Abstract
Multiple sclerosis (MS) is considered to be an autoimmune disease with an unknown cause and with immune system dysregulation. Among environmental factors, viruses are most often connected with the etiology of MS. Human endogenous retroviruses (HERVs) constitute 5 to 8% of human genomic DNA and have been detected as transcripts and proteins in the central nervous system (CNS) and peripheral blood, frequently in the context of neuroinflammation. HERV-Fc1, which belongs to the HERV-H/F family, has received our attention largely because of the genetic association with MS. We studied the expression of a capsid (Gag) protein of HERV-H/F origin by flow cytometry in peripheral blood mononuclear cells (PBMCs) from healthy controls and from MS patients with nonactive or active disease. There was a significant increase in HERV-H/F Gag expression in CD4+ (P < 0.001) and CD8+ (P < 0.001) T lymphocytes and in monocytes (P = 0.0356) in PBMCs from MS patients with active disease. Furthermore, we have undertaken the first rigorous SYBR green-based absolute quantitative PCR (Q-PCR) evaluation approach to quantify extracellular HERV-Fc1 RNA viral loads in plasma from MS patients and healthy controls. We found a 4-fold increase in extracellular HERV-Fc1 RNA titers in patients with active MS compared with healthy controls (P < 0.001). These findings strengthen the link between HERV-Fc1 and the pathology of MS. The cause and biological consequences of these differential expression levels will be the subject of further investigation. HERV-Fc1 biology could be a compelling area for understanding the pathology of MS and possibly other autoimmune disorders.
INTRODUCTION
Multiple sclerosis (MS) is an inflammatory disease targeting the central nervous system (CNS), leading to demyelination, axon degeneration, and severe disability as the disease progresses. MS is the most common cause of acquired neurological disability among young adults. In Denmark, approximately 10,000 people suffer from MS, and 350 new cases are diagnosed every year (31). Twin studies have indicated that MS has a concordance rate of approximately 30% in monozygotic and 5% in dizygotic twins, suggesting a genetic component in MS susceptibility (18, 25). Epidemiological studies strongly suggest that although there is a genetic component in MS, environmental factors also play a significant role in the development of the disease (53). Therefore, MS is thought to develop in genetically susceptible individuals as a consequence of environmental exposures. Among environmental factors, viral infections are most often linked to the etiology of MS (23, 24). Predominantly, human herpesviruses (type 6A and -B [HHV-6A and -B]), varicella-zoster virus (VZV), Epstein-Barr virus (EBV), and human endogenous retroviruses (HERVs) are implicated as etiologic agents in MS (7, 12, 54, 56, 58). The concept that retroviruses contribute to MS originated in the realization that demyelinating diseases in animals were caused by such viruses (20, 47). Also, most cases of MS show episodic attacks with intervening periods of recovery, a pattern that has often been observed with retroviral infections (33, 59). Even though it is widely accepted that there is strong evidence for association of endogenous and exogenous components with the etiology of MS, extensive studies failed to incriminate a single endogenous or exogenous agent (32, 44). The casual relationship of any given agent to the etiology of MS is difficult to establish, as putative agents infect most humans long before the disease outcome.
HERVs are remnants of ancient retroviral infections and constitute up to 8% of the human genome. They are transmitted vertically through the germ line and are thus inherited by successive generations in a Mendelian manner. A large proportion of endogenous retroviral genes have lost their coding potential due to accumulation of mutations, frameshifts, and deletions.
Phylogenetic analyses of structural genes (gag, pol, and env) indicate that the HERV-Fc family is closely related to the HERV-H family (30). Recently, HERV-H/F, a HERV-H subfamily, was characterized from several human leukemia cell lines (48). HERV-Fc1, which is part of the expanded HERV-H/F family, was identified with a full-length coding envelope gene in primates (3). HERV-Fc has a very limited expansion in primates, and there are only six HERV-Fc elements present in the human genome (3). The relatively high level of divergence for elements of the same family could be relevant to the high rate of mutation that is observed in the course of retroviral replication in an infectious process. The HERV-Fc1 element might have passed through an extracellular/infectious stage before being fixed in the gorilla/chimpanzee/human branch (3). HERV-Fc1 is the most intact member of this entire group, with almost complete gag and pol and intact pro and env genes (3, 27, 30). The gag open reading frame (ORF) is terminated by two stop codons compared to the common single stop in exogenous retroviruses. The pol frame is interrupted by a frameshift mutation and a premature stop codon (3, 43). Consequently, though it has high potency, the occurrence of a functional capsid particle should still be considered hypothetical. Transcripts from HERV-Fc1 env genes have been detected in different human tissues from testis, skin, and trachea, indicating that their promoters are active (48). Activation and expression of HERV-H/F elements may result in unpredictable and deleterious mobilization of the genetic material of retroviral origin.
