A new classification system that is more sensitive and specific to the defining features of geographic atrophy detects affected areas more reliably and significantly earlier than previously possible with color fundus photography alone.
Abstract
Purpose.
To evaluate new grading criteria for geographic atrophy (GA), as detected by annual stereoscopic color fundus photographs and fluorescein angiograms, and to assess whether application of the revised criteria provides earlier identification of GA than previous criteria involving only color fundus photography.
Methods.
Annual fundus image sets from 114 CAPT patients who developed GA in the untreated eye during 5 to 6 years of follow-up were reassessed for the presence of GA, using revised grading criteria, in which GA was defined by (1) the presence of hyperfluorescence on fluorescein angiography; and (2) at least one other characteristic indicative of involution of the retinal pigment epithelium (i.e., sharp edges, excavation of the retina, or visible choroidal vessels on either color images or fluorescein angiograms). Reliability and time of initial detection of GA using the revised criteria were assessed.
Results.
The revised criteria are reliable (97.8% intragrader, 93.3% intergrader agreement) and accurate (false-positive rate, 0.8%) for detecting individual early GA lesions. Using this revised method, individual GA lesions were identified 1-year earlier on average than was possible with criteria used in previous CFP studies. The use of two imaging modalities was more sensitive in detecting GA and its features than either imaging modality alone (P ≤ 0.0001).
Conclusions.
Early GA areas can be reliably identified when defining criteria are based on both color photographs and fluorescein angiograms. These methods can be used to investigate the natural history of GA earlier in the course of disease than previously possible and to facilitate the design of future clinical trials of treatments for GA. (ClinicalTrials.gov number, NCT00000167.)
Geographic atrophy (GA) is one of the advanced forms of age-related macular degeneration and a leading cause of vision loss in the United States. Nearly 1 million individuals in the United States have GA in at least one eye.1 Because the prevalence of GA rises sharply with age, the number of Americans with GA is projected to rise rapidly in the future as the U.S. population ages.2 Despite its prevalence, there is little information available regarding the earliest stages in the natural history of this progressive disease.
Histopathologically, GA is a loss of the retinal pigment epithelial (RPE) cell layer and overlying retinal photoreceptors.3 It is associated with deterioration of the underlying choriocapillaris and the reduction in density of the underlying choroidal vessels.4 GA often develops as one or several small parafoveal lesions, which enlarge and coalesce over time, often sparing the fovea until late in the course of the disease.5 In its earliest stages, these small areas of evolving GA have been described, though briefly, using multiple different imaging modalities including color fundus photography (CFP), optical coherence tomography (OCT), and fundus autofluorescence (FAF).6–9
However, despite numerous studies describing the natural progression of GA for large, well-established lesions, none have systematically investigated the characteristics, natural history, and progression of the earliest stages of disease. Treatments designed to halt the progression of GA are currently under investigation. These candidate treatments would be most beneficial if targeted toward the earliest stages of disease, before large scotomas develop and while central vision is preserved. Therefore, data on the initial appearance and natural progression rates of early GA are essential to the design of a clinical trial involving these early lesions.
Color fundus photographs have been used in most of the standard grading systems developed for categorizing GA for observational studies and clinical trials. The definitions of GA developed for studies using photographs alone do not lend themselves well to description of very early GA. According to the standard color fundus photograph criteria, an area of depigmentation with a diameter at least 175 μm must be present as well as at least two of the following three features: a more or less circular shape, sharply demarcated edges, and visible choroidal vessels.6,10–17 These features may not be present when the RPE cell layer first begins to recede in a discrete area of the retina as GA first develops. Furthermore, very early GA is difficult to differentiate from its precursors.18
The standard CFP criteria are not specific enough to differentiate GA from its precursors, such as depigmentation and drusen, which also appear roughly circular and have a pigment change from the background fundus. To study the earliest stages of GA, criteria that are more sensitive and specific for GA are required.
One way to improve the criteria for detecting GA is to modify them to be more selective for features specific to GA. Sunness et al.,18 have noted that GA appears as an area of excavation or decreased retinal thickness when viewed stereoscopically. This characteristic of excavation can distinguish GA from its precursors, such as drusen or depigmentation, which appear elevated and flat, respectively, when viewed in stereo. By using this clinical feature in the criteria that define GA on color fundus photos, we may be able to aid in the identification of GA and improve on the CFP criteria used previously in standard grading protocols.
Color fundus photography has historically been the main means of defining GA. In 1995, an international group was formed and came to a consensus on a unified definition of GA as seen in stereoscopic color photographs.19 One of the advantages of defining lesions using this imaging modality is that CFP correlates well with the clinical examination. However, color fundus photographs do not convey any direct information about the presence or absence of the RPE, because absence of the RPE does not have a consistent clinical appearance—a problem that is exacerbated by the vast variation in fundus pigmentation across subjects.18 Other imaging modalities that have been used more recently to characterize GA (i.e., fluorescein angiography [FA], FAF, spectral-domain [SD], and scanning laser ophthalmoscopy [SLO] microperimetry [MP]) do provide such information. The loss of the RPE cell layer in a discrete area appears as an absolute scotoma (retinal sensitivity of 0) on MP,20,21 as a hyporeflective area on FAF (absence of lipofuscin-laden RPE cells),22–24 as the absence of the outer hyperreflective bands (which are anatomically consistent with the RPE cell layer) in the SD-OCT signal,7,25 and as a hyperfluorescent area or window defect (absence of the RPE cell layer, which normally blocks the underlying fluorescence of the choroid) on FA.26–28 Using one of these other imaging modalities, we may be able to identify areas of GA with greater sensitivity and specificity than features that are only variably present on color photography. Authors of textbooks describe delineation of GA from FA, and one recent study has categorized GA based on FA appearance, but there are no standard criteria (Apte RS, et al. IOVS 2002;43:E-Abstract 2505).26–28
We propose a new classification system (referred to herein as revised criteria) that uses features from two imaging modalities simultaneously—color fundus photographs and fluorescein angiograms that are sensitive and specific to GA. In this report we evaluate the reliability and accuracy of the revised criteria for detecting early GA areas; compare CFP, FA, and dual-imaging modalities in the detection of the characteristic features of GA; and assess whether application of the revised criteria leads to earlier detection of GA than the previous criteria with CFP alone.
Methods
Included were eyes of patients who participated in CAPT, a multicenter randomized clinical trial to evaluate low-intensity laser treatment in the prevention of vision loss from AMD. The CAPT protocol was reviewed and approved by the institutional review board associated with each clinical center and adhered to the tenets of the Declaration of Helsinki. An informed consent form was signed by each study participant, and all data management was HIPAA-compliant. The design of CAPT is described in detail elsewhere.12 In short, 1052 participants with bilateral large drusen (≥10 drusen at least 125 μm in diameter) were enrolled from 22 clinical centers from 1999 to 2001 and followed annually for 5 to 6 years with stereoscopic fundus photographs and fluorescein angiograms. Both eyes of each patient were enrolled in CAPT. One eye of each patient was randomized to laser treatment and the contralateral eye to observation. Each eye had to have visual acuity ≥20/40 at entry. Neither eye could have GA within 500 μm of the foveal center or >1 disc area (DA) in total size or any choroidal neovascularization. For each annual visit, graders at the CAPT Photograph Reading Center recorded the presence or absence of GA on stereoscopic fundus photographs as one of the secondary outcome measures. According to the original CAPT grading protocol, “GA was considered present when the color fundus photographs showed an area of atrophy >250 μm in diameter accompanied by 2 of the following 3 features: visible choroidal vessels, sharp edges, and approximately circular shape.”
Study Participants
Of the 1052 subjects enrolled in CAPT, GA was noted in 306 untreated eyes by the reading center graders, and these eyes were considered for inclusion in the Early GA substudy. Of those, 114 eyes were included in the current analyses. Eyes were excluded if the first visit where GA was identified was (1) CAPT baseline—in these cases, the length of time that a GA lesion had been present could not be accurately assessed, or (2) the final CAPT visit—in these cases, the presence of GA could not be confirmed on later images, which might skew the false-positive rate. Eyes were also excluded if any annual images were missing or unsuitable for grading due to inadequate photo quality.
Image Grading
Revised Definition of GA.
Grading was based on features observed in the stereoscopic fundus photographs and fluorescein angiograms. According to the revised criteria, GA was defined as an area in which the RPE was absent, as evidenced by hyperfluorescence on late-stage fluorescein angiograms plus one additional feature indicative of RPE atrophy, specifically: visible choroidal vessels, sharp edges, or marked excavation on either CFP or FA. Atrophic drusen (i.e., degenerating drusen associated with RPE atrophy at its margins) were not considered GA unless the drusenoid material was completely encircled by a 360° rim of atrophy. This distinction was made to include regressing drusen located underneath a larger area of atrophy and exclude individual drusen or areas of confluent drusen that are associated with early atrophic changes.
Grading Protocol.
Photographic sets for each patient were graded sequentially. Candidate areas of GA were identified from stereoscopic color films viewed on a light box. For each atrophic area, the presence or absence of five features (visible choroidal vessels, sharp edges, circular shape, excavation, and depigmentation) was noted based on the color photographs. Similarly, film negatives of fluorescein angiograms were reviewed for candidate areas of GA, and the presence or absence of three features (sharp borders, visible choroidal vessels, and excavation) was noted for each candidate area. Final determination of whether a candidate lesion constituted GA was based on the combined features from the color fundus photographs and fluorescein angiograms. Size and shape were not used as criteria in this revised GA definition.
Unlike the original grading in the CAPT study, each area of GA was assessed independently from other areas when GA was multifocal in a given fundus image. Year 0 was assigned to the first year in which a specific GA lesion was detected in an eye, and that may or may not have been the first year in which any GA was detected in that eye. Each GA lesion was assigned an identification number, for monitoring changes over time. Monitoring involved classifying each lesion as new (not present at previous visit), previously detected, or merged (formed from two or more previously distinct atrophic areas), as well as tracking the characteristic features present on CFP and FA over time.
Assessment of Accuracy and a Reliability Study
A sample of 15 photographic sets, some of which included lesions that met the new criteria but not the previously used criteria, was reviewed by the CAPT study chair. In all instances, he confirmed the presence or absence of GA from a clinical perspective. Six months after the initial grading with the revised criteria, a random sample of 25 photographs was independently regraded by both the original grader (HSB) and a senior CAPT reading center grader (ERM), to assess inter- and intragrader agreements.
Data Analysis
All data were collected on paper forms and then entered into a database (Access; Microsoft, Redmond, WA). Inter- and intragrader agreements were assessed as a percentage of exact agreement and kappa coefficients (κ), interpreted as follows: 0 < κ ≤ 0.4, indicating marginal reproducibility; 0.4 < κ ≤ 0.75, indicating good reproducibility; and κ > 0.75 indicating excellent reproducibility.29 McNemar's test was used for the comparison of percentage of GA features identified by color photographs and those identified by fluorescein angiograms. The Kaplan-Meier method was used to estimate the cumulative incidence of GA when defined by the revised and the standard GA criteria and the difference in curves was evaluated with the log rank statistic (data analysis performed in STATA 10; StataCorp, College Station, TX, or SAS ver. 9.1, SAS Inc, Cary, NC).
Results
Study Participants
Of the 306 CAPT participants identified with GA in the untreated eye by the CAPT Reading Center, 114 were included in this analysis. Among the 192 excluded patients, 30 (15.6%) had GA at the initial CAPT visit, 100 (52.1%) first developed GA at the final CAPT visit, 43 (22.4%) had photographs missing or unsuitable for grading, and on re-review, 19 (9.9%) had no areas that met the original CAPT criteria (i.e., error in the first CAPT grading).
There were 310 distinct areas of GA identified over time in the 114 study eyes. Of these, 255 (82.3%) lesions had at least 1 year of additional follow-up. Although all individual areas of GA included in these analyses were followed up for at least 2 years, the number of lesions present at year 1 was smaller than the number present at year 0, because some lesions merged.
Accuracy and Reliability of the Revised GA Criteria as Assessed by Consistency in Lesion Detection over Time
The consistency in identification of specific retinal areas as GA over follow-up time is an indicator of the accuracy of the definition of GA used in this study. Of the 255 distinct areas of GA, 253 (99.2%) were identified at every subsequent visit. Only two (0.8%) lesions failed to meet the revised criteria for GA at some point during follow-up and may be regarded as false positives.
Ninety-two (36.4%) of 253 new GA lesions had only one of the additional three features (choroidal vessels, sharp edges, or excavated appearance) necessary to meet the revised criteria. Among these 92 areas, 74 (80.5%) developed additional clinical features of GA in later years and would have fulfilled CFP criteria for GA in most of the previous major GA studies.
There were 45 individual areas of GA identified on at least one assessment among the 25 eyes selected for repeat grading. As summarized in Table 1, exact agreement was 97.8% for intragrader assessments and 93.3% for intergrader assessments for the presence or absence of these 45 lesions. Reliability of detection was excellent (κ = 0.845) for intragrader and good (κ = 0.536) for intergrader determinations. Missed lesions were either very small peripheral lesions or were present on lower quality photographs.
Table 1.
Intra- and Intergrader Agreement on Presence of Individual GA Lesions Using Revised Criteria
| Comparison | Presence of GA on Repeat Grading |
Exact Agreement (%) | κ Statistic (95% CI) | |||
|---|---|---|---|---|---|---|
| Yes, Both Gradings | No, Both Gradings | Yes on 1st Grading, No on 2nd | No on 1st Grading, Yes on 2nd | |||
| Intragrader | 41 | 3 | 0 | 1 | 97.8% | 0.84 (0.55–1.14) |
| Intergrader | 40 | 3 | 1 | 1 | 93.3% | 0.54 (0.03–1.04) |
Data are the number of exact matches and the percentage of exact agreement between graders.
Relative Performance of CFP, FA, and Combined Imaging in Detecting GA Features
As shown in Table 2, sharp edges and excavation were detected more frequently on FA than on CFP (P < 0.0001). However, for some lesions, these features were present only on CFP, so that the percentage of lesions with the feature was highest using the combined imaging modalities. In contrast, choroidal vessels were detected more frequently by CFP than by FA (P < 0.0001). Choroidal vessels were identified only on FA for some lesions so that, again, the percentage is highest using the combined imaging modalities.
Table 2.
Defining Features of GA on Color Photographs and Fluorescein Angiograms by Time Since Detection
| Characteristic/Year | Color Photograph n (%) | Fluorescein Angiogram n (%) | Combined FA + CFP n (%) |
|---|---|---|---|
| Sharp edges | |||
| 0 (n = 253) | 115 (45.9) | 176 (69.6) | 206 (81.4) |
| 1 (n = 215) | 119 (55.4) | 155 (72.1) | 188 (87.4) |
| 2 (n = 126) | 85 (67.5) | 104 (82.5) | 118 (93.7) |
| 3 (n = 55) | 36 (65.5) | 50 (90.9) | 52 (94.6) |
| 4 (n = 26) | 21 (80.8) | 22 (84.6) | 24 (92.3) |
| 5 (n = 7) | 7 (100) | 7 (100) | 7 (100) |
| Excavation | |||
| 0 (n = 253) | 83 (32.8) | 111 (43.9) | 144 (56.9) |
| 1 (n = 215) | 105 (48.8) | 142 (66.1) | 170 (79.1) |
| 2 (n = 126) | 76 (60.3) | 96 (76.2) | 109 (86.5) |
| 3 (n = 55) | 35 (63.6) | 45 (81.8) | 50 (90.9) |
| 4 (n = 26) | 18 (69.2) | 23 (88.5) | 24 (92.3) |
| 5 (n = 7) | 6 (85.7) | 7 (100) | 7 (100) |
| Visible choroidal vessels | |||
| 0 (n = 253) | 132 (52.2) | 31 (12.3) | 137 (54.2) |
| 1 (n = 215) | 131 (60.9) | 33 (15.4) | 132 (61.4) |
| 2 (n = 126) | 94 (74.6) | 30 (23.8) | 95 (75.4) |
| 3 (n = 55) | 44 (80.0) | 15 (27.3) | 46 (83.6) |
| 4 (n = 26) | 24 (92.3) | 8 (30.8) | 24 (92.3) |
| 5 (n = 7) | 7 (100) | 3 (42.9) | 7 (100) |
All P < 0.0001 except difference between dual modality vs. color only for vessels, where P = 0.0039.
Assessment of Early Detection with the Revised Criteria
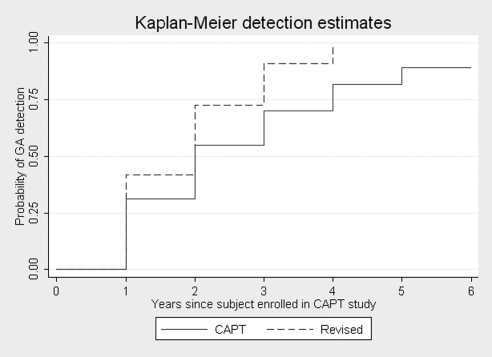
Table 3 summarizes the number of early lesions that would have been missed by the standard CFP criteria or alternatively, by using only one imaging modality and the revised criteria. Only 55.7% of new areas (year 0) of GA identified using the revised criteria would have been detected using standard CFP criteria. In other words, previously used methodologies for identifying GA in color photographs alone miss 44.3% of incident lesions in comparison with the revised criteria. Over time, early GA lesions develop additional characteristic features, resulting in greater numbers of lesions meeting standard CFP criteria in every year of follow-up (Tables 2, 3). A Kaplan-Meier estimation showed that the median time between initial detection of a previously missed lesion according to the revised GA criteria and fulfillment of standard CFP criteria was 1 year (95% confidence interval 1 to 2 years; log rank P ≤ 0.0001). The probability of detection by year since enrollment in the CAPT study using the revised versus CAPT methodologies is illustrated in Figure 1.
Table 3.
Lesions That Would Not Be Detected with Standard GA Criteria or if Using Only a Single Imaging Modality
| Year | Standard Criteria CFP Alone n (%) Missed | Revised Criteria CFP Alone n (%) Missed | Revised Criteria FA Alone n (%) Missed |
|---|---|---|---|
| 0 (n = 253) | 112 (44.3) | 59 (22.3) | 48 (19.0) |
| 1 (n = 215) | 74 (34.4) | 34 (15.8) | 32 (14.9) |
| 2 (n = 126) | 26 (20.6) | 12 (9.5) | 10 (7.9) |
| 3 (n = 55) | 9 (16.4) | 3 (5.5) | 3 (5.5) |
| 4 (n = 26) | 4 (15.4) | 2 (7.7) | 1 (3.9) |
| 5 (n = 7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Figure 1.
Comparison of the cumulative probability of GA detection by year since enrollment in the original CAPT study using the revised criteria versus the standard criteria (CAPT definition).
Discussion
The ability to detect new and very early GA has been limited due to the grading criteria used in previous studies involving CFP alone, which required a lesion to be at least 175 to 500 μm in diameter and have at least two of the following features: circular shape, sharp borders, and visible choroidal vessels. These criteria are well suited for large, well-developed lesions, but not for early GA. To detect new and very early GA lesions, the grading criteria must be revised to include characteristic features that have increased sensitivity and specificity for GA. We developed a revised classification system that requires: (1) FA hyperfluorescence, a characteristic necessary and therefore sensitive for GA, and (2) only one of three additional characteristics, all of which have a histopathologic basis that distinguishes GA lesions from precursors, to increase the specificity for GA.
Need for Increased Sensitivity
Many of the early GA lesions identified through the revised criteria are missed when the standard CFP criteria are used. Detection of features associated with GA is frequently limited by the quality of the fundus images themselves and the variable degree of image clarity and stereoscopy; thus, the greater number of features that must be identified by CFP alone under the standard criteria reduces sensitivity. In addition, many early GA lesions have fewer characteristic features than more established areas of atrophy. Previously used criteria of sharp borders, visible choroidal vessels, and circular shape are present in color photographs in only 46%, 52%, and 66% of new GA lesions, respectively.
For the revised GA criteria, no minimum size was imposed, as this characteristic is neither sensitive nor specific for GA. There is no histopathologic necessity for a minimum size criterion; and, as evidenced by the diversity of minimum size criteria used in previous studies, the choice of minimum size has been arbitrary. Previous definitions of GA include minimum sizes of 175, 180, 189, 250, and 500 μm.10–12,16,19 Although most of the areas of incident GA that were identified were larger than the minimum size used in the AREDS and the original grading for CAPT (99.4% and 98.8%, respectively), nearly half (43.8%) of these lesions would have been excluded if the most stringent minimum size criterion (500 μm in diameter) used previously had been applied to the eyes in this report.30
In contrast, hyperfluorescence on FA is exquisitely sensitive for RPE atrophy. The absence of the RPE layer produces a window defect through which the fluorescence of the underlying structures can be seen. A lesion cannot be GA in the absence of this characteristic. Although hyperfluorescence is not specific for GA, as many other fundus features including drusen can present with hyperfluorescence, it is very sensitive for GA.
Need for Increased Specificity
One of the challenges in identifying early GA involves distinguishing atrophy from its precursors. GA often develops after a sequence of drusen formation, drusen regression, and RPE depigmentation.6 These precursors share features with GA, such as a change in color from the background fundus on color photographs and a generally circular shape. Although the majority of early GA lesions (67%) are circular, this feature is not specific for GA and does not aid in distinguishing the earliest of GA lesions from precursors to atrophy. For this reason, “circular shape” was not used as one of the defining features of GA.
The three additional factors that were used in the revised criteria were chosen because of their specificity for GA or, in other words, the ability to differentiate between GA and its precursors. However, all these “specific” characteristics are only variably present (54%–81% for incident GA lesions and 65%–87% overall) on fundus images.
The criterion of sharply defined borders is dependent on photo quality, and can be difficult to assess when the border area is small, as is often the case with small early GA lesions. Furthermore, GA often develops before complete drusen regression, and the borders of new GA areas are often obscured by drusen and pigment migration. However, this criterion distinguishes GA from its precursors, which tend to have soft borders.
The criterion of visible choroidal vessels is convincing when they are present, but often they are not present in early lesions for two reasons: First, early GA lesions are often small with limited probability of occurring directly over a choroidal vessel. Second, the visibility of choroidal vessels is not only dependent on the atrophy of the RPE, but also on involution of the overlying choriocapillaris. Early RPE atrophy is often associated with early choroidal changes, in which the choriocapillaris remains intact, and this opaque, salmon-colored layer obscures the view of the underlying large choroidal vessels. It is only after the choriocapillaris has atrophied that the choroidal vessels are exposed.31
The criterion of an excavated or “punched-out” appearance is novel to this revised GA criteria substudy and has not been part of previous definitions of GA, although this feature has been cited by investigators and reading center graders as a distinguishing characteristic of GA.18 This feature reflects the underlying histopathology of GA—namely, absence of the RPE. When the opaque pigment epithelial layer is absent in an area of the retina, the first visible structure is the underlying choroid, which appears lower (more posterior) than the surrounding intact RPE. This criterion also helps to distinguish atrophy (excavated) from its precursors such as drusen (raised) and depigmentation (flat). However, this characteristic is highly dependent on image quality and the presence of stereoscopy. Therefore, an excavated appearance is convincing when present but is frequently undetectable because of the limits of photography.
Because these “specific” GA characteristics are only variably present, especially with regard to early GA, the revised criteria require the presence of only one of these three additional features. This method allows for much greater sensitivity in lesion detection than previous studies which required at least two variably present criteria.
By using a combination of a sensitive characteristic for GA (hyperfluorescence) and requiring only one more atrophy-specific characteristic, we identified early GA lesions with greater accuracy than in previous studies using CFP alone. As evidenced by the low rate of false-positive identifications, the revised GA criteria are able to detect GA with greater sensitivity without sacrificing specificity.
CFP Alone Is Insufficient for Identifying Early GA Lesions
In this study we used simultaneously information from more than one imaging modality to identify GA. Similar to color photographs, FA allows identification of additional characteristic features of GA such as sharp edges, visible choroidal vessels, and an excavated appearance, thus providing a second opportunity to observe these features when color photographs are of poor quality. In roughly 60% of the photograph sets, the photo quality was less than good, meaning that the images were either not clear or significantly lacked stereopsis.
In this study, FA was more sensitive at detecting sharp edges and excavation than was CFP. Perhaps this is because the high-contrast nature of a window defect on a monochromatic FA image is much easier to detect than the subtle low-contrast color change of GA against the background fundus in a color photograph, especially when the image is blurred (Fig. 2). In contrast, CFP was much more sensitive for detecting the presence of choroidal vasculature than late-stage FA. As anticipated, the use of a combination of the two imaging modalities outperformed the use of either alone.
Figure 2.
Comparison of CFP and FA for detecting and measuring GA. (A) Color photograph of an eye with GA, in which the fundus is lightly colored, allowing the choroidal vessels to be seen under areas of GA as well as intact RPE. In this case, it is difficult to delineate GA from color photography alone. (B) The areas of atrophy and depigmentation are more clearly distinguished on the corresponding fluorescein angiogram. (C) Color photograph from an eye with several areas of GA with multiple drusen and areas of depigmentation and hyperpigmentation obscuring the borders of atrophy. It is difficult to determine the exact margins of GA from color photography alone. (D) The borders of GA and the spared areas of the fovea in between the lesions are more apparent on the corresponding fluorescein angiogram.
The presence of hyperfluorescence reliably distinguishes depigmentation from atrophy. However, FA may not help in distinguishing soft drusen from atrophy, because soft drusen can stain and appear hyperfluorescent.32 In these circumstances, color photography often aids in the distinction. Thus, both FA and CFP perform essential roles in the detection of early GA. This concept is supported by the data from this substudy, which demonstrate a significant benefit in using dual-imaging modalities over either CFP or FA alone.
We used FA as the adjunctive measure to standard color fundus images to increase sensitivity of early GA detection. However, this “additional opportunity” to detect areas of early geographic atrophic is not limited to FA. Other secondary imaging modalities could fulfill the same role, such as FAF, SD-OCT, or SLO MP. In fact, these alternative imaging methods have advantages over FA as a secondary modality for future studies. All three methods are noninvasive and do not carry with them the risks posed by FA; and, in addition, they have advantages over FA in clinical trials. For example, in addition to decreased autofluorescence providing clear demarcation of the borders of GA, increased autofluorescence at the margins of GA on FAF provides prognostic information about progression of GA.9,23,24,33–35 There have been multiple studies, in which FAF was used as the primary or sole means of detecting and measuring GA9,23,24,33,35–39; however, there have been none to date, of which we are aware, that were designed to detect incident and early GA lesions. The development of new atrophic lesions in eyes with preexisting GA has been described anecdotally using FAF, detected as areas of decreased FAF signal, similar in appearance to well-established GA,7,9 and the presence of this incident GA was confirmed with OCT in one of the studies (absence of the hyperreflective band representing Bruch's membrane).7 Although FAF detects areas of GA with essentially 100% sensitivity, areas of decreased autofluorescence are not specific for GA. Drusen present with variable autofluorescence patterns, including decreased or absent autofluorescence.40 Therefore, similar to FA, color photography or another second imaging modality, such as OCT or MP, may still be needed as an adjunct to accurately distinguish between drusen and GA, especially when considering small areas of early GA that are often not much larger than a patch of confluent drusen (manuscript in preparation).
Similarly, MP may be a helpful adjunct in clinical trials, in that it is able to determine actual changes in retinal sensitivity and can be used in confirming specific areas on the retina that are associated with absolute scotomas. In many ways, MP may be the best measure of visual function associated with dry AMD in clinical trials, as it is uniquely responsive to small areas of visual dysfunction and is a much better measure of the visual impairment associated with small parafoveal areas of GA than is global VA measurement such as ETDRS testing.20,42 However, MP cannot be practically implemented as the sole imaging modality for large-scale studies, where the entire fundus must be evaluated for new and multifocal lesions. As promising as this modality appears to be, MP may not be feasible as a screening tool used to identify subtle or multifocal macular lesions, at least with current technology.
SD-OCT has the advantage of providing in vivo cross-sectional imaging of the actual structural layers of the retina, so that the presence or absence of RPE can be determined anatomically, rather than presumed by using an indirect measure such as window defect hyperfluorescence or other two-dimensional measures obtained from imaging modalities such as FA, FAF, or CFP. In addition, en face imaging can be used to identify the exact location of areas of atrophy to align follow-up images and track changes over time.7 Several studies have used OCT as the primary imaging modality for characterizing GA lesions and monitoring disease progression over time.7,25,41 In one such study, the investigators were able to detect the evolution of new atrophic lesions that formed during the course of the study, which suggests that SD-OCT is capable of detecting incident and early GA.7 Moving forward, it would also be of interest to investigate the feasibility and reliability of using SD-OCT to detect incident and early GA lesions on a large scale, as this method is less invasive than one involving FA.
In this early GA substudy, the relative benefit of the revised criteria (using two imaging modalities) over CFP alone was inversely proportional to the age of the GA lesions. Roughly 20% of new areas of GA are missed when CFP alone is used; however, this number decreases to less than 10% for areas of GA present for at least 2 years and 5.5% for areas present for 3 years. This result applies not only to the first incidence of GA in an eye but also to new satellite lesions that develop in an eye with established GA. Therefore, using the revised GA criteria has merit not only for early GA studies but also for any study monitoring or measuring individual areas of GA over time.
Conclusion
Using these revised criteria, GA is detected earlier than with previous criteria using CFP alone. Current and future treatments under investigation may be most effective if administered in the early stage the disease, before development of large scotomas and loss of central vision. These revised criteria can facilitate the design of clinical trials of new therapies for GA as well as provide a rich description of GA early in its course.
Acknowledgments
The authors thank Janet Sunness for invaluable discussions and guidance on the fine points of delineating GA from photographic images, Judy Alexander for helpful discussions and generous support of the work; and Stuart Fine for reviewing a sample of the images, to assess the agreement of the new criteria with clinical assessment.
Footnotes
Supported by Grants EY012211, EY012261, and EY012279 from the National Eye Institute, National Institutes of Health, Department of Health and Human Services; an unrestricted grant from Research to Prevent Blindness, and a grant from the Doris Duke Charitable Foundation.
Disclosure: H.S. Brader, None; G. Ying, None; E.R. Martin, None; M.G. Maguire, None
References
- 1. Friedman DS, O'Colmain BJ, Muñoz B, et al. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572 [DOI] [PubMed] [Google Scholar]
- 2. Rein DB, Wittenborn JS, Zhang X, et al. Vision Health Cost-Effectiveness Study Group. Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127:533–540 [DOI] [PubMed] [Google Scholar]
- 3. Solomon SD, Sunness JS. Geographic atrophy. In: Lim JI. ed. Age-Related Macular Degeneration. 2nd ed New York: Informa Healthcare; 2008;111–123 [Google Scholar]
- 4. McLeod DS, Taomoto M, Otsuji T, et al. Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2002;43(6):1986–1993 [PubMed] [Google Scholar]
- 5. Sunness JS. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis. 1999;5:25. [PubMed] [Google Scholar]
- 6. Klein ML, Ferris FL, 3rd, Armstrong J, et al. AREDS Research Group. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115:1026–1031 [DOI] [PubMed] [Google Scholar]
- 7. Fleckenstein M, Schmitz-Valckenberg S, Adrion C, et al. Tracking progression with spectral-domain optical coherence tomography in geographic atrophy caused by age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:3846–3852 [DOI] [PubMed] [Google Scholar]
- 8. Fleckenstein M, Adrion C, Schmitz-Valckenberg S, et al. Concordance of disease progression in bilateral geographic atrophy due to AMD. Invest Ophthalmol Vis Sci. 2010;51:637–642 [DOI] [PubMed] [Google Scholar]
- 9. Holz FG, Bellman C, Staudt S, et al. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1051–1056 [PubMed] [Google Scholar]
- 10. Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134 [DOI] [PubMed] [Google Scholar]
- 11. Age-Related Eye Disease Study Research Group The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132:668–681 [DOI] [PubMed] [Google Scholar]
- 12. Complications of Age-Related Macular Degeneration Prevention Trial Research Group Laser treatment in patients with bilateral large drusen: the complications of age-related macular degeneration prevention trial. Ophthalmology. 2006;113:1974–1986 [DOI] [PubMed] [Google Scholar]
- 13. Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21 [DOI] [PubMed] [Google Scholar]
- 14. Klaver CC, Assink JJ, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2001;42:2237–2241 [PubMed] [Google Scholar]
- 15. Mitchell P, Wang JJ, Foran S, Smith W. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002;109:1092–1097 [DOI] [PubMed] [Google Scholar]
- 16. Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106:1768–1779 [DOI] [PubMed] [Google Scholar]
- 17. Sunness JS, Margalit E, Srikumaran D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114:271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sunness JS, Bressler NM, Tian Y, et al. Measuring geographic atrophy in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 1999;40:1761–1769 [PubMed] [Google Scholar]
- 19. Bird AC, Bressler NM, Bressler SB, et al. The International ARM Epidemiological Study Group. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995;39:367–374 [DOI] [PubMed] [Google Scholar]
- 20. Hartmann KI, Bartsch DUG, Cheng L, et al. Scanning laser ophthalmoscope imaging stabilized microperimetry in dry age-related macular degeneration. Retina. 2011;31:1323–1331 [DOI] [PubMed] [Google Scholar]
