Abstract
The induction of proinflammatory proteins in stimulated endothelial cells (EC) requires activation of multiple transcription programs. The homeobox transcription factor HOXA9 has an important regulatory role in cytokine induction of the EC-leukocyte adhesion molecules (ELAM) E-selectin and vascular cell adhesion molecule 1 (VCAM-1). However, the mechanism underlying stimulus-dependent activation of HOXA9 is completely unknown. Here, we elucidate the molecular mechanism of HOXA9 activation by tumor necrosis factor alpha (TNF-α) and show an unexpected requirement for arginine methylation by protein arginine methyltransferase 5 (PRMT5). PRMT5 was identified as a TNF-α-dependent binding partner of HOXA9 by mass spectrometry. Small interfering RNA (siRNA)-mediated depletion of PRMT5 abrogated stimulus-dependent HOXA9 methylation with concomitant loss in E-selectin or VCAM-1 induction. Chromatin immunoprecipitation analysis revealed that PRMT5 is recruited to the E-selectin promoter following transient HOXA9 binding to its cognate recognition sequence. PRMT5 induces symmetric dimethylation of Arg140 on HOXA9, an event essential for E-selectin induction. In summary, PRMT5 is a critical coactivator component in a newly defined, HOXA9-containing transcription complex. Moreover, stimulus-dependent methylation of HOXA9 is essential for ELAM expression during the EC inflammatory response.
INTRODUCTION
Activation of quiescent endothelial cells (EC) results in the transient induction of a variety of proinflammatory endothelial-leukocyte adhesion molecules (ELAM), including E-selectin and vascular cell adhesion molecule 1 (VCAM-1), on the EC surface at sites of inflammation (6, 11, 20). While E-selectin acts as a mediator of initial adhesion of leukocytes to the endothelium, VCAM-1 promotes firm adhesion and infiltration (28, 32). Also, both support adhesion of circulating tumor cells to EC and transendothelial migration during metastasis (2). Exposure of EC to an inflammatory signal causes histone modifications and chromatin remodeling to allow both sequence-specific DNA binding and associations among transcription factors/cofactors for the induction of these molecules, including E-selectin (19). The homeobox gene HOXA9 plays a key transcriptional role in this activation (4, 44), which also requires other necessary transcription factors/cofactors, including NF-κB, ATF-2/Jun, HMG-I(Y), and p300 (14, 22, 50).
HOX genes are well known for their regulation of developmental gene expression in cell lineage differentiation (36, 59). HOX proteins, characterized by the presence of a unique 60-amino-acid highly conserved DNA-binding homeodomain, bind to specific DNA sequences in the promoter regions to regulate target gene expression (31). Importantly, they play a regulatory role in EC function, including differentiation from embryonic stem cells (3), EC maturation and vascular development from mesoderm-derived precursor cells (23, 66), proliferation, migration, and angiogenesis (9, 10, 12, 13, 39, 51). Recently, the pursuit of HOXA9 in EC gene regulation has led to the recognition of important new concepts (4, 8, 44, 53, 63). Apart from its role in E-selectin (4) or VCAM-1 induction, HOXA9 is necessary for endothelial tube formation during angiogenesis (8) and is a key regulator of adult progenitor cell commitment to the endothelial lineage (53). HOXA9's role in promoting expression of EC activation genes suggests that its dysregulation and/or modification at the protein level may contribute to the development of vascular defects and diseases. Although little is known regarding posttranslational modifications of HOXA9 in EC, its phosphorylation and ubiquitination play regulatory roles in hematopoietic differentiation (64, 67). While protein kinase C (PKC)-mediated phosphorylation reduces HOXA9-DNA binding activity to modulate gene transcription (64), ubiquitination of HOXA9 leads to its degradation (67). Protein methylation of HOXA9 or any other related family member has not been described.
Here, we show that HOXA9 interacts specifically with protein arginine methyltransferase 5 (PRMT5) in activated EC. PRMT5 is a member of the PRMT family of enzymes, which regulate diverse cellular processes by catalyzing protein arginine methylation (5, 30, 40, 45). PRMT5 methylates arginine residues either in isolation or in glycine- and arginine-rich (GAR) motifs to form monomethylarginine or symmetrical dimethylarginine (MMA/sDMA) (7). The expression of PRMT5 is regulated developmentally, and its deletion in mouse models leads to zygotic death (30, 61). PRMT5 is a component of multiple protein complexes and contributes to essential cellular processes, such as RNA transport and splicing (38), cell cycle regulation, tumor growth (1, 26, 27), and chromatin remodeling, leading to either gene silencing or activation (18, 41). As a cofactor in transcription, PRMT5 is implicated in the repression of several genes, including cyclin E1, interleukin-8 (IL-8), and IκBα (21, 33, 68); in contrast, it enhances IL-2 promoter induction upon T-cell activation (52) and myogenin expression in muscle differentiation (18).
The context-dependent function of HOXA9 often involves other interacting factors, such as PBX, MEIS, CBP-p300, or Smad4 (48, 55, 56). Therefore, identification of a HOXA9-binding partner(s) may lead to a better understanding of the role of HOXA9 in EC activation. The focus of the current study is on the identification and functional characterization of PRMT5 as a new binding partner of HOXA9 in activated EC. Importantly, we describe a new posttranslational modification of HOXA9 in EC. PRMT5-mediated methylation of HOXA9 at Arg140 is stimulated by tumor necrosis factor alpha (TNF-α) and plays an important role in the induction of specific ELAM. These results demonstrate a novel mechanism for the regulation of the proinflammatory function of HOXA9 by PRMT5 that may provide important mechanistic insight into the pathobiology of inflammation and cardiovascular inflammatory diseases, such as atherosclerosis.
MATERIALS AND METHODS
cDNA construction and protein expression.
Mammalian expression vectors containing appropriate cDNA, including HOXA9, were subcloned as described previously (4). A Myc tag epitope sequence was introduced upstream of the initiating ATG codon in the PRMT5 cDNA purchased from Origene Technologies (Rockville, MD) by PCR and subcloned into pcDNA3. cDNA constructs were verified for protein expression using a TNT-coupled in vitro translation system from Promega Corporation (Madison, WI). Reporter luciferase constructs containing a 510-bp fragment of the E-selectin promoter (E-selectin-510) were generated as described earlier (4). The plasmid pCMVβ (Clontech) containing the β-galactosidase gene driven by the cytomegalovirus (CMV) promoter was used as a control in the transfection experiments.
Cell culture and transfection.
Human umbilical vein EC (HUVEC) were routinely cultured in MCDB 107 medium supplemented with 15% fetal bovine serum (FBS), 150 μg/ml endothelial cell growth supplement (ECGS), and 90 μg/ml heparin (54). Transfections of effector, reporter, and control plasmids were carried out with either Lipofectin (Life Technologies, Inc.) or Targefect F-2/peptide enhancer (Targeting Systems, CA) in cultured cells as described earlier (4). Cells were replenished with fresh culture medium and allowed to recover for 16 to 20 h before harvest or TNF-α treatment. Luciferase activity was measured for reporter expression and corrected for transfection efficiency by cotransfecting with the plasmid vector pCMVβ and measuring β-galactosidase activity. We routinely obtained 25 to 40% transfection efficiency using Targefect F-2 in human EC.
Western blot analysis.
The level of protein expression, either endogenous or recombinant, was assessed by SDS-PAGE and immunoblotting (4). EC lysates were separated on 10 to 12% SDS-PAGE gels by electrophoresis and then transferred onto Immobilon-P membrane. The blots were incubated with protein-specific primary antibodies followed by horseradish peroxidase (HRP)-conjugated specific secondary antibodies according to the manufacturer's instructions. Immunocomplexes were detected by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech). Polyclonal anti-HOXA9, anti-PRMT5, anti-green fluorescent protein (GFP), and anti-FLAG antibodies from Millipore (Billerica, MA) and/or Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) were used. Monoclonal anti-E-selectin, anti-intercellular adhesion molecule 1 (anti-ICAM-1), anti-PRMT1, anti-β-actin, anti-α-tubulin, and anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) were obtained from Santa Cruz, and anti-FLAG (M2) antibody was from Sigma (St. Louis, MO). Monoclonal anti-dimethyl arginine antibody was obtained from Abcam (Cambridge, MA).
Immunoprecipitation and co-IP assays.
To identify HOXA9-binding proteins in human EC by a coimmunoprecipitation (co-IP) approach, we overexpressed FLAG-HOXA9 or GFP in human EC. Total lysates from control and 1-h TNF-α-treated (5 ng/ml) EC were prepared in buffer A (25 mM HEPES, pH 7.4, 400 mM NaCl, 1% Nonidet P-40, 10% glycerol, and protease inhibitor cocktail from Life Technologies). The NaCl concentration was adjusted to 150 mM by dilution with the same buffer without NaCl (buffer B). Cell lysates were first precleared and then incubated with polyclonal anti-FLAG antibody (Santa Cruz) overnight at 4°C. Next, protein A/G-agarose beads were added to supernatants and further mixed for 4 h at 4°C. Beads were washed three times with buffer B plus 150 mM NaCl (buffer C) and three times with modified buffer C (without NP-40). Beads were boiled with 2× Laemmli protein sample buffer (Sigma), and eluted proteins were subjected to SDS-PAGE for silver staining and Western blot analysis. Complementary experiments were carried out in which EC lysates were precipitated with anti-PRMT5 antibody, and blots were probed with anti-FLAG antibody. FLAG-HOXA9, GFP, and endogenous PRMT5 were detected by Western blot analysis. In vivo protein-protein interactions were also demonstrated by coimmunoprecipitation assays. Human EC were treated with TNF-α (5 ng/ml) as described in the text. EC nuclear extracts were prepared in buffer A as described earlier (4) or using a Nuclear Complex Co-IP kit (Active Motif, CA) and subjected to immunoprecipitation with specific antibody as described above. Proteins were separated by SDS-PAGE using bis-Tris gels (similar to NuPAGE gel system; Life Tech, NY) for higher resolution and lower backgrounds in subsequent Western blot analyses. Thermo Scientific (Waltham, MA) Clean-Blot IP detection reagents were used for specific protein detection without interference in the blots. For protein arginine methylation analysis by mass spectrometry, lysates of 1-h TNF-treated HEK293 cells containing overexpressed FLAG-HOXA9 were prepared in radioimmunoprecipitation assay (RIPA) buffer, immunoprecipitated with anti-FLAG antibody, and separated by SDS-PAGE.
Mass spectrometry.
Silver-stained bands that specifically appeared in the FLAG-HOXA9-containing sample in the gel were excised for digestion. Tryptic peptides of specific bands were analyzed using liquid chromatography-electrospray ionization/ matrix-assisted laser desorption ionization–time of flight mass spectrometry (LC-ESI/MALDI-TOF MS). The Finnigan LTQ (linear trap quadrupole) linear ion trap mass spectrometer (ThermoFinnigan, San Jose, CA) equipped with an Eksigent nano-one-dimensional (1D) high-performance liquid chromatography (HPLC) system was used for the LC-MS system. Data were analyzed using the NCBI nonredundant protein database. The interpretation process was aided by additional searches using the programs Sequest and BLAST as needed. Chymotryptic peptides of HOXA9 were analyzed for arginine methylation by mass spectrometry as above. The chymotryptic digestion was carried out overnight at room temperature. The digests were analyzed in a data-dependent manner to identify the chymotryptic peptides present in the sample, and this step was followed by a targeted selected reaction monitoring (SRM) experiment looking for specific peptides.
RT-PCR assay.
First-strand cDNA was synthesized from total RNA (1 μg) isolated from untreated EC or EC treated with TNF-α (5 ng/ml) using a SuperScript First-Strand Synthesis system from Life Technologies (Carlsbad, CA) as described earlier (4). Real-time PCR (RT-PCR) was performed in a 25-μl reaction volume with specific primer pairs corresponding to a particular gene of interest using SYBR green PCR core reagents (PE Applied Biosystems, United Kingdom) and a PerkinElmer ABI Prism 7700 sequence detector, according to the manufacturer's instructions. All results were verified by multiple independent experiments. Details of primer sequences are available upon request.
RNA interference.
Specific small interfering RNAs (siRNA) for PRMT5 target sequence (AAGAGGGAGUUCAUUCAGGAA) and scrambled sequence (AAGUGAUAGGAAGUCAGUACG) were designed and synthesized using a Silencer siRNA construction kit (Ambion, TX). Experimental results were verified with On-Target plus Smart pool PRMT5 siRNA, purchased from Dharmacon (Lafayette, CO). The HOXA9 siRNA was described earlier (4). A second HOXA9 siRNA targeting the 3′ untranslated region (UTR; AACUAGGCUGUUAACCUAUGA) was synthesized as described above. The nontargeting Silencer negative-control siRNA was purchased from Ambion (Austin, TX). PRMT1 siRNA was purchased from Dharmacon (Lafayette, CO). Human EC (>90% confluent) were transfected with 50 nM siRNA for 4 h, using Targefect F-2 (5 μl/ml) and peptide enhancer (5 μl/ml) in Dulbecco's modified Eagle's medium (DMEM) with greater than 90% transfection efficiency, as described earlier (4). Cells were replenished with fresh EC culture medium and, following a 24-h incubation period, treated with TNF-α (2 ng/ml) for both 2 h and 5 h. Cells were harvested for subsequent RNA and protein analyses.
Monocyte attachment to TNF-α-stimulated EC.
Monocytic cell adhesion to human EC was determined by fluorescent labeling of U937 cells with 2.5 μM calcein-AM (Molecular Probes, OR) in RPMI medium as described previously (58). The labeled cells (2.5 × 105/ml) were then added to confluent monolayers of human EC treated with TNF-α (1 ng/ml) for 5 h in 24-well plates. After incubation for 45 min at 37°C, unbound cells were removed by washing, and the number of U937 cells adhering to EC was assessed by quantifying the labeled cells using NIH ImageJ.
In vitro methyltransferase assay.
A methyltransferase assay was performed using total EC lysate or immunoprecipitated myc-tagged PRMT5 expressed in HEK293 cells as described earlier (7), with modification. The PRMT5 substrate myelin basic protein (MBP; 1 to 2 μg) was incubated in a final reaction volume of 30 μl containing 25 mM HEPES (pH 7.6), 5 mM MgCl2, 100 mM NaCl, 2 mM dithiothreitol (DTT), 2.75 μCi of 3H-labeled S-adenosyl-l-methionine ([3H]SAM; PerkinElmer Life Sciences, Boston, MA) at 37°C for 90 min. The proteins were separated on a 10 or 12% SDS-polyacrylamide gel and visualized by enhanced (EN3HANCE; PerkinElmer Life Sciences) fluorography. For HOXA9 methylation, immunoprecipitated FLAG-tagged HOXA9 expressed in HEK293 cells was used as a substrate. The enzymatic reaction was stopped by trichloroacetic acid (TCA) precipitation, followed by acetone wash. [3H]SAM incorporation was measured by scintillation counting.
EMSA.
An electrophoretic mobility shift assay (EMSA) was performed with in vitro translated protein as described previously (4). Control rabbit reticulocyte lysate (5 μl) or lysate expressing FLAG-tagged HOXA9 protein, either the wild-type (WT) or R140A mutant, was used in a 25-μl final reaction volume in a buffer containing 20 mM HEPES (pH 7.5), 75 mM NaCl, 2 mM MgCl2, 1 mM DTT, 500 μg/ml bovine serum albumin (BSA), 5% glycerol, and 2.5 μg of poly(dI-dC). Next, DNA probes labeled using T4 polynucleotide kinase and [γ-32P]ATP were incubated with this reaction mixture at room temperature for 20 min. DNA-protein complexes were resolved on a 6% polyacrylamide gel in 0.25× Tris-borate-EDTA (TBE) buffer, followed by autoradiography or phosphorimage analysis. For supershift assays, FLAG antibody was used. The sequences of E-selectin promoter DNA probes used for EMSA are as follows: Abdominal B (Abd-B)-like site forward, GTATATGCAATTTTATTAATAT, and reverse, ATATTAATAAAATTGCATATAC (core sequences are underlined). The sequences from −213 to −193 of VCAM-1 promoter containing a putative HOXA9-binding site were used as DNA probes in EMSA. The sequences are as follows: forward, AAGCCTGTATTTTATAGTCTT, and reverse, AAGACTATAAAATACAGGCTT.
ChIP assay.
A chromatin immunoprecipitation (ChIP) assay was performed using a kit from Millipore as described earlier (4). Human EC treated with TNF-α (5 ng/ml) were cross-linked by the addition of formaldehyde, lysed, and sonicated using a Sonicator 3000 (Misonix Inc., Farmingdale, NY). The chromatin-protein complex was subjected to chromatin immunoprecipitation using appropriate antibody as described in the text. The genomic DNA fragments were purified, amplified by PCR, and analyzed on 2% agarose gel. Three primer sets for PCR were designed to cover different regions of the E-selectin promoter. The product of the first set of primers spanned the sequence from −238 to −110 (129 bp) in the E-selectin promoter. The primer sequences are 5′-CATGCATTTTTGTCATATTAATAAAAATTG-3′ and 5′-CTTTCCCGGGAATATCCACGATGC-3′. The product of the second set of primers spanned the sequence from −326 to −110 (217 bp). The primer sequences are 5′-CTACCACAACTACATGAGAGACACTAC-3′ and 5′-CTTTCCCGGGAATATCCACGATGC-3′. Both the primer sets cover the Abd-B-like site located in the E-selectin primer. The third product spanned the sequence from −1632 to −1449 (184 bp) serving as a control. The primer sequences were 5′-ATCTACCTTGTGAGTCATTC-3′ and 5′-TAGTTGTGGTAGTAATTAGAAT-3′.
EC surface expression of E-selectin and ICAM-1.
An enzyme-linked immunosorbent assay (ELISA) was used to quantify expression of E-selectin or ICAM-1 on the EC surface as described earlier (49), with modification. Confluent cultures of human EC in 96-well plates were treated with TNF-α (2 ng/ml) for 4 h and fixed with 2% paraformaldehyde. The cells were then washed and incubated with the primary anti-ICAM-1 or anti-E-selectin antibody, followed by washing. The secondary antibody (rabbit anti-mouse IgG–horseradish peroxidase conjugate; Dako Corp., Carpinteria, CA) was then added for 30 min and washed, followed by the addition of the substrate tetramethylbenzidine (TMB). The reaction was stopped after 30 min with 0.3 M sulfuric acid, and the optical density was determined at 490 nm in an enzyme-linked immunosorbent assay reader (Bio-Tek Instruments, Inc., Winooski, VT). TNF-α-induced E-selectin surface expression in specific siRNA-transfected EC was also determined by this method.
E-selectin surface expression in oxidized adenosine (AdOx; Sigma)-treated human EC was determined by flow cytometry. Cells were incubated with AdOx (20 μM) for 6 h prior to TNF-α treatment (5 ng/ml) for 4 h and collected by centrifugation after being dislodged from the dish by use of a cell dissociation solution (Sigma). For detection of surface expression of E-selectin, cells were incubated with anti-human E-selectin monoclonal antibody (R & D Systems, MN) at a 1:100 dilution for 30 min at room temperature, washed, and subsequently incubated for 30 min with phycoerythrin (PE)-conjugated secondary antibody at a 1:200 dilution. Cells, either fresh or fixed with 2% paraformaldehyde, were then analyzed by fluorescence-activated cell sorting ([FACS] Becton Dickinson and Co., NJ) for E-selectin protein.
Statistics.
Results are expressed as means ± standard deviation (SD). Statistical comparisons between two groups were evaluated by Student t test and analysis of variance (ANOVA) for multiple comparisons. A P value of <0.05 was considered statistically significant.
RESULTS
HOXA9 associates with PRMT5 in TNF-α-activated EC.
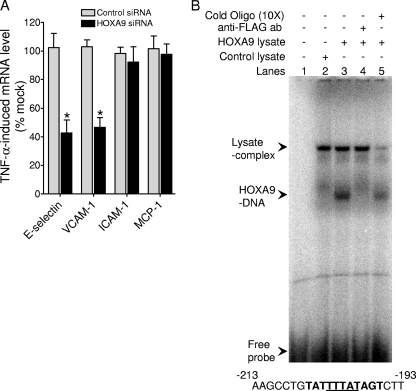
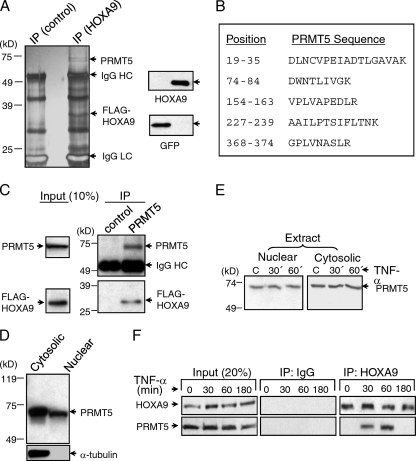
We have previously shown that HOXA9 participates in the transcriptional induction of E-selectin by cytokines in EC (4). We have recently identified a similar role for HOXA9 in cytokine induction of VCAM-1 (Fig. 1A) via a putative Abd-B-like binding site in the VCAM-1 proximal promoter region (bp −196 to −206). We verified this element as a HOXA9-binding site by EMSA (Fig. 1B). To identify binding partners/cofactors that might interact with HOXA9 for the transcriptional induction of E-selectin and VCAM-1, we overexpressed FLAG epitope-tagged HOXA9 or GFP as control and undertook a coimmunoprecipitation approach (Fig. 2A). Cell lysates from TNF-α-treated EC were immunoprecipitated with anti-FLAG antibody and separated by SDS-PAGE and silver staining. Subsequent mass spectrometric analysis of specific silver-stained bands identified an ∼72-kDa candidate HOXA9-interacting protein (Fig. 2A). The sequence identity of multiple tryptic peptides of this protein revealed the presence of protein arginine methyltransferase 5 (PRMT5) (Fig. 2B). Simultaneously, we performed reciprocal co-IP experiments and verified the association between HOXA9 and endogenous PRMT5 in anti-PRMT5 antibody-mediated immunoprecipitate from TNF-α-treated EC lysates (Fig. 2C). Thus, we conclude that FLAG epitope-tagged HOXA9 associates with PRMT5 in TNF-α-stimulated EC. These two proteins most likely interact in the EC nucleus, as the transcription factor HOXA9 is exclusively present in the nucleus. Although PRMT5 was principally present in the cytoplasm, we detected significant PRMT5 in nuclear preparations (Fig. 2D), the level of which did not change upon TNF-α-treatment (Fig. 2E).
Fig 1.
(A) HOXA9 is required for the maximal induction of E-selectin and VCAM-1. The induction of E-selectin, VCAM-1, ICAM-1, or MCP-1 mRNA, isolated from control or HOXA9 siRNA-transfected EC treated with TNF-α for 2 h, was measured by real-time PCR, using gene-specific primer sets. The percent change was calculated relative to mock-transfected EC. Each mRNA expression was normalized to GAPDH mRNA expression. Data represent the means ± SD of three replicate experiments. *, P < 0.05 versus mock transfections. (B) HOXA9 associates with the Abd-B-like putative binding site in the VCAM-1 promoter. An EMSA was performed by incubating in vitro translated FLAG-tagged HOXA9 in rabbit reticulocyte lysate and double-stranded 32P-labeled oligonucleotide, as described in Materials and Methods. Lane 1, no lysate; lane 2, control lysate; lane 3, lysate expressing FLAG-HOXA9; lane 4, FLAG-HOXA9 lysate incubated with monoclonal anti-FLAG antibody; lane 5, FLAG-HOXA9 lysate incubated with 10-fold excess cold oligonucleotide. The putative HOXA9 recognition element is shown in bold in the oligonucleotide sequence (bp −196 to −206). The core sequence is underlined.
Fig 2.
HOXA9 binds to PRMT5 in TNF-α-treated EC. (A) Lysates of FLAG-HOXA9 or GFP-transfected EC treated with TNF-α for 1 h were immunoprecipitated (IP) with anti-FLAG antibody and analyzed by SDS-PAGE. PRMT5 (∼72 kDa) is indicated in the silver-stained gel (left). Western blot analysis of EC total lysate shows the expression of either FLAG-HOXA9 or GFP (right). The experiment was repeated three times with similar results. (B) Protein sequence of PRMT5. Specific silver-stained bands, following trypsin digestion, were analyzed using LC-ESI/MALDI-TOF mass spectroscopy. The positions of five peptides that matched with the sequence of PRMT5 are shown. (C) FLAG-HOXA9 is coimmunoprecipitated with PRMT5 in TNF-α-treated EC. Human EC were transfected with the vector expressing FLAG-HOXA9 and analyzed by Western blotting for the presence of either FLAG-HOXA9 or PRMT5 in total extracts and anti-PRMT5 antibody-mediated immunoprecipitates as described in the text. Representative data from three replicate experiments are shown. (D) PRMT5 is present both in the nucleus and cytoplasm of human EC as shown by Western blot analyses of respective preparations. The purity of the nuclear preparation was determined by testing for the presence of α-tubulin by Western blotting. (E) The levels of PRMT5 protein present both in the nucleus and cytoplasm of human EC remain unchanged in EC treated with TNF-α for 30 or 60 min versus untreated EC as shown by Western analyses of respective preparations. (F) PRMT5 transiently associates with HOXA9 in the nucleus of TNF-α-treated EC. Coimmunoprecipitation was performed with human EC untreated or following TNF-α treatment for the indicated times, using anti-HOXA9 or control IgG. The immunoprecipitates were analyzed using protein-specific antibodies as indicated. Data are representative of three independent, highly reproducible experiments.
We then investigated the direct physical association between endogenous HOXA9 and PRMT5 in the EC nucleus by coimmunoprecipitating HOXA9-containing complexes from nuclear extracts of TNF-α-treated EC, using anti-HOXA9-specific antibody. The immunoprecipitated complexes were analyzed for the presence of both HOXA9 and PRMT5. HOXA9 was readily detected in all immunoprecipitates (Fig. 2F). However, PRMT5 was detected only in immunoprecipitates of 30-min or 1-h TNF-α-treated EC. These results suggest that HOXA9 transiently associates with PRMT5 in the nucleus of TNF-α-stimulated EC.
PRMT5 contributes to TNF-α-induced expression of E-selectin and VCAM-1 in EC.
Identification of PRMT5 as a binding partner of HOXA9 led us to explore protein arginine methylation in the induction of EC inflammatory genes, including the HOXA9 target gene E-selectin. EC were pretreated with the methyltransferase inhibitor AdOx for 4 to 6 h prior to TNF-α stimulation. FACS analysis of AdOx-treated EC revealed a significant reduction in TNF-α-induced E-selectin surface expression (Fig. 3A and B). A similar inhibition by AdOx of TNF-α induction of VCAM-1 was observed (data not shown); however, TNF-α-induced surface expression of ICAM-1 showed little change (Fig. 3B). Taken together, these results demonstrate a specific and positive functional role of protein methylation in E-selectin and VCAM-1 induction by TNF-α.
Fig 3.
Oxidized adenosine (AdOx) blocks the induction of E-selectin by TNF-α. (A) Human EC were treated with AdOx (20 μM) for 4 h prior to TNF-α (5 ng/ml) treatment for 4 h. The cells were dislodged and analyzed for the surface expression of E-selectin by FACS analysis using an anti-human E-selectin antibody, as described in Materials and Methods. This experiment was independently repeated three times with similar results. (B) Human EC were treated with AdOx (20 μM) for 6 h prior to the addition of TNF-α (5 ng/ml) to the culture medium. The cells were fixed and analyzed for the surface expression of either E-selectin or ICAM-1 with appropriate antibodies in ELISAs. Data represent the means ± SD for three replicate experiments.
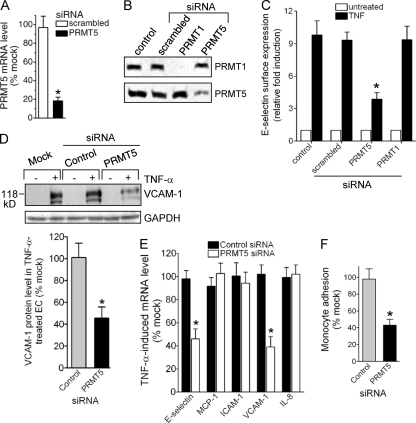
To address directly the role of PRMT5 in the induction of EC inflammatory genes, we used PRMT5-specific siRNA, which is effective in reducing both message and protein levels of PRMT5 (Fig. 4A and B). Both TNF-α-induced E-selectin and VCAM-1 in EC were markedly reduced in PRMT5-deficient EC (Fig. 4C and D). In contrast, depletion of PRMT1 (Fig. 4B) did not affect induction of E-selectin (Fig. 4C). Inhibition of E-selectin or VCAM-1 mRNA was observed, consistent with regulation at the level of transcription (Fig. 4E). Importantly, TNF-α-induced expression of other EC activation genes, i.e., ICAM-1, MCP-1, and IL-8, was not reduced by PRMT5 siRNA (Fig. 4E). These results as well as results from subsequent experiments using siRNA to reduce gene expression in EC were verified with a second gene-specific siRNA (data not shown), as described in detail in Materials and Methods. Therefore, PRMT5 is specifically required for E-selectin and VCAM-1 induction in activated EC. In view of the important role of these adhesion molecules in the interaction of EC with leukocytes, we examined whether downregulation of PRMT5 in EC affected TNF-induced monocyte adhesion. Treatment of human EC with PRMT5-specific siRNA resulted in a strong reduction (>50%) in U937 monocyte adhesion to stimulated EC, whereas control siRNA had no effect (Fig. 4F, and data not shown).
Fig 4.
PRMT5 is required for the induction of E-selectin and VCAM-1 in EC. Human EC were transfected with PRMT5, PRMT1, and scrambled or negative-control siRNA for 24 h and treated with TNF-α (5 ng/ml) for an additional 2 h and 5 h. (A) PRMT5 mRNA, isolated from specific siRNA-transfected EC either untreated or treated with TNF-α for 2 h, was measured by quantitative real-time PCR. The percent change was calculated relative to mock-transfected EC and normalized to GAPDH mRNA expression. Data represent the means ± SD for three independent experiments. *, P < 0.01 versus mock transfections. (B) Both PRMT5 and PRMT1 levels were detected in lysates from control EC or EC transfected with the indicated siRNA by Western blot analysis. A representative experiment is shown. (C) EC surface protein expression of E-selectin was detected in control or the indicated siRNA-transfected EC both untreated and treated with TNF-α for 5 h by ELISA. Data represent the means ± SD for three replicate experiments. *, P < 0.01 versus control-treated EC. (D) VCAM-1 protein level was detected by Western blot analysis of mock, control, or PRMT5 siRNA-transfected EC both untreated and treated with TNF-α for 5 h. GAPDH served as loading control. VCAM-1 levels were quantified by densitometry, and the percent change was calculated relative to mock-transfected EC. Data represent the means ± SD of three replicate experiments. *, P < 0.05 versus mock transfections. (E) E-selectin, MCP-1, ICAM-1, VCAM-1, or IL-8 mRNA, isolated from control or PRMT5 siRNA-transfected EC treated with TNF-α for 2 h, was measured by real-time PCR. The percent change was calculated relative to mock-transfected EC. Each mRNA expression was normalized to GAPDH mRNA expression. Data represent the means ± SD of three replicate experiments. *, P < 0.05 versus mock transfection. (F) PRMT5 contributes to monocyte adhesion to TNF-α-stimulated EC. U937 monocytes, fluorescently labeled with calcein-acetoxymethyl ester (2.5 μM), were added to control or PRMT5-specific siRNA-transfected human EC (1.2 × 105 cells), which were treated with TNF-α (1 ng/ml) for 5 h. The number of labeled cells adhering to EC was quantified using NIH ImageJ. Data represent the means ± SD of three replicate experiments. *, P < 0.05 versus control siRNA.
HOXA9 is required for TNF-α-dependent binding of PRMT5 to the E-selectin promoter in EC.
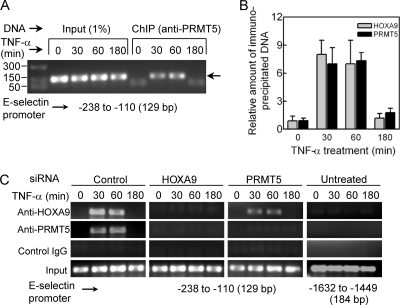
PRMT5 is a component of distinct nuclear complexes that function to regulate the transcriptional activity of specific gene promoters (29). To explore possible PRMT5 association with the E-selectin promoter, we performed chromatin immunoprecipitation (ChIP) assays using cellular lysates from TNF-α-treated human EC. The E-selectin promoter region encompassing the Abd-B-like HOXA9-recognition site was specifically amplified from anti-PRMT5 chromatin immunoprecipitates from cells treated with TNF-α for 30 or 60 min (Fig. 5A). In contrast, immunoprecipitates from untreated cells or cells treated with TNF-α for 180 min showed little or no DNA amplification. Notably, our ChIP analysis failed to detect association of PRMT5 with EphB4 promoter (8), an actively transcribed promoter expressed in EC that constitutively binds HOXA9 (data not shown). These results demonstrate a transient association of PRMT5 with the E-selectin promoter region containing the HOXA9 DNA-binding site in activated EC.
Fig 5.
To associate with the E-selectin promoter in TNF-α-treated EC, PRMT5 requires HOXA9. (A) PRMT5 transiently associates with the E-selectin promoter in response to TNF-α. ChIP assays were performed with the cross-linked chromatin from human EC both untreated and treated with TNF-α for 30, 60, or 180 min using anti-PRMT5 antibody. The specific E-selectin promoter region spanning bp −238 to −110 was amplified from the de-cross-linked immunoprecipitated DNA or input DNA by PCR with specific primer sets. The 129-bp products were analyzed by agarose-gel electrophoresis on a 2% agarose gel and verified by sequencing. Representative data from three independent experiments are shown. (B) Relative enrichment of E-selectin promoter levels as measured by quantitative real-time PCR. Cross-linked chromatin was extracted from human EC treated with TNF-α for 0, 30, 60, or 180 min and precipitated with antibodies to either HOXA9 or PRMT5. The amplification of the specific E-selectin promoter region as shown above was monitored by real-time PCR. The recovered DNA was evaluated relative to DNA enriched by control IgG immunoprecipitation. Data represent the means ± SDs from three experiments. (C) HOXA9 is required for TNF-α-dependent PRMT5 association with the E-selectin promoter. ChIP assays were performed, using appropriate IgG as indicated, with human EC, untreated or treated with TNF-α for the indicated times, following a 24-h transfection with a negative control (Ambion, TX), HOXA9-specific, or PRMT5-specific siRNA. The PCR amplified DNA fragments were analyzed using a 2% agarose gel and sequenced for verification (data not shown). Data represent one of three replicate experiments.
Next, we investigated the potential coordination of binding of HOXA9 and PRMT5 to the E-selectin promoter by ChIP analysis. After 30 min or 60 min of TNF-α treatment, both HOXA9 and PRMT5 associated with the promoter in EC (Fig. 5B) or EC transfected with the control siRNA (Fig. 5C). No DNA binding was observed in untreated control or after a 3-h TNF-α treatment (Fig. 5B and C). Therefore, the kinetics of PRMT5 and HOXA9 binding to the E-selectin promoter are comparable. Strikingly, TNF-α-dependent binding of PRMT5 to the promoter was inhibited in cells treated with HOXA9-specific siRNA (Fig. 5C). In contrast, siRNA-mediated depletion of PRMT5 did not inhibit HOXA9 binding to the promoter (Fig. 5C). Notably, HOXA9 siRNA did not alter PRMT5 expression, nor did PRMT5 siRNA change HOXA9 expression in EC (data not shown). Neither HOXA9 nor PRMT5 was found to bind to the 1.5-kb upstream region lacking the putative HOXA9-binding site. Thus, binding of HOXA9 to its recognition sequence is necessary prior to recruitment of PRMT5 to the E-selectin promoter in TNF-α-activated EC.
PRMT5 acts as a coactivator for HOXA9 in E-selectin promoter transactivation.
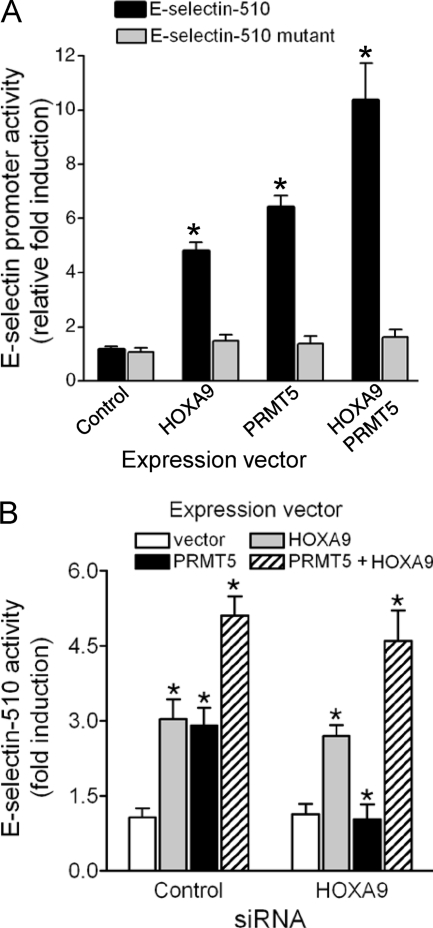
We previously demonstrated that HOXA9 overexpression was sufficient to transactivate the E-selectin promoter-reporter in EC (4). To determine whether PRMT5 cooperates with HOXA9 in induction of E-selectin, we cotransfected the E-selectin promoter-luciferase (E-selectin-510) with either the control (empty CMV vector), HOXA9, PRMT5, or both expression vectors in EC and measured reporter activity. Either HOXA9 or PRMT5 expression elicited a 4- to 6-fold induction in E-selectin promoter activity; however, expression of both resulted in a 10-fold induction (Fig. 6A). Neither HOXA9 nor PRMT5 by itself, or in combination, stimulated expression of a reporter bearing a HOXA9 DNA-binding site mutation (E-selectin-510 mutant) that abrogates binding (Fig. 6A). Therefore, PRMT5 cooperates with HOXA9 in activating the E-selectin promoter through the HOXA9 DNA-binding site.
Fig 6.
PRMT5 transactivates the E-selectin promoter in a HOXA9-dependent manner. (A) PRMT5-mediated induction of the E-selectin promoter (E-selectin-510) was abrogated upon mutation of the Abd-B-like site in the promoter (E-selectin-510 mutant). FLAG-HOXA9 or Myc-PRMT5 cDNA in the pcDNA3 vector (0.2 μg/ml), either alone or in combination (1:1), was cotransfected with the promoter/luciferase reporter construct (0.1 μg/ml) either pGL3-E-selectin-510 or pGL3-E-selectin-510 mutant into human EC. The reporter luciferase activity was normalized by cotransfecting pCMV-βGal (10 ng/ml). Relative normalized luciferase activity is expressed as fold induction compared to control vector transfection. Data represent the mean ± SD of three independent experiments. *, P < 0.05 versus control. (B) Overexpression of HOXA9 restores PRMT5-mediated transactivation of the E-selectin promoter in HOXA9-deficient EC. Human EC were transfected with HOXA9 siRNA or scrambled RNA for 24 h and then washed and allowed to recover for 6 h prior to a second round of cotransfection using either a control or PRMT5 expression vector and the reporter construct E-selectin-510. The reporter luciferase activity was normalized by cotransfecting pCMV-βGal (10 ng/ml). Relative normalized luciferase activity is expressed as fold induction compared to control vector transfection. Data represent the mean ± SD of three replicate experiments. *, P < 0.05 versus control vector.
We confirmed the transcriptional coactivator role of PRMT5 using siRNA-mediated knockdown of HOXA9 followed by overexpression of PRMT5. Following treatment with control or HOXA9-specific siRNA, EC were cotransfected with the reporter E-selectin-510 and either the vector control, HOXA9, PRMT5, or both expression vectors. In contrast to the upregulation of reporter expression by HOXA9 alone, PRMT5 by itself had no impact on reporter activity in HOXA9-deficient EC, but in combination with HOXA9, stimulated expression was restored (Fig. 6B).
TNF-α induces PRMT5-mediated methylation of HOXA9.
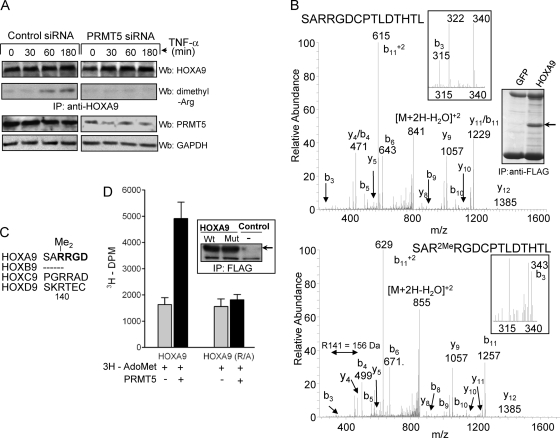
We investigated whether the interaction of PRMT5 and HOXA9 results in arginine methylation of HOXA9. After EC treatment with TNF-α for up to 180 min, HOXA9 was immunoprecipitated and analyzed for methylation (Fig. 7A). Robust, signal-dependent arginine methylation of HOXA9 was observed after a 60-min treatment with TNF-α; the methylated form was present even after 180 min. The requirement for PRMT5 was established by the absence of HOXA9 methylation in cells treated with siRNA that targets PRMT5. Together with ChIP data (Fig. 5), these results led us to conclude that E-selectin promoter-bound HOXA9 undergoes arginine methylation by PRMT5 during the inflammatory response. Our observed time lag between the presence of PRMT5 on the promoter and HOXA9 methylation may be due to delayed activation of PRMT5. It is been recently reported that dephosphorylation of PRMT5 is essential for its ability to methylate its substrates (35).
Fig 7.
TNF-α induces PRMT5-mediated methylation of HOXA9 at Arg140. (A) Human EC transfected with either control or PRMT5-specific siRNA were treated with TNF-α as indicated. Cell lysates of transfected EC were immunoprecipitated with HOXA9-specific antibody and analyzed for total HOXA9 by Western blotting (Wb; upper panel). HOXA9 arginine methylation was detected by anti-dimethyl arginine antibody (upper panel). The presence of PRMT5 or GAPDH for a loading control was detected using protein-specific antibody (lower panel). (B) HOXA9 is methylated on Arg140. Lysates of FLAG-HOXA9-transfected HEK293 cells were immunoprecipitated with anti-FLAG antibody and analyzed by SDS-PAGE and Coomassie blue staining (right). Chymotryptic digests of HOXA9 were analyzed by LC-ESI/MALDI-TOF mass spectrometry. Collision-induced dissociation (CID) spectra for the chymotryptic (138SARRGDCPTLDTHTL152) HOXA9 peptide are shown. The unmodified peptide has a mass of 1699.8 Da (upper panel). The dimethylated peptide has a mass of 1727.8 Da, and it contains two potential sites of R methylation, R140 and R141 (lower panel). The site of modification can be determined by the mass of the b3 ion (343 Da), which corresponds to the SAR portion of the peptide (insets). This indicates that the site of dimethylation is R140. (C) Among the members of HOX paralogue group 9, only HOXA9 has the unique RRGD sequence (in bold) containing the dimethylated Arg140. (D) HOXA9 is methylated on Arg140 by PRMT5. Wild-type or an R140A mutant of FLAG-tagged HOXA9 immunoprecipitated from HEK293 cells (right) was incubated with 3H-labeled S-adenosylmethionine (AdoMet) and immunoprecipitate of Myc-tagged PRMT5 (250 ng). Incorporation of methyl groups was measured by scintillation counting (disintegrations per minute [dpm]) of TCA precipitates of the reaction mixture. The means of data from three independent experiments is shown here.
HOXA9 is methylated on Arg140 by PRMT5.
To identify the arginine residue(s) methylated by PRMT5, we overexpressed FLAG-HOXA9 in HEK293 cells, which are known to upregulate the E-selectin promoter in response to HOXA9 (data not shown). HOXA9 was immunoprecipitated and separated on an SDS-PAGE gel (Fig. 7B). The Coomassie-stained HOXA9 band was digested with chymotrypsin and subjected to LC-mass spectrometric analysis. The principal target site for HOXA9 dimethylation was identified to be Arg140 (Fig. 7B), which is present in a sequence R140RGD in the transactivation domain of HOXA9. This highly conserved sequence is uniquely present in HOXA9 among the members of HOX paralogue group 9 (Fig. 7C) and is distinct from the glycine-arginine-rich motif (GAR) in other PRMT5 target proteins. To test whether methylation of HOXA9 was catalyzed by PRMT5, we generated HOXA9 bearing an Arg140Ala (R140A) point mutation (Fig. 7D, inset) and determined its susceptibility to in vitro arginine methylation. Contrary to the efficient methylation of wild-type HOXA9 by PRMT5, the Ala mutant was not methylated above the background level (Fig. 7D). Thus, Arg140 in HOXA9 is the major site of methylation by PRMT5.
HOXA9 Arg140 methylation by PRMT5 is critical for E-selectin induction.
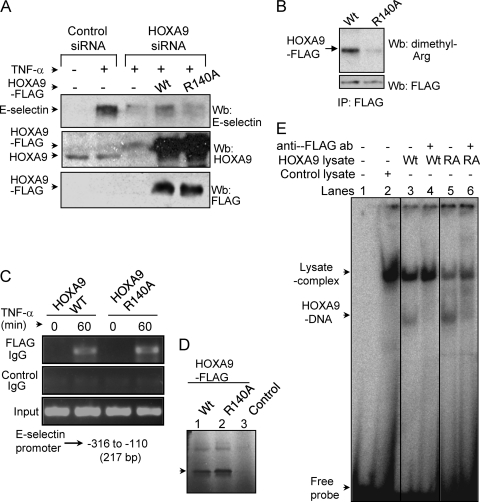
We then investigated the role of HOXA9 methylation in E-selectin induction by 3′-UTR-specific siRNA-mediated knockdown of endogenous HOXA9 followed by overexpression of the wild type or Arg140Ala mutant to reconstitute protein levels. As seen previously, E-selectin induction was impaired with HOXA9-specific siRNA treatment. Restoring HOXA9 protein levels to the EC, using the wild-type HOXA9 cDNA, recovered the induction to a level that would be expected with the 25 to 40% transfection efficiency of the expression vector in these cells. In contrast, the mutant HOXA9 had little impact (Fig. 8A). Although the overexpressed HOXA9 levels were comparable, only the wild-type protein was efficiently methylated in TNF-α-treated EC (Fig. 8B). Notably, compared to the wild type, this mutation in the transactivation domain of HOXA9 did not show any impairment in its ability to recognize the E-selectin promoter in TNF-α-treated EC (Fig. 8C) or bind to the Abd-B-like site in vitro (Fig. 8D and E). Thus, HOXA9 arginine methylation, which is not necessary for DNA binding, contributes to the transcriptional activation of E-selectin.
Fig 8.
HOXA9 methylation on Arg140 is important for E-selectin induction. (A) TNF-α-stimulated induction of E-selectin requires HOXA9 methylation at Arg140. Human EC were cotransfected simultaneously with control or HOXA9 siRNA targeting the 3′ UTR and wild-type or mutant HOXA9 for 24 h and treated with TNF-α for an additional 5 h. E-selectin induction in EC was analyzed by Western blotting (upper panel). HOXA9 protein levels, both endogenous and FLAG-tagged, in control versus HOXA9 siRNA-transfected EC were analyzed by Western blotting (middle panel). Western blot analysis of EC total lysate shows the expression of FLAG-tagged wild-type HOXA9 or its Arg140Ala mutant version (lower panel). Data from a representative of three replicate experiments are shown here. (B) Western blot analysis of EC immunoprecipitate shows TNF-α-dependent methylation of wild-type FLAG-HOXA9 only (upper panel). The presence of both the wild type and the R140A mutant of FLAG-HOXA9 in TNF-α-treated EC was detected in the immunoprecipitate using a monoclonal anti-FLAG antibody by Western blotting (lower panel). FLAG-HOXA9, either WT or mutant, was not pulled down with control IgG (data not shown). (C) Both the wild type and the R140A mutant of HOXA9 associates with the E-selectin promoter in 1-h TNF-α-treated EC. A ChIP assay was performed with the crossed-linked chromatin isolated from EC transfected with either wild-type or mutant HOXA9 construct. The primers spanning the E-selectin sequence from −326 to −110, containing the Abd-B-like site, were used for PCR amplification of both input and immunoprecipitated DNA as described in Materials and Methods. PCR products of both input and ChIP samples were loaded as indicated and analyzed using a 2% agarose gel. The amplified fragments were verified by sequencing. (D) In vitro translation of HOXA9. [35S]methionine-labeled HOXA9 was synthesized using a pCDNA3 construct in rabbit reticulocyte lysate by T7 polymerase in a coupled transcription-translation system (Promega). An autoradiogram of SDS-polyacrylamide gel shows the presence of a ∼32-kDa FLAG-tagged HOXA9 protein, wild type (lane 1) and Arg140Ala mutant (lane 2). The control reaction using the pCDNA3 vector is also shown (lane 3). (E) Arg140Ala mutation does not affect the specific binding of HOXA9 to the Abd-B-like site in the E-selectin promoter. An EMSA was performed by incubating in vitro translated FLAG-tagged HOXA9, either wild type (WT) or Arg140Ala (RA), and double-stranded 32P-labeled Abd-B-like site oligonucleotide, as described in Materials and Methods. Lane 1, no lysate control; lane 2, control lysate; lane 3, lysate expressing FLAG-tagged WT HOXA9; lane 4, WT incubated with monoclonal anti-FLAG antibody; lane 5, lysate expressing FLAG-tagged Arg140Ala (RA) mutant of HOXA9; lane 6, RA mutant incubated with monoclonal anti-FLAG antibody.
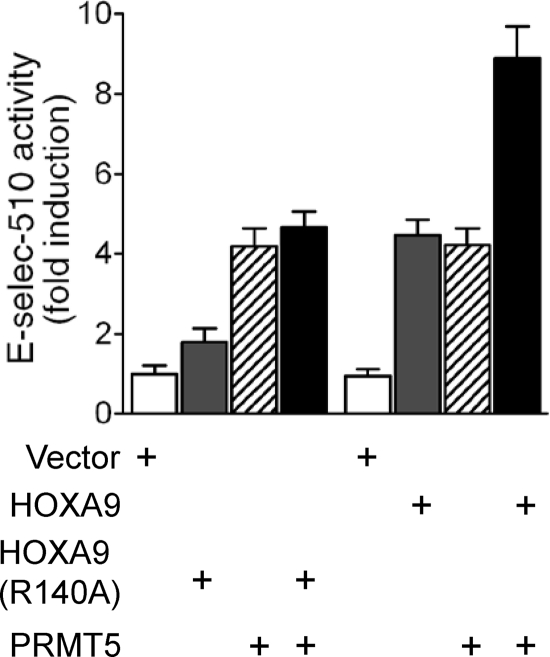
To determine the role of HOXA9 arginine methylation in promoter transactivation, E-selectin-510 was cotransfected with either the control (empty CMV vector), HOXA9 (either wild-type or Arg140Ala mutant), PRMT5, or both HOXA9 and PRMT5 in human EC. Wild-type HOXA9 showed a positive impact on the E-selectin promoter activity, and when it was combined with PRMT5, an additive effect was observed (Fig. 9). However, apart from its own contribution to promoter activity, PRMT5 failed to show any stimulatory effect with mutant HOXA9 (Fig. 9), a result consistent with the inability of PRMT5 to methylate the mutant construct. Thus, our results demonstrate the requirement for arginine methylation of HOXA9 by PRMT5 for maximal E-selectin promoter transactivation.
Fig 9.
The mutation Arg140Ala abrogates HOXA9-mediated induction of the E-selectin promoter. The wild-type or Arg140Ala mutant FLAG-HOXA9 cDNA expression construct in the pcDNA3 vector (0.2 μg/ml), either alone or in combination with Myc-PRMT5 (1:1), was cotransfected with the promoter/luciferase reporter construct pGL3-E510 (0.1 μg/ml) into human EC. The reporter luciferase activity was normalized by cotransfecting pCMV-βGal (10 ng/ml). Relative normalized luciferase activity is expressed in fold induction compared to control vector transfection. Data represent the means ± SDs for three replicate experiments.
DISCUSSION
This report uncovers a mechanism by which HOXA9 positively regulates a subset of EC inflammatory genes via interaction with PRMT5. Protein arginine methylation by the PRMT family of enzymes regulates diverse aspects of cellular processes, including signal transduction, chromatin remodeling, and gene expression. Although a few recent reports revealed a link between protein arginine methylation and endothelial dysfunction (46, 47), little is known about the role of PRMT enzymes in cytokine-activated EC. Here, we show for the first time the physiological importance of TNF-α-induced HOXA9 arginine methylation by PRMT5 in the induction of leukocyte adhesion molecules by EC. Extending our previous studies, we have elucidated a mechanism that involves a transient association of PRMT5 with HOXA9 on selective-target gene promoters, leading to symmetric dimethylation of Arg140 on HOXA9. This posttranslational modification is required for TNF-α induction of E-selectin and VCAM-1. Alternatively, PRMT5 could bind and methylate HOXA9 in TNF-α-stimulated EC, resulting in the formation of an active complex that participates in E-selectin and VCAM-1 gene induction.
There is an emerging body of evidence that PRMT enzymes play an important pathogenic role in inflammation (43). For example, both PRMT1 and PRMT4 have been implicated in the inflammatory response in association with NF-κB (17, 24). Although cytoplasmic PRMT5 was reported to interact with death receptors to induce multiple NF-κB target genes, it had no effect on the TNF-α-dependent NF-κB pathway (60). Despite its predominant localization in the cytoplasm, the nuclear function of PRMT5 is critical with regard to the finer regulation of gene expression. Among the identified nuclear substrates of PRMT5 are histones H3 and H4, involved in transcriptional regulation (18, 34), and p53, involved in regulating tumor suppression and cell cycle (27). We now report on our identification of PRMT5 as a HOXA9-binding protein in TNF-α-stimulated EC, using both in vitro and in vivo approaches (25, 27, 33, 62).
PRMT5 is a new addition to the growing list of factor/cofactor(s) that may be required for the context-dependent transcriptional regulatory function of HOXA9 (37, 57, 65). These factors may dictate whether HOXA9 activates or represses transcription. Interestingly, while DNA-binding partners Pbx1 and Meis1 facilitate HOXA9 to select correct binding for target gene activation in myeloid cells (65), PRMT5 posttranslationally modifies HOXA9 to influence a positive functional outcome without affecting its target DNA recognition in EC. PRMT5 selectively contributes to E-selectin induction, which requires TNF-α-dependent specific and transient binding of HOXA9 to its DNA-binding site in the promoter. In contrast, PRMT5 appears to be inconsequential for the constitutive impact of HOXA9 toward other EC target genes, such as the tyrosine kinase receptor EphB4 (8). Furthermore, induction of other inflammatory genes, including ICAM-1, is unaltered in the absence of PRMT5. This is consistent with the fact that, while the E-selectin and VCAM-1 promoters contain HOXA9-binding sites, the ICAM-1 promoter does not. Our evidence depicting the role of PRMT5 in specific ELAM induction is a new addition to other reports describing its positive impact (18, 52) and the first to show its function as a modifier of a HOX protein in EC.
In cytokine-treated EC, the E-selectin promoter undergoes dynamic changes, allowing histone modifications and highly regulated sequence-specific DNA-binding and associations of transcription factors/cofactors, including NF-κB, ATF-2/Jun, HOXA9, HMG-I(Y), and p300 for gene induction. Notably, PRMT5 is recruited in a stimulus-dependent manner to the HOXA9-bound E-selectin promoter. Our results suggest that PRMT5 recruitment to this promoter depends on HOXA9 and follows HOXA9 binding to DNA. Identification of PRMT5-mediated HOXA9 methylation strengthens this speculation. Finally, results based on our current as well as previous studies (4, 44) suggest additional possible roles of PRMT5 in the active downregulation of E-selectin induction. It has been shown that PRMT5-mediated methylation of histone H4R3 leads to DNA methylation in promoting gene silencing (68). Moreover, PRMT5 is known to be present in chromatin-remodeling repressor complexes (41, 42). Thus, in addition to methylating HOXA9 as a positive regulator, PRMT5 may subsequently methylate H4R3 or interact with other functionally critical factors, including the chromatin-modifying factors SWI/SNF (41). Such events may constitute the “off-switch” circuitry to shut down E-selectin and VCAM-1 gene induction.
Symmetric dimethylation of arginine residues by PRMT5 can act as both a positive and negative regulator of protein-protein interaction. The methylation of splicing protein SmB is required for its interaction with Tudor domain-containing proteins, e.g., SMN, SPF30, or TDRD3, and is an example of positive regulation (15). In contrast, methylation of the elongation factor Spt5 reduces its affinity for RNA polymerase II and hampers transcription elongation (33). In this respect, the functional consequence of context-dependent HOXA9 arginine methylation is quite intriguing. Between the adjacent arginines in the R140R141GD motif in the HOXA9 transactivation domain, Arg140 is the target site for methylation by PRMT5 (Fig. 5). This preferential selection must be influenced by sequence specificity and/or structural context. It seems plausible that the addition of a hydrophobic moiety, i.e., two methyl groups, might prevent the binding or accelerate the release of an inhibitory protein, as the contiguous R141GD sequence is well known for protein-protein interaction (16). Alternatively, HOXA9 methylation may stabilize its association with other component(s) of the transcriptional machinery that eventually is responsible for the persistence of E-selectin transcription.
In summary, our findings demonstrate that stimulus-dependent HOXA9 arginine methylation by PRMT5 regulates its proinflammatory role in activated EC and contributes to specific ELAM induction to promote leukocyte binding to EC. PRMT5-mediated methylation may orchestrate alterations in HOXA9-mediated gene expression required for EC activation. Such changes may be independent of the normal transcriptional activities of homeodomain proteins, including HOXA9. Understanding a potential role for PRMT5-HOXA9 interactions and subsequent methylation of HOXA9 in the cytokine-induced expression of ELAM may provide important mechanistic insight into both EC inflammatory processes and leukocyte infiltration at sites of inflammation.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant HL29582 (P.E.D.).
We thank Lisa Dechert, Kristy Waite, Lori Mavrakis, and Sean Steenberge for cell culture assistance. We also thank Michael Kinter for mass spectrometric analysis. Human umbilical vein endothelial cells were isolated from the cords collected by the Perinatal Clinical Research Center (supported by National Institutes of Health Research Center Award RR-00080) at the Cleveland Metro Health Hospital.
Footnotes
Published ahead of print 23 January 2012
REFERENCES
- 1. Aggarwal P, et al. 2010. Nuclear cyclin D1/CDK4 kinase regulates CUL4 expression and triggers neoplastic growth via activation of the PRMT5 methyltransferase. Cancer Cell 18:329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Auguste P, et al. 2007. The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. Am. J. Pathol. 170:1781–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bahrami SB, Veiseh M, Dunn AA, Boudreau NJ. 2011. Temporal changes in Hox gene expression accompany endothelial cell differentiation of embryonic stem cells. Cell Adh. Migr. 5:133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bandyopadhyay S, Ashraf MZ, Daher P, Howe PH, DiCorleto PE. 2007. HOXA9 participates in the transcriptional activation of E-selectin in endothelial cells. Mol. Cell. Biol. 27:4207–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bedford MT, Clarke SG. 2009. Protein arginine methylation in mammals: who, what, and why. Mol. Cell 33:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bevilacqua MP, Stengelin S, Gimbrone MA, Jr, Seed B. 1989. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science 243:1160–1165 [DOI] [PubMed] [Google Scholar]
- 7. Branscombe TL, et al. 2001. PRMT5 (Janus kinase-binding protein 1) catalyzes the formation of symmetric dimethylarginine residues in proteins. J. Biol. Chem. 276:32971–32976 [DOI] [PubMed] [Google Scholar]
- 8. Bruhl T, et al. 2004. Homeobox A9 transcriptionally regulates the EphB4 receptor to modulate endothelial cell migration and tube formation. Circ. Res. 94:743–751 [DOI] [PubMed] [Google Scholar]
- 9. Burridge KA, Friedman MH. 2010. Environment and vascular bed origin influence differences in endothelial transcriptional profiles of coronary and iliac arteries. Am. J. Physiol. Heart Circ. Physiol. 299:H837–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cantile M, Schiavo G, Terracciano L, Cillo C. 2008. Homeobox genes in normal and abnormal vasculogenesis. Nutr. Metab. Cardiovasc. Dis. 18:651–658 [DOI] [PubMed] [Google Scholar]
- 11. Carlos TM, Harlan JM. 1994. Leukocyte-endothelial adhesion molecules. Blood 84:2068–2101 [PubMed] [Google Scholar]
- 12. Carrio M, Arderiu G, Myers C, Boudreau NJ. 2005. Homeobox D10 induces phenotypic reversion of breast tumor cells in a three-dimensional culture model. Cancer Res. 65:7177–7185 [DOI] [PubMed] [Google Scholar]
- 13. Charboneau A, East L, Mulholland N, Rohde M, Boudreau N. 2005. Pbx1 is required for Hox D3-mediated angiogenesis. Angiogenesis 8:289–296 [DOI] [PubMed] [Google Scholar]
- 14. Collins T, et al. 1995. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 9:899–909 [PubMed] [Google Scholar]
- 15. Cote J, Richard S. 2005. Tudor domains bind symmetrical dimethylated arginines. J. Biol. Chem. 280:28476–28483 [DOI] [PubMed] [Google Scholar]
- 16. Courter D, et al. 2010. The RGD domain of human osteopontin promotes tumor growth and metastasis through activation of survival pathways. PLoS One 5:e9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Covic M, et al. 2005. Arginine methyltransferase CARM1 is a promoter-specific regulator of NF-κB-dependent gene expression. EMBO J. 24:85–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dacwag CS, Ohkawa Y, Pal S, Sif S, Imbalzano AN. 2007. The protein arginine methyltransferase Prmt5 is required for myogenesis because it facilitates ATP-dependent chromatin remodeling. Mol. Cell. Biol. 27:384–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edelstein LC, Pan A, Collins T. 2005. Chromatin modification and the endothelial-specific activation of the E-selectin gene. J. Biol. Chem. 280:11192–11202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Endemann DH, Schiffrin EL. 2004. Endothelial dysfunction. J. Am. Soc. Nephrol. 15:1983–1992 [DOI] [PubMed] [Google Scholar]
- 21. Fabbrizio E, et al. 2002. Negative regulation of transcription by the type II arginine methyltransferase PRMT5. EMBO Rep. 3:641–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerritsen ME, et al. 1997. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc. Natl. Acad. Sci. U. S. A. 94:2927–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gorski DH, Walsh K. 2000. The role of homeobox genes in vascular remodeling and angiogenesis. Circ. Res. 87:865–872 [DOI] [PubMed] [Google Scholar]
- 24. Hassa PO, Covic M, Bedford MT, Hottiger MO. 2008. Protein arginine methyltransferase 1 coactivates NF-κB-dependent gene expression synergistically with CARM1 and PARP1. J. Mol. Biol. 377:668–678 [DOI] [PubMed] [Google Scholar]
- 25. Hou Z, et al. 2008. The LIM protein AJUBA recruits protein arginine methyltransferase 5 to mediate SNAIL-dependent transcriptional repression. Mol. Cell. Biol. 28:3198–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsu JM, et al. 2011. Crosstalk between Arg 1175 methylation and Tyr 1173 phosphorylation negatively modulates EGFR-mediated ERK activation. Nat. Cell Biol. 13:174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jansson M, et al. 2008. Arginine methylation regulates the p53 response. Nat. Cell Biol. 10:1431–1439 [DOI] [PubMed] [Google Scholar]
- 28. Kansas GS. 1996. Selectins and their ligands: current concepts and controversies. Blood 88:3259–3287 [PubMed] [Google Scholar]
- 29. Karkhanis V, Hu YJ, Baiocchi RA, Imbalzano AN, Sif S. 2011. Versatility of PRMT5-induced methylation in growth control and development. Trends Biochem. Sci. 36:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krause CD, et al. 2007. Protein arginine methyltransferases: evolution and assessment of their pharmacological and therapeutic potential. Pharmacol. Ther. 113:50–87 [DOI] [PubMed] [Google Scholar]
- 31. Krumlauf R. 1994. Hox genes in vertebrate development. Cell 78:191–201 [DOI] [PubMed] [Google Scholar]
- 32. Kunkel EJ, Ley K. 1996. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ. Res. 79:1196–1204 [DOI] [PubMed] [Google Scholar]
- 33. Kwak YT, et al. 2003. Methylation of SPT5 regulates its interaction with RNA polymerase II and transcriptional elongation properties. Mol. Cell 11:1055–1066 [DOI] [PubMed] [Google Scholar]
- 34. Lacroix M, et al. 2008. The histone-binding protein COPR5 is required for nuclear functions of the protein arginine methyltransferase PRMT5. EMBO Rep. 9:452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu F, et al. 2011. JAK2V617F-mediated phosphorylation of PRMT5 downregulates its methyltransferase activity and promotes myeloproliferation. Cancer Cell 19:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mallo M, Wellik DM, Deschamps J. 2010. Hox genes and regional patterning of the vertebrate body plan. Dev. Biol. 344:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mann RS, Lelli KM, Joshi R. 2009. Hox specificity unique roles for cofactors and collaborators. Curr. Top. Dev. Biol. 88:63–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meister G, Fischer U. 2002. Assisted RNP assembly: SMN and PRMT5 complexes cooperate in the formation of spliceosomal UsnRNPs. EMBO J. 21:5853–5863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Myers C, Charboneau A, Boudreau N. 2000. Homeobox B3 promotes capillary morphogenesis and angiogenesis. J. Cell Biol. 148:343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pal S, Sif S. 2007. Interplay between chromatin remodelers and protein arginine methyltransferases. J. Cell. Physiol. 213:306–315 [DOI] [PubMed] [Google Scholar]
- 41. Pal S, Vishwanath SN, Erdjument-Bromage H, Tempst P, Sif S. 2004. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol. Cell. Biol. 24:9630–9645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pal S, et al. 2003. mSin3A/histone deacetylase 2- and PRMT5-containing Brg1 complex is involved in transcriptional repression of the Myc target gene cad. Mol. Cell. Biol. 23:7475–7487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parry RV, Ward SG. 2010. Protein arginine methylation: a new handle on T lymphocytes? Trends Immunol. 31:164–169 [DOI] [PubMed] [Google Scholar]
- 44. Patel CV, Sharangpani R, Bandyopadhyay S, DiCorleto PE. 1999. Endothelial cells express a novel, tumor necrosis factor-alpha-regulated variant of HOXA9. J. Biol. Chem. 274:1415–1422 [DOI] [PubMed] [Google Scholar]
- 45. Pollack BP, et al. 1999. The human homologue of the yeast proteins Skb1 and Hsl7p interacts with Jak kinases and contains protein methyltransferase activity. J. Biol. Chem. 274:31531–31542 [DOI] [PubMed] [Google Scholar]
- 46. Polotskaia A, et al. 2007. Regulation of arginine methylation in endothelial cells: role in premature senescence and apoptosis. Cell Cycle 6:2524–2530 [DOI] [PubMed] [Google Scholar]
- 47. Pope AJ, Karuppiah K, Cardounel AJ. 2009. Role of the PRMT-DDAH-ADMA axis in the regulation of endothelial nitric oxide production. Pharmacol. Res. 60:461–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Quere R, et al. 2011. Smad4 binds Hoxa9 in the cytoplasm and protects primitive hematopoietic cells against nuclear activation by Hoxa9 and leukemia transformation. Blood 117:5918–5930 [DOI] [PubMed] [Google Scholar]
- 49. Rasmussen LM, et al. 2001. Diverse effects of inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase on the expression of VCAM-1 and E-selectin in endothelial cells. Biochem. J. 360:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Read MA, et al. 1997. Tumor necrosis factor alpha-induced E-selectin expression is activated by the nuclear factor-κB and c-JUN N-terminal kinase/p38 mitogen-activated protein kinase pathways. J. Biol. Chem. 272:2753–2761 [DOI] [PubMed] [Google Scholar]
- 51. Rhoads K, et al. 2005. A role for Hox A5 in regulating angiogenesis and vascular patterning. Lymphat. Res. Biol. 3:240–252 [DOI] [PubMed] [Google Scholar]
- 52. Richard S, Morel M, Cleroux P. 2005. Arginine methylation regulates IL-2 gene expression: a role for protein arginine methyltransferase 5 (PRMT5). Biochem. J. 388:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rossig L, et al. 2005. Histone deacetylase activity is essential for the expression of HoxA9 and for endothelial commitment of progenitor cells. J. Exp. Med. 201:1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Scarpati EM, DiCorleto PE. 1996. Identification of a thrombin response element in the human platelet-derived growth factor B-chain (c-sis) promoter. J. Biol. Chem. 271:3025–3032 [DOI] [PubMed] [Google Scholar]
- 55. Shen WF, Krishnan K, Lawrence HJ, Largman C. 2001. The HOX homeodomain proteins block CBP histone acetyltransferase activity. Mol. Cell. Biol. 21:7509–7522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shen WF, et al. 1999. HOXA9 forms triple complexes with PBX2 and MEIS1 in myeloid cells. Mol. Cell. Biol. 19:3051–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shi X, Bai S, Li L, Cao X. 2001. Hoxa-9 represses transforming growth factor-beta-induced osteopontin gene transcription. J. Biol. Chem. 276:850–855 [DOI] [PubMed] [Google Scholar]
- 58. Sung MJ, et al. 2005. Protective effect of alpha-lipoic acid in lipopolysaccharide-induced endothelial fractalkine expression. Circ. Res. 97:880–890 [DOI] [PubMed] [Google Scholar]
- 59. Svingen T, Tonissen KF. 2006. Hox transcription factors and their elusive mammalian gene targets. Heredity 97:88–96 [DOI] [PubMed] [Google Scholar]
- 60. Tanaka H, Hoshikawa Y, Oh-hara T, et al. 2009. PRMT5, a novel TRAIL receptor-binding protein, inhibits TRAIL-induced apoptosis via nuclear factor-kappaB activation. Mol. Cancer Res. 7:557–569 [DOI] [PubMed] [Google Scholar]
- 61. Tee WW, et al. 2010. Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev. 24:2772–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Teng Y, et al. 2007. AS1411 alters the localization of a complex containing protein arginine methyltransferase 5 and nucleolin. Cancer Res. 67:10491–10500 [DOI] [PubMed] [Google Scholar]
- 63. Trivedi CM, Patel RC, Patel CV. 2008. Differential regulation of HOXA9 expression by nuclear factor kappa B (NF-kappaB) and HOXA9. Gene 408:187–195 [DOI] [PubMed] [Google Scholar]
- 64. Vijapurkar U, et al. 2004. Protein kinase C-mediated phosphorylation of the leukemia-associated HOXA9 protein impairs its DNA binding ability and induces myeloid differentiation. Mol. Cell. Biol. 24:3827–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang GG, Pasillas MP, Kamps MP. 2006. Persistent transactivation by Meis1 replaces Hox function in myeloid leukemogenesis models: evidence for co-occupancy of Meis1-Pbx and Hox-Pbx complexes on promoters of leukemia-associated genes. Mol. Cell. Biol. 26:3902–3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wu Y, Moser M, Bautch VL, Patterson C. 2003. HoxB5 is an upstream transcriptional switch for differentiation of the vascular endothelium from precursor cells. Mol. Cell. Biol. 23:5680–5691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang Y, et al. 2003. CUL-4A stimulates ubiquitylation and degradation of the HOXA9 homeodomain protein. EMBO J. 22:6057–6067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao Q, et al. 2009. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat. Struct. Mol. Biol. 16:304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]