A genetic epidemiology study in MS patients (42) indicated a possible specific association between HERV-Fc1 and MS. One marker neighboring HERV-Fc1 (rs391745) had a P value of 1.3 × 10−6 for disease association in a trend test. Other markers in that region were also associated with MS. In addition, similar genetic evidence suggests that retroviral replication is involved in MS development: The host gene TRIM5, known to restrict the replication of a broad range of retroviruses, influences the risk of getting MS (42). In the past, animal studies have demonstrated that retroviral replication is associated with increased levels of viral proteins in organs and blood (1, 35, 61, 63), which again impinged on disease development. These observations were the driving force behind the present attempts to quantify HERV-H/F in plasma and circulating white blood cells.
HERVs have previously been hypothesized to play a role in neuropathogenesis, both as putative susceptibility genes and as pathogenic viruses (8, 11, 28, 37). Studies on brain tissue from individuals with MS show increased levels of HERV-H, HERV-W, and HERV-K RNA (28) and HERV-W Env and Gag proteins (50). Prominent HERV-W Gag expression was also detected in endothelial cells of MS lesions from acute or actively demyelinating cases, a pattern not detected in any controls (50). Long-term cultures of B lymphoblastoid cells from the peripheral blood of MS patients have been shown to produce reverse transcriptase (RT)-positive virions containing HERV-H/F and/or HERV-W/multiple sclerosis-associated retrovirus (MSRV) sequences (9, 14, 22, 39, 50). Moreover, such sequences were found to be more prevalent in peripheral blood of MS patients than in samples from controls (13, 22). Increased levels of antibodies to HERV-H-derived peptide epitopes are consistently found in both serum and cerebrospinal fluid (CSF) samples from MS patients compared with controls (7, 13), and the antibody serum levels decrease with beta interferon (IFN-β) therapy (51). Additionally, a HERV-H protease-env splice variant is more frequent in peripheral blood mononuclear cell (PBMC) mRNA from MS patients than in controls (13).
Of particular relevance to the present study is the recent demonstration of increased expression of HERV-H- and HERV-W-derived Env epitopes on PBMCs from MS patients, particularly from MS patients with active disease, and predominantly on B cells and monocytes (6).
MATERIALS AND METHODS
Blood samples.
As summarized in Table 1, the study group consisted of 22 patients with active MS (19 females and 3 males; mean age, 39 years; range, 24 to 59 years), 19 patients with nonactive MS (15 females and 4 males; mean age, 47 years; range, 29 to 67 years), and 30 healthy volunteers (15 females and 15 males; mean age, 39 years; range, 25 to 60 years). In the group of patients with active MS, the median number of months after relapse was 3.2, and the range was between 1 and 8 months. In the group of patients with nonactive MS, the median number of months after relapse was 36 months and the range was between 12 and 360 months. Among patients with active MS, 14 were not treated with any immunomodulating treatment during blood collection, 5 were treated with Tysabri (Elan Pharma), 2 with Betaferon (Bayer Schering Pharma), and 1 with Rebif22 (Merck Serono). Among patients with nonactive MS, 10 were not treated with any immunomodulating treatment during blood collection and 6 were treated with Tysabri, 1 with Betaferon, 1 with Copaxone (Teva), and 1 with Rebif (Merck Serono). The MS patients were recruited at the Neurology Clinic, Aarhus University Hospital, Aarhus, Denmark. The control samples were obtained from Skejby Sygehus, Aarhus University Hospital, Aarhus, Denmark. All samples were collected between September 2009 and March 2010. The Central Denmark Committee on Biomedical Research Ethics gave ethical approval for this protocol, and all patients and healthy volunteers gave their consent. Blood samples were collected in cell preparation tubes (BD Vacutainers, BD Diagnostics, NJ) and processed within 1 h according to the manufacturer's protocol (two CPT tubes for each individual). The tubes/blood samples were centrifuged at room temperature in a horizontal rotor for a minimum of 30 min at 1,800 × g (relative centrifuge force). After centrifugation, mononuclear cell layers were collected and transferred to 15-ml conical tubes. Following two washing steps, the cell pellets were resuspended in the desired medium for subsequent procedures or cryopreserved at −135°C until use. Plasma samples were collected after the CPT tube centrifugation and were stored at −70°C. The disease activities of the MS patients were stratified according to the standard criteria into two groups: patients with active MS and patients with nonactive MS. Active MS is defined as at least one relapse within 1 year prior to blood collection, while nonactive MS is defined as the absence of disease activity for at least 1 year as determined by standard clinical criteria.
Table 1.
Clinical data for MS patients and healthy controls
| Parameter | Value |
||
|---|---|---|---|
| Patients with active MS | Patients with nonactive MS | Healthy controls | |
| No. | 22 | 19 | 30 |
| Sex (no. of women/men) | 19/3 | 15/4 | 15/15 |
| Age (yr) [median (range)] | 39 (24–59) | 47 (29–67) | 39 (25–60) |
| EDSSa (range) | 3 (0–6) | 3 (1.5–8.5) | |
EDSS, Expanded Disability Status Scale.
Antibodies and primers.
Polyclonal anti-HERV-H/F Gag antibodies (number 2319) were raised in a New Zealand White rabbit under contract with Sigma-Genosys (United Kingdom) against the peptide DIRKKLKKVEEGPQT. The equivalent positions in the Gag ORF of HERV-Fc1 are amino acids (aa) 380 to 395, and the putative stop codon position is aa 470. This peptide is translated from the long putative gag ORF of the HERV-Fc1 sequence (GenBank accession number AL354685) in a region with very high similarity to the gag sequence of known HERV-H copies with complete Env ORFs: HERV-H env62/H19, HERV-H env60, and HERV-H env59 (16). Preimmune serum was collected before immunization. The rabbit was immunized with the peptide and boosted 3 times, and after the final boost, peripheral blood was collected for subsequent measuring of anti-peptide antibodies. The specificity and cross-reactivity of the anti-HERV-H/F Gag antiserum was analyzed in both enzyme-linked immunosorbent assay (ELISA) and time-resolved immunofluorimetric assay (TRIFMA). The anti-HERV-H/F Gag epitope antiserum was at least 600,000 times more reactive to the HERV-H/F Gag peptide than the corresponding preimmune serum (data not shown). The anti-CD4, -CD8, and -CD19 antibodies (numbers 15-0049-42, 12-0199-42, and 25-0088-42) and mouse isotype controls (numbers 15-4714-42, 25-4714-42, and 12-4714-42) were purchased from eBioscence, San Diego, CA. Secondary anti-rabbit IgG F(ab)2 antibody was purchased from Pierce. Primers were designed using the online software PrimoPro 3.4 and were synthesized at MWG Eurofins Operon (Germany) at 0.01-μmol scale. Selective amplification of HERV-Fc1 gag (AL354685) sequences was obtained by using the corresponding primer pairs HERV-Fc1 extracellular RNA Fc1 1F (TGCAGAAGACAAGGCAATG) and Fc1 1R (AGTGTTCCCTTGGACAGGTG) and HERV-Fc1 DNA copy numbers Fc1 2F (TTAAGCGGAAGCGACTCATT) and Fc1 2R (CACCTGTCCAAGGGAACACT). The high level of divergence for elements of the same family and for a gene that is normally conserved excludes the possibility of amplifying other proviruses, e.g., Fc2 master element. The specificity of the amplicons was confirmed by sequencing. The correctness of the sequence obtained was verified by alignment with HERV-Fc1 sequence retrieved from the Human Genome Browser. Homology searches were performed using the BLASTN program at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi?PAGE=Nucleotides).
Genomic DNA extraction.
Total genomic DNA was extracted from PBMCs using a standard proteinase K (Roche, Mannhein, Germany) digestion (overnight at 55°C), followed by phenol-chloroform extraction and DNA precipitation using ethanol. DNA was recovered by centrifugation, dissolved in TE (10 mM Tris, 1 mM EDTA, pH 8), and stored at −20°C.
Extracellular viral RNA extraction.
Plasma samples (200 μl) were subjected to 0.45-μm filtration (Ultra Free-MC; Millipore, Bedford, MA), and viral RNAs were isolated from 0.2 ml of the flowthrough using the High Pure Viral RNA kit (Roche, Denmark) according to the manufacturer's instructions. RNA was eluted in 50 μl RNase-free water and stored at −70°C until analysis.
Construction of HERV-Fc1 standards.
A HERV-Fc1 gag-pol pcDNA3.1-Zeo plasmid was used to create HERV-Fc1 standards. After confirming the authenticity of the plasmid by sequencing (MWG Eurofins Operon, Germany), we linearized the construct with XhoI, which cuts a sequence downstream from the HERV-Fc1 gag-pol PCR insert and the T7 promoter. HERV-Fc1 RNA standards were produced using T7 RNA polymerase and the DuraScribe T7 Transcription Kit (Epicentre Biotechnologies, Madison, WI). These in vitro RNA standards were treated with RNase-free DNase for 2 h at 37°C and purified twice by ethanol (EtOH) precipitation in the presence of 3 M sodium acetate, pH 5.2, at −20°C. The purified in vitro RNA was quantified spectrophotometrically at 260 nm and diluted serially to obtain RNA concentrations ranging from 2.76 × 102 to 2.76 × 108 copies per μl. Furthermore, in control experiments, we included DNA standards obtained from the same plasmid after linearization. The DNA standards were quantified by spectrophotometry. The ratio of absorbances at 260 nm and 280 nm was used to assess the purity of DNA. A ratio of >1.8 was accepted as “pure” for DNA. DNA was diluted to obtain DNA concentrations ranging from 1.0 × 101 to 1.0 × 108 copies per μl. To calculate the RNA and DNA volumes required for the desired dilutions, we used the online copy number calculator available from Finnzymes.
Quantification of HERV-Fc1 extracellular RNA loads by SYBR green absolute Q-PCR.
To determine the extracellular HERV-Fc1 RNA load, absolute quantitative PCR (Q-PCR) was undertaken using the QuantiTect SYBR green RT-PCR kit (Qiagen, Valencia, CA). A total of 5 μl of extracted viral RNA or control RNA and 0.5 μM forward and reverse primers were used in a final 50-μl master mix volume. A reverse transcription step of 20 min at 50°C was included prior to PCR. The PCR conditions were as follows: an initial enzyme activation step of 15 min at 95°C, cycling (50 cycles) denaturation at 94°C for 15 s, annealing at 58°C for 30 s, extension at 72°C for 15 s, and an optimized data acquisition step at 78°C for 5 s. All samples and RNA standards were run in duplicate. No-RT controls were included for each experimental sample by omitting the RT/RNase block from the reaction. The no-RT controls generated no amplification of genomic DNA, verifying that the viral-RNA preparation was free of genomic DNA from lysed cells and that the primers were specific for the cDNA. Prevention measures against cross-contamination were employed; in particular, sample processing and PCR amplification were carried out in separate laboratories with different equipment. Some of the PCR products were subsequently cloned and sequenced to confirm the amplified target.
PCRs were run on a LightCycler 480 instrument (Roche, Denmark). Data were collected and recorded by the LightCycler software and expressed as a function of the threshold cycle (CT), which represents the number of cycles at which the fluorescence intensity of the SYBR green dye is significantly above the background fluorescence. The CT is directly correlated with the log10 copy number of the RNA standards. RNA copies were extrapolated from standard curves (CT versus log10 copy number) representing at least eight-point serial dilutions of standard RNA. RNA standards were used as calibrators for the quantification of the product generated in the exponential phase of the amplification curve. The results were accepted for standard curves with correlation coefficients greater than 0.95.
Sensitivity and specificity evaluation of the SYBR green assay for HERV-Fc1 DNA copy number detection in PBMCs and detection of extracellular viral RNA in plasma.
Scalar (2.0 × 101 to 2.0 × 108) dilutions of reference DNA, HERV-Fc1 plasmid pcDNA3.1, were prepared and then amplified using a HERV-Fc1 gag gene-specific primer pair, resulting in the synthesis of a 207-bp specific HERV-Fc1 gag product. In our system, 2.0 × 101 copies were detected in 100% of the samples. Table 2 demonstrates that repeated testing (four replicates in each run of all dilutions tested) disclosed the detection limit of our technique as 2.0 × 101 copies/reaction. The DNA assay included at least 8 orders of magnitude with a high linear relationship (r > 0.99) between the CT values and the standard DNA input copies. For RNA standards, serial dilutions were prepared, ranging from 5.52 × 101 to 5.52 × 108 copies/reaction. We failed to detect, in many cases, 5.52 × 101 copies of RNA standards. Thus, in RNA calibration curve assays, 5.52 × 102 copies were set up as a detection limit. In all assays, the sizes of the PCR products were confirmed further by 2% agarose electrophoresis of the PCR-amplified products and ethidium bromide staining. No signal was detected in negative-control samples (distilled water) in the analysis of fluorescence for SYBR green reactions or for electrophoresis of the PCR products. Examples of amplification curves with the DNA and RNA standards (2.0 × 101 to 2.0 × 108 and 5.52 × 102 to 5.52 × 108) plotted by the Light Cycler software are shown in Fig. S1A and B in the supplemental material. The reproducibility of the techniques both intra- and interassay using serial dilutions of standard HERV-Fc1 RNA or DNA was also tested. In particular, intra-assay reproducibility was evaluated. We compared the CT values for DNA or RNA standard dilutions from 2.0 × 101 to 2.0 × 108 and from 5.52 × 102 to 5.52 × 108, respectively, using four replicates of each point of stock dilutions. The coefficient of variation (CV) for all standard dilutions in one run was less than 3%. Similar results could be observed when comparing different runs (Table 3). The interassay reproducibility was obtained using three different experiments performed in duplicate. The CT CV was <2% for two selected standards from the DNA or RNA standard scalar dilutions (Table 3).
Table 2.
Comparison of CT values for standard curves and intra-assay analyses
| Reference standard (copies/reaction, 2 μl) | Mean CT valuea | SD | CV |
|---|---|---|---|
| DNA dilutions | |||
| 2.0E8 | 8.56 | 0.14 | 1.64 |
| 2.0E7 | 12.49 | 0.02 | 0.16 |
| 2.0E6 | 16.36 | 0.29 | 1.7 |
| 2.0E5 | 20.09 | 0.05 | 0.24 |
| 2.0E4 | 23.87 | 0.02 | 0.08 |
| 2.0E3 | 27.62 | 0.5 | 1.8 |
| 2.0E2 | 31.79 | 0.17 | 0.53 |
| 2.0E1 | 34.61 | 0.06 | 0.17 |
| RNA dilutions | |||
| 5.52E8 | 13.56 | 0.02 | 0.15 |
| 5.52E7 | 17.03 | 0.05 | 0.3 |
| 5.52E6 | 22.61 | 0.7 | 3.1 |
| 5.52E5 | 26.49 | 0.51 | 1.9 |
| 5.52E4 | 31.43 | 0.2 | 0.6 |
| 5.52E3 | 34.46 | 0.31 | 0.89 |
| 5.52E2 | 39.78 | 0.4 | 1 |
| 5.52E1 | ND |
For each sample, the CT value is an average of results from four replicates. ND, not determined.
Table 3.
Interassay analysis of mean CT values
| Reference standard (copies/reaction, 2 μl) | Mean CT valuea | SD | CV |
|---|---|---|---|
| DNA dilutions | |||
| 2.0E4 | 23.95 | 0.1 | 0.4 |
| 2.0E3 | 27.23 | 0.3 | 1.1 |
| RNA dilutions | |||
| 5.52E6 | 22.81 | 0.2 | 0.8 |
| 5.52E5 | 26.79 | 0.31 | 1.1 |
For each sample, the CT value is an average of three different experiments performed in duplicate.
Flow cytometry analyses and quantification of HERV-H/F Gag protein expression in human PBMCs from healthy controls and patients with active or nonactive disease.
Three million PBMCs from each individual were stained for flow cytometry analyses as follows. The cells were resuspended in growth medium (RPMI 1640 and 10% fetal bovine serum) and washed twice with phosphate-buffered saline (PBS) (pH 7.4). The cells in 100-μl aliquots were moved to a V-bottom 96-well plate. They were pelleted at 600 × g for 5 min and washed twice in PBS. Next, the samples were incubated with 500 μg/ml of human IgG (Beriglobin; Statens Serum Institute, Copenhagen, Denmark) for 60 min at 4°C. Further, the cells were stained for 30 min with either fluorescent anti-CD4 (phycoerythrin [PE]-Cy5), anti-CD8a (PE-Cy7), or anti-CD19 (PE) antibody at a dilution of 1:20 at 4°C. The cells were spun down at 270 × g for 5 min and washed once with PBS. Subsequently, the cells were fixed with PBS plus 0.1% formaldehyde for 10 min at room temperature on a rocking platform. The samples were spun down at 270 × g for 5 min and resuspended in 0.2% Tween 20 in PBS (pH 7.4). The cells were incubated for 15 min at room temperature with rocking. Further, the cells were incubated for 30 min on ice with rabbit anti-HERV-H/F Gag antibody 2319 or preimmune serum from the same rabbit at a final dilution of 1:1,000. After washing, the cells were incubated with secondary fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG antibody (1:200; Pierce, IL) for 30 min on ice. In the next step, the samples were spun down at 270 × g for 5 min and washed three times with PBS. Each sample was resuspended in 400 μl PBS and 0.1% formaldehyde and moved to a Falcon flow cytometry tube. The samples were placed at 4°C until analysis. In all experiments, the following samples were analyzed: unstained control PBMCs; PBMCs stained only with secondary antibody; PBMCs stained with mouse IgG1; K isotype controls (PE, PE-Cy5, and PE-Cy7); PBMCs stained with anti-CD4, -CD8a, and -CD19 antibodies and preimmune rabbit antiserum; and PBMCs stained with anti-CD4, -CD8a, and -CD19 and rabbit anti-HERV-F/H Gag antibodies. The samples were analyzed on the Cytomics FC500 instrument (Beckman Coulter, Brea, CA). At least 50,000 events were recorded and analyzed for each sample. Monocytes were identified by gating on side/forward scatter. The data were analyzed using the FlowJo software (TreeStar Inc., Ashland, OR). The results from the relative quantification of HERV-H/F Gag epitope expression are presented as fluorescence indices (FI). The FI were calculated for CD4+, CD8+, and CD19+ lymphocytes and for monocytes as the mean fluorescence obtained with the anti-HERV-H/F Gag antibodies divided by the mean fluorescence obtained with the HERV-H/F preimmune serum for each sample.
Statistical analysis.
Statistical analyses were performed using the nonparametric Mann-Whitney U test in the Graphpad Prism software v4.03 (Graphpad Software Inc., La Jolla, CA). Spearman's rank correlation coefficients were also calculated. Differences were considered to be significant at a P value of <0.05. For statistical calculations of HERV-H/F Gag expression analyses, Mann-Whitney testing and multiple regression testing were performed using Graphpad Prism software v4.03.
RESULTS
Quantification of HERV-Fc1 DNA copy numbers in DNA samples from control subjects.
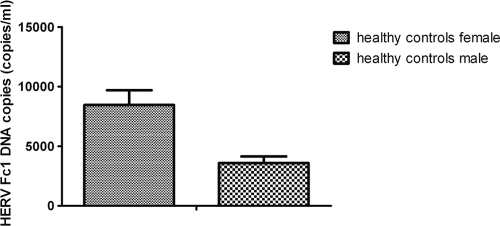
The median HERV-Fc1 DNA copy numbers in PBMCs from healthy subjects were estimated. We assessed the ability of our absolute Q-PCR strategy to accurately quantify HERV-Fc1 copy numbers in human DNA. Moreover, this experiment was performed to further confirm the specificity of the primers used for amplifying HERV-Fc1 gag gene sequences. When we determined the HERV-Fc1 gag gene copy number per haploid human genome, we obtained figures of approximately 9.3 × 103 copies per human female genome in the 40-ng/μl sample and 4.5 × 103 copies per human male genome in the 40-ng/μl sample (Fig. 1). Figure 1 shows that the female DNA against the male reference yields a ratio close to 2:1 for the HERV-Fc1 gag-specific sequence (P < 0.001 for HERV-Fc1 gag). Given that the HERV-Fc1 provirus is located on the X chromosome (ChrX) (96,982,818 to 96,994,075), this confirmed the specificity of our absolute Q-PCR assay. There were no significant differences in the HERV-Fc1 provirus copy number between healthy subjects and MS patients on the DNA level (data not shown). This suggests that the mechanism by which HERV-Fc1 might trigger pathogenesis of multiple sclerosis relies on the occurrence of activated HERV-Fc1 or differences in virus expression without new provirus integrations.
Fig 1.
HERV-Fc1 gag DNA copy number calculations in the control group for the two genders. Shown are SYBR green absolute Q-PCR assay analyses of HERV-Fc1-positive healthy controls; HERV-Fc1 gag gene copy numbers are calculated per human genome equivalent (30 subjects for each gender). The data are expressed as median copy numbers/reaction plus standard deviation (SD), quantified on the basis of an external DNA calibration curve obtained from serial dilutions of plasmids containing the amplicon of interest, as described in Materials and Methods. The P value was calculated using the nonparametric Mann-Whitney U test.
Quantification of HERV-Fc1 extracellular RNA in plasma samples from healthy controls and from multiple sclerosis patients.
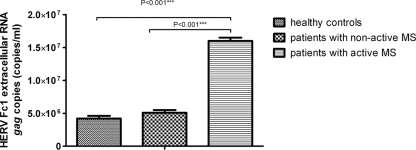
Extracellular HERV-Fc1 gag RNAs were detected in all of the MS patients (22 patients with active MS and 19 patients with nonactive MS) and in all healthy controls (30 healthy controls), indicating that healthy controls, as well as MS patients, both transcribe and release the HERV-Fc1 gag sequences. Healthy controls had a median extracellular gag RNA titer of 4.2 × 106 HERV-Fc1 gag extracellular copies/ml, whereas patients with nonactive MS had a median titer of 5.1 × 106 HERV-Fc1 gag extracellular copies/ml (Fig. 2). Patients with active MS had a median extracellular RNA titer of 1.6 × 107 HERV-Fc1 gag copies/ml, significantly higher than healthy controls (P < 0.001). On average, a 4-fold increase was observed (Fig. 2). Moreover, we found that patients with active MS had a median extracellular gag RNA titer significantly higher than that of patients with nonactive MS (P < 0.001).
Fig 2.
Absolute quantification of extracellular HERV-Fc1 gag RNA molecule copy numbers in plasma samples from healthy controls and patients with MS. The data are expressed as a median RNA copy number/reaction (1 ml) plus SD. Experimental values were quantified on the basis of the external RNA calibration curve obtained from serial dilutions of plasmid containing the amplicon of interest transcribed in vitro (see Materials and Methods). P values were calculated using the nonparametric Mann-Whitney U test.
HERV-H/F Gag protein expression in circulating T cells, B cells, and monocytes in healthy controls and patients with nonactive MS.
Flow cytometry analysis was performed on permeabilized PBMCs to determine HERV-H/F Gag expression in samples from patients with nonactive MS.
We analyzed PBMC samples from healthy controls (n = 30) and patients with nonactive MS (n = 19) using 4-color flow cytometry analyses. One sample from each group (two in total) had to be excluded from the analyses because cell numbers were too low to acquire 50,000 events. For detection of HERV-H/F Gag expression, PBMCs were analyzed using a triple-labeling procedure as described in Materials and Methods. Appropriate fluorochrome-conjugated mouse isotype-matched monoclonal antibodies were used as controls for background staining in each flow acquisition. Initially, a fluorescence compensation experiment was performed to exclude an overlap of emission spectra (see Fig. S3 in the supplemental material). This was done by running samples that were individually stained with the antibody-fluorochrome components of the multicolor samples. The anti-HERV-H/F specificity of the rabbit antiserum was first tested in control human PBMCs, and experimental conditions were optimized by flow cytometry analyses (see Fig. S2 in the supplemental material). The fluorescence peak from the anti-HERV-H/F Gag-specific immune serum and from the preimmune serum as a negative control showed no overlap, confirming the specificity of our assay. Each analysis was performed using at least 50,000 cells that were gated in the region of the lymphocyte-monocyte population, as determined by light scatter properties, forward scatter versus side scatter. Additionally, we prepared cytospins for fluorescence microscopy evaluation of the flow cytometry samples. Representative cytospins from 3 different individuals (PBMCs) stained with anti-HERV-H/F Gag antibodies or with preimmune serum are shown in Fig. S4A in the supplemental material.
Lymphocytes were further stratified in different subsets on the basis of expression of phenotypic markers: CD4+ (expressed on a subpopulation of mature T cells and T helper cells and low levels of monocytes and dendritic cells), CD8+ (expressed on a subpopulation of mature cytotoxic T cells and NK cells), and CD19+ (expressed on B cells at all stages of development, excluding the terminally differentiated plasma cells) cells.
To analyze the expression of HERV-H/F Gag in the lymphocyte subsets, permeabilized cells were gated in both the lymphocytes and one of the regions (CD4+/CD8+/CD19+). Mean fluorescence intensity was calculated only for positive events after subtraction of the appropriate isotype control. Earlier studies had demonstrated that permeabilization does not affect the staining of the CD4, CD8, and CD19 molecules on the cell surface (57).
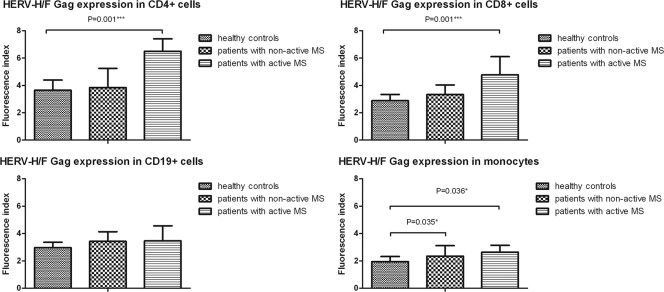
We found higher HERV-H/F Gag expression in monocytes and a tendency toward higher HERV-H/F Gag expression in CD19+ cells from patients with nonactive MS than in cells from healthy controls [P(monocytes) = 0.035 and P(CD19+) = 0.14] (Fig. 3).
Fig 3.
Flow cytometry analysis of intracellular HERV-H/F Gag expression in PBMCs from healthy controls and patients with nonactive and active MS. The bar graphs show comparative analysis of HERV-H/F Gag expression in CD4+ T cells, CD8+ T cells, CD19+ B cells, and monocytes in peripheral blood of healthy controls and patients with nonactive and active MS. The y axis represents FI. The bars show the median values and SD. The P values show statistical differences (<0.05) between the different groups. P values were calculated using the nonparametric Mann-Whitney U test. *, P ≤ 0.05; ***, P ≤ 0.001.
We did not find any significant difference in HERV-H/F Gag expression in CD4+ and CD8+ cells from patients with nonactive MS compared with cells from healthy controls.
HERV-H/F Gag expression in circulating T cells, B cells, and monocytes from MS patients during the active disease phase.
Flow cytometry analysis was also performed on permeabilized PBMCs to determine HERV-H/F Gag expression in samples from MS patients during the active disease phase. Here, we analyzed 22 patients in the active phase of MS. Previous claims that differences in the absolute numbers of circulating T suppressor cells were a consistent marker of disease activity (55) led us to look at the absolute numbers of CD8+ cells. Furthermore, we determined the ratios of CD4+ to CD8+ T cells in the group of patients with active MS compared with healthy controls. We found no consistent differences in the numbers of circulating CD8+ cells or the CD4+/CD8+ T-cell ratios between the groups of patients with active MS and healthy controls (data not shown).
In CD4+ T cells, HERV-H/F Gag expression was significantly higher in cells from patients with active MS than in cells from healthy controls (P < 0.001), as shown in Fig. 3. Moreover, we also found higher HERV-H/F expression in CD8+ T cells and monocytes from this patient group than in cells from healthy controls (P < 0.001 and P = 0.0356, respectively). A comparison of two groups—patients with nonactive MS and patients with active MS—showed that HERV-H/F Gag expression was significantly higher in CD4+ and CD8+ T cells in patients with recent disease activity than in patients with nonactive disease (P = 0.002 and P = 0.003, respectively) (Fig. 3). There were no significant differences in HERV-H/F Gag expression in CD19+ cells or in monocytes between patients with active MS and patients with nonactive MS.
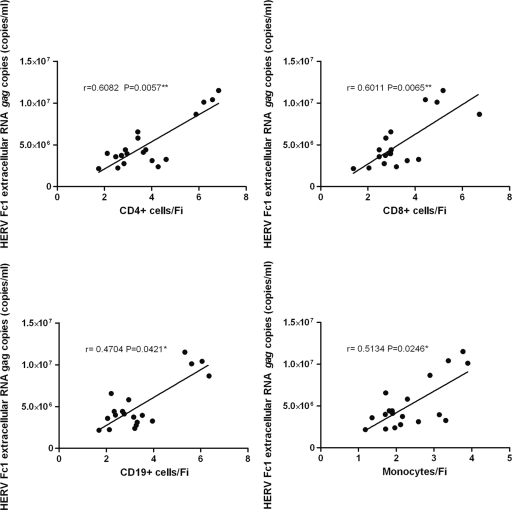
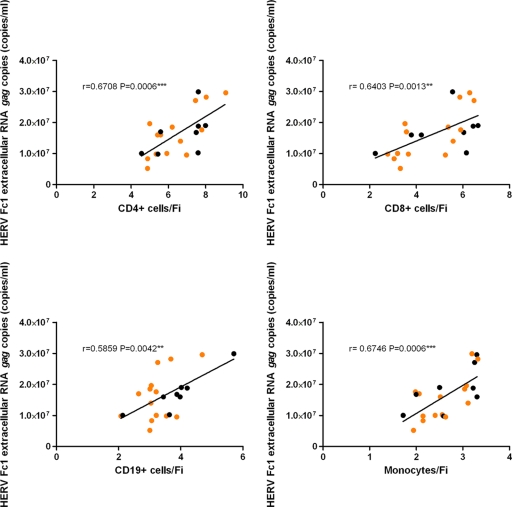
Furthermore, to determine whether an association exists between HERV-H/F Gag expression in PBMCs and HERV-Fc1 gag extracellular RNA, we evaluated the relationship between flow cytometry data and Q-PCR data. We found a strong positive correlation between the HERV-Fc1 gag extracellular-RNA titers in plasma and HERV-H/F Gag expression in PBMCs for all tested samples (Fig. 4 and 5), indicating that anti-HERV-H/F Gag antibody reactivities may be used for assessment of HERV-Fc1 Gag expression. Additionally, in the group of patients with active MS, we did not observe any tendencies toward higher or lower expression levels between patients without or with drug treatment in the course of blood sampling (Fig. 5) or any significant correlations with the time of the last relapse.
Fig 4.
Correlation between HERV-H/F Gag expression and levels of extracellular HERV-Fc1 gag RNA in patients with nonactive MS. The graphs show a positive correlation between levