Abstract
In Streptococcus thermophilus, the ComRS regulatory system governs the transcriptional level of comX expression and, hence, controls the early stage of competence development. The present work focuses on the posttranslational control of the activity of the sigma factor ComX and, therefore, on the late stage of competence regulation. In silico analysis performed on the S. thermophilus genome revealed the presence of a homolog of mecA (mecASt), which codes for the adaptor protein that is involved in ComK degradation by ClpCP in Bacillus subtilis. Using reporter strains and microarray experiments, we showed that MecASt represses late competence genes without affecting the early competence stage under conditions that are not permissive for competence development. In addition, this repression mechanism was found not only to act downstream of comX expression but also to be fully dependent on the presence of a functional comX gene. This negative control was similarly released in strains deleted for clpC, mecA, and clpC-mecA. Under artificial conditions of comX expression, we next showed that the abundance of ComX is higher in the absence of MecA or ClpC. Finally, results of bacterial two-hybrid assays strongly suggested that MecA interacts with both ComX and ClpC. Based on these results, we proposed that ClpC and MecA act together in the same regulatory circuit to control the abundance of ComX in S. thermophilus.
INTRODUCTION
Streptococcus thermophilus is of major economical value due to its extensive use for the production of yogurt and hard cooked cheese by the dairy industry (3, 21). Recently, S. thermophilus was shown to be able to develop natural competence for transformation, a transitory physiological state enabling the capture and stable integration of naked DNA in the chromosome of this species (2, 14, 15, 18).
It is well established that natural competence regulation usually involves a set of regulators, which together form a cascade from the early stage to the late stage of competence development (8, 20). For S. thermophilus, competence was shown to be naturally induced when S. thermophilus cells were grown in a chemically defined medium. This induction results in the transitory expression of the comX gene, coding for the master regulator of late competence (com) genes, which are necessary for DNA uptake, protection, and chromosomal integration (14, 18). ComX is an alternative sigma factor (σX) that binds to the RNA polymerase in order to transcribe specifically the late com genes by recognizing a small sequence (Com box) in their upstream regions (31, 33, 46). Recently, we have shown that the activation of comX expression in S. thermophilus relies on a novel quorum-sensing system, named ComRS (14). The proposed activation model starts with the intracellular production of a precursor peptide (pre-ComS), which is then secreted and matured (ComS*) (14, 34). When its concentration in the extracellular medium increases and reaches a threshold, ComS* binds to the oligopeptide-binding protein AmiA3 and is internalized by the multicomponent oligopeptide transporter AmiCDEF. Inside the cell, ComS* activates the transcriptional regulator ComR. The ComRS complex in turn activates comX and comS transcription, resulting in an autoamplification loop (14). Homologs of the ComRS system were also found in all sequenced genomes of streptococci belonging to the mutans, pyogenic, and bovis groups (34). Furthermore, this system was shown to be functional in Streptococcus mutans as the most proximal regulatory system for comX activation and competence development (34).
Besides the transcriptional control of comX, the ComX protein was shown to be the target of posttranslational control in streptococci. In Streptococcus pneumoniae, the amount of ComX is positively regulated by ComW and negatively controlled by ClpEP and ClpCP. ComW is produced in the early competence phase to stabilize and activate ComX. ClpEP and ClpCP are protease machineries that degrade ComX and ComW, respectively (32, 36, 42, 46). In Streptococcus pyogenes, the mechanism of ComX stabilization/degradation is still unknown, except for the implication of the ClpP protease (38). For S. thermophilus, previous extensive in silico analyses did not allow the identification of a homolog of ComW, but during the course of that study, the ClpC ATPase subunit was shown to act as a negative regulator of competence development in this species (1). For Bacillus subtilis, the posttranslational control of the master regulator of competence development, ComK, has also been highlighted (25–27, 30, 36, 37, 40, 43, 45, 47–49). To prevent competence development under inappropriate conditions, ComK is sequestered by MecA, an adaptor protein which targets ComK to the ClpCP protease system (25, 40, 43, 47–49). ComK is released from the inhibitory complex by the small antiadaptor peptide ComS, which interacts directly with MecA and is produced when the cell density increases, leading to the activation of ComK transcription and of the late com genes (11, 12, 37, 40, 43). B. subtilis also contains a paralog of MecA, YpbH, which binds ClpC and affects both competence and sporulation by an unknown mechanism (39).
In silico analyses revealed the existence of a unique MecA-like protein in S. thermophilus. The aim of this study was to investigate its role in the control of competence development. We showed using reporter strains and microarray analyses that MecA represses the expression of a large set of late com genes under nonpermissive competence conditions. Importantly, the expression of early com genes, including comX, was not affected. In addition, we found evidence that MecA, together with ClpC, but not ClpE, exerts posttranslational control on the abundance of ComX. We propose that MecA may act as an anti-sigma factor for targeting ComX to the ClpCP degradation machinery, similarly to the posttranslational control of ComK in B. subtilis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli was grown with shaking at 37°C in Luria-Bertani (LB) broth (44). S. thermophilus strains were grown anaerobically (BBL GasPak systems; Becton Dickinson, Franklin Lakes, NJ) at 29°C, 37°C, or 42°C in M17 broth, Todd-Hewitt broth (THB) (Difco Laboratories Inc., Detroit, MI) or CDML (29). These media were supplemented with either 1% (wt/vol) glucose (M17 broth with glucose [M17G] and THB with glucose [THBG]) or 1% (wt/vol) lactose (CDML). Solid-agar plates were prepared by adding 2% (wt/vol) agar to the medium. When necessary, antibiotics were added to the media at the following concentrations: ampicillin at 250 μg ml−1 for E. coli, kanamycin at 50 μg ml−1 for E. coli, erythromycin at 250 μg ml−1 for E. coli and at 2.5 or 5 μg ml−1 for S. thermophilus, and chloramphenicol at 5 μg ml−1 and spectinomycin at 75 μg ml−1 for S. thermophilus.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| EC1000 | Kmr RepA+; MC1000 containing a copy of the repA gene of pWV01 in its chromosome | 28 |
| BTH101 | F′ cya-99 araD139 galE15 galK16 rpsL1hsdR2 mcrA1 mcrB1 | 23 |
| S. thermophilus | ||
| LMD-9 | Wild type | ATCC |
| CB003 | LMD-9 ΔcomX | 14 |
| CB001 | LMD-9 blpD-blpX::PcomX-luxAB | 14 |
| CB0011 | CB001 mecA::lox66-P32-cat-lox71 | This study |
| CB0012 | CB001 mecA::lox72 | This study |
| CB007 | LMD-9 blpD-blpX::PcomGA-luxAB | This study |
| CB0071 | CB007 mecA::lox66-P32-cat-lox71 | This study |
| CB0072 | CB007 mecA::lox72 | This study |
| CB0078 | CB007 ΔcomX | This study |
| CB0051 | LMD-9 mecA::lox66-P32-cat-lox71 | This study |
| CB0052 | LMD-9 mecA::lox72 | This study |
| CB0053 | LMD-9 comX::StrepTagII | This study |
| CB0054 | CB0011 comX::StrepTagII | This study |
| AW001 | LMD-9 clpC::spc | This study |
| AW002 | CB007 clpC::spc | This study |
| AW003 | LMD-9 clpE::spc | This study |
| AW004 | CB007 clpE::spc | This study |
| AW005 | CB0072 clpC::spc | This study |
| AW006 | CB0072 clpE::spc | This study |
| AW007 | CB0072 comX::P32-cat | This study |
| CB006 | LMG18311 blpD-blpX::PcomGA-luxAB | This study |
| CB0061 | CB006 mecA::lox66-P32-cat-lox71 | This study |
| Plasmids | ||
| pGICB001 | Emr TS; pJIM4900 derivative containing the PcomX-luxAB transcriptional fusion flanked by the upstream and downstream sequences of blpD and blpX, respectively | 14 |
| pGICB007 | Emr TS; pGICB001 derivative in which the PcomX-luxAB fusion was replaced by a PcomGA-luxAB fusion | This study |
| pGICB002 | Emr TS; pJIM4900 derivative containing the luxAB genes flanked by the upstream and downstream sequences of comX | 14 |
| pGICB003 | Emr TS; pJIM4900 derivative containing the upstream and downstream sequences of comX; this plasmid was used to delete comX | 14 |
| pUC18Cm | Apr Cmr; pUC18 derivative containing the P32-cat cassette from pGIZ850 | 19 |
| pGIBD001 | Emr Cmr TS; pGICB002 derivative for chromosomal exchange of comX by P32-cat | This study |
| pGIBD002 | Emr TS; pGICB002 derivative for chromosomal exchange of comX by comX::StrepTagII | This study |
| pXL | Emr; pTRKH2 derivative containing a PblpU-comXLMG18311 fusion | 2 |
| pGICB005 | Emr; pXL derivative in which comX has been replaced by mecA and which contains a PblpU-mecALMD-9 fusion | This study |
| pXLΔ1 | Emr; pXL derivative containing a deletion of comX | This study |
| pMG36eT | Emr; pMG36e derivative for constitutive expression under the control the P32 promoter | 16 |
| pMGXstrep | Emr; pMG36eT derivative containing a P32-comXLMD9::StrepTagII fusion | This study |
| pGhostcre | Emr TS; pG+host9 derivative containing a P1144-cre fusion | 16 |
| pUT18 | Apr; pUC19 derivative containing the T18 fragment of CyaA under the control of the Plac promoter for in-frame X-T18 fusions | 24 |
| pUT18C | Apr; pUC19 derivative containing the T18 fragment under the control of the Plac promoter for in-frame T18-X fusions | 24 |
| pKNT25 | Kmr; pSU40 derivative encoding the T25 fragment of CyaA under the control of the Plac promoter for in-frame X-T25 fusions | 22 |
| pKT25 | Kmr; pSU40 derivative encoding the T25 fragment of CyaA under the control of the Plac promoter for in-frame T25-X fusions | 24 |
Kmr, kanamycin resistance; Emr, erythromycin resistance; Apr, ampicillin resistance; Cmr chloramphenicol resistance; TS, thermosensitive RepA protein.
Detection of absorbance and luminescence.
Growth (optical density at 600 nm [OD600] and luciferase (Lux) activity (expressed in relative light units [RLU]) were monitored at 10-min intervals with a Varioskan Flash multimode reader (ThermoFisher Scientific, Zellic, Belgium) as previously described (14).
DNA techniques and electrotransformation.
For general molecular biology techniques, we followed methods reported previously by Sambrook et al. (44). The preparation of S. thermophilus chromosomal DNA and the electrotransformation of E. coli and S. thermophilus were performed as previously described (10, 13, 15). The primers used in this study were purchased from Eurogentec (Seraing, Belgium) and are listed in Table S1 in the supplemental material. PCRs were performed with Phusion high-fidelity DNA polymerase (Finnzymes, Espoo, Finland) with a GeneAmp 2400 PCR system (Applied Biosystems, Foster City, CA).
Construction of the PcomGA-luxAB reporter strains.
Reporter strains CB007 and CB006 were constructed by replacing part of the blp locus of strains LMD-9 and LMG18311, respectively, with the transcriptional fusion PcomGA-luxAB carried by plasmid pGICB007, as previously described (14). Strains CB007 and CB006 were confirmed by PCR with primers located upstream and downstream of the recombination regions (primers are listed in Table S1 in the supplemental material). Plasmid pGICB007 is a derivative of pGICB001 (14), where the comX expression signals (PcomX) were replaced with the comGA expression signals (PcomGA) amplified with primer pair DPGA1-DPGA2.
Construction of mecA deletion mutants.
LMD-9 derivative strains CB0011, CB0071, and CB0051 and LMG18311 derivative strain CB0061 were constructed by replacing the sequence between the start and stop codons of mecA with the chloramphenicol resistance cassette lox66-P32-cat-lox71 (mecA::lox66-P32-cat-lox71) according to procedures described previously by Fontaine et al. (16). The primers used to construct these strains are listed in Table S1 in the supplemental material. Strains CB0012, CB0072, and CB0052 were constructed by excising the chloramphenicol marker from strains CB0011, CB0071, and CB0051, respectively, using the Cre-loxP system. This procedure was performed by using plasmid pGhostcre as described previously (16).
Construction of comX deletion mutants.
Strain CB0078 (ΔcomX) was obtained by the double homologous recombination of plasmid pGICB003 in strain CB007, as previously described (14). Double mutant strain AW007 (mecA::lox72 comX::P32-cat) was obtained by the natural transformation of strain CB0072 (mecA::lox72) using plasmid pGIBD001 as the donor DNA. pGIBD001 is a derivative of pGICB002 (14), in which the luxAB genes have been replaced by a chloramphenicol resistance cassette (P32-cat). To obtain plasmid pGIBD001, plasmid pGICB002 was reverse amplified by PCR with primers pJUDdelcomX1 and pJUDdelcomX2, which hybridize upstream and downstream of luxAB, respectively, and then ligated to the P32-cat cassette obtained as a PvuII restriction fragment from pUC18Cm (19).
Construction of clpC and clpE deletion mutants.
LMD-9 derivative strains AW001 (ClpC−) and AW003 (ClpE−), CB007 derivative reporter strains AW002 (ClpC−) and AW004 (ClpE−), and CB0072 derivative reporter strains AW005 (MecA− ClpC−) and AW006 (MecA− ClpE−) were constructed by replacing the coding sequence of clpC or clpE with a spectinomycin resistance cassette (spc). This was achieved by natural competence (16) using PCR fragments amplified from chromosomal DNA extracted from the corresponding mutant strains of S. thermophilus LMG 18311 (a generous gift from L. S. Håvarstein) (1). The primers used are listed in Table S1 in the supplemental material.
Construction of comX::StrepTagII replacement strains.
LMD-9 and CB0072 derivative strains CB0053 and CB0054 (MecA−), respectively, were constructed by the chromosomal exchange of comX with a comX::StrepTagII fusion (purification affinity tag StrepTagII [IBA, Germany] fused at the C terminus of ComX) carried by plasmid pGIBD002, as previously described (14). Plasmid pGIBD002 is a derivative of pGICB002 (14), where the luxAB genes have been replaced by comX::StrepTagII. Plasmid pGIBD002 was obtained as follows. Plasmid pGICB002 was reverse amplified with primers pJUDdelcomX1 and pJUDdelcomX2. The open reading frame (ORF) of comX was amplified by PCR from the LMD-9 chromosome with primers IntcomXSTREP1 and IntcomXSTREP2 to generate the comX::StrepTagII fusion. Both PCR products were restricted by NcoI and HindIII, ligated, and then transformed into E. coli EC1000 cells.
Construction of the mecA complementation vector.
mecA expression plasmid pGICB005 contains a transcriptional fusion between the promoter PblpU and mecA. This plasmid was constructed as follows. Plasmid pXL was reverse amplified with primers pXLdeltacomX1 and pXLdeltacomX2, which hybridize the upstream and downstream regions of comX, respectively. The promoterless mecA gene was amplified by PCR from the LMD-9 chromosome with primers SURTRADMECA1 and SURTRADMECA2. Both PCR products were restricted by ApaI and XmaI, ligated, and then transformed into E. coli EC1000. The control plasmid (pXLΔ1) used in complementation experiments is a pXL derivative containing a comX deletion. This plasmid was constructed by the reverse amplification of pXL with primers DelcomX1 and DelcomX2. The resulting PCR fragment was restricted by NcoI, ligated, and transformed into E. coli EC1000. The induction of the promoter PblpU was achieved by the addition of 250 ng ml−1 D9C-30 synthetic peptide to the cultures at the beginning of growth, as reported previously (15).
Construction of the pMGXstrep expression vector.
In plasmid pMGXstrep, the comX::StrepTagII fusion is cloned under the control of the constitutive P32 promoter. The ORF of comX::StrepTagII was amplified by PCR from the CB0053 chromosome with primers MX1 and MX2. The PCR product was restricted by SalI and SmaI and then cloned into pMG36eT (16) digested by the same restriction enzymes.
Detection of ComX-StrepTagII by Western analysis.
