Abstract
Tetherin/BST-2 forms a proteinaceous tether that restricts the release of a number of enveloped viruses following viral budding. Tetherin is an unusual membrane glycoprotein with two membrane anchors and an extended coiled-coil ectodomain. The ectodomain itself forms an imperfect coil that may undergo conformational shifts to accommodate membrane dynamics during the budding process. The coiled-coil ectodomain is required for restriction, but precisely how it contributes to the restriction of particle release remains under investigation. In this study, mutagenesis of the ectodomain was used to further define the role of the coiled-coil ectodomain in restriction. Scanning mutagenesis throughout much of the ectodomain failed to disrupt the ability of tetherin to restrict HIV particle release, indicating a high degree of plasticity. Targeted N- and C-terminal substitutions disrupting the coiled coil led to both a loss of restriction and an alteration of subcellular distribution. Two ectodomain mutants deficient in restriction were endocytosed inefficiently, and the levels of these mutants on the cell surface were significantly enhanced. An ectodomain mutant with four targeted serine substitutions (4S) failed to cluster in membrane microdomains, was deficient in restriction of particle release, and exhibited an increase in lateral mobility on the membrane. These results suggest that the tetherin ectodomain contributes to microdomain localization and to constrained lateral mobility. We propose that focal clustering of tetherin via ectodomain interactions plays a role in restriction of particle release.
INTRODUCTION
Host restriction factors have evolved to limit the replication and spread of pathogenic microorganisms. Acute viral infection induces type I interferon responses, stimulating the expression of a number of innate immune defense factors (25, 31). Tetherin (BST-2/CD317/HM1.24) was recently identified as an interferon-inducible host restriction factor, linking nascent HIV-1 to the plasma membrane of infected cells and preventing the spread of cell-free virus (25, 37). Tetherin has been shown to inhibit not only HIV-1 particle release but is active against a wide variety of enveloped viruses, including members of the lentivirus, arenavirus, herpesvirus, and filovirus families (14, 15, 22, 32, 37).
HIV-1 overcomes tetherin-mediated restriction through expression of the accessory protein Vpu (16, 38). Vpu is a 16-kDa type I integral membrane protein (4, 35) that performs two distinct functions in HIV-1-infected cells (21). Vpu leads to surface downregulation and proteasomal degradation of CD4 in infected T cells and macrophages (23, 34, 40, 41) and enhances viral particle release in restrictive cell types (16, 38). Vpu overcomes tetherin-mediated restriction via an interaction facilitated through their respective transmembrane (TM) domains (12, 13, 24, 30). The potential mechanisms accounting for tetherin surface downregulation by Vpu include proteasomal and lysosomal degradation together with sequestration of tetherin within the trans-Golgi network (TGN) (5). The relevance of tetherin to host-pathogen biology is emphasized by the finding that viruses have evolved distinct mechanisms to alleviate tetherin-mediated restriction of particle release. These include the K5 protein of Kaposi's sarcoma-associated herpesvirus (27), the Nef protein of SIV (13, 44), and the envelope glycoproteins of SIV, HIV-2, and Ebola virus (8, 15, 20).
Tetherin is a type II integral membrane glycoprotein with a short N-terminal cytoplasmic domain, a single transmembrane-spanning region, an ∼110-residue extracellular coiled-coil domain, and a C-terminal glycosyl-phosphatidylinositol (GPI) anchor (18). Tetherin's subcellular distribution is punctate on the plasma membrane and is also present within intracellular endosomal membranes, particularly the TGN (6, 18). Due to the C-terminal GPI modification, tetherin is concentrated within lipid raft microdomains at the plasma membrane (18). Tetherin plasma membrane concentrations within lipid rafts may indeed facilitate the capture of nascent HIV-1 virions. Several recent structural studies have strongly supported that tetherin forms a parallel homodimer, stabilized through ectodomain coiled-coil interactions further strengthened by disulfide linkages at residue positions C53, C63, and C91 (10, 33, 42). Tetherin may form a tetramer, as suggested by crystal structures in two published studies (33, 42), although tetramer formation is not required for antiviral activity (42). Tetherin has been proposed to span both the viral and the cellular membranes through the action of its two membrane anchors (25, 39). In addition, disulfide linkages conferred by any of the three cysteines in the N-terminal portion of the ectodomain and contributions from the coiled-coil region of the ectodomain are required for antiviral activity (25, 28). However, the nature of the contribution from the coiled-coil ectodomain has remained obscure.
In the present study, we show that targeted destabilization of the tetherin extracellular coiled-coil region results in the loss of particle release restriction, while the majority of substitutions throughout the ectodomain had no discernible phenotypic effect. Serine substitution mutations at key ectodomain positions predicted to destabilize the coiled coil resulted in a complete loss of restriction. These mutants displayed enhanced cell surface levels, a diffuse plasma membrane distribution, and enhanced lateral mobility, suggesting that failure to restrict correlates with failure to cluster within a constrained plasma membrane microdomain.
MATERIALS AND METHODS
Cell lines and transfection.
HT1080 and HEK293T cells were obtained from the American Type Culture Collection (ATCC). Cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin-streptomycin. HEK293T and HT1080 cells were transfected with either Fugene HD (Roche Applied Science) or Lipofectamine 2000 (Invitrogen) in Opti-MEM medium (Invitrogen).
Plasmids.
An N-terminal FLAG-tagged tetherin construct (pFLAG-tetherin) was generated by PCR amplification of human tetherin from a commercially available cDNA clone and inserted in-frame into the BamHI/XhoI sites of FLAG-Hsp90α (17). Tetherin ectodomain alanine scanning and serine substitution mutants were generated using pFLAG-tetherin. Nucleotide substitutions were introduced using a QuikChange II site-directed mutagenesis kit (Stratagene) and primer sequences as indicated in Table S1 in the supplemental material. The N-terminal, green fluorescent protein (GFP)-tagged tetherin ectodomain mutant constructs were generated by PCR amplification from pFLAG-tetherin, followed by insertion into pEGFP-C1 (Clontech) using the Sal1and BamHI restriction sites. The vpu-deficient HIV-1 molecular clone, pNL4.3/Udel, has been described elsewhere (16). A GPI-linked version of GFP (GPI-GFP) was obtained from Jennifer Lippincott-Schwartz and Ann Kenworthy (26), and ICAM-1 molecule tagged with GFP was constructed by cloning the ICAM-1 cDNA into pEGFP-C1 (Clontech), as described by others (3).
HIV-1 particle release assay.
Transfected HEK 293T supernatants were harvested, clarified by low-speed centrifugation, and subsequently concentrated by ultracentrifugation through a 20% sucrose cushion (100,000 × g for 2 h, 4°C). Viral pellets were lysed in 1× radioimmunoprecipitation assay (RIPA) buffer supplemented with protease inhibitors (RIPA-PI). 293T cells were washed with phosphate-buffered saline (PBS) prior to detachment using prewarmed EDTA (0.2g/liter EDTA-4NA in PBS; Invitrogen). Cells were then pelleted by low-speed centrifugation, washed with PBS, and lysed with 1× RIPA-PI for 30 min at 4°C. Lysates were clarified by centrifugation at 15,000 × g for 30 min at 4°C. Analysis of cell lysates and concentrated supernatants was performed by Western blotting using anti-p24 hybridoma 183-H12-5C (obtained from Bruce Chesboro and Hardy Chen through the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program) supernatants (1:1,000) and rabbit anti-tetherin antisera (1:2,000) (9).
HIV-1 infectivity assay.
293T cells were cotransfected with pNL4-3 or pNL4-3/Udel and 0, 50, and 100 ng of tetherin expression plasmids. Virus was harvested from transfected cell supernatants at 36 h posttransfection, clarified, and assayed for infectivity using TZM-bl indicator cells in 96-well plates. Cells were incubated for 48 h, and 100 μl of supernatant was removed from each well prior to the addition of 100 μl of Bright Glo substrate (Promega, Madison, WI). Measurement of infectivity involved transfer of 150 μl of cell/substrate mixture to black 96-well solid plates and measurement of luminescence using a Packard TopCount plate luminometer.
Flow cytometry.
A total of 6 × 105 293T cells/well were propagated overnight in six-well dishes. On the following day, the cells were cotransfected with the appropriate tetherin and GFP expression constructs and incubated for 24 h prior to analysis. Transfected cell monolayers were washed with prewarmed PBS and detached using EDTA. The cells were then pelleted and washed repeatedly with ice-cold PBS. The cells were resuspended in PBS–2% bovine serum albumin (BSA) and allowed to incubate on ice for 10 min prior to addition of primary antibody (rabbit α-tetherin) for 1 h at 4°C. Cells were then pelleted by low-speed centrifugation and washed twice with PBS–2% BSA, followed by the addition of allophycocyanin (APC)-conjugated anti-rabbit F(ab′)2 for 30 min at 4°C. For whole-cell tetherin measurements, pellets were fixed and permeabilized using a BD Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions. Staining procedures were then identical to those of nonpermeabilized cell samples. Cotransfected GFP-expressing cells were assayed for tetherin cell surface and whole-cell expression by flow cytometry using a FACSCanto (BD Biosciences). Subsequent data analyses were performed using FlowJo 7.6.5 (Tree Star).
Tetherin endocytosis assay.
Transfected 293T cells were washed with PBS and treated with EDTA in order to detach cells. The cells were resuspended in PBS–0.2% BSA at a concentration of 106 cells/ml, followed by incubation with rabbit anti-tetherin antisera for 1 h at 4°C. After several washes with PBS–0.2% BSA, the cells were incubated at 37°C in prewarmed DMEM supplemented with 5% FBS for the appropriate time period. At each interval, cells were harvested and immediately placed on ice. The cells were then washed with cold PBS–0.2% BSA and stained with a secondary APC-conjugated anti-rabbit F(ab′)2 for 30 min at 4°C. Cotransfected GFP-expressing cells were assayed for tetherin cell surface expression by flow cytometry.
Immunoelectron microscopy.
HT1080 monolayer cells expressing wild-type or 4S tetherin were grown on coverslips and fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.2) overnight at 4°C. The cells were then washed and treated with 1% sodium borohydrate, incubated in blocking buffer (PBS containing 5% normal goat serum, 5% BSA and 0.1% cold water fish gelatin), and then further incubated with the primary rabbit anti-antisera overnight at 4°C with gentle agitation. After six washes with the rinsing buffer, PBS containing 0.1% acetylated BSA (BSA-c; Aurion, Netherlands), the cells were incubated overnight in 10-nm colloidal gold conjugated secondary antibody (Aurion) at 4°C. After additional washes, cells were fixed with 2.5% glutaraldehyde in 0.1 M PB for 2 h followed by 1% osmium tetroxide in the same buffer for 1 h. Cells were then dehydrated in graded ethanol, and infiltrated with Eponate 12 resin for embedding. After resin polymerization, blocks were separated from coverslips, leaving monolayer cells on the block surface. Ultrathin sections (70 nm) were cut parallel to the cell surface on a Leica UC6rt ultramicrotome and collected onto 200-mesh copper grids. Sections were then counter stained with 4% aqueous uranyl acetate and lead citrate. Sections were examined on a Hitachi H-7500 transmission electron microscope. Images were taken primarily in the region where cell membrane was cut parallel or tangentially to show the distribution of gold particles.
Immunofluorescence microscopy.
HT1080 cells were seeded on poly-d-lysine-coated glass coverslips in six-well dishes and transfected with the appropriate tetherin expression construct using Lipofectamine 2000 (Invitrogen). On the following day, transfected cells were fixed with 4% formaldehyde in sodium phosphate buffer (PBS), permeabilized with 0.1% Triton X-100, and blocked with Dako blocking buffer (Dako). Cells were incubated with rabbit anti-tetherin antisera (1:2,000), sheep anti-TGN46, washed thoroughly, and incubated with the appropriate secondary antibodies. In order to visualize the nucleus, cells were subsequently stained with DAPI (4′,6′-diamidino-2-phenylindole) at 300 nM in PBS for 5 min at room temperature, washed several times with PBS, and mounted. To study the colocalization between clathrin and tetherin, HT1080 cells on coverslips were transfected with clathrin-GFP and wild-type (WT) or 4S tetherin, fixed in 4% formaldehyde, and stained for tetherin in the absence of permeabilization. Confocal images were acquired using a DeltaVision RT deconvolution microscope (Applied Precision), and data analyses were performed with Volocity software (Perkin-Elmer). For super-resolution studies, nonpermeabilized HeLa or HT1080 cells were fixed and incubated with anti-tetherin antisera as described above to stain the ectodomain on the cell surface. Fixed samples were examined on an OMX imaging station (Applied Precision) using three-dimensional structured illumination microscopy to define tetherin clustering. ImageJ software version 1.45s (NIH) was utilized to derive line plots of signal intensity in some experiments.
FRAP.
For Fluorescence recovery after photobleaching (FRAP) experiments, 8 × 104 HT1080 cells were seeded onto collagen coated, 35-mm MatTek (MatTek Corp.) dishes, incubated overnight at 37°C/5% C02, and then transfected with 100 ng of the appropriate GFP-tagged tetherin expression construct using Fugene HD (Roche). In order to measure membrane mobility, a defined tetherin positive membrane region was bleached (70% power, 32% transmission, 0.250 s) using a 488-nm argon laser. Fluorescence recovery was monitored by scanning the bleached area for an additional 30 s postbleaching. Images were processed using Volocity software (Perkin-Elmer). FRAP experimental analysis was accomplished by measuring the fluorescence intensity of the bleached region prior to, immediately after, and throughout recovery from bleaching. The half-life duration was calculated by using the subsequent equation: t1/2 = ln (0.5)/(−τ). Diffusion coefficients were calculated by using the following equation: D = 0.88·w2/(4·t1/2) (2, 11).
RESULTS
Alanine scanning mutagenesis of the human tetherin ectodomain.
In order to identify regions of the tetherin ectodomain required for restriction of particle release, we generated a series of alanine scanning mutants starting with amino acid position 47 and continuing through to position 150 (Fig. 1A). Each mutant in the panel incorporates four alanine residues. Permissive HEK293T cells were cotransfected with a vpu-deficient provirus, pLN4-3/Udel (NLUdel), together with each member of a panel of FLAG-tagged tetherin constructs. After incubation for 24 h, cell lysates and virus-containing supernatants were harvested. Particle retention efficiency was then assessed qualitatively by Western blotting. Tetherin expression from the mutant panel was relatively consistent throughout the panel of mutants. The electrophoretic mobility of mutants spanning the conserved glycosylation sites at amino acid positions 65 (63-66A, 67-70A) and 92 (91-94A) demonstrated an ∼3.5-kDa reduction in size, which is consistent with the mass associated with mammalian N-linked glycosylation (Fig. 1B). The majority of ectodomain scanning mutants displayed no particle retention deficit (Fig. 1B), which is consistent with the idea that much of the ectodomain may serve as a loose coil and flexible spacer and that primary sequence may not be important for restriction (10). However, tetherin ectodomain scanning mutants 47-50A and 135-138A exhibited significantly reduced particle retention efficiency, while 55-58A and 63-66A demonstrated an intermediate level of disruption of restriction (Fig. 1B, p24 bands). One of the constructs demonstrating intermediate loss of restriction overlapped the glycosylation site at residue 65 (63-66A). The most striking defect, that of 135-138A, indicated that this region of the coiled coil may be crucial for restriction and warrant further evaluation. We therefore focused on this construct and designed additional point substitutions overlapping this region and combined them with mutations of the more N-terminal portion of the coiled coil.
Fig 1.
Alanine scanning mutagenesis of the human tetherin ectodomain. HEK293T cells were transfected with 100 ng of FLAG-tagged tetherin expression plasmids and 1 μg of pNL4.3/Udel (NLUdel). At 24 h posttransfection, cells and supernatants were collected and processed for Western blot analysis. (A) Schematic representation of tetherin ectodomain alanine scanning mutagenesis. FLAG, N-terminal FLAG tag; CD, cytoplasmic domain, TM, transmembrane domain; GPI, glycosyl-phosphatidylinositol anchor. (B) Viral and cell lysate protein detection using anti-p24 and tetherin specific antibodies. Numbers above the blots correspond to the position of alanine substitutions. Sup, particles pelleted from supernatants.
Serine point substitutions within the tetherin coiled coil impair retention of nascent HIV-1 and enhance tetherin levels on the plasma membrane.
Recently, several studies have generated crystal structures of the human and murine tetherin ectodomains in both oxidized and reduced states (10, 33, 42). Using protein modeling supplemented by this structural information, we designed polar substitutions for core hydrophobic residues within the coiled-coil domain of human tetherin in an attempt to disrupt local hydrophobic interactions. Specifically, we generated tetherin constructs incorporating serine mutations at amino acid positions 74 and 84 (74.84S) in the N-terminal portion of the coiled coil, amino acid positions 137 and 144 (137.144S) near the C terminus of the coil, and all four serine substitutions in a single construct termed 4S tetherin. The position of these changes is shown schematically in Fig. 2A. To determine the effect of serine substitutions on HIV-1 particle retention, permissive HEK293T were cotransfected with NLUdel along with 74.84S, 137.144S, 4S tetherin, and the 135-138A tetherin mutant described above. The strategic substitution of polar serine residues for hydrophobic residues at the “a” and “d” positions of the alpha helix within the coiled-coil region of human tetherin resulted in a decreased efficiency of HIV-1 particle retention (Fig. 2B). Under these conditions, wild-type tetherin fully restricted particle release, whereas 4S and tetherin 135-138A failed to restrict. The individual paired mutations (74.84S, 137.144S) demonstrated an intermediate phenotype of restriction (Fig. 2B). The relative differences in restriction of tetherin mutants were further examined by measurement of the reduction of infectivity in infected cell supernatants using the TZM-bl indicator cell line. Wild-type tetherin potently restricted the release of infectious HIV, as did an alanine substitution ectodomain mutant selected from the panel that had not altered restriction, as indicated by Western blotting results (tetherin 79-82A) (Fig. 2C). 4S and tetherin 135-138A were only minimally able to restrict infectivity release in this assay, whereas the individual 74.84S and 137.144S constructs again demonstrated an intermediate phenotype. We conclude that 4S and tetherin 135-138A represent ectodomain substitution mutants that can serve as useful tools to dissect the role of the ectodomain in restriction of particle release by tetherin.
Fig 2.
Targeted serine substitutions within the tetherin coiled-coil region exhibit loss of HIV particle retention. HEK293T cells were transfected with 100 ng of FLAG-tagged tetherin expression plasmids and 1 μg of NLUdel. At 24 h posttransfection, cells and supernatants were collected and processed for Western blot analysis. (A) Schematic representation of tetherin ectodomain serine substitution mutations. (B) Viral and cell lysate protein detection using anti-p24 and tetherin specific antibodies. NLUdel alone (no tetherin, leftmost lane) and NLUdel + wild-type tetherin (second lane from left) served as controls for no restriction and wild-type restriction, respectively. (C) Infectivity measurement of pelleted particles from tetherin and NLUdel-transfected cells. Transfection of increasing amount of tetherin is shown on the x axis, with relative light units measured in the TZM-bl reporter cell line on the y axis. (D) Analysis of dimer formation by tetherin serine mutants under nonreducing (left four lanes) and reducing conditions (right four lanes). Position of tetherin monomers versus dimers is indicated on the right.
We considered that specific disruption of the coiled coil might have disrupted tetherin dimer formation. To determine whether this was the case, we performed electrophoretic analysis of wild-type and mutant tetherin under reducing and nonreducing conditions. As shown in Fig. 2D, wild-type and serine mutant tetherin constructs formed dimers. The 4S mutant was equally capable of dimer formation, indicating that the targeted disruption of coiled-coil interactions in this construct had not prevented dimer formation.
We next examined cell surface levels of the entire panel of tetherin ectodomain substitution constructs. A dimeric structure of the human tetherin ectodomain expressed from HEK293T cells is shown in Fig. 3A (PDB 3MQC) (9) as a guide for the position of each mutant. The position of serine substitutions in the 4S construct are shown in red and underlined in the sequence. Both total (permeabilized) and cell surface (nonpermeabilized) tetherin levels were measured by flow cytometry. Relative cell surface expression for wild type was set at 1.0, and the cell surface levels were examined in three individual experiments using flow cytometry to derive a mean and standard deviation (Fig. 3B). Elevated surface expression was observed with constructs clustered around glycosylation sites at amino acids 65 and 92 (peaks in black bars). Somewhat contrary to results previously published with individual glycosylation mutants (25), we had observed no loss of particle retention phenotype for alanine scanning mutants that included loss of glycosylation at residue 92 (Fig. 1, 91-94A), while there was a partial loss of restriction when residue 65 was replaced (Fig. 1, 63-66A). The most marked elevation of tetherin surface expression compared to total cellular tetherin was exhibited by 135-138A (2.3-fold increase), 74.84S (2.1-fold increase), and 4S tetherin (3.1-fold increase) (Fig. 3B, stippled bars). Thus, the ectodomain mutants that most potently disrupted restriction (135-138A, 4S) also demonstrated markedly increased cell surface levels. Individual fluorescence-activated cell sorting (FACS) plots of cell surface tetherin for these mutants demonstrated a consistent shift in mean fluorescence intensity in nonpermeabilized cells (Fig. 3C), except for the tetherin 135-138A construct. Tetherin 135-138A demonstrated two populations, one with increased and one with decreased cell surface tetherin compared to the wild type. The reason for this mixed population is not clear. In this analysis, tetherin 79-82A is included as an example of an ectodomain substitution construct that did not alter restriction and did not alter cell surface levels. Taken together, these data suggest that targeted disruption of coiled-coil interactions in the tetherin ectodomain that are important for restriction of particle release also lead to enhanced amounts of tetherin on the cell surface. The increase in cell surface tetherin exhibited by ectodomain mutants that failed to restrict was somewhat counterintuitive, since the cell surface levels of wild-type tetherin diminish upon relief of restriction by Vpu (1, 37).
Fig 3.
Tetherin ectodomain mutants display altered cell surface expression. (A) Structure of coiled-coil ectodomain (from Schubert et al. [33]) for reference to alanine substitution positions. Positions altered in serine mutants designed to disrupt hydrophobic interactions are shown in red and bolded. (B) Cell surface proportion of tetherin constructs. 293T cells were cotransfected with the indicated flag-tagged tetherin expression construct together with a GFP-expression plasmid. At 24 h posttransfection, the cells were harvested, and GFP+ were cells analyzed for tetherin cell surface expression by flow cytometry. Both cell surface (nonpermeabilized) and total tetherin expression was measured; cell surface expression was normalized to total tetherin expression in permeabilized cells, with the wild type set at 1.0. Error bars represent the standard deviation of three separate experiments. (C) FACS plots of wild-type and serine mutant cell surface expression. The dotted plot represents the isotype control, the gray plot is the wild-type cell surface expression, and the dark unfilled plot represents the mutants indicated.
Altered endocytosis of ectodomain mutants.
Next, we sought to define the mechanism accounting for elevated tetherin cell surface levels for a subset of our mutants. We hypothesized that disruption of the coiled coil may have altered the rate of internalization of tetherin. In order to test this hypothesis, we performed a kinetic endocytosis assay to determine differences in rates exhibited by wild-type, 4S, and 135-138A tetherin and a subset of other panel members. To measure endocytosis, HEK293T cells were cotransfected with a tetherin and a marker GFP expression plasmid, placed on ice, stained with anti-tetherin antisera, and incubated in a 37°C water bath for designated intervals prior to measurement of cell surface tetherin by flow cytometry. Wild-type tetherin was rapidly endocytosed as measured by this assay (Fig. 4, filled squares and dashed line). Most of the ectodomain mutants examined demonstrated rates of endocytosis similar to wild type by this assay (Fig. 4). However, 74.84S and 4S tetherin exhibited a significant reduction in the rate of internalization from the plasma membrane (Fig. 4, diamonds and triangles). Endocytosis of 4S tetherin was markedly impaired, with ca. 10% of the total cellular tetherin endocytosed at 20 min, compared to 30% of wild-type tetherin. The 74.84S mutant, representing the N-terminal heptad repeat disruption in 4S, was intermediate in its rate of endocytosis. Thus, while much of the ectodomain sequence can be substituted without harming the ability of tetherin to restrict and without altering cell surface levels, targeted disruption of the coiled coil represented by the 4S mutant disrupted restriction and slowed the endocytosis of tetherin. Notably, tetherin 135-138A demonstrated a wild-type rate of endocytosis, necessitating an alternative explanation for the enhanced cell surface levels of this mutant.
Fig 4.

Endocytosis rates of tetherin and tetherin ectodomain mutants. HEK293T cells were cotransfected with the indicated tetherin expression construct and a GFP expression plasmid. At 24 h posttransfection, the cells were detached with EDTA and surface labeled with rabbit anti-tetherin antisera at 4°C. Cell aliquots were incubated at 37°C for the indicated time intervals in order to facilitate endocytosis. Cells were then rapidly cooled to 4°C and stained with the appropriate secondary antibody. Relative surface tetherin levels in GFP+ cell populations were set to 100% at time zero. Error bars represent results from three concurrent experiments. Wild-type tetherin is indicated by filled squares and the dashed line.
Immunofluorescence and immunoelectron microscopic localization of alanine-scanning and targeted tetherin mutants.
Tetherin has been described as forming puncta on the plasma membrane, while the predominant intracellular population of tetherin resides in the TGN (6, 18). On the basis of our flow cytometry findings that tetherin coiled-coil mutants that display reduced particle retention efficiency were increased on the plasma membrane, we wanted to examine the altered distribution of tetherin by microscopic analysis. Wild-type tetherin and tetherin mutants were exogenously expressed in HT1080 cells and immunostained for tetherin and the trans-Golgi marker TGN46. Wild-type tetherin displayed a typical subcellular localization pattern, with predominance within the TGN and less apparent plasma membrane staining (Fig. 5A). Next, we examined the subcellular distribution of one of the tetherin ectodomain alanine scanning mutants that exhibited wild-type restriction of particle release, tetherin 79-82A. Subcellular distribution of 79-82A was indistinguishable from the wild type, with the most pronounced distribution in the TGN (Fig. 5B). This established that the substitution of alanines followed in our scanning strategy did not inherently alter tetherin subcellular distribution or colocalization with TGN markers. In contrast, the 4S mutant and tetherin 135-138A both demonstrated a marked increase in plasma membrane fluorescence, while retaining some TGN localization (Fig. 5C and D). The images are representative of more than 100 cells examined for each construct. The plasma membrane fluorescence appeared diffuse rather than punctate, suggesting that some degree of plasma membrane focal clustering was lost. These findings confirm that targeted substitutions within the coiled-coil region of the tetherin ectodomain that negatively affect restriction also redistribute the overall tetherin population, with a higher concentration of tetherin on the plasma membrane in a diffuse pattern.
Fig 5.
Subcellular distribution of tetherin ectodomain mutants. HT1080 cells were transfected with wild-type (A), 79.82A (B), 4S (C), and 135-138A (D) tetherin expression plasmids. At 20 h posttransfection, cells were fixed, permeabilized, and costained with anti-tetherin (green), anti-TGN46 (red), and nuclei with DAPI (blue). Cells were visualized using wide-field fluorescence deconvolution microscopy on a Deltavision imaging station (Applied Precision). The cells shown for each construct are representative of 100 cells examined.
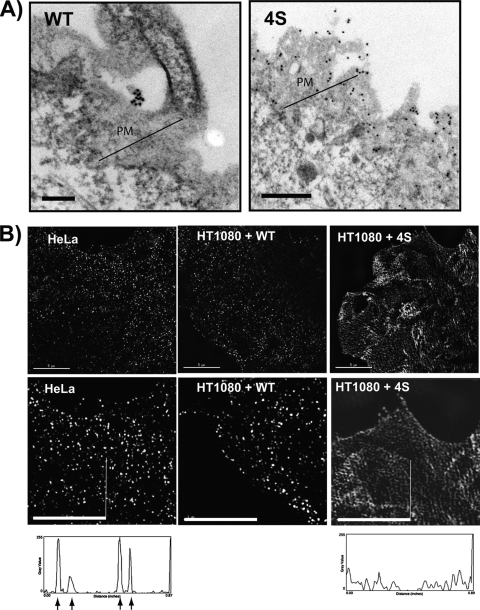
To further analyze cell surface clustering of wild-type and mutant tetherin, we performed immunoelectron microscopic analysis and super-resolution microscopy of cell surface tetherin. Wild-type tetherin was frequently located in focal clusters on small cellular microvilli (Fig. 6A, WT), as has been previously described (7, 9). In contrast, 4S tetherin staining at the plasma membrane revealed a diffuse pattern, in agreement with our immunofluorescence results (Fig. 6A, 4S). To provide some quantitation of these observations, we examined 25 sequential images of stained membranes and counted the number of images in which focal tetherin clusters (defined arbitrarily as >5 clustered/overlapping gold beads surrounded by plasma membrane lacking beads as in Fig. 6, WT). By this measurement, 16% of images for 4S demonstrated focal clusters, whereas 56% of the images with WT tetherin yielded focal clusters. To further test the hypothesis that focal plasma membrane clustering was lost with the 4S mutant, we examined cell surface tetherin using super-resolution microscopy employing three-dimensional structured illumination technology. Endogenous tetherin on the surface of HeLa cells revealed a distribution of tetherin in puncta 70 to 150 nm in diameter (Fig. 6B, left panels). When wild-type tetherin was expressed in HT1080 cells, the distribution and sizes of the focal puncta were identical to that seen in HeLa cells (Fig. 6B, center panels). The distribution observed with 4S tetherin was markedly different. Here, the signal was more diffuse, although accentuated in a corrugated pattern that appeared to follow the surface of the plasma membrane. The corrugated pattern is sometimes seen for diffuse fluorescence signal on the membrane when examined by structured illumination microscopy and may be partly artifactual (personal communication with engineers at Applied Precision). Line plots of signal intensity provide an additional means of presenting the focal clustering of tetherin versus 4S tetherin (Fig. 6B, below images). We conclude that the concentration of tetherin in bright puncta on the cell surface was disrupted by the 4S ectodomain substitutions.
Fig 6.
Immunoelectron and super-resolution microscopic analysis of plasma membrane tetherin distribution. (A) HT1080 cells grown on coverslips were fixed and stained with anti-tetherin antibody and anti-rabbit immunogold beads prior to further fixation and sectioning. Sectioning was performed tangential to the plasma membrane as described in Materials and Methods. Wild-type (WT) tetherin cluster on cellular extension, representative of focal clustering. 4S distribution on plasma membrane demonstrates the higher expression and diffuse nature of 4S tetherin. Scale bars, 200 nm. (B) Super-resolution microscopic images of tetherin on the plasma membrane of HT1080 cells were gathered using structured illumination microscopy. Cells were stained with anti-tetherin antisera in the absence of permeabilization, and a z-stack of images gathered on the OMX structured illumination imaging station version 3 (Applied Precision). Image acquisition and reconstruction was performed with the SoftWorx software package from Applied Precision, and an individual section at the cell surface is shown in the panels at two different magnifications. Endogenous tetherin in HeLa cells is shown in leftmost panels. HT1080 cells expressing WT tetherin are shown in middle panels, and HT1080 cells with 4S tetherin are shown on the right. Vertical bars on leftmost and rightmost panels indicate lines used to generate line plots of signal intensity shown below corresponding images. Arrows indicate peaks representing WT tetherin clusters. Scale bars, 5 μm.
Colocalized puncta of WT but not 4S tetherin with clathrin and with Gag.
The discrete puncta of tetherin on the cell surface, the involvement of clathrin and clathrin adaptor proteins in tetherin-mediated endocytosis (19), and the localization of tetherin in apparent pits on the membrane by immunoelectron microscopy studies (7, 9) suggested that tetherin puncta at the cell surface may be associated with clathrin. We stained HT1080 cells with tetherin antisera in the absence of permeabilization and then examined segments of the thin cellular periphery for colocalization with clathrin-GFP. As anticipated, cell surface tetherin puncta colocalized significantly with clathrin-GFP (Fig. 7A). In cells expressing 4S, we noted again a more diffuse tetherin staining, and even the areas of brighter intensity of stained 4S tetherin representing plasma membrane folds and extensions did not correspond to the bright puncta of clathrin-GFP (Fig. 7B). We next sought to determine whether 4S tetherin differed in the ability to concentrate at the particle budding site in cells expressing Vpu-deficient virus. Wild-type tetherin puncta at the periphery of cells colocalized strongly with Gag (Fig. 7C). While bright Gag puncta were similarly observed at the periphery of cells expressing 4S tetherin, there was no corresponding focal intensification of tetherin signal at these sites, but instead a diffuse distribution of tetherin was apparent with some concentration on filopodial extensions unrelated to sites of accumulation of Gag (arrows, Fig. 7D). These results support the concept that formation of discrete puncta of tetherin on the cell surface is an element associated with restriction and support the notion that the 4S ectodomain mutant has lost this ability and no longer concentrates at the particle budding site.
Fig 7.
WT and 4S tetherin colocalization with clathrin-GFP, Gag, and subcellular localization of additional tetherin mutants. (A) Clathrin-GFP (green) and plasma membrane tetherin (red) were examined at the periphery of transfected HT1080 cells for punctate colocalization. Square in leftmost image indicates area examined for colocalization. Colocalized puncta are shown in rightmost panel. After appropriate thresholding for each wavelength to measure only those pixels associated with intense puncta, a colocalization coefficient of 0.84 was obtained (green puncta colocalizing with red puncta). Size bars: 11 μm (far left images) and 2.7 μm (right three images). (B) Clathrin-GFP and 4S tetherin colocalization. Diffuse nature of 4S precludes meaningful colocalization quantitation, but there was no obvious concentration of red pixels where green signal is concentrated. (C) HT1080 cells expressing NLUdel and WT tetherin were stained with anti-tetherin antisera and then permeabilized and stained with anti-Gag monoclonal antibody. Colocalized puncta on plasma membrane are indicated by arrows. Size bars, 5.4 μm. (D) HT1080 cells expressing 4S tetherin were treated as in panel C above. Location of Gag puncta reveals no concentration of 4S tetherin (red). Size bars, 5.4 μm. (E) Surface staining for tetherin comparing 4S, tetherin 71-74A, and tetherin 95-98A. Size bars, 11 μm.
We had noted that several of the ectodomain alanine-scanning mutants were increased in cell surface distribution while retaining the ability to restrict HIV particle release (Fig. 1 and 3). We considered the possibility that these mutants might also be diffusely distributed on the cell surface and lack the ability to cluster. We examined tetherin 71-74A and tetherin 95-98A, both of which were found at increased concentration on the cell surface, and performed immunostaining for tetherin in nonpermeabilized cells to highlight cell surface tetherin distribution. Notably, these mutants retained a punctate distribution of tetherin, more closely resembling wild-type tetherin than 4S (Fig. 7E). Thus, the lack of punctate clustering observed with 4S correlates with its lack of restriction, while constructs that were increased on the cell surface but retained the ability to restrict particle release remained punctate.
Lack of plasma membrane mobility correlates with ability to restrict particle release.
Results presented above established that tetherin ectodomain substitution constructs 4S and tetherin 135-138A resulted in a significant reduction in particle retention and an altered localization of cellular tetherin on the plasma membrane. We hypothesized that tetherin might form large oligomers in microdomains on the plasma membrane and that ectodomain mutants that could not restrict may have altered plasma membrane lateral mobility. In order to perform dynamic assays of tetherin mobility, we generated several N-terminal GFP-tagged tetherin constructs for further characterization. These included 74.84S, 137.144S, 4S, 79-82A (control), 135-138A, and WT tetherin. WT GFP-tagged tetherin and tetherin-GFP mutants were expressed at consistent levels (Fig. 8A). Wild-type tetherin-GFP restricted the release of NLUdel from HT1080 cells, as indicated by minimal pelletable p24. Control alanine scanning construct tetherin 79-82A-GFP restricted particle release in a manner equivalent to that of the wild type. Notably, 4S, tetherin 135-138A, and the two serine substitution constructs 74.84S and 137.144S all demonstrated defects in restriction that correlated closely with their untagged counterparts, although there appeared to be some variation in the relative disruption of 74.84S-GFP versus 137.144S-GFP (Fig. 8A). GFP-tagged tetherin expression plasmids were next examined for subcellular distribution by wide-field deconvolution microscopy. Differences in tetherin subcellular localization patterns that had been seen by immunostaining in Fig. 6 and 7 were maintained for the GFP-tagged constructs, as represented by wild-type and 4S-GFP (Fig. 8B). The increased and diffuse plasma membrane appearance of 4S-GFP was clearly evident and contrasted with the bright puncta of wild-type tetherin-GFP.
Fig 8.
Characterization of N-terminal GFP-tagged tetherin ectodomain mutants. HEK 293T cells were transfected with 100 ng of GFP-tagged tetherin expression plasmids and 1 μg of NLUdel. At 24 h posttransfection, the cells and supernatants were collected and processed for Western blot analysis. (A) Viral and cell lysate protein detection were performed using anti-p24 and GFP-specific antibodies. Pelleted NLUdel particles are shown below as p24 band. (B) Subcellular distribution of GFP-tetherin (WT) and GFP-4S tetherin was examined on a wide-field deconvolution imaging system. Size bars: 11 μm (left) and 16 μm (right).
Mutations within the tetherin ectodomain (74.84S, 137.144S, 4S, and 135-138A) resulted in a prominent diffuse plasma membrane distribution rather than concentrated puncta, suggesting that targeted ectodomain substitutions had altered the distribution of tetherin in microdomains on the cell surface. To determine whether lateral mobility differed between wild-type and ectodomain mutant tetherins, we used fluorescence recovery after photobleaching (FRAP). HT1080 cells were cultured in 35-mm2 MatTek dishes and transfected the following day with the appropriate GFP-tetherin expression plasmids. After overnight incubation, cells were visualized by live-cell, wide-field microscopy. Immobile puncta in close proximity to the coverslip were selected and bleached using a targeted 488-nm laser. We noted that wild-type GFP-tetherin was localized to discrete foci on the plasma membrane that were amenable to analysis by FRAP. Photobleached foci of WT GFP-tetherin displayed very minimal recovery (Fig. 9A) and exhibited a mean membrane diffusion rate (D) of 0.019 μm2/s (Fig. 9B and C). 4S tetherin was not observed to form discrete plasma membrane foci, but instead was present diffusely on the plasma membrane, as previously noted. In order to perform kinetic analysis of this mutant, we chose to bleach points along the plasma membrane lying between cells (Fig. 9A). In sharp contrast to recovery seen with wild-type GFP-tetherin, 4S GFP-tetherin displayed rapid and obvious fluorescence recovery within the first 30 s of the photobleaching experiment (Fig. 9A and B). 4S exhibited a mean membrane diffusion rate (D) of 0.210 μm2/s, a 10-fold increase compared to WT (Fig. 8B and C). GPI-GFP and ICAM-1 GFP were used as controls for our FRAP experiments and exhibited mean D values of 0.33 and 0.10 μm2/s, respectively. The membrane mobility exhibited by GPI-GFP correlates well to established membrane diffusion rates previously reported for this construct (11). All of the ectodomain mutants in this panel with a particle retention deficit displayed greater membrane diffusion rates than ICAM-1-GFP (Fig. 9C). Finally, tetherin 79-82A was examined as a control construct with particle retention and surface expression levels similar to that of WT tetherin. The diffusion rate (D) for 79-82A was measured at 0.03 μm2/s (Fig. 9C), which was not significantly different than wild-type tetherin-GFP. Together, these data indicate that WT tetherin exists in discrete plasma membrane foci with minimal plasma membrane exchange. In contrast, serine substitution mutants 74.84S, 137.144S, 4S, and tetherin 135-138A displayed diffusion rates comparable to well-defined integral plasma membrane proteins. Mutations within the tetherin ectodomain that resulted in the loss of particle retention efficiency thus allowed for enhanced diffusion and were no longer constrained to defined microdomains on the plasma membrane.
Fig 9.
Fluorescence recovery after photobleaching for wild-type and ectodomain mutant tetherin. HT1080 cells were transfected with 100 ng of indicated GFP-tagged tetherin expression plasmid. At 24 posttransfection a membrane region of interest (ROI) close to the coverslip was bleached using high intensity laser settings. Fluorescence recovery was measured every second for a total of 30 s postbleaching. (A) Representative image series for wild-type tetherin (top panels) and 4S tetherin (lower panels). A larger field view is at left. Dashed circle indicates targeted region for bleaching. The 1.0-s time point represents the first postbleaching image. Bleached area is indicated by dashed circle. Scale bar, 10 μm. (B) Representative FRAP curves are shown for wild-type and 4S tetherin. An arrow indicates the time of laser pulse. (C) From the fluorescence recovery half times, the diffusion coefficient was calculated from FRAP measurements of 10 cells each. Statistical significance was performed using an unpaired t test, comparing to WT levels. *, P < 0.05.
DISCUSSION
Tetherin/BST-2 is an intriguing type II membrane protein with an unusual topology. The cytoplasmic tail is involved in AP-2-dependent endocytosis of the molecule (28, 43), while the transmembrane domain provides specificity for interaction with and downregulation by Vpu (11, 12, 23, 24, 30). Disulfide-linked dimerization of tetherin is required for restriction (28). The function of the coiled coil that forms the majority of the ectodomain is less clear. It is known that deletion of the entire ectodomain deletion greatly diminishes restriction by tetherin (28), and disruption of heptad repeat residues within the region marked by residues 62 to 73 leads to a loss of restriction (10). Perez-Caballero et al. created an artificial chimeric protein pieced together with the cytoplasmic tail, transmembrane, and membrane-proximal ectodomain of the transferrin receptor, the coiled coil from dystrophia myotonica protein kinase, and a C-terminal GPI anchor from urokinase plasminogen activator receptor (28). The resulting parallel homodimer with no sequence homology to tetherin was capable of restricting particle release. Thus, the structural arrangement of tetherin, rather than its primary sequence, was sufficient to confer restriction, although wild-type tetherin was more potent in restricting particle release than the artificial molecule. Four reports of the ectodomain structure of tetherin have shown that the ectodomain forms a loose or imperfect coiled coil (10, 33, 36, 42). The loose coil may confer flexibility on the ectodomain that is essential for tethering in the context of the dynamic shifts in membrane curvature occurring at the particle budding site. In the present study, we took a scanning alanine mutagenesis approach and performed targeted heptad repeat disruption in order to further study the characteristics of restrictive versus nonrestrictive molecules that differed only in the coiled coil, with the goal of deriving additional insights into the mechanism of particle tethering.
The majority of a panel of 26 ectodomain alanine substitution mutants restricted particle release in a manner identical to that of wild-type tetherin. These results are consistent with the idea that there is significant flexibility within the primary sequence of the ectodomain as long as the dimer and loose coiled-coil structure is maintained. However, two regions of the ectodomain were identified that were required for restriction and could not tolerate alanine substitution; a segment at the N terminus of the coiled-coil domain (represented by tetherin 47-50A) and a segment nearer the C terminus (tetherin 135-138A). We focused on the C-terminal segment, along with a four-serine mutant (4S) designed to disrupt the coil that partly overlapped this segment and included N-terminal segment substitutions. The results provide several interesting findings regarding the contribution of the coiled-coil ectodomain to restriction. First, mutants that lost tethering ability were enhanced in cell surface expression. 4S was endocytosed more slowly than wild-type tetherin, which we believe at least partly accounts for the increase in its plasma membrane distribution. There are several possibilities to explain how an ectodomain alteration may alter endocytosis. One possibility is that tetherin may form higher-order oligomers at the particle budding site that are more readily endocytosed than tetherin dimers. While the 4S mutant retained dimer formation, the 74S and 84S substitutions within this construct lie within the region that contributed to tetramer formation in the reduced form of the ectodomain solved by Schubert et al. (33), so it is possible that these substitutions, while not preventing dimer formation, inhibited tetramer formation. A second possibility is that portions of the ectodomain interact with an additional plasma membrane protein that influences tetherin endocytosis. This notion is supported by the finding that HIV-2 ROD10 Env can regulate cell surface tetherin in a manner that is dependent upon the SU subunit of Env. The extracellular orientation of SU indicates that the responsible interactions must occur between SU and the tetherin ectodomain (20). While HIV-2 ROD10 Env does not alter endocytic rates of wild-type tetherin (19), the proposed SU-tetherin ectodomain interaction may lead to diminished recycling of tetherin to the particle budding site and trapping in the TGN or recycling compartment. This model for the interaction of HIV-2 ROD10 Env with the ectodomain suggests that in the absence of Env, the ectodomain may interact with other cellular glycoproteins at the particle budding site. Clustering or retention of tetherin in areas of active clathrin-mediated endocytosis may be required for the normal endocytic rate of tetherin. We hypothesize that such interactions contribute to the rate of endocytosis of wild-type tetherin and that the 4S mutant no longer is able to interact with a putative cellular protein partner and, as a result, is endocytosed at a lower rate.
Hinz et al. examined a series of mutations within the tetherin ectodomain (10) and identified combinations of mutations (set1, set2, and set4) capable of disrupting restriction of release of Vpu-deficient virus. These researchers found that disruption of the coiled coil near the N terminus (set 1) or the C terminus (set 2) eliminated HIV-1 retention, without disrupting the appearance of tetherin on the plasma membrane. The results presented here with the 4S mutant similarly disrupted particle retention through disruption of key residues involved in coiled-coil interactions. We extended these findings by demonstrating that the loss of restriction with 4S was accompanied by an alteration in plasma membrane punctate localization, loss of colocalization with clathrin, and loss of concentration at sites of viral budding.
We interpret the loss of punctate membrane localization seen with 4S to indicate that the ectodomain of wild-type tetherin contributes to specific membrane microdomain association. Indeed, immunoelectron microscopy and immunofluorescence microscopy of the cell surface demonstrated a very diffuse distribution of 4S on the cell surface and little evidence of clustering, while more typical focal clusters of tetherin were identified with wild-type tetherin. The altered distribution of the nonrestricting mutants on the plasma membrane also correlated with an increase in lateral mobility of the molecules as measured by FRAP. Together, these data support a model in which tetherin oligomers cluster in focal membrane microdomains and are constrained within these domains via interactions that are ectodomain dependent. Punctate cell surface localization of tetherin has been demonstrated previously by immunofluorescence and immunoelectron microscopy techniques and may be critical in positioning tetherin molecules at the particle budding site where retention of virions occurs (7, 9, 28). Rollason et al. have described the attachment of tetherin to the underlying cytoskeleton through interactions mediated by RICH2, EBP50, and ezrin (29). It is possible that the ectodomain contributes to anchoring of tetherin to underlying actin through promoting higher-order oligomerization and the subsequent enhanced contribution of multiple attachment sites, while ectodomain mutants such as 4S in the form of dimers become more laterally mobile because their connections to cortical actin are insufficient to retain them within the microdomain. Alternatively, other components of the microdomain architecture may interact with the tetherin ectodomain and constrain its lateral mobility. The fact that the lateral mobility of 4S was quite similar to that of GPI-GFP suggests that the GPI anchor alone and the corresponding lipid raft microdomain association of tetherin are not sufficient to account for the constrained mobility of wild-type tetherin in the observed puncta.
In summary, we demonstrate that the coiled-coil ectodomain of tetherin is an important contributor to the restriction of HIV particle release and that endocytosis, clustering into punctate microdomains, and constrained lateral exchange of tetherin on the plasma membrane are features of tetherin biology that are regulated by the ectodomain. Future work in this area will be directed at dissecting the differences between 4S and wild-type tetherin in the ability to form higher-order oligomers, interact with additional membrane components, and in interacting with the underlying actin cytoskeleton. This avenue of research is likely to provide further insights into the contribution of the tetherin ectodomain to the restriction of particle release.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH AI058828 and by funds from Children's Healthcare of Atlanta. The work was partly supported by the Flow Cytometry/Cell Sorting core of Children's Healthcare of Atlanta and by assistance from the Emory Center for AIDS Research (P30 AI050409).
We thank the James B. Pendleton Charitable Trust for the gift of the Deltavision microscopy system. We thank Gang Bao and Annie Zheng at Georgia Institute of Technology and the Center for Pediatric Nanomedicine for the use of the OMX imaging station and the Robert P. Apkarian Integrated Electron Microscopy Core Facility of Emory University for assistance with electron microscopy. Becky Kinkead and the Grants Editing and Manuscript Support Core provided editorial assistance with the manuscript.
The authors declare that they have no competing financial interests or commercial affiliations.
Footnotes
Published ahead of print 30 November 2011
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Andrew AJ, Miyagi E, Strebel K. 2011. Differential effects of human immunodeficiency virus type 1 Vpu on the stability of BST-2/tetherin. J. Virol. 85:2611–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Axelrod D, Koppel DE, Schlessinger J, Elson E, Webb WW. 1976. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16:1055–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen WH, Da WM, Gao CJ. 2009. Construction of ICAM-1-GFP and its binding with Molt-4 cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 17:650–655 [PubMed] [Google Scholar]
- 4. Cohen EA, Terwilliger EF, Sodroski JG, Haseltine WA. 1988. Identification of a protein encoded by the vpu gene of HIV-1. Nature 334:532–534 [DOI] [PubMed] [Google Scholar]
- 5. Douglas JL, et al. 2010. The great escape: viral strategies to counter BST-2/tetherin. PLoS Pathog. 6:e1000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dube M, et al. 2009. Suppression of Tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J. Virol. 83:4574–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fitzpatrick K, et al. 2010. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 6:e1000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta RK, et al. 2009. Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc. Natl. Acad. Sci. U. S. A. 106:20889–20894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hammonds J, Wang JJ, Yi H, Spearman P. 2010. Immunoelectron microscopic evidence for tetherin/BST2 as the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 6:e1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hinz A, et al. 2010. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell Host Microbe 7:314–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann C, et al. 2010. Caveolin limits membrane microdomain mobility and integrin-mediated uptake of fibronectin-binding pathogens. J. Cell Sci. 123:4280–4291 [DOI] [PubMed] [Google Scholar]
- 12. Iwabu Y, et al. 2009. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J. Biol. Chem. 284:35060–35072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jia B, et al. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5:e1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jouvenet N, et al. 2009. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83:1837–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. 2009. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 106:2886–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klimkait T, Strebel K, Hoggan MD, Martin MA, Orenstein JM. 1990. The human immunodeficiency virus type 1-specific protein vpu is required for efficient virus maturation and release. J. Virol. 64:621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koga F, et al. 2006. Hsp90 inhibition transiently activates Src kinase and promotes Src-dependent Akt and Erk activation. Proc. Natl. Acad. Sci. U. S. A. 103:11318–11322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kupzig S, et al. 2003. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic 4:694–709 [DOI] [PubMed] [Google Scholar]
- 19. Lau D, Kwan W, Guatelli J. 2011. Role of the endocytic pathway in the counteraction of BST-2 by human lentiviral pathogens. J. Virol. 85:9834–9846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le Tortorec A, Neil SJ. 2009. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 83:11966–11978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malim MH, Emerman M. 2008. HIV-1 accessory proteins: ensuring viral survival in a hostile environment. Cell Host Microbe 3:388–398 [DOI] [PubMed] [Google Scholar]
- 22. Mansouri M, et al. 2009. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83:9672–9681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Margottin F, et al. 1998. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1:565–574 [DOI] [PubMed] [Google Scholar]
- 24. McNatt MW, et al. 2009. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 5:e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neil SJ, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430 [DOI] [PubMed] [Google Scholar]
- 26. Nichols BJ, et al. 2001. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153:529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pardieu C, et al. 2010. The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog. 6:e1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perez-Caballero D, et al. 2009. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139:499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rollason R, Korolchuk V, Hamilton C, Jepson M, Banting G. 2009. A CD317/tetherin-RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. J. Cell Biol. 184:721–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rong L, et al. 2009. The transmembrane domain of BST-2 determines its sensitivity to down-modulation by human immunodeficiency virus type 1 Vpu. J. Virol. 83:7536–7546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sadler AJ, Williams BR. 2008. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sakuma T, Sakurai A, Yasuda J. 2009. Dimerization of tetherin is not essential for its antiviral activity against Lassa and Marburg viruses. PLoS One 4:e6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schubert HL, et al. 2010. Structural and functional studies on the extracellular domain of BST2/tetherin in reduced and oxidized conformations. Proc. Natl. Acad. Sci. U. S. A. 107:17951–17956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schubert U, et al. 1998. CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J. Virol. 72:2280–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strebel K, Klimkait T, Martin MA. 1988. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science 241:1221–1223 [DOI] [PubMed] [Google Scholar]
- 36. Swiecki M, et al. 2011. Structural and biophysical analysis of BST-2/tetherin ectodomains reveals an evolutionary conserved design to inhibit virus release. J. Biol. Chem. 286:2987–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Damme N, et al. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Varthakavi V, Smith RM, Bour SP, Strebel K, Spearman P. 2003. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc. Natl. Acad. Sci. U. S. A. 100:15154–15159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vigan R, Neil SJ. 2010. Determinants of tetherin antagonism in the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein. J. Virol. 84:12958–12970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Willey RL, Maldarelli F, Martin MA, Strebel K. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66:7193–7200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Willey RL, Maldarelli F, Martin MA, Strebel K. 1992. Human immunodeficiency virus type 1 Vpu protein regulates the formation of intracellular gp160-CD4 complexes. J. Virol. 66:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang H, et al. 2010. Structural insight into the mechanisms of enveloped virus tethering by tetherin. Proc. Natl. Acad. Sci. U. S. A. 107:18428–18432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang F, et al. 2011. SIV Nef proteins recruit the AP-2 complex to antagonize Tetherin and facilitate virion release. PLoS Pathog. 7:e1002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang F, et al. 2009. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6:54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.