i. (exposure to 5 log10 PFU) appear to have succumbed from acute-onset hepatitis, whereas marmosets euthanized on day 11 p.i. (exposure to 7 log10 PFU) and day 12 p.i. (exposure to 5 log10 PFU) succumbed from late-developing encephalitis. The route of neuroinvasion in these marmosets is unclear and will require serial sampling studies to evaluate the spread of the virus over time. The meningoencephalitic form of RVF occurs in less than 1% of natural human cases (25), but residual central nervous system (CNS) symptoms are common in neurological cases and may be severe. In the context of the tens of thousands of human cases that may arise during outbreaks, even a low percentage of neurological cases can provide a significant medical challenge. Therefore, future studies with the marmoset model would be useful to evaluate the route of neuroinvasion in hopes of better understanding this severe form of human RVF disease.
The marmoset exposed to RVFV s.c. that succumbed on day 11 p.i. also exhibited widespread positive immunohistochemical staining in the retina of the eye, although there was no associated inflammation. Even though virus was detected in the retinas of several other marmosets by a plaque assay, this was the only case detected by immunohistochemistry. This is likely due to the differing sensitivities of the assays and should be addressed further to determine if the marmoset model will be useful to investigate RVFV-associated retinitis.
The highest levels of viremia were found for the fatally infected marmosets exposed s.c. to 5 log10 PFU of RVFV. It is interesting that the animals that received the lower dose of virus (5 log10 PFU versus 7 log10 PFU) developed a higher level of viremia. RVFV-infected mosquitoes have been shown to transmit primarily around 3 log10 PFU of virus extravascularly (45), so our results suggest that a lower virus dose delivered peripherally could potentially cause a higher level of viremia. We speculate that this could be due to the virus actively replicating to higher levels at the inoculation site prior to an active immune response being initiated to clear the virus. The neutralizing antibody titers were slightly higher in the marmosets exposed to RVFV s.c. at the lower dose. Marmosets that were exposed to RVFV s.c. and succumbed or were euthanized seemed to develop slightly delayed neutralizing antibody and RVFV IgG responses than did marmosets that survived infection, which may have influenced their survival.
Marmosets exposed s.c. had the lowest body temperatures at the time of euthanasia. Fevers were never noted for these animals but could have been missed, since temperature was not continually monitored and also because body temperature is known to decrease as major organ systems begin to shut down. In fact, the temperature decrease was drastic shortly before death and neared room temperature in fatally infected marmosets exposed s.c. to the higher virus dose. These animals also had the most significant increases in levels of the liver enzymes ALT and ALP, which signified that widespread hepatocellular damage occurred in these animals and contributed to lethality.
Interestingly, marmosets exposed i.n. had the highest rates of morbidity and mortality compared to the other exposure routes. All of the animals infected i.n. succumbed from a late-developing encephalitis and interstitial pneumonia, and marmosets exposed i.n. were the only cohort where a consistent decrease in weight and increase in WBC was observed. Most of these animals also had a decrease in body temperature and an increase in levels of liver enzymes similarly to marmosets exposed s.c. The observed leukocytosis was most likely due to the high degree of inflammation caused by the virus. Leukocytosis is known to occur during the early phase of recovery in RVFV-infected livestock (41).
Although RVFV is transmitted most often through the bite of an infected mosquito, it can also be transmitted by aerosol, which is evident from the number of laboratory workers who have become infected (43) and the potential for infection of veterinarians and abattoir workers who handle infected animals (although infection can also occur through mucosal contact in this setting). The amount of virus associated with infected animal tissues, body fluids, aborted fetal materials, and placental membranes is very high (>6 log10 PFU/ml) and allows for the adequate generation of aerosols and contact contamination, which can account for many human infections (37). The incidence of CNS manifestations resulting from an aerosol infection has been speculated to be much greater than is typical with peripheral infection, given the potential for neuroinvasion to occur more easily through aerosol exposure. Our results with the marmoset model suggest that these animals are more susceptible to neurological manifestations when exposed by aerosol than by peripheral exposure routes. Marmosets exposed i.n. offer a novel model to further investigate the specific mechanism of neuroinvasion by RVFV and could be compared to the mechanism of neuroinvasion caused by peripheral exposure (i.e., s.c.).
In conclusion, this newly described marmoset model represents a novel NHP model that mimics the severe manifestations of human RVF for the evaluation of potential therapeutics and vaccines. The major pathological features of hemorrhagic disease, hepatitis, encephalitis, and possibly retinitis are reproduced in marmosets depending on the route of exposure and can be exploited to further understand RVFV-induced pathology. Future work should monitor the progression of disease in this model and further evaluate the immune response to RVFV in marmosets.
Supplementary Material
ACKNOWLEDGMENTS
We thank Bobbie Rae Erickson for technical assistance, Jeff Brubaker, Neil Davis, Angela Grove, Gale Krietz, and Chris Mech for pathology support, and Joseph Shaw for critically reading the manuscript. We also thank Sarah Norris for statistical analysis support and Cindy Rossi for kindly providing the ELISA cell lysate.
This project was funded through an interagency agreement with the Science and Technology Directorate of the U.S. Department of Homeland Security under award number HSHQDC-09-00568.
The views of the authors do not necessarily reflect the position of the Department of Defense or the Department of the Army.
Footnotes
Published ahead of print 7 December 2011
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Adams AP, et al. 2008. Common marmosets (Callithrix jacchus) as a nonhuman primate model to assess the virulence of eastern equine encephalitis virus strains. J. Virol. 82:9035–9042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson GW, Jr, Slayter MV, Hall W, Peters CJ. 1990. Pathogenesis of a phleboviral infection (Punta Toro virus) in golden Syrian hamsters. Arch. Virol. 114:203–212 [DOI] [PubMed] [Google Scholar]
- 3. Anderson GW, Jr, Slone TW, Jr, Peters CJ. 1988. The gerbil, Meriones unguiculatus, a model for Rift Valley fever viral encephalitis. Arch. Virol. 102:187–196 [DOI] [PubMed] [Google Scholar]
- 4. Anderson GW, Jr, Slone TW, Jr, Peters CJ. 1987. Pathogenesis of Rift Valley fever virus (RVFV) in inbred rats. Microb. Pathog. 2:283–293 [DOI] [PubMed] [Google Scholar]
- 5. Avila MM, Samoilovich SR, Laguens RP, Merani MS, Weissenbacher MC. 1987. Protection of Junin virus-infected marmosets by passive administration of immune serum: association with late neurologic signs. J. Med. Virol. 21:67–74 [DOI] [PubMed] [Google Scholar]
- 6. Battles JK, Dalrymple JM. 1988. Genetic variation among geographic isolates of Rift Valley fever virus. Am. J. Trop. Med. Hyg. 39:617–631 [DOI] [PubMed] [Google Scholar]
- 7. Beaty BJ, Calisher CH, Shope RE. 1989. Arboviruses, p 797–855 In Schmidt NJ, Emmons RW. (ed), Diagnostic procedures for viral, rickettsial and chlamydial infections, 6th ed American Public Health Association, Washington, DC [Google Scholar]
- 8. Bird BH, et al. 2008. Rift valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J. Virol. 82:2681–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bird BH, Albarino CG, Nichol ST. 2007. Rift Valley fever virus lacking NSm proteins retains high virulence in vivo and may provide a model of human delayed onset neurologic disease. Virology 362:10–15 [DOI] [PubMed] [Google Scholar]
- 10. Bird BH, Khristova ML, Rollin PE, Ksiazek TG, Nichol ST. 2007. Complete genome analysis of 33 ecologically and biologically diverse Rift Valley fever virus strains reveals widespread virus movement and low genetic diversity due to recent common ancestry. J. Virol. 81:2805–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bird BH, Ksiazek TG, Nichol ST, Maclachlan NJ. 2009. Rift Valley fever virus. J. Am. Vet. Med. Assoc. 234:883–893 [DOI] [PubMed] [Google Scholar]
- 12. Carrion R, Jr, et al. 2007. Lassa virus infection in experimentally infected marmosets: liver pathology and immunophenotypic alterations in target tissues. J. Virol. 81:6482–6490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cosgriff TM, et al. 1989. Hemostatic derangement produced by Rift Valley fever virus in rhesus monkeys. Rev. Infect. Dis. 11(Suppl 4):S807–S814 [DOI] [PubMed] [Google Scholar]
- 14. Daubney R, Hudson J, Garnham P. 1931. Enzootic hepatitis or Rift Valley fever: an undescribed virus disease of sheep, cattle and man from East Africa. J. Pathol. Bacteriol. 34:545–579 [Google Scholar]
- 15. Davies FG, Clausen B, Lund LJ. 1972. The pathogenicity of Rift Valley fever virus for the baboon. Trans. R. Soc. Trop. Med. Hyg. 66:363–365 [DOI] [PubMed] [Google Scholar]
- 16. Easterday BC. 1965. Rift valley fever. Adv. Vet. Sci. 10:65–127 [PubMed] [Google Scholar]
- 17. Findlay GM. 1932. The infectivity of Rift Valley fever for monkeys. Trans. R. Soc. Trop. Med. Hyg. 26:161–168 [Google Scholar]
- 18. Findlay GM. 1932. Rift Valley fever or enzootic hepatitis. Trans. R. Soc. Trop. Med. Hyg. 25:229–265 [Google Scholar]
- 19. Findlay GM, Daubney R. 1931. The virus of Rift Valley fever or enzootic hepatitis. Lancet 221:1350–1351 [Google Scholar]
- 20. Fisher AF, et al. 2003. Induction of severe disease in hamsters by two sandfly fever group viruses, Punta toro and Gabek Forest (Phlebovirus, Bunyaviridae), similar to that caused by Rift Valley fever virus. Am. J. Trop. Med. Hyg. 69:269–276 [PubMed] [Google Scholar]
- 21. Flick R, Bouloy M. 2005. Rift Valley fever virus. Curr. Mol. Med. 5:827–834 [DOI] [PubMed] [Google Scholar]
- 22. Greenough TC, et al. 2005. Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am. J. Pathol. 167:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacob JR, Lin KC, Tennant BC, Mansfield KG. 2004. GB virus B infection of the common marmoset (Callithrix jacchus) and associated liver pathology. J. Gen. Virol. 85:2525–2533 [DOI] [PubMed] [Google Scholar]
- 24. Keegan K, Collett MS. 1986. Use of bacterial expression cloning to define the amino acid sequences of antigenic determinants on the G2 glycoprotein of Rift Valley fever virus. J. Virol. 58:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laughlin LW, Meegan JM, Strausbaugh LJ, Morens DM, Watten RH. 1979. Epidemic Rift Valley fever in Egypt: observations of the spectrum of human illness. Trans. R. Soc. Trop. Med. Hyg. 73:630–633 [DOI] [PubMed] [Google Scholar]
- 26. Lee DR, Doane CJ. 2011. Association of Primate Veterinarians, primate formulary. Alamogordo Primate Facility, Alamogordo, NM [Google Scholar]
- 27. Madani TA, et al. 2003. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin. Infect. Dis. 37:1084–1092 [DOI] [PubMed] [Google Scholar]
- 28. Mansfield K. 2003. Marmoset models commonly used in biomedical research. Comp. Med. 53:383–392 [PubMed] [Google Scholar]
- 29. McIntosh BM, Russell D, dos Santos I, Gear JH. 1980. Rift Valley fever in humans in South Africa. S. Afr. Med. J. 58:803–806 [PubMed] [Google Scholar]
- 30. Meegan JM, Bailey CL. 1989. Rift Valley fever, p 51–76 In Monath TP. (ed), The arboviruses: epidemiology and ecology, vol 4 CRC Press, Boca Raton, FL [Google Scholar]
- 31. Miller WS, Demchak P, Rosenberger CR, Dominik JW, Bradshaw JL. 1963. Stability and infectivity of airborne yellow fever and Rift Valley fever viruses. Am. J. Hyg. 77:114–121 [Google Scholar]
- 32. Mims CA. 1956. Rift Valley fever virus in mice. I. General features of the infection. Br. J. Exp. Pathol. 37:99–109 [PMC free article] [PubMed] [Google Scholar]
- 33. Morrill JC, et al. 1990. Pathogenesis of Rift Valley fever in rhesus monkeys: role of interferon response. Arch. Virol. 110:195–212 [DOI] [PubMed] [Google Scholar]
- 34. National Institute for Communicable Diseases 2011. Interim report on the 2011 Rift Valley fever (RVF) outbreak in South Africa. National Institute for Communicable Diseases, Sandringham, Johannesburg, South Africa [Google Scholar]
- 34a. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 35. Peters CJ, Anderson GW. 1981. Pathogenesis of Rift Valley fever. Contrib. Epidemiol. Biostat. 3:21–41 [Google Scholar]
- 36. Peters CJ, et al. 1988. Experimental Rift Valley fever in rhesus macaques. Arch. Virol. 99:31–44 [DOI] [PubMed] [Google Scholar]
- 37. Peters CJ, Linthicum KJ. 1994. Rift Valley fever, p 125–138 In Beran GW, Steele JH. (ed), Handbook of zoonoses, section B. Viral, 2nd ed CRC Press, Boca Raton, FL [Google Scholar]
- 38. Peters CJ, Liu CT, Anderson GW, Jr, Morrill JC, Jahrling PB. 1989. Pathogenesis of viral hemorrhagic fevers: Rift Valley fever and Lassa fever contrasted. Rev. Infect. Dis. 11(Suppl 4):S743–S749 [DOI] [PubMed] [Google Scholar]
- 39. Prophet EB, Mills B, Arrington JB, Sobin LH. 1992. Laboratory methods for histotechnology. Armed Forces Institute of Pathology, Washington, DC [Google Scholar]
- 40. Ritter M, et al. 2000. Resistance to Rift Valley fever virus in Rattus norvegicus: genetic variability within certain ‘inbred’ strains. J. Gen. Virol. 81:2683–2688 [DOI] [PubMed] [Google Scholar]
- 41. Schmaljohn CS, Nichol ST. 2007. Bunyaviridae, p 1766 In Knipe DM, et al. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 42. Smith DR, et al. The pathogenesis of Rift Valley fever virus in the mouse model. Virology 407:256–267 [DOI] [PubMed] [Google Scholar]
- 43. Smithburn K, Mahaffy A, Haddow A. 1949. Rift Valley fever: accidental infections among laboratory workers. J. Immunol. 62:213–227 [PubMed] [Google Scholar]
- 44. Snoy PJ. 2010. Establishing efficacy of human products using animals: the US Food and Drug Administration's “animal rule.” Vet. Pathol. 47:774–778 [DOI] [PubMed] [Google Scholar]
- 45. Turell MJ, Spielman A. 1992. Nonvascular delivery of Rift Valley fever virus by infected mosquitoes. Am. J. Trop. Med. Hyg. 47:190–194 [DOI] [PubMed] [Google Scholar]
- 46. van Velden DJ, Meyer JD, Olivier J, Gear JH, McIntosh B. 1977. Rift Valley fever affecting humans in South Africa: a clinicopathological study. S. Afr. Med. J. 51:867–871 [PubMed] [Google Scholar]
- 47. Yarbrough LW, Tollett JL, Montrey RD, Beattie RJ. 1984. Serum biochemical, hematological and body measurement data for common marmosets (Callithrix jacchus jacchus). Lab. Anim. Sci. 34:276–280 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.