- 21. Meleth AD, Mettu P, Agron E, et al. Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Invest Ophthalmol Vis Sci. 2011;52:1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Ruckmann A, Fitzke FW, Bird AC. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci. 1997;38:478–486 [PubMed] [Google Scholar]
- 23. Bearelly S, Cousins SW. Fundus autofluorescence imaging in age-related macular degeneration and geographic atrophy. Adv Exp Med Biol. 2010;664:395–402 [DOI] [PubMed] [Google Scholar]
- 24. Holz FG, Bellmann C, Margaritidis M, et al. Patterns of increased in vivo fundus autofluorescence in the junctional zone of geographic atrophy of the retinal pigment epithelium associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1999;237:145–152 [DOI] [PubMed] [Google Scholar]
- 25. Bearelly S, Chau FY, Koreishi A, et al. Spectral domain optical coherence tomography imaging of geographic atrophy margins. Ophthalmology. 2009;116:1762–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spaide RF, Eter N. Fundus Angiography: Nonneovascular age-related macular degeneration. In: Holz FG, Bindewald A, Pauleikhoff D, et al., eds. Age-Related Macular Degeneration. Heidelberg, Germany: Springer-Verlag; 2004;94–96 [Google Scholar]
- 27. Coscas G, Coscas F, Zourdani A. Atlas of Indocyanine Green Angiography: Fluorescein Angiography, ICG Angiography and OCT Correlations. Paris, France: Elsevier SAS: 2005;172–179 [Google Scholar]
- 28. Johnson TM, Johnson MW. Macular diseases: age-related macular degeneration: nonexudative. In: Quillen DA, Blodi BA. eds. Clinical Retina. Chicago: AMA Press; 2002;86–89 [Google Scholar]
- 29. Fleiss JL. The measurement of interrater agreement. Statistical Methods for Rates and Proportions. 3rd ed New York: Wiley; 2003 [Google Scholar]
- 30. Sunness JS, Applegate CA, Bressler NM, Hawkins BS. Designing clinical trials for age-related geographic atrophy of the macula: enrollment data from the geographic atrophy natural history study. Retina. 2007;27:204–210 [DOI] [PubMed] [Google Scholar]
- 31. Spaide RF, Eter N. Fundus angiography: non-neovascular age-related macular degeneration. In: Holz FG, Bindewald A, Pauleikhoff D, et al., eds. Age-Related Macular Degeneration. Heidelberg, Germany: Springer-Verlag; 2004;96 [Google Scholar]
- 32. Coscas G, Coscas F, Zourdani A. Atlas of Indocyanine Green Angiography: Fluorescein Angiography, ICG Angiography and OCT Correlations. Paris, France: Elsevier SAS; 2005;82 [Google Scholar]
- 33. Holz FG, Bindewald-Wittich A, Fleckenstein M, et al. FAM-Study Group. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463–472 [DOI] [PubMed] [Google Scholar]
- 34. Schmitz-Valckenberg S, Jorzik J, Unnebrink K, Holz FG. FAM Study Group Analysis of digital scanning laser ophthalmoscopy fundus autofluorescence images of geographic atrophy in advanced age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2002;240:73–78 [DOI] [PubMed] [Google Scholar]
- 35. Schmitz-Valckenberg S, Bindewald-Wittich A, Dolar-Szczasny J, et al. Fundus Autofluorescence in Age-Related Macular Degeneration Study Group. Correlation between the area of increased autofluorescence surrounding geographic atrophy and disease progression in patients with AMD. Invest Ophthalmol Vis Sci. 2006;47:2648–2654 [DOI] [PubMed] [Google Scholar]
- 36. Dreyhaupt J, Mansmann U, Pritsch M, et al. Modelling the natural history of geographic atrophy in patients with age-related macular degeneration. Ophthalmic Epidemiol. 2005;12:353–362 [DOI] [PubMed] [Google Scholar]
- 37. Brar M, Kozak I, Cheng L, et al. Correlation between spectral-domain optical coherence tomography and fundus autofluorescence at margins of geographic atrophy. Am J Ophthalmology. 2009;143:439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schmitz-Valckenberg S, Fleckenstein M, Gobel AP, Hohman TC, Holz FG. Optical coherence tomography and autofluorescence findings in area with geographic atrophy due to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1–6 [DOI] [PubMed] [Google Scholar]
- 39. Kellner U, Kellner S, Weinitz S. Fundus autofluorescence (488 nm) and near-infrared autofluorescence (787 nm) visualize different retinal pigment epithelium alterations in patients with age-related macular degeneration. Retina. 2010;30:6–15 [DOI] [PubMed] [Google Scholar]
- 40. Sunness JS, Ziegler MD, Applegate CA. Issues in quantifying atrophic macular disease using retinal autofluorescence. Retina. 2006;26:666–672 [DOI] [PubMed] [Google Scholar]
- 41. Wolf-Schnurrbusch UEK, Enzmann V, Brinkmann CK, Wolf S. Morphologic changes in patients with geographic atrophy assessed with a novel spectral OCT-SLO combination. Invest Ophthalmol Vis Sci. 2008;49:3095–3099 [DOI] [PubMed] [Google Scholar]
- 42. Sunness JS, Rubin GS, Applegate CA, Bressler NM, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104:1677–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]