Plasmid pMGXstrep was electroporated into wild-type (WT) strain LMD-9 and its derivative strains deficient in MecA, ClpC, and ClpE (CB0051, AW001, and AW003, respectively). Lactococcus lactis overproducing ComX-StrepTagII (A. Wahl, unpublished data) and WT LMD-9 carrying plasmid pMGX (producing ComX without StrepTagII) (16) were used as positive and negative controls, respectively. These strains were grown overnight in THBG containing 5 μg ml−1 erythromycin at 37°C. The OD600 was then adjusted to 0.05 in THBG, and cells were grown until they reached an OD600 of 0.4 at 37°C or 42°C. Cells from 50-ml cultures were resuspended in 1 ml lysis buffer (100 mM Tris [pH 8.0], 150 NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride [PMSF]) containing 1/5 (vol/vol) glass beads (G8893, ≤106 μm; Sigma). Bacterial cells were then lysed by using a Precellys24 homogenizer (three times for 30 s at 5,000 rpm). Eighty microliters of total cell extract was mixed with 20 μl of 5× Laemmli buffer and heated at 98°C for 10 min. Samples (25 μg of total protein) were loaded onto gradient 4 to 20% SDS-PAGE precast gels (Thermo-Scientific), separated by electrophoresis, and transferred onto a nitrocellulose membrane. ComX-StrepTagII was detected by using a mouse monoclonal antibody against StrepTagII (StrepMAB-Classic) as the primary antibody and a horseradish peroxidase-conjugated rabbit anti-mouse polyclonal antibody (pAb) as a secondary antibody, according to the manufacturer's instructions (IBA, Germany).
B2H plasmid construction and bacterial two-hybrid assay.
Methods used for bacterial two-hybrid (B2H) plasmid construction and the bacterial two-hybrid assay were described previously by Karimova et al. (22–24). The coding sequences of comX (ster_0189), mecA (ster_0216), clpC (ster_0109), and clpE (ster_0648) were amplified by PCR from S. thermophilus LMD-9 genomic DNA by using primers reported in Table S1 in the supplemental material and inserted into plasmids pUT18, pUT18C, pKT25, and pKNT25. The full list of constructed plasmids for B2H assays is shown in Table S1 in the supplemental material. To perform a two-hybrid assay, a combination of two recombinant plasmids was electrotransformed into E. coli BTH101 (cya-99). Six clones from each combination were tested on indicator MacConkey (Difco) agar plates containing kanamycin and ampicillin and supplemented with 1% (wt/vol) maltose and 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside). Plates were incubated at 30°C for 36 h.
RNA extraction.
For transcriptome comparisons between the LMD-9 ΔmecA:: lox72 mutant (CB052) and WT LMD-9, strains were grown during 16 h at 37°C in THBG. For the comparison between WT LMD-9 and the LMD-9 ΔcomX mutant (CB003), strains were grown at 37°C during 16 h in M17G, washed twice (5,000 × g for 9 min at room temperature) in 1 volume of CDML, and resuspended in 1 volume of CDML. Cultures were then diluted 30-fold in their respective media (either THBG or CDML). When cells reached the mid-log phase (OD600 of 0.4), 25-ml aliquots were collected. Cells were harvested by centrifugation (7,000 × g for 4 min) and mechanically broken with 0.18-mm-diameter glass beads in a Braun homogenizer (three 1-min periods of homogenization with 1-min intervals on ice). Total RNA was extracted by using the High Pure RNA isolation kit (Roche, Basel, Switzerland).
Microarray experiments and data analysis.
The slide for S. thermophilus LMD-9 was a custom-designed Agilent Technologies oligonucleotide microarray containing eight 15K arrays (Gene Expression Omnibus [GEO] accession number GPL13365), and each coding sequence was represented by one to five 60-mer probes. Microarray experiments were performed as previously described (14). Gene expression analysis was carried out by using GeneSpring GX v11.0 software (Agilent Technologies). Data were filtered for outliers, negative and positive controls, and flagged signals as previously described (14). Probes from triplicates were filtered by a t test for significance at a threshold of a P value of <0.05. Significantly regulated probes were then defined based on a fold change (FC) higher than 1.5. Significantly regulated genes were defined as genes for which at least 50% of the probes were significantly regulated and with a mean absolute FC of total probes of at least 1.5. Significantly induced probes adjacent to an induced coding DNA sequence (CDS) were also retained if the FC of the total probes of the adjacent CDS was at least of 1.5.
Microarray data accession numbers.
The normalized transcriptome data have been deposited in the GEO database under accession numbers GSE28371 and GSE28372.
RESULTS
The genome of S. thermophilus contains a unique homolog of MecA from B. subtilis.
Five S. thermophilus genomes (strains LMD-9, LMG18311, CNRZ1066, ND03, and JIM8232) have been sequenced so far. In silico analyses of the 5 genomes identified a unique gene encoding a putative homolog of MecA from B. subtilis (MecABs) in each of them. The MecA-like protein of S. thermophilus (MecASt [Ster_0216]; 249 amino acids [aa]) is more closely related to MecABs (60.6% and 26.0% similarity and identity, respectively) than YpbH (53.5% and 20.4%) of B. subtilis (see Fig. S1 and S2 in the supplemental material). Among streptococci, MecA-like proteins were identified in all streptococcal groups, where the closest orthologs of MecASt were found in the genomes of Streptococcus vestibularis (94.8% identity), S. salivarius (94.0%), and S. gallolyticus (60.7%) (see Fig. S1 in the supplemental material). These in silico data are consistent with work reported previously by Persuh et al. (40), which showed that MecA was widespread among Gram-positive bacteria. Both MecA and YpbH are organized into two domains but display different lengths of the interdomain linker region (39, 40). In the ClpC-MecA-ComK complex, the N-terminal domain (NTD) was shown to interact with ComK, while the C-terminal domain (CTD) is the site of recognition of the ClpC ATPase subunit of the Clp machinery (35, 40, 43, 50). An alignment between a set of streptococcal MecA proteins including MecASt, MecABs, and YpbH revealed a high level of conservation in both the NTD and CTD, with the NTD being the most conserved, while the interdomain linker region exhibited a very low level of conservation and is longer in streptococcal proteins (see Fig. S2 in the supplemental material). Since MecASt shows a significant level of identity with MecABs and the same organization in two domains, we hypothesized that it could play a role in S. thermophilus similar to that found for B. subtilis, i.e., a repressor of competence development.
MecA is a repressor of the late comGA operon under conditions that are not permissive for competence development.
In the first set of experiments, the functional role of MecA from S. thermophilus was evaluated by growing cells in THBG medium. These growth conditions were shown previously to be nonpermissive for spontaneous competence development, since the level of comX expression is very low (1, 2, 16).
To assess the role of MecA in the regulation of early and late competence genes, the mecA open reading frame was replaced by a chloramphenicol resistance cassette (lox66-P32-cat-lox71) in LMD-9 reporter strains harboring either PcomX-luxAB or a PcomGA-luxAB inserted into the blp locus (strains CB0011 and CB0071, respectively). To avoid polar effects on adjacent genes, the chloramphenicol resistance marker was removed by using the Cre-loxP system as previously reported (16) (yielding strains CB0012 and CB0072, respectively). The promoter of the comX gene was chosen since it integrates all the signals originating from the early stage of competence, while the promoter of comGA controls one of the most important late com operons, which codes for a part of the DNA uptake machinery (7). All the constructed reporter strains were then grown in THBG medium in order to monitor the luciferase (Lux) activity. The specific Lux activities (RLU/OD600) of the control reporter strains (CB001 and CB007) were compared to those of their isogenic mecA::lox66-P32-cat-lox71 (CB0011 and CB0071) and mecA::lox72 (CB0012 and CB0072) mutant strains (Fig. 1 and Table 2). The inactivation of MecA had no impact on the expression of the PcomX-luxAB fusion (Fig. 1A). In contrast, the maximum Lux activities of the two MecA-deficient strains (CB0071 and CB0072) harboring the PcomGA-luxAB fusion were similarly increased more than 100-fold compared to that of the control strain (CB007) (Fig. 1B). To enlarge our investigation of the functional role of MecA, the corresponding gene was deleted in LMG18311 derivative strains harboring a PcomGA-luxAB fusion (CB006 and CB0061). Similarly to LMD-9, specific PcomGA activity was highly induced (200-fold) in the MecA-deficient strain (CB0061) (Table 2). To further support that the observed phenotype results from the mecA deletion solely, a mecA complementation plasmid (the pXL derivative pGICB005) was constructed and electrotransformed into MecA-deficient reporter strain CB0071. Plasmid pGICB005 fully restored PcomGA activity to a level similar to that of WT reporter strain CB007 (Table 2). In contrast, the control strain harboring the empty vector [CB0071(pXLΔ1)] showed a maximum Lux activity similar to that of the parental MecA-deficient strain (CB0071) (Table 2).
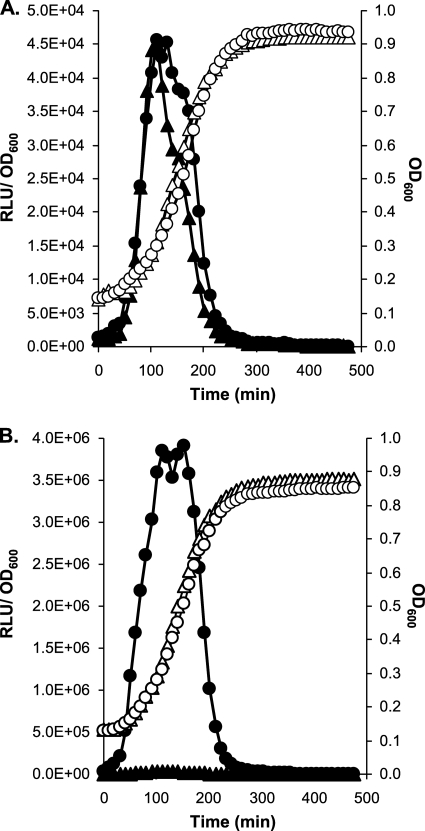
Fig 1.
Growth (OD600) and luciferase activities (RLU/OD600) of LMD-9 reporter strains and their isogenic MecA-deficient strains grown in THBG at 37°C. (A) Growth (white symbols) and specific luciferase activities (black symbols) of PcomX-luxAB fusions of strains CB001 (MecA+) (triangles) and CB0012 (MecA−) (circles). (B) Growth (white symbols) and specific luciferase activities (black symbols) of PcomGA-luxAB fusions of strains CB007 (MecA+) (triangles) and CB0072 (MecA−) (circles).
Table 2.
Maximum luciferase activities of S. thermophilus strains
| Strain | Promoter | Relevant feature(s)b | Mean max luciferase activity ± SEMa |
|
|---|---|---|---|---|
| THBG conditions (RLU/OD600 × 104) | CDML conditions (RLU/OD600 × 106) | |||
| LMD-9 derivatives | ||||
| CB007 | PcomGA | Wild type | 3.5 ± 1.0 | 14.6 ± 0.5 |
| CB0071 | PcomGA | MecA− (Cmr) | 353.6 ± 32.1 | 14.5 ± 0.3 |
| CB0072 | PcomGA | MecA− | 389.0 ± 77.3 | 16.7 ± 0.5 |
| CB0078 | PcomGA | ComX− | 1.6 ± 0.5 | NT |
| AW007 | PcomGA | MecA− ComX− | 1.7 ± 0.4 | NT |
| AW002 | PcomGA | ClpC− | 280.6 ± 26.1 | NT |
| AW004 | PcomGA | ClpE− | 4.2 ± 1.3 | NT |
| AW005 | PcomGA | MecA− ClpC− | 300.0 ± 33.5 | NT |
| AW006 | PcomGA | MecA− ClpE− | 318.0 ± 49.7 | NT |
| CB0071(pXLΔ1) | PcomGA | MecA−, empty vector | 337.1 ± 8.5 | NT |
| CB0071(pGICB005) | PcomGA | MecA−, complementation vector | 4.1 ± 0.6 | NT |
| CB001 | PcomX | Wild type | 6.3 ± 0.6 | 33.1 ± 2.0 |
| CB0011 | PcomX | MecA− (Cmr) | 6.7 ± 0.7 | 28.6 ± 5.9 |
| CB0012 | PcomX | MecA− | 8.0 ± 0.5 | 16.2 ± 2.7 |
| LMG18311 derivatives | ||||
| CB006 | PcomGA | Wild type | 0.2 ± 0.02 | 0.2 ± 0.04 |
| CB0061 | PcomGA | MecA− (Cmr) | 39.2 ± 1.6 | 5.3 ± 0.4 |
Mean values from triplicate experiments ± SEM. NT, not tested.
Cmr, chloramphenicol resistance resulting from the replacement of mecA with cat.
We next studied whether the repression activity exerted by MecA on PcomGA expression was dependent on the presence of a functional ComX protein. To this end, we compared the PcomGA-luxAB activities of a comX mutant strain (CB0078) to those of a comX mecA double mutant strain (AW007). The deletion of comX solely or in combination with mecA similarly decreased the level of activity of PcomGA compared to that of the WT reporter strain (Table 2).
Altogether, these results show that MecA is a negative regulator of a major late com operon in S. thermophilus. Notably, MecA exerts its control without affecting the transcriptional activity of comX. Moreover, the role of MecA as a repressor of late competence gene expression absolutely requires the presence of an intact ComX protein, strongly suggesting that MecA acts on the ComX protein to achieve its regulatory function.
MecA is not a repressor of the comGA operon under permissive competence conditions.
In a second set of experiments, the functional role of MecA from LMD-9 and LMG18311 strains was evaluated by growing cells in CDML medium. These growth conditions were shown previously to be permissive for spontaneous competence development at high and low levels for LMD-9 and LMG18311, respectively (16).
LMD-9 and LMG18311 PcomGA-luxAB reporter strains were grown in CDML, and PcomGA activity was measured (Table 2). For LMD-9, the maximum Lux activities were similar between the two MecA-deficient strains (CB0071 and CB0072) and the control (CB007). In contrast, the MecA-deficient strain of LMG18311 (CB0061) displayed a 27-fold-higher level of Lux activity (Table 2).
These data strongly suggest that when the level of competence induction reached a certain threshold, such as that which was observed for strain LMD-9 under CDML conditions, the depletion of MecA has no impact. In contrast, MecA could still act as a repressor in LMG18311, which is poorly competent under these conditions. These results suggest that when the transcription of comX is strongly activated by the ComRS system, the latter represents the dominant mechanism for the control of competence development.
The inactivation of MecA partially activates the late com regulon.
To further investigate the functional role of MecA, we decided to evaluate the overlap between the late com regulon controlled by ComX and the MecA regulon in S. thermophilus LMD-9 using microarrays.
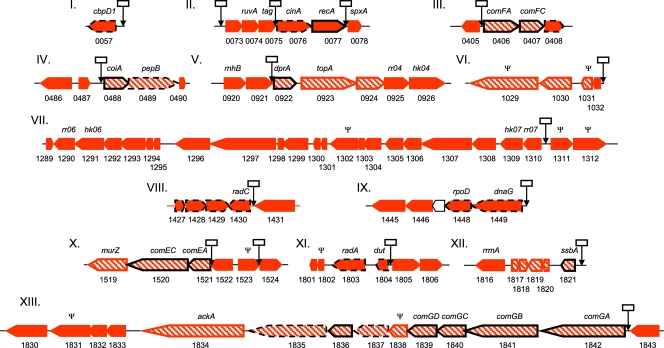
In order to determine the late com regulon, we compared the transcriptomes of WT LMD-9 and its isogenic comX mutant strain (ComX−) (CB003) under natural comX-inducing conditions (CDML). Microarray experiments were performed as described in Materials and Methods. RNAs were extracted when cultures reached the mid-log phase, when the maximum PcomGA activity of reporter strain CB007 was measured (data not shown). We identified 135 genes that were upregulated (FC ≥ 1.5; P < 0.05) in the wild type compared to the ComX-deficient strain (see Table S2 in the supplemental material). From this gene list, 93 genes were distributed into 13 clusters that include at least one predicted ComX box (therefore defined here as the ComX-regulated loci). All late com genes that were identified previously as being essential for competence development in S. pneumoniae (41) were harbored in the ComX-regulated loci, except pilD, giving confidence in the extent of the regulon that was established (Fig. 2, and see Table S3 in the supplemental material).
Fig 2.
Organization of the ComX-regulated loci in S. thermophilus LMD9. The selected set of upregulated genes (red and red-hatched pentagons) is based on the microarray experiment performed on WT LMD-9 versus the LMD-9 comX mutant (loci I to XIII) (see Table S2 in the supplemental material). These genes are organized into 13 loci on the basis of the presence of predicted ComX boxes (arrows with white rectangles) (see Table S3 in the supplemental material). Shared upregulated genes resulting from mecA inactivation and obtained from the microarray experiment with CB0052 (mecA::lox72) versus WT LMD-9 (Table 3) are shown as red-hatched pentagons. Pentagons surrounded by a dotted line and a black line correspond to late and essential late com genes in S. pneumoniae TIGR4, respectively (41). The ORF orientation in each locus is indicated by the direction of the pentagons. The number below each pentagon corresponds to the STER locus tag of LMD-9. Ψ, pseudogene.
