This study showed that mouse retina and retinal pigment epithelial (RPE) cells express the heme transporters FLVCR, BCRP, and PCFT. FLVCR is localized to the apical membrane, and BCRP and PCFT are localized to the basolateral membrane in RPE cells. Hemochromatosis, a genetic disease with iron overload, is associated with upregulation of FLVCR and PCFT, but with downregulation of BCRP in retina and RPE.
Abstract
Purpose.
FLVCR, BCRP, and PCFT/HCP-1 represent the three heme transporters identified thus far in mammalian cells, but there is very little known about their expression and regulation in the retina. In this study, the expression of these transporters in mouse retina and retinal pigment epithelium (RPE) and their regulation in the iron-overload disease hemochromatosis were examined.
Methods.
The expression of FLVCR, BCRP, and PCFT in mouse retina and primary mouse RPE cells was studied by RT-PCR and immunofluorescence. Polarized localization of the transporters in RPE was studied by co-localization using a specific marker of the RPE apical membrane. Uptake of heme in primary RPE cells was determined using zinc-mesoporphyrin, a fluorescent heme analogue. The regulation of heme transporters by iron overload was studied in two genetic models of hemochromatosis (HFE-null mouse and HJV-null mouse) and in two nongenetic models of iron overload (cytomegalovirus infection and treatment with ferric ammonium citrate).
Results.
All three heme transporters were expressed in the retina and RPE. In the RPE, the expression of FLVCR was restricted to the apical membrane, and the expression of BCRP and PCFT was restricted to the basolateral membrane. In all cases of iron overload, the expression of FLVCR and PCFT was upregulated and that of BCRP was downregulated.
Conclusions.
Hemochromatosis is associated not only with excessive accumulation of free iron in the retina and RPE but also with excessive accumulation of heme. Since heme is toxic at high levels, as is free iron, heme-induced oxidative damage may also play a role in hemochromatosis-associated retinal pathology.
Heme (iron-protoporphyrin IX) plays an essential role in diverse biological functions such as oxygen delivery and storage, oxygen metabolism, synthesis of signaling molecules such as nitric oxide and cGMP, and gene regulation.1,2 To date, three different plasma membrane transporters that recognize heme as a substrate have been identified in mammalian cells. These are heme carrier protein (HCP)-1 (also known as SLC46A1),3 breast cancer resistance protein (BCRP, also known as ATP-binding cassette transporter G2; ABCG2),4 and feline leukemia virus subgroup C receptor (FLVCR).5 Recent studies have shown that HCP-1 is also able to transport the vitamin folate.6,7 Because of the dependence of folate transport via this transporter on a transmembrane H+ gradient as the driving force, HCP-1 is also known as proton-coupled folate transporter (PCFT).6 HCP-1/PCFT is an influx transporter for heme, meaning that heme is transported into cells from extracellular fluid.1,2 BCRP/ABCG2 transports not only heme4 but also protoporphyrin IX.8 BCRP functions solely as an exporter, meaning that it transports its substrates, including heme, out of the cells. The transport process is active, fueled directly by the hydrolysis of ATP. FLVCR is also an exporter of heme, and its function is facilitated by the heme-binding protein hemopexin, which interacts with the transporter on the exoplasmic surface.9
Iron is obligatory for normal function of the retina; therefore, retinal cells express all proteins known to be involved in the regulation of iron homeostasis.10 In mouse models of hemochromatosis, a genetic disease associated with excessive accumulation of iron to toxic levels in systemic organs, retina has also been shown to be a target organ for iron overload with consequent disruption of retinal morphology.11–13 To date, three different mouse models of hemochromatosis have been used for the assessment of retinal involvement in this disease: HFE-null mouse,11 hepcidin-null mouse,12 and HJV-null mouse.13 In all three mouse models, iron accumulates throughout the retina. In HFE- and HJV-null mice, significant loss of ganglion cells and disruption of the inner and outer nuclear layers occur; in addition, there is evidence of RPE cell hypertrophy and hyperproliferation.11,13 Additional features of retinal changes such as autofluorescent RPE, photoreceptor cell death, and subretinal neovascularization have been described in hepcidin-null mice.12 In humans, loss-of-function mutations in HFE, an important iron-regulatory protein, cause classic hemochromatosis with excessive iron accumulation seen in systemic organs at ≥50 years of age.14 In contrast, loss-of-function mutations in hepcidin, an iron-regulatory hormone, and HJV, an important iron-regulatory protein, cause juvenile hemochromatosis with excessive iron accumulation seen in systemic organs at a much younger age.15 Similar to free iron, heme is also toxic when present in excessive amounts inside the cells. Therefore, the cellular levels of iron as well as heme need to be regulated tightly to avoid their excessive build up. The retina is separated from systemic circulation by blood–retinal barriers. The outer blood–retinal barrier is composed of the retinal pigment epithelium (RPE), and the inner blood–retinal barrier is composed of endothelial cells of the retinal vasculature.16,17 RPE supplies nutrients to the outer one third of the neural retina, and one of the functions of this cell layer is to transport iron from systemic blood into the neural retina. Whether this cell also transports heme from systemic blood into the neural retina is not known. Recent studies have shown that RPE cells synthesize hemoglobin for delivery into neural retina to facilitate oxygen transfer,18 highlighting the biological need for heme in these cells.
Among the three plasma membrane heme transporters, only the expression and function of PCFT has been investigated in RPE. It is expressed in RPE where it is co-expressed with folate receptor α in the basolateral membrane as well as in endosomal membranes.19,20 There is no information available on the expression of BCRP and FLVCR in this cell. The genetic disease hemochromatosis is associated with iron overload, not only in systemic organs but also in RPE.11–13 Since iron is functionally connected to heme, it is likely that the disease is also associated with alterations in cellular levels of heme, but the link has not been examined. Thus, the purpose of the present study was twofold: first, to study the expression of BCRP and FLVCR in mouse retina and RPE, and, second, to investigate the regulation of all three heme transporters in genetic and nongenetic mouse models of retinal iron overload.
Methods
Materials
Antibodies were obtained from the following sources: rabbit polyclonal anti-FLVCR (Santa Cruz Biotechnology Inc, Santa Cruz, CA), rat anti-BCRP (Enzo Life Sciences International Inc., Farmingdale, NY), and chicken anti-MCT1 (Alpha Diagnostic International, San Antonio, TX), goat anti-rabbit IgG coupled to Alexa Fluor 568 (red fluorescence), goat anti-rat IgG coupled to Alexa Fluor 568 (red fluorescence), goat anti-chicken IgG coupled to Alexa Fluor 568 (red fluorescence), and goat anti-rabbit and anti-rat IgG coupled to Alexa Fluor 488 (green fluorescence) (Molecular Probes, Carlsbad, CA) and Zn(II)-mesoporphyrin IX (Frontier Scientific Inc, Logan, UT).
Animals
Breeding pairs of HFE+/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME); breeding pairs of HJV+/− mice were obtained from Nancy C. Andrews (Duke University, Durham, NC). Other mouse strains were obtained from Harlan-Sprague-Dawley, Inc. (Indianapolis, IN). All procedures involving mice were approved by the Institutional Committee on Animal Use for Research and Education and were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Primary RPE, Müller, and Ganglion Cell Cultures from Mouse Eyes
Primary cultures of RPE were prepared as described previously.11,13 Age-matched wild type and HFE−/− and HJV−/− mice were obtained from the same litter originating from the mating of heterozygous mice. Three-week-old mice were then used to establish primary cultures of RPE. The purity of the culture was verified by immunodetection of RPE-65 (retinal pigment epithelial protein 65) and CRALBP (cellular retinaldehyde binding protein), proteins known to be expressed in RPE. Müller cells were prepared from 7- to 10-day-old C57BL/6 mice by a method adapted from Hicks and Courtois21 and described previously by our laboratory.22 The purity of the primary cultures was confirmed by staining for the Müller cell markers glutamine synthetase, glutamate transporter EAAT1, and CRALBP. Retinal ganglion cells were isolated by immunopanning from 2-day-old C57BL/6 mice by the method of Barres et al.,23 as described previously by our laboratory.24,25 The purity of the cultures was confirmed by immunostaining for Thy-1, a ganglion cell marker.
Infection of Mouse Eyes with Cytomegalovirus
Ocular inoculation of mouse cytomegalovirus (MCMV) was performed as described previously.26 Mice were anesthetized by intramuscular injection of a mixture of 42.9 mg/mL ketamine, 8.57 mg/mL xylazine, and 1.43 mg/mL acepromazine at a dose of 0.5 to 0.7 mL/kg body weight. The left eyes of the mice were injected with 5 × 103 PFUs of MCMV (or PBS in control mice) in a volume of 2 μL by way of the supraciliary route. Mice were killed on day 7 after infection, and retinas were processed for preparation of RNA and tissue sections.
Semiquantitative and Real-Time RT-PCR
For the initial expression analysis of FLVCR and BCRP by semiquantitative RT-PCR, we used RNA samples isolated separately from neural retina and RPE eyecup. Neural retina was separated from rest of the retina (RPE eyecup) with the aid of a dissecting microscope. The changes in the levels of mRNA for different target genes in response to various experimental manipulations were analyzed by semiquantitative RT-PCR as well as by real-time RT-PCR of RNA samples prepared from whole retina. RT-PCR was performed under optimal conditions specific for PCR primer pairs. The following primers were used: mouse FLVCR forward primer 5′-CTGCACATCAACTGGCTGTC-3′ and reverse primer 5′-AGCATGGTGACCCAGAAGAG-3′; mouse BCRP forward primer 5′-AGCAGCAAGGAAAGATCCAA-3′ and reverse primer 5′-CCCATCACAACGTCATCTTG-3′; mouse PCFT forward primer 5′-CTCATGTTCACAGGGTACGGATT-3′ and reverse primer 5′-ACAGCAGAGAACAGAGCACCCT-3′. 18S was used as an internal control for the PCR reaction. The expression levels of target mRNA were quantified by densitometry and normalized with the corresponding internal control. Each PCR experiment was repeated at least three times with similar results. Real-time amplifications, using SYBR green detection chemistry, were run in triplicate on 96-well reaction plates. Statistical analysis was performed by Student's t-test, comparing the expression levels between control and experimental samples. P < 0.05 was statistically significant.
Immunofluorescence Analysis
Localization of specific proteins in retina or RPE cells was monitored by immunofluorescence. Cryosections of mouse eyes were fixed in 4% paraformaldehyde for 10 minutes, washed with 0.01 M PBS (pH 7.4), and blocked with 1× blocking reagent (Power Block; Biogenex, Fremont, CA) for 60 minutes. Sections were then incubated overnight at 4°C with the appropriate primary antibodies. Negative controls involved omission of the primary antibodies. Sections were rinsed and incubated for 1 hour with appropriate secondary antibodies. For primary RPE cells, the cells were cultured on coverslips. In both cases, the coverslips were mounted with antifade mounting medium (Vectashield Hardset; Vector Laboratories, Burlingame, CA) with Hoechst (a nuclear stain), and the sections were examined by epifluorescence microscopy (Axioplan-2 microscope, equipped with an HRM camera and the Axiovision imaging program; Carl Zeiss, Jena, Germany). Polarized expression of FLVCR and BCRP in RPE was investigated by double labeling with a specific marker of RPE apical membrane (MCT1),27 and the slides were examined by laser scanning confocal microscopy (model MRC-600; Bio-Rad, Hercules, CA).
Zinc-Mesoporphyrin (ZnMP) Uptake in RPE Cells
Primary RPE cells were grown on 12-mm coverslips for 24 hours. The cells were then incubated with ZnMP (5 μM) for 30 minutes at 37°C in a buffer (25 mM Hepes/Tris [pH 7.4], 130 mM NaCl, 10 mM KCl, 1 mM CaCl2, and 1 mM MgSO4) containing 2.5 μM bovine serum albumin. Cells were then washed with ice-cold 5% bovine serum albumin in the same buffer followed by two more washes with uptake buffer. Fluorescence in cells was then monitored using a confocal microscope (Carl Zeiss) with excitation at 540 to 580 nm.
Treatment of ARPE-19 Cells and Primary Mouse RPE Cells with Ferric Ammonium Citrate
ARPE-19 cells (a human RPE cell line) and primary mouse RPE cells were treated with ferric ammonium citrate (100 μg/mL; 72 hours), as described previously, to load the cells with free iron.11
Western Blot Analysis
Protein lysates were prepared from the retinas of Sv/129, BALB/c, and C57BL/6 mice and subjected to SDS-PAGE using 10% polyacrylamide gel. The proteins were then transferred onto a polyvinylidene fluoride membrane and probed with the anti-FLVCR antibody. The positive bands were detected with appropriate secondary antibodies coupled to horseradish peroxidase. The signals were developed with an enhanced chemiluminescence detection kit. This experiment was repeated twice with comparable results.
Results
Expression of FLVCR in Mouse Retina and Its Polarized Localization in Mouse RPE Cells
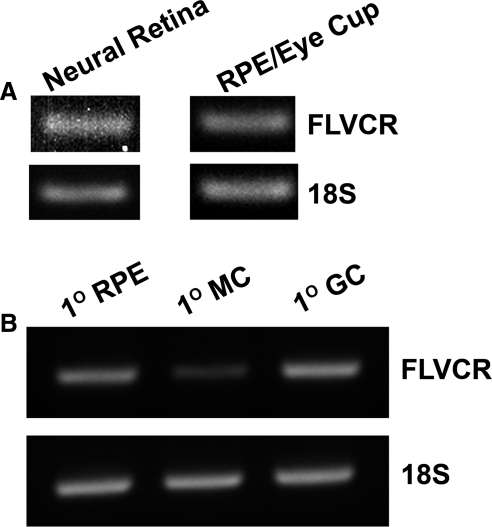
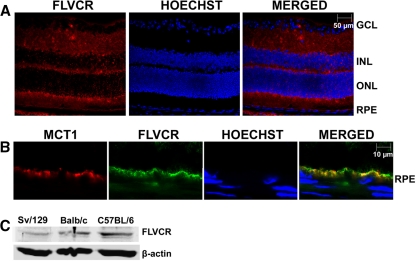
RT-PCR analysis revealed the presence of FLVCR mRNA in neural retina as well as in the RPE eyecup (Fig. 1A). FLVCR expression was also detectable at the mRNA level in primary cultures of mouse RPE cells, Müller cells, and ganglion cells (Fig. 1B). Immunofluorescence analysis of mouse (BALB/c) retinal sections showed expression of FLVCR protein throughout the retina (Fig. 2A). The expression was evident in ganglion cells, other neuronal cells in the neural retina, photoreceptor cells, and RPE. Since RPE cells are polarized with its basolateral membrane facing the choroidal circulation and apical membrane facing the subretinal space, we examined the differential localization of FLVCR in these cells using MCT1 (monocarboxylate transporter 1) as a marker for the RPE apical membrane. We found that FLVCR co-localized with the apical membrane marker (Fig. 2B), demonstrating the polarized localization of FLVCR to the apical membrane. We used BALB/c mice for the immunofluorescence localization of FLVCR because of the nonpigmented nature of the retina in this particular mouse strain. The absence of pigment makes it easier to interpret the immunospecific fluorescence signals because of the lack of nonspecific fluorescence that normally occurs due to the retinal pigment. However, the hemochromatosis mouse models used in the present study were on either a C57BL/6 background (HFE+/+ and HFE−/−) or an Sv/129 background (HJV+/+ and HJV−/−). Therefore, to confirm that the expression of FLVCR in mouse retina is not strain-selective, we performed Western blot analysis of retinal lysates from all three mouse strains. FLVCR protein was indeed expressed in the retina, irrespective of the mouse strain (Fig. 2C).
Figure 1.
Expression of FLVCR mRNA in mouse retina and in primary cultures of RPE, Müller, and ganglion cells. (A) RNA prepared from 3-month-old mouse (C57BL/6) neural retina and RPE eyecup was used for RT-PCR with 18S RNA as the internal control. (B) RNA prepared from primary cultures of mouse RPE cells (1° RPE), Müller cells (1° MC), and ganglion cells (1° GC) was used for RT-PCR with 18S RNA as the internal control. The age of the mice used for the preparation of the primary cells varied, depending on the cell type.
Figure 2.
Expression of FLVCR protein in mouse retina and its polarized localization in RPE. (A) Immunohistochemical localization of FLVCR in 3-month-old mouse (BALB/c) retina. Hoechst was used as a nuclear stain. BALB/c mice were used because of the absence of pigment in the RPE (the RPE pigment autofluoresces, thus making the interpretation of immunofluorescence data difficult). GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. (B) Co-localization of MCT1 (an apical membrane marker in RPE) and FLVCR in RPE. (C) Western blot analysis of FLVCR protein in lysates prepared from retinas of Sv/129, BALB/c, and C57BL/6 mice.
Regulation of FLVCR in Mouse Retina and RPE Cells in Hemochromatosis
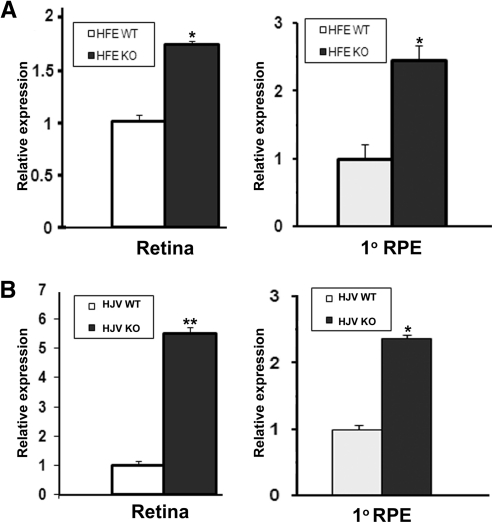
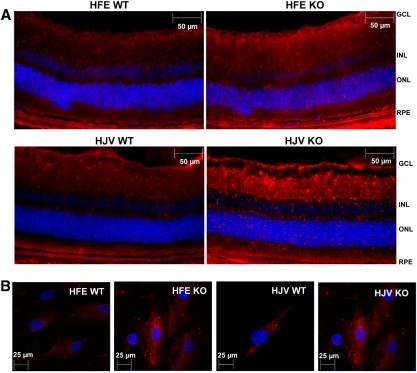
We used two different knockout mouse models of hemochromatosis, HFE- HJV-null mice, to examine the influence of excessive iron accumulation in the retina on the expression of FLVCR. Since these two mouse models were on different genetic backgrounds, we used the corresponding wild-type controls in our analysis to eliminate any strain-dependent differences in the expression levels of this gene. With real-time RT-PCR, we found that the steady state levels of FLVCR mRNA were elevated in retina as well as in primary cultures of RPE cells in both models of hemochromatosis (Fig. 3). In the whole retina, the increase was higher in the HJV-null mice than in the HFE-null mice (5.5 ± 0.2-fold versus 1.7 ± 0.1-fold), whereas in the primary RPE cells the increase was similar (∼2.5-fold) in both mouse models. The increase in FLVCR protein was demonstrated by immunocytochemistry in retinal sections (Fig. 4A) as well as in primary RPE cells prepared from the HFE- and HJV-null mice (Fig. 4B).
Figure 3.
Upregulation of FLVCR mRNA expression in hemochromatosis mouse retina. (A) Steady state levels of FLVCR mRNA in whole retina and in primary RPE cells prepared from wild-type and HFE-null mice. Whole retina was obtained from 20-month-old mice, and primary RPE cells were prepared from 3-week-old mice. Analysis was done by real-time RT-PCR, with 18S RNA levels as the internal control. The experiment was repeated with three different RNA preparations from different mice, and the data are given as the mean ± SE. (B) Steady state levels of FLVCR mRNA in whole retina and in primary RPE cells prepared from wild-type and HJV-null mice. The experimental details are the same as those described in (A) for HFE-null mice. *P < 0.05; **P < 0.001.
Figure 4.
Immunohistochemical analysis of FLVCR protein in HFE- and HJV-null retina and RPE with their corresponding wild-type controls. (A) Immunostaining of FLVCR protein in HFE- and HJV-null mice retina with the corresponding strain and age-matched (18- to 20-months) wild-type controls. (B) Immunostaining of FLVCR protein in HFE- and HJV-null primary RPE cells and the corresponding wild-type RPE cells.
Regulation of FLVCR in Mouse Retina in Nongenetic Models of Iron Overload
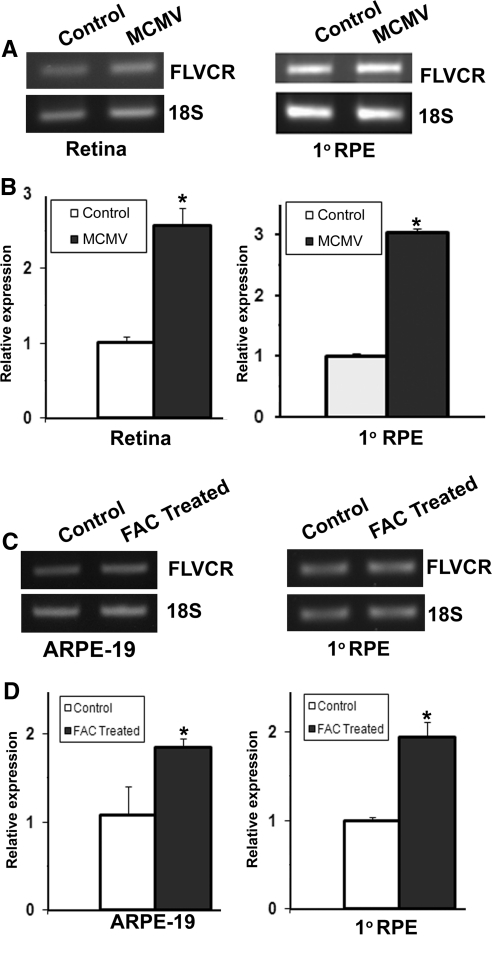
We also used two nongenetic models of iron overload in mice to determine whether the upregulation of FLVCR is a general phenomenon under any conditions of excessive iron. First, we used a model involving cytomegalovirus infection of the eye. We have shown previously that infection of mouse eyes with this virus leads to depletion of HFE, with consequent iron overload.26 In the present study, we found that the expression of FLVCR was upregulated in the mouse retina as a consequence of CMV infection as evident by RT-PCR and real-time RT-PCR (Figs. 5A, 5B). The same phenomenon also occurred when mouse primary RPE cells were infected with CMV (Figs. 5A, 5B). Second, we caused iron overload in ARPE-19 cells and primary mouse RPE cells by culturing the cells in the presence of ferric ammonium citrate and found that the expression of FLVCR was upregulated in this experimental model of iron overload (Figs. 5C, 5D).
Figure 5.
Regulation of FLVCR in mouse retina and RPE cells in nongenetic models of iron overload. RNA was prepared from whole retina of 3-month-old mice infected in vivo with mouse cytomegalovirus (MCMV) and from primary mouse RPE cells infected in vitro with MCMV and used for semiquantitative RT-PCR (A) and real-time RT-PCR (B). In both cases, 18S RNA was used as the internal control. ARPE-19 cells (a human RPE cell line) and primary mouse RPE cells were treated with ferric ammonium citrate (FAC; 100 μg/mL; 72 hours) to cause iron overload in cells. RNA was then isolated from these cells and used for semiquantitative (C) and real-time (D) RT-PCR with 18S RNA as the internal control. *P < 0.05.
Rationale for Studies of BCRP Expression in Retina
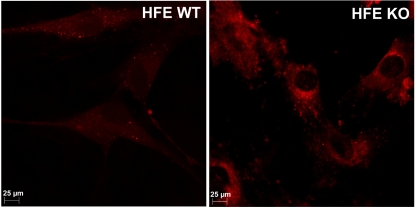
Since our studies showed upregulation of FLVCR in retina and RPE cells in hemochromatosis, we expected the cells to have reduced accumulation of heme based on the known function of this transporter as a heme exporter. To determine whether this is true, we compared the accumulation of the fluorescent heme analogue ZnMP in wild-type and HFE-null primary mouse RPE cells (Fig. 6). Surprisingly, the results were the opposite of what we had expected. The accumulation of ZnMP was higher in the HFE-null RPE cells than in the wild-type RPE cells. This result shows that heme accumulated in HFE-null RPE cells more than in wild-type RPE cells, despite the upregulation of the heme exporter FLVCR, suggesting possible downregulation of another heme exporter and/or upregulation of a heme importer in hemochromatosis.
Figure 6.
Uptake of ZnMP in wild-type and HFE-null RPE cells. Primary RPE cells prepared from wild-type and HFE-null mice were incubated with 5 μM ZnMP (a fluorescent heme analogue) for 30 minutes, and the cellular levels of fluorescence were monitored with a fluorescence microscope.
Expression of BCRP in Mouse Retina and Its Polarized Localization in Mouse RPE Cells
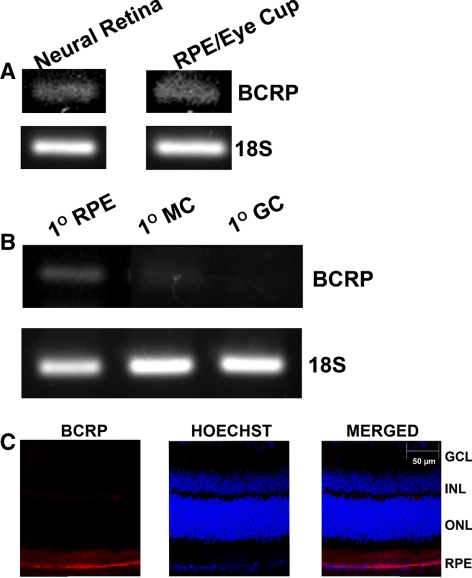
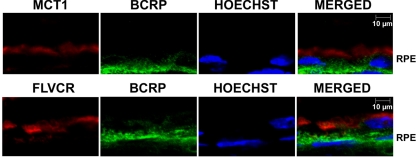
BCRP is known to function as a heme exporter, but there are no reports in the literature on the expression of this transporter in the retina. Studies with cultured RPE cell lines have shown that BCRP is expressed only at very low levels.28 Therefore, we asked first whether this heme exporter is expressed in mouse retina and RPE cells and, if it is, whether it is located differentially in the apical versus basolateral membrane in the RPE. By RT-PCR, we found evidence of the presence of BCRP mRNA in neural retina as well as in RPE eyecup (Fig. 7A). The expression was also evident in primary mouse RPE cells (Fig. 7B). However, there was no expression of this transporter in primary mouse Müller cells and ganglion cells (Fig. 7B). BCRP may be expressed in the retinal vasculature that is present in the neural retina preparation. Immunofluorescence analysis of the whole retina with an antibody specific for BCRP showed the expression to be almost exclusively restricted to the RPE cell layer (Fig. 7C). These retinal sections are not suitable to image protein expression in retinal vasculature, thereby raising the question of whether BCRP is expressed in retinal vessels unanswered. We then examined the polarized expression of BCRP in RPE by using MCT1 as a marker of the apical membrane. These studies showed that BCRP did not co-localize with the apical membrane marker, indicating that the expression was restricted to the basolateral membrane (Fig. 8). Since FLVCR is also expressed solely in the apical membrane of RPE, we performed double-labeling studies using anti-FLVCR and anti-BCRP antibodies. These studies also showed the differential localization of the two proteins in RPE (Fig. 8), thus corroborating the conclusion that BCRP expression is restricted to the basolateral membrane in RPE.
Figure 7.
Expression of BCRP in mouse retina and in primary cultures of mouse RPE cells, Müller cells, and ganglion cells. (A) BCRP mRNA was analyzed by RT-PCR using RNA samples prepared from neural retina and RPE-eyecup from 3-month-old C57BL/6 mice. (B) BCRP mRNA was analyzed by RT-PCR using RNA samples prepared from primary mouse RPE cells (1° RPE), Müller cells (1° MC), and ganglion cells (1° GC). (C) Analysis of BCRP protein by immunofluorescence in mouse (BALB/c) retina. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
Figure 8.
Polarized localization of BCRP in mouse RPE. Double-labeling of MCT1 (an apical membrane marker in RPE) (top) or FLVCR (bottom) and BCRP in the RPE cell layer in the whole mouse (BALB/c; 3-month-old) retina. Hoechst was used as the nuclear stain.
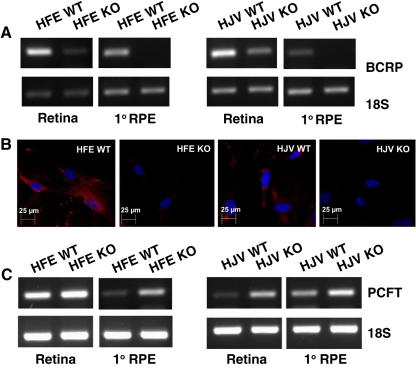
Regulation of BCRP in Mouse Retina and RPE Cells in Hemochromatosis
Since our present studies demonstrated the expression of BCRP in mouse RPE, we asked whether the expression of this heme exporter is regulated in hemochromatosis. To address this question, we compared the steady state levels of BCRP mRNA in retina and primary RPE cells from wild-type mice and the two knockout mouse models of hemochromatosis (HFE- and HJV-null). These studies showed that the levels of BCRP mRNA decreased markedly in the whole retina as well as in RPE cells in both mouse models of hemochromatosis (Fig. 9A). The protein levels also decreased markedly in RPE cells from HFE- and HJV-null mice compared to corresponding wild-type cells as determined by immunocytochemistry (Fig. 9B).
Figure 9.
Differential regulation of BCRP and PCFT in hemochromatosis mouse retina and RPE cells. (A) Steady state levels of BCRP mRNA in the whole retina and in primary RPE cells prepared from HFE- and HJV-null mice. Analysis was performed by semiquantitative RT-PCR with 18S RNA as the internal control. (B) Immunocytochemical analysis of BCRP protein in primary RPE cells prepared from HFE- and HJV-null mice and their corresponding wild-type controls. (C) Steady state levels of PCFT mRNA in the whole retina and in primary RPE cells prepared from HFE- and HJV-null mice. Analysis was done by semiquantitative RT-PCR with 18S RNA as the internal control. In all cases, whole retina for preparation of RNA and cryosections was obtained from 20-month-old mice, and primary RPE cells were prepared from 3-week-old mice.
Regulation of PCFT in Mouse Retina in Hemochromatosis
PCFT is predominantly a transporter for folate, but it also accepts heme as a substrate.29 Therefore, we thought that it would be of interest to see whether the expression of this transporter in the retina and RPE is regulated by iron overload. We found that the expression of PCFT was markedly upregulated in both knockout mouse models of hemochromatosis (HFE- and HJV-null; Fig. 9C).
Discussion
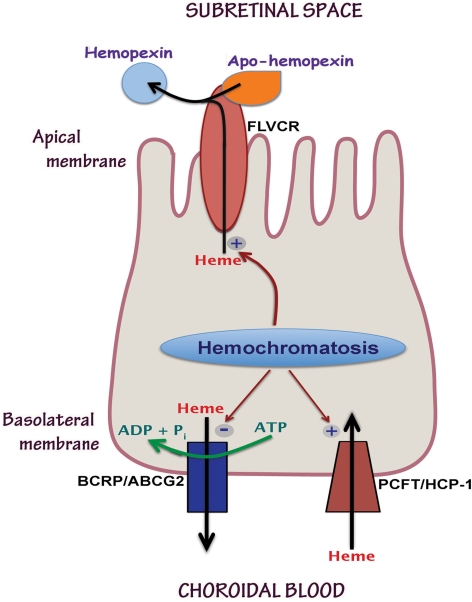
The findings of the present study are summarized in Figure 10. This study showed for the first time the expression of the heme transporters FLVCR and BCRP in retina. The expression of FLVCR was widespread in mouse retina; in the RPE, its expression was restricted to the apical membrane. This transporter requires the binding of another protein, known as apo-hemopexin, on the exoplasmic surface to facilitate the export of heme from the cells.9 Apo-hemopexin binds heme exported by FLVCR and becomes hemopexin. This process is obligatory for the ability of FLVCR to function as a heme exporter. In the absence of apo-hemopexin, the ability to FLVCR to export heme is markedly impaired. Studies from other laboratories have already demonstrated the expression and secretion of apo-hemopexin by photoreceptor cells.30 Therefore, the apical membrane localization of FLVCR in RPE is suitably located for interaction with apo-hemopexin present in the subretinal space to facilitate the export of heme from RPE cells into the subretinal space. The basolateral membrane of RPE cells expresses the other two heme transporters: the heme exporter BCRP and the heme importer PCFT. Excessive iron accumulation in RPE cells, either due to the genetic disease hemochromatosis or due to nongenetic causes such as CMV infection, has differential effects on the expression of these three heme transporters. The expression of FLVCR and PCFT are upregulated by iron overload, but the expression of BCRP is downregulated under the same conditions. We speculate that the net result of these effects is excessive accumulation of heme in RPE cells and increased transfer of heme from RPE cells into the subretinal space. Since RPE cells synthesize hemoglobin, these cells must possess the biosynthetic enzymes for de novo synthesis of heme. We speculate that RPE cells express the heme exporters FLVCR and BCRP as a means to protect themselves from excessive heme accumulation. The relevance of PCFT in RPE cells as a heme importer is not immediately apparent. Neither free heme nor hemoglobin is present in plasma at any significant concentrations under normal physiological conditions. However, there are some pathologic conditions in which intravascular hemolysis occurs, including various genetic disorders such as sickle cell disease and glucose-6-phosphate dehydrogenase deficiency.31 Free hemoglobin in plasma binds to haptoglobin, and the hemoglobin-haptoglobin complex is endocytosed by the scavenger receptor CD163.32 Once heme is released from hemoglobin in the endosomes, it may be transported into the cytoplasm via PCFT. In retinal Müller cells, PCFT is present not only in the plasma membrane, but also in the endosomal membranes, where it is believed to play a role in the absorption of folate, a process involving the folate receptor-mediated endocytosis.20 The upregulation of PCFT in RPE in hemochromatosis suggests that this process may occur at a much higher rate in patients with this disease than in normal subjects.
Figure 10.
The polarized localization of the three heme transporters (FLVCR, BCRP, and PCFT) in apical membrane versus basolateral membrane in RPE cells and their differential regulation by iron overload associated with hemochromatosis.
Based on these studies, we speculate that hemochromatosis is associated not only with an overload of free iron in the retina and RPE but also an overload of heme. Since excessive heme is also toxic, similar to excessive free iron, we speculate that heme overload is also likely to contribute to the hemochromatosis-associated oxidative damage in RPE and retina. The present study providing information on the expression of the heme transporters in the retina and RPE is also relevant to other retinal diseases in which hemorrhage and heme release play a pathologic role.
The present studies describing the expression of heme transporters in retina and RPE may also be relevant to age-related macular degeneration. There is evidence of a role of porphyrin-induced photosensitization in the thickening of Bruch's membrane, RPE atrophy, and RPE hyperplasia that may occur in age-related macular degeneration.33 Protoporphyrin IX is highly photoactive and produces superoxide and singlet oxygen, which can cause damage to the retina, thus contributing to the pathogenesis and/or progression of age-related macular degeneration. It is important to note that all three heme transporters expressed in RPE can also transport protoporphyrin IX. Therefore, it is likely that these transporters play a critical role in regulating the cellular levels of this photoactive compound in RPE and that dysregulation of these transporters has potential to cause oxidative damage in the cell. As mentioned earlier, RPE cells must have the ability to synthesize protoporphyrin IX in significant amounts because of this cell's newly found role as a hemoglobin producer. Therefore, defective function of the protoporphyrin IX exporters BCRP and FLVCR in RPE may increase the cellular levels of this phototoxin, thus contributing to oxidative stress and enhancing the progression of retinal diseases such as age-related macular degeneration.
Acknowledgments
The authors thank Preethi S. Ganapathy and Renee Bozard for RNA samples from primary mouse ganglion cells and Müller cells, and Sally S. Atherton and Ming Zhang for studies related to cytomegalovirus infection of mice in vivo and mouse RPE cells in vitro.
Footnotes
Supported by National Eye Institute Grant EY019672.
Disclosure: J.P. Gnana-Prakasam, None; S.K. Reddy, None; R. Veeranan-Karmegam; None; S.B. Smith, None; P.M. Martin, None; V. Ganapathy, None
References
- 1. Krishnamurthy P, Xie T, Schuetz JD. The role of transporters in cellular heme and porphyrin homeostasis. Pharmacol Ther. 2007;114:345–358 [DOI] [PubMed] [Google Scholar]
- 2. Khan AA, Quigley JG. Control of intracellular heme levels: heme transporters and heme oxygenases. Biochim Biophys Acta. 2011;1813:668–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shayeghi M, Latunde-Dada GO, Oakhill JS, et al. Identification of an intestinal heme transporter. Cell. 2005;122:789–801 [DOI] [PubMed] [Google Scholar]
- 4. Krishnamurthy P, Ross DD, Nakanishi T, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279:24218–24225 [DOI] [PubMed] [Google Scholar]
- 5. Quigley JG, Yang Z, Worthington MT, et al. Identification of a human heme exporter that is essential for erythropoiesis. Cell. 2004;118:757–766 [DOI] [PubMed] [Google Scholar]
- 6. Qiu A, Jansen M, Sakaris A, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917–928 [DOI] [PubMed] [Google Scholar]
- 7. Umapathy NS, Gnana-Prakasam JP, Martin PM, et al. Cloning and functional characterization of the proton-coupled electrogenic folate transporter and analysis of its expression in retinal cell types. Invest Ophthalmol Vis Sci. 2007;48:5299–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou S, Zong Y, Ney PA, Nair G, Stewart CF, Sorrentino BP. Increased expression of the Abcg2 transporter during erythroid maturation plays a role in decreasing cellular protoporphyrin IX levels. Blood. 2005;105:2571–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang Z, Philips JD, Doty RT, et al. Kinetics and specificity of feline leukemia virus subgroup C receptor (FLVCR) export function and its dependence on hemopexin. J Biol Chem. 2010;285:28874–28882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gnana-Prakasam JP, Martin PM, Smith SB, Ganapathy V. Expression and function of iron-regulatory proteins in retina. IUBMB Life. 2010;62:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gnana-Prakasam JP, Thangaraju M, Liu K, et al. Absence of iron-regulatory protein Hfe results in hyperproliferation of retinal pigment epithelium: role of cystine/glutamate exchanger. Biochem J. 2009;424:243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hadziahmetovic M, Song Y, Ponnuru P, et al. Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice. Invest Ophthalmol Vis Sci. 2011;52:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gnana-Prakasam JP, Tawfik A, Romej M, et al. Iron-mediated retinal degeneration in hemojuvelin knockout mice. Biochem J. Published online September 26 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleming RE, Britton RS, Waheed A, Sly WS, Bacon BR. Pathophysiology of hereditary hemochromatosis. Semin Liver Dis. 2005;25:411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wallace DF, Subramaniam VN. Non-HFE hemochromatosis. World J Gastroenterol. 2007;13:4690–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Runkle EA, Antonetti DA. The blood-retinal barrier: structure and functional significance. Methods Mol Biol. 2011;686:133–148 [DOI] [PubMed] [Google Scholar]
- 17. Tachikawa M, Ganapathy V, Hosoya K. Systemic route for retinal drug delivery: role of the blood-retinal barrier. In: Kompella UB, Edelhauser HF. eds. Drug Product Development for the Back of the Eye. New York: AAPS Press-Springer; 2011:85–109 [Google Scholar]
- 18. Tezel TH, Geng L, Lato EB, et al. Synthesis and secretion of hemoglobin by retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2009;50:1911–1919 [DOI] [PubMed] [Google Scholar]
- 19. Chancy CD, Kekuda R, Huang W, et al. Expression and differential polarization of the reduced-folate transporter-1 and the folate receptor alpha in mammalian retinal pigment epithelium. J Biol Chem. 2000;275:20676–20684 [DOI] [PubMed] [Google Scholar]
- 20. Bozard BR, Ganapathy PS, Duplantier J, et al. Molecular and biochemical characterization of folate transport proteins in retinal Müller cells. Invest Ophthalmol Vis Sci. 2010;51:3226–3235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hicks D, Courtois Y. The growth and behaviour of rat retinal Müller cells in vitro. 1. An improved method for isolation and culture. Exp Eye Res. 1990;51:119–129 [DOI] [PubMed] [Google Scholar]
- 22. Umapathy NS, Li W, Mysona BA, Smith SB, Ganapathy V. Expression and function of glutamine transporters SN1 (SNAT3) and SN2 (SNAT5) in retinal Müller cells. Invest Ophthalmol Vis Sci. 2005;46:3980–3987 [DOI] [PubMed] [Google Scholar]
- 23. Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803 [DOI] [PubMed] [Google Scholar]
- 24. Dun Y, Mysona B, Van Ells T, Amarnath L, Ola MS, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x−c) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res. 2006;324:189–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ganapathy PS, White RE, Ha Y, et al. The role of N-methyl-D-aspartate receptor activation in homocysteine-induced death of retinal ganglion cells. Invest Ophthalmol Vis Sci. 2011;52:5515–5524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gnana-Prakasam JP, Zhang M, Martin PM, Atherton SS, Smith SB, Ganapathy V. Expression of the iron-regulatory protein hemojuvelin in retina and its regulation during cytomegalovirus infection. Biochem J. 2009;419:533–543 [DOI] [PubMed] [Google Scholar]
- 27. Philp NJ, Wang D, Yoon H, Hjelmeland LM. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci. 2003;44:1716–1721 [DOI] [PubMed] [Google Scholar]
- 28. Mannermaa E, Vellonen KS, Ryhanen T, et al. Efflux protein expression in human retinal pigment epithelium cell lines. Pharm Res. 2009;26:1785–1791 [DOI] [PubMed] [Google Scholar]
- 29. Laftah AH, Latunde-Dada GO, Fakih S, Hider RC, Simpson RJ, McKie AT. Haem and folate transport by proton-coupled folate transporter/haem carrier protein 1 (SLC46A1). Br J Nutr. 2009;101:1150–1156 [DOI] [PubMed] [Google Scholar]
- 30. Hunt RC, Hunt DM, Gaur N, Smith A. Hemopexin in the human retina: protection of the retina against heme-mediated toxicity. J Cell Physiol. 1996;168:71–80 [DOI] [PubMed] [Google Scholar]
- 31. Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–1662 [DOI] [PubMed] [Google Scholar]
- 32. Nielsen MJ, Moller HJ, Moestrup SK. Hemoglobin and heme scavenger receptors. Antioxid Redox Signal. 2010;12:261–273 [DOI] [PubMed] [Google Scholar]
- 33. Gottsch JD, Pou S, Bynoe LA, Rosen GM. Hematogenous photosensitization: a mechanism for the development of age-related macular degeneration. Invest Ophthalmol Vis Sci. 1990;31:1674–1682 [PubMed] [Google Scholar]