In order to identify the set of MecA-regulated genes, the transcriptomes of the LMD-9 mecA::lox72 mutant (CB0052) and WT LMD-9 were compared under THBG growth conditions (mid-log phase). As expected, mecA was included in the list of downregulated genes (FC = 67.7) (see Table S4 in the supplemental material). We also noticed that comX expression was not altered, confirming the results obtained with PcomX-luxAB reporter strain CB001. Moreover, none of the early com genes previously identified were up- or downregulated (14) (Table 3, and see Table S4 in the supplemental material). The set of upregulated genes with an FC of ≥1.5 (P < 0.05) consists of 47 genes, including 27 late com genes and 25 genes distributed in ComX-regulated loci (Table 3 and Fig. 2, and see Table S2 in the supplemental material). Among ComX-regulated loci, the gene cluster (ster_1834 to ster_1842) that includes comGA (FC = 46.7) is the cluster most strongly induced by the inactivation of MecA. All late com genes that were identified as being essential for competence development in S. pneumoniae, except pilD and recA, are members of the MecA regulon. In addition, 22 upregulated genes are unique to MecA inactivation.
Table 3.
Genes upregulated in the LMD-9 MecA− strain compared to WT strain LMD-9 grown under THBG conditions
| Category and locus tag in LMD-9 | Gene | Descriptione | Strand | FCa |
Late com gene in S. pneumoniaeb | Presence of: |
||
|---|---|---|---|---|---|---|---|---|
| MecA− strain | ComX− strain | Essential com in S. pneumoniaec | ComX boxd | |||||
| MecA-regulated genes shared with the late com regulon | ||||||||
| STER_0406 | comFA | Late competence protein required for DNA uptake | + | 1.6 | 7.2 | SP_2208 | Y | Y |
| STER_0407 | comFC | Late competence protein | + | 1.9 | 7.6 | SP_2207 | Y | |
| STER_0488 | coiA | Competence protein, transcription factor | + | 27.0 | 42.0 | SP_0978 | Y | Y |
| STER_0489 | pepB | Oligopeptidase | + | 2.4 | 18.9 | SP_0979 | ||
| STER_0922 | dprA | DNA-processing protein, Smf family | + | 31.3 | 72.2 | SP_1266 | Y | Y |
| STER_0923 | topA | DNA topoisomerase I | + | 2.6 | 29.9 | |||
| STER_1029 | Pseudo | 1.8 | 11.3 | |||||
| STER_1030 | Hypothetical protein | − | 1.8 | 13.4 | ||||
| STER_1031 | Pseudo | 1.6 | 8.7 | |||||
| STER_1519 | murZ | UDP-N-Acetylglucosamine 1-carboxyvinyltransferase | − | 2.0 | 6.8 | |||
| STER_1520 | comEC | Late competence protein required for DNA uptake | − | 22.1 | 23.2 | SP_0955 | Y | |
| STER_1521 | comEA | Late competence protein required for DNA binding and uptake | − | 15.1 | 10.7 | SP_0954 | Y | Y |
| STER_1818 | Hypothetical protein | + | 1.5 | 7.1 | ||||
| STER_1819 | Cytidine/deoxycytidylate deaminase family protein, putative | − | 1.6 | 16.3 | ||||
| STER_1820 | Hypothetical protein | − | 2.3 | 21.1 | ||||
| STER_1821 | ssbA | Single-strand DNA-binding protein | − | 1.7 | 23.3 | SP_1908 | Y | Y |
| STER_1834 | ackA | Acetate kinase | − | 3.4 | 25.9 | |||
| STER_1835 | Hypothetical protein | − | 8.4 | 72.6 | SP_2045 | |||
| STER_1836 | Hypothetical protein | − | 62.0 | 181.4 | SP_2047 | Y | ||
| STER_1837 | Competence protein, putative | − | 67.2 | 163.2 | SP_2048 | |||
| STER_1838 | Pseudo | 77.0 | 190.4 | |||||
| STER_1839 | comGD | Competence protein | − | 72.8 | 151.3 | SP_2050 | Y | |
| STER_1840 | comGC | Late competence protein, exogenous DNA-binding protein | − | 83.5 | 203.3 | SP_2051 | Y | |
| STER_1841 | comGB | Late competence protein, ABC transporter subunit | − | 77.4 | 147.3 | SP_2052 | Y | |
| STER_1842 | comGA | Late competence protein, ABC transporter subunit | − | 46.7 | 50.0 | SP_2053 | Y | Y |
| STER_1677 | ABC transporter, permease protein | − | 1.8 | 2.8 | ||||
| STER_1678 | ABC-type multidrug transport system, ATPase component | − | 1.8 | 2.7 | ||||
| Unique MecA-regulated genes | ||||||||
| STER_0596 | Pyridine nucleotide-disulfide oxidoreductase | + | 1.9 | <1.5 | ||||
| STER_0588 | Hypothetical protein | + | 2.3 | <1.5 | ||||
| STER_0870 | ptpS | 6-Pyruvoyl tetrahydrobiopterin synthase | + | 1.5 | <1.5 | |||
| STER_0871 | Putative coenzyme PQQ synthesis protein | + | 1.6 | <1.5 | ||||
| STER_0872 | 7-Cyano-7-deazaguanine reductase | + | 1.6 | <1.5 | ||||
| STER_1022 | High-affinity Fe2+/Pb2+ permease | − | 4.4 | <1.5 | ||||
| STER_1023 | Hypothetical protein | − | 4.4 | <1.5 | ||||
| STER_1024 | Hypothetical protein | − | 5.3 | <1.5 | ||||
| STER_1025 | ABC-type Fe3+-hydroxamate transport system, periplasmic component | − | 7.3 | <1.5 | ||||
| STER_1026 | fatA | ABC-type spermidine/putrescine transport system, ATPase component | − | 7.7 | <1.5 | |||
| STER_1027 | fatC | ABC-type Fe3+-siderophore transport system, permease component | − | 7.4 | <1.5 | |||
| STER_1028 | fatD | ABC-type Fe3+-siderophore transport system, permease component | − | 6.8 | <1.5 | |||
| STER_1257 | ldh | l-Lactate dehydrogenase | + | 1.8 | <1.5 | |||
| STER_1601 | Hypothetical protein | + | 1.9 | <1.5 | ||||
| STER_1602 | Pseudo | 1.8 | <1.5 | |||||
| STER_1639 | Hypothetical protein | − | 1.5 | <1.5 | ||||
| STER_1683 | Hypothetical protein | − | 3.4 | <1.5 | ||||
| STER_1684 | Response regulator | − | 3.6 | <1.5 | ||||
| STER_1709 | scrK | Fructokinase | − | 1.6 | <1.5 | |||
| STER_1710 | scrA | Sucrose PTS, enzyme IIBCA | − | 2.0 | <1.5 | |||
Mean absolute fold change (FC) with a cutoff value of ≥1.5 and a P value of <0.05 for the LMD-9 MecA− strain (WT versus CB0052) and the LMD-9 ComX− strain (WT versus CB003).
Late com genes identified in S. pneumoniae TGIR4 (41). Orthologous genes with an identity of >40% were identified by BlastP.
Late com genes identified in S. pneumoniae TGIR4 as being essential for competence (41). Y, presence of an orthologous gene in LMD-9.
Presence (Y) of a conserved ComX box ([T/A]NCGAAT[A/G]) in the upstream intergenic region.
Pseudo, pseudogene; PQQ, pyrroloquimoline quinone; PTS, phosphotransferase system.
Taken together, these microarray results show that the absence of MecA activates to some extent the late com regulon without affecting the early stage of competence.
ClpC and MecA display similar repressing effects on the expression of the late comGA operon.
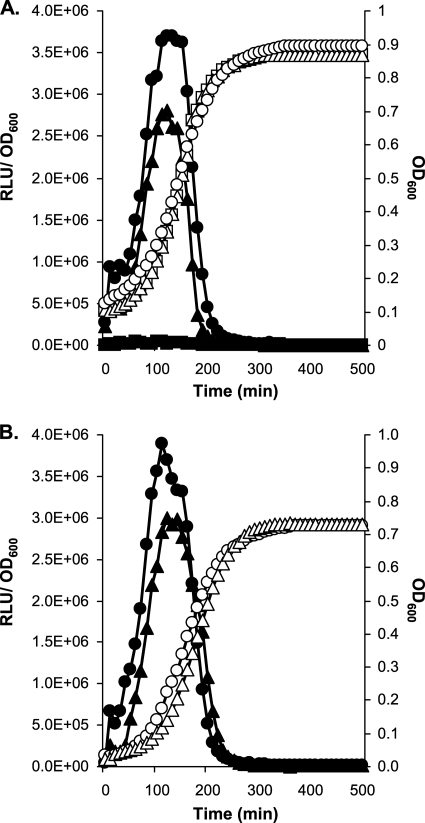
For S. thermophilus LMG18311, both ClpC and ClpE were recently shown to repress the activity of a late com promoter (1). Furthermore, the depletion of ClpC in this strain had no impact on the activity of PcomX, but the abundance of ComX was shown previously to be increased in the clpC mutant (1). In order to compare the effects of the deprivation of MecA, ClpC, and ClpE on the activity of PcomGA in LMD-9, a range of reporter strains (CB007 derivatives) were constructed, and their Lux activities were compared under conditions not permissive for competence (THBG). Both MecA- and ClpC-deficient strains (strains CB0072 and AW002, respectively) showed similar maximum specific Lux activities and similar kinetics of activation of the PcomGA-luxAB fusion (Fig. 3A and Table 2). In contrast, the inactivation of ClpE in strain LMD-9 (AW004) had nearly no impact on the expression of the PcomGA-luxAB fusion (Fig. 3A and Table 2). In order to evaluate the synergetic or cumulative effects of the inactivation of MecA and Clp, double MecA-ClpC- and MecA-ClpE-deficient reporter strains were constructed (AW005 and AW006, respectively). Notably, both double mutants showed behaviors similar to those of the single clpC and mecA mutants (Fig. 3B and Table 2).
Fig 3.
Growth (OD600) and luciferase activities (RLU/OD600) of LMD-9 reporter strains deficient for MecA, ClpC, ClpE, and MecA-ClpC grown in THBG at 37°C. (A) Growth (white symbols) and specific luciferase activities (black symbols) of PcomGA-luxAB fusions of strains CB0072 (MecA−) (circles), AW002 (ClpC−) (triangles), and AW004 (ClpE−) (squares). (B) Growth (white symbols) and specific luciferase activities (black symbols) of PcomGA-luxAB fusions of strains CB0072 (MecA−) (circles) and AW005 (MecA−-ClpC−) (triangles).
These genetic results strongly suggest that MecA and ClpC, but not ClpE, are involved in the same regulatory circuit for the transcriptional control of the comGA operon in strain LMD-9.
MecA and ClpC negatively affect ComX accumulation in vivo.
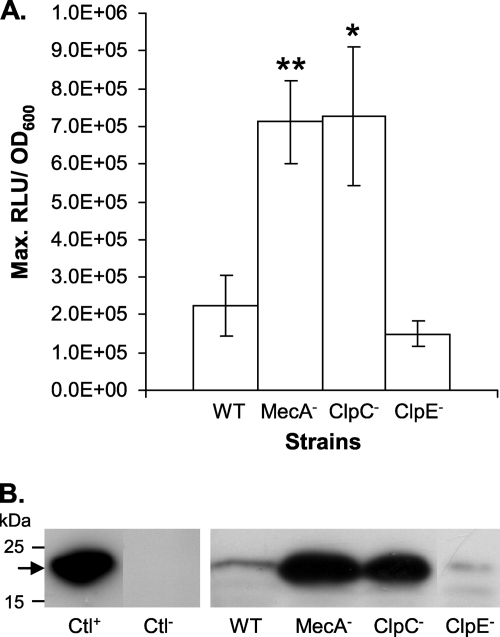
Analogously to the role played by MecA and ClpC in B. subtilis, the results collected so far were in favor of a direct control of the abundance of ComX by the MecA and ClpC proteins. In the first approach to support this hypothesis, we tagged the chromosomal copy of ComX with StrepTagII fused at its C terminus in order to monitor its abundance using anti-StrepTagII antibodies in Western blotting experiments. Although ComX-StrepTagII was not altered in its ability to develop competence, we were unable to detect the fusion protein in the wild type and its isogenic MecA-deficient strain (CB0053 and CB0054) under conditions not permissive for competence (THBG) (data not shown). This suggests that the abundance of ComX under these conditions remains very low, even when MecA is inactivated. To circumvent this problem, the comX::StrepTagII fusion was constitutively expressed under the control of the P32 promoter on a multicopy plasmid (pMGXstrep). pMGXstrep was then transferred to PcomGA reporter strain CB007 and its derivatives deficient for MecA (CB0072), ClpC (AW002), and ClpE (AW004). At 37°C, the maximum specific Lux activities were similar between the WT and mutant strains (data not shown). Similarly to the situation with LMD-9 growing in CDML, it is probable that the level of ComX-StrepTagII production is too high under these conditions to observe the negative effect of MecA and ClpC on PcomGA expression. As expected, Western blotting experiments showed that the abundance of ComX-StrepTagII remained unchanged between all these strains (data not shown). For Gram-positive bacteria, the level of the ClpCP machinery was shown previously to be increased in response to high temperatures (6, 9). Therefore, the same experiments were reproduced at 42°C instead of at 37°C. In this experimental setup, the levels of specific Lux activities of the MecA- and ClpC-deficient strains were ∼4-fold increased compared to those of their isogenic parental strain (CB007), while the inactivation of ClpE had no effect (Fig. 4A). Consistently, Western blotting experiments showed that the abundance of the ComX-StrepTagII protein was similarly increased when MecA or ClpC was inactivated, compared to the wild-type and ClpE-defective strains (Fig. 4B).
Fig 4.
Luciferase activity (RLU/OD600) and detection of ComX-StrepTagII by Western blotting of WT strain LMD-9 and strains deficient for MecA, ClpC, and ClpE expressing comX::streptagII constitutively from pMGXstrep and grown in THBG at 42°C (OD600 = ∼0.4). (A) Maximum specific luciferase activities of PcomGA-luxAB fusions of strains CB007 (WT), CB0072 (MecA−), AW002 (ClpC−), and AW004 (ClpE−). Shown are mean values from triplicate experiments ± standard errors of the means (SEM). Significance between WT and mutant strains is based on a t test. ∗∗, P value of <0.01; ∗, P value of <0.05. (B) Detection of ComX-StrepTagII in strains LMD-9 (WT), CB0052 (MecA−), AW001 (ClpC−), and AW003 (ClpE−). Lactococcus lactis overproducing ComX-StrepTagII and WT LMD-9 producing ComX without StrepTagII were used as a positive control (Ctl+) and a negative control (Ctl−), respectively.
These data show that MecA and ClpC, but not ClpE, negatively affect the abundance of ComX in vivo in S. thermophilus LMD-9, confirming the above-described expression results obtained with the reporter strains.
MecA interacts with both ComX and ClpC in bacterial two-hybrid assays.
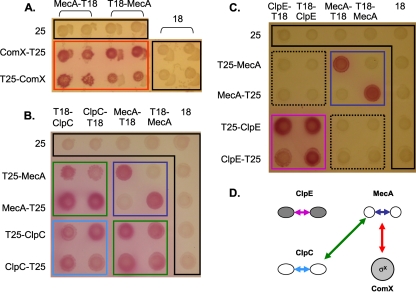
To obtain the first evidence of direct physical interactions between the MecA, ComX, and Clp machineries, a range of bacterial two-hybrid (B2H) system experiments was set up. In the B2H system, the T25 and T18 fragments of the Bordetella pertussis adenylate cyclase are used as fusion partners. When the fusion proteins interact with each other, a functional complementation between the T25 and T18 fragments takes place, resulting in adenylate cyclase activity and cyclic AMP (cAMP) production, which turns on the transcription of the lac operon (23).
Plasmids allowing the production of all possible combinations of N and C fusion proteins between T18 and T25 and full-length MecA and ComX were constructed. The production of adenylate cyclase from transformants of the cya mutant of E. coli (BTH101) was detected on MacConkey agar plates. As shown in Fig. 5, strain BTH101, producing the following pairs of fusion proteins, formed red colonies on MacConkey indicator plates: T18-MecA and T25-ComX, T18-MecA and ComX-T25, MecA-T18 and T25-ComX, and MecA-T18 and ComX-T25 (Fig. 5A, red rectangle). In contrast, the negative-control strains (T18 and T25, T18-MecA and T25, MecA-T18 and T25, T18 and T25-ComX, and T18 and ComX-T25) remained white on the indicator plates (Fig. 5A, black rectangles). The T18 fusions with ComX carried by high-copy-number plasmid pUT18 were excluded due either to their activation in the negative-control strains, possibly by aggregation with the partnerless T25, or to not-reproducible results (data not shown). Concerning MecA self-interactions, positive signals were observed for the following two pairs of fusions proteins: MecA-T18 and T25-MecA, and T18-MecA and MecA-T25 (Fig. 5B and C, dark blue squares). Therefore, only two out of four possible combinations of MecA fusion partners gave positive signals. This finding may be explained by the fact that both the NTD and CTD of MecA are involved in the oligomerization process in B. subtilis, and therefore, fusion partners could affect the self-interaction (40). Next, all combinations of fusions proteins between MecA and ClpC or MecA and ClpE were tested in the B2H system as described above (Fig. 5B and C). All MecA and ClpC fusion proteins gave positive interaction signals with each other (Fig. 5B, green squares), while all MecA and ClpE fusions proteins were negative with each other (Fig. 5C, dotted black squares). Both ClpC and ClpE showed strong self-interactions, as expected, and all the control strains remained negative (Fig. 5B, light blue square, and C, magenta square, respectively).
Fig 5.
Interactions between MecA and ComX, ClpC, or ClpE evaluated by B2H assays. (A to C) Matrices of bacterial two-hybrid interactions between MecA and ComX (A), MecA and ClpC (B), and MecA and ClpE (C) on MacConkey indicator plates supplemented with 1% maltose. Plates were incubated at 30°C for 36 h. Red, green, and dotted black rectangles indicate MecA-ComX, MecA-ClpC, and MecA-ClpE interaction assays, respectively. Dark blue, light blue, and magenta rectangles indicate MecA, ClpC, and ClpE self-interaction assays, respectively. Controls are surrounded by a black line; 25 and 18 correspond to the empty vectors pKT25 and pUT18, respectively. (D) Summary of interactions detected by B2H assays suggesting a ternary complex, ClpC-MecA-ComX. The arrow color code refers to the interaction assays described above for panels A, B, and C.
Altogether, these B2H results were the first indication that MecA could interact in vivo with ComX and ClpC to form a ternary complex (Fig. 5D).
DISCUSSION
Although previous studies unraveled the regulatory cascade leading to the transcriptional activation of comX, encoding the master regulator of competence development in S. thermophilus, the posttranslational control of ComX activity in this species remained nearly unexplored. In this work, we show by a genetic approach that the adaptor protein MecA acts as a negative regulator of competence development by a mechanism that requires the presence of ComX. Using luciferase reporter strains and transcriptome comparisons, we demonstrate that the inactivation of MecA has no effect on the expression of early com genes such as comX but largely affects the expression of late com genes. However, the inactivation of mecA is insufficient to activate natural transformation under these conditions (data not shown), while the overexpression of comX led to a low but detectable transformation rate (16). Similarly, no improvement in the transformation rate of a ClpC-deficient strain of LMG18311 grown under nonpermissive THBG conditions was reported (1). Various hypotheses could explain this apparent discrepancy, such as the absence of an induction of one or more key late com genes that could be required for natural transformation or an indirect effect of the inactivation of MecA that could negatively alter the cell status for natural transformation.
Similarly to the role played by MecA in B. subtilis, our results strongly suggest that MecA of S. thermophilus interacts with ComX to target ComX to the ClpCP machinery for its degradation. This hypothesis is supported by the following results: (i) MecA, ClpC, and combined MecA-ClpC deprivations release the transcriptional control of the late comGA operon with a similar response and without cumulative effects; (ii) the inactivation of MecA or ClpC showed a similar in vivo accumulation of the ComX protein; and (iii) B2H experiments showed that MecA interacts separately with ComX and ClpC, suggesting the formation of a ternary complex, ClpC-MecA-σX, analogous to the ClpC-MecA-ComK complex of B. subtilis.
In a recent study, Biornstad and Havarstein reported that both ClpC and ClpE act as negative regulators of the activity of a late com promoter in S. thermophilus LMG18311 (1). The implication of ClpC in the posttranslational control of ComX was clearly demonstrated, while the role of ClpE in competence development was not further investigated (1). Concerning the role of ClpE, our results based on reporter strains, Western blotting experiments, and B2H assays show that ClpE does not play a significant role in the control of competence development in strain LMD-9 of S. thermophilus. In S. pneumoniae, the posttranslational control of ComX involves mainly ComW and the ClpEP degradation machinery. The role of ComW as a possible adaptor protein for ComX stabilization or as an antiadaptor protein has yet to be clarified (42). Concerning ClpC, it seems to play an indirect role in the stabilization of ComX by degrading ComW (42). Interestingly, a MecA homolog is present in S. pneumoniae, but its implication in the posttranslational control of ComX has never been reported. Altogether, these results support the hypothesis that ClpC and ClpE independently interact with different actors to control the abundance of ComX in streptococci.
Remarkably, a similar mechanism for the posttranslational control of the master regulator of competence development involving both MecA and ClpC seems to be conserved between relatively distant species, such as B. subtilis and S. thermophilus. This reinforces the importance of a strict control of competence development that has been positively selected through evolution. In the case of S. thermophilus, the MecA adaptor protein seems to act as an anti-sigma factor to control competence development. The implication of an adaptor protein targeting a sigma factor to the Clp machinery was reported previously in the case of the RssB adaptor protein, which specifically interacts with σS for its targeting to the ClpXP machinery, thereby controlling the master regulator of the stationary phase and the general stress response in E. coli (4, 5). Other adaptor proteins were also shown previously to play the role of anti-sigma factors, such as RsiW in the control of σW during alkaline stress or SpoIIAA/SpoIIAB in the control of σH during the sporulation of B. subtilis (17).
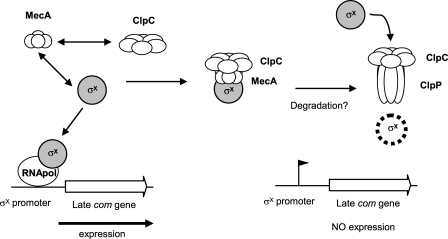
Based on the results presented in this study and current knowledge of the functional role of MecA in B. subtilis (48), we propose the following model for the posttranslational control of ComX in S. thermophilus (Fig. 6). Under inappropriate growth conditions, ComX is produced at a low level but sequestered by the anti-sigma factor MecA in a ternary complex with the ATPase subunit ClpC, which itself binds to the serine protease subunit ClpP, resulting in ComX degradation. In contrast, the negative control exerted by MecA would be bypassed when conditions are suitable for competence development, possibly via MecA saturation, due to a high level of accumulation of ComX. Consequently, ComX (σX) would be free to associate with the core RNA polymerase and specifically recognize the Com box in front of the late com genes to activate their transcription. In order to strengthen this model, future work will be dedicated to confirming the interactions between the different partners of the ternary complex and to investigating ComX degradation by ClpCP using in vitro studies.
Fig 6.
Model of the role of MecA in competence regulation of S. thermophilus. The alternative sigma factor σX, encoded by comX, is recognized and sequestrated by MecA, which also binds the ClpC ATPase subunit of the Clp machinery. This ternary complex interacts with the serine protease ClpP, leading to the degradation of σX by ClpCP, resulting in a small amount of free σX, which is insufficient to induce the transcription of late com genes. However, when conditions are appropriate for competence development, σX accumulates above a critical threshold, allowing its association with the core RNA polymerase for the specific transcription of late com genes.
Supplementary Material
ACKNOWLEDGMENTS
This research was carried out with financial support from FNRS.
We are grateful to E. Bouveret for helpful discussions on the use of the B2H system. We warmly thank L. S. Håvarstein for providing plasmid pXL and chromosomal DNA from Clp mutants. We are grateful to M. Wels for his help in the design of custom Agilent microarrays of S. thermophilus LMD-9.
C.B. held a doctoral fellowship from FRIA. L.F. and A.W. are postdoctoral researchers at FNRS. P.H. is research associate at FNRS.
Footnotes
Published ahead of print 27 January 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Biornstad TJ, Havarstein LS. 2011. ClpC acts as a negative regulator of competence in Streptococcus thermophilus. Microbiology 157:1676–1684 [DOI] [PubMed] [Google Scholar]
- 2.Blomqvist T, Steinmoen H, Havarstein LS. 2006. Natural genetic transformation: a novel tool for efficient genetic engineering of the dairy bacterium Streptococcus thermophilus. Appl. Environ. Microbiol. 72:6751–6756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolotin A, et al. 2004. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat. Biotechnol. 22:1554–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bougdour A, Cunning C, Baptiste PJ, Elliott T, Gottesman S. 2008. Multiple pathways for regulation of sigmaS (RpoS) stability in Escherichia coli via the action of multiple anti-adaptors. Mol. Microbiol. 68:298–313 [DOI] [PubMed] [Google Scholar]
- 5.Bougdour A, Wickner S, Gottesman S. 2006. Modulating RssB activity: IraP, a novel regulator of sigma(S) stability in Escherichia coli. Genes Dev. 20:884–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chastanet A, Prudhomme M, Claverys JP, Msadek T. 2001. Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J. Bacteriol. 183:7295–7307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claverys JP, Martin B, Polard P. 2009. The genetic transformation machinery: composition, localization, and mechanism. FEMS Microbiol. Rev. 33:643–656 [DOI] [PubMed] [Google Scholar]
- 8.Claverys JP, Prudhomme M, Martin B. 2006. Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu. Rev. Microbiol. 60:451–475 [DOI] [PubMed] [Google Scholar]
- 9.Derre I, Rapoport G, Msadek T. 1999. CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria. Mol. Microbiol. 31:117–131 [DOI] [PubMed] [Google Scholar]
- 10.Dower WJ, Miller JF, Ragsdale CW. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127–6145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Souza C, Nakano MM, Frisby DL, Zuber P. 1995. Translation of the open reading frame encoded by comS, a gene of the srf operon, is necessary for the development of genetic competence, but not surfactin biosynthesis, in Bacillus subtilis. J. Bacteriol. 177:4144–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Souza C, Nakano MM, Zuber P. 1994. Identification of comS, a gene of the srfA operon that regulates the establishment of genetic competence in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 91:9397–9401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferain T, et al. 1996. Knockout of the two ldh genes has a major impact on peptidoglycan precursor synthesis in Lactobacillus plantarum. J. Bacteriol. 178:5431–5437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontaine L, et al. 2010. A novel pheromone quorum-sensing system controls the development of natural competence in Streptococcus thermophilus and Streptococcus salivarius. J. Bacteriol. 192:1444–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontaine L, et al. 2007. Quorum-sensing regulation of the production of Blp bacteriocins in Streptococcus thermophilus. J. Bacteriol. 189:7195–7205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontaine L, et al. 2010. Development of a versatile procedure based on natural transformation for marker-free targeted genetic modification in Streptococcus thermophilus. Appl. Environ. Microbiol. 76:7870–7877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frees D, Savijoki K, Varmanen P, Ingmer H. 2007. Clp ATPases and ClpP proteolytic complexes regulate vital biological processes in low GC, Gram-positive bacteria. Mol. Microbiol. 63:1285–1295 [DOI] [PubMed] [Google Scholar]
- 18.Gardan R, Besset C, Guillot A, Gitton C, Monnet V. 2009. The oligopeptide transport system is essential for the development of natural competence in Streptococcus thermophilus strain LMD-9. J. Bacteriol. 191:4647–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goffin P, Lorquet F, Kleerebezem M, Hols P. 2004. Major role of NAD-dependent lactate dehydrogenases in aerobic lactate utilization in Lactobacillus plantarum during early stationary phase. J. Bacteriol. 186:6661–6666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamoen LW, Venema G, Kuipers OP. 2003. Controlling competence in Bacillus subtilis: shared use of regulators. Microbiology 149:9–17 [DOI] [PubMed] [Google Scholar]
- 21.Hols P, et al. 2005. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol. Rev. 29:435–463 [DOI] [PubMed] [Google Scholar]
- 22.Karimova G, Dautin N, Ladant D. 2005. Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J. Bacteriol. 187:2233–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karimova G, Pidoux J, Ullmann A, Ladant D. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 95:5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karimova G, Ullmann A, Ladant D. 2001. Protein-protein interaction between Bacillus stearothermophilus tyrosyl-tRNA synthetase subdomains revealed by a bacterial two-hybrid system. J. Mol. Microbiol. Biotechnol. 3:73–82 [PubMed] [Google Scholar]
- 25.Kirstein J, et al. 2006. Adaptor protein controlled oligomerization activates the AAA+ protein ClpC. EMBO J. 25:1481–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong L, Dubnau D. 1994. Regulation of competence-specific gene expression by Mec-mediated protein-protein interaction in Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 91:5793–5797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong L, Siranosian KJ, Grossman AD, Dubnau D. 1993. Sequence and properties of mecA, a negative regulator of genetic competence in Bacillus subtilis. Mol. Microbiol. 9:365–373 [DOI] [PubMed] [Google Scholar]
- 28.Law J, et al. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011–7018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Letort C, Juillard V. 2001. Development of a minimal chemically-defined medium for the exponential growth of Streptococcus thermophilus. J. Appl. Microbiol. 91:1023–1029 [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Zuber P. 1998. A molecular switch controlling competence and motility: competence regulatory factors ComS, MecA, and ComK control sigmaD-dependent gene expression in Bacillus subtilis. J. Bacteriol. 180:4243–4251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo P, Li H, Morrison DA. 2003. ComX is a unique link between multiple quorum sensing outputs and competence in Streptococcus pneumoniae. Mol. Microbiol. 50:623–633 [DOI] [PubMed] [Google Scholar]
- 32.Luo P, Li H, Morrison DA. 2004. Identification of ComW as a new component in the regulation of genetic transformation in Streptococcus pneumoniae. Mol. Microbiol. 54:172–183 [DOI] [PubMed] [Google Scholar]
- 33.Luo P, Morrison DA. 2003. Transient association of an alternative sigma factor, ComX, with RNA polymerase during the period of competence for genetic transformation in Streptococcus pneumoniae. J. Bacteriol. 185:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mashburn-Warren L, Morrison DA, Federle MJ. 2010. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol. Microbiol. 78:589–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mei Z, et al. 2009. Molecular determinants of MecA as a degradation tag for the ClpCP protease. J. Biol. Chem. 284:34366–34375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Msadek T, et al. 1998. ClpP of Bacillus subtilis is required for competence development, motility, degradative enzyme synthesis, growth at high temperature and sporulation. Mol. Microbiol. 27:899–914 [DOI] [PubMed] [Google Scholar]
- 37.Ogura M, Liu L, Lacelle M, Nakano MM, Zuber P. 1999. Mutational analysis of ComS: evidence for the interaction of ComS and MecA in the regulation of competence development in Bacillus subtilis. Mol. Microbiol. 32:799–812 [DOI] [PubMed] [Google Scholar]
- 38.Opdyke JA, Scott Jr, Moran CP., Jr 2003. Expression of the secondary sigma factor sigmaX in Streptococcus pyogenes is restricted at two levels. J. Bacteriol. 185:4291–4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persuh M, Mandic-Mulec I, Dubnau D. 2002. A MecA paralog, YpbH, binds ClpC, affecting both competence and sporulation. J. Bacteriol. 184:2310–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Persuh M, Turgay K, Mandic-Mulec I, Dubnau D. 1999. The N- and C-terminal domains of MecA recognize different partners in the competence molecular switch. Mol. Microbiol. 33:886–894 [DOI] [PubMed] [Google Scholar]
- 41.Peterson SN, et al. 2004. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol. Microbiol. 51:1051–1070 [DOI] [PubMed] [Google Scholar]
- 42.Piotrowski A, Luo P, Morrison DA. 2009. Competence for genetic transformation in Streptococcus pneumoniae: termination of activity of the alternative sigma factor ComX is independent of proteolysis of ComX and ComW. J. Bacteriol. 191:3359–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prepiak P, Dubnau D. 2007. A peptide signal for adapter protein-mediated degradation by the AAA+ protease ClpCP. Mol. Cell 26:639–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 45.Schlothauer T, Mogk A, Dougan DA, Bukau B, Turgay K. 2003. MecA, an adaptor protein necessary for ClpC chaperone activity. Proc. Natl. Acad. Sci. U. S. A. 100:2306–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sung CK, Morrison DA. 2005. Two distinct functions of ComW in stabilization and activation of the alternative sigma factor ComX in Streptococcus pneumoniae. J. Bacteriol. 187:3052–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turgay K, Hahn J, Burghoorn J, Dubnau D. 1998. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 17:6730–6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turgay K, Hamoen LW, Venema G, Dubnau D. 1997. Biochemical characterization of a molecular switch involving the heat shock protein ClpC, which controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes Dev. 11:119–128 [DOI] [PubMed] [Google Scholar]
- 49.Turgay K, Persuh M, Hahn J, Dubnau D. 2001. Roles of the two ClpC ATP binding sites in the regulation of competence and the stress response. Mol. Microbiol. 42:717–727 [DOI] [PubMed] [Google Scholar]
- 50.Wang F, et al. 2009. Crystal structure of the MecA degradation tag. J. Biol. Chem. 284:34376–34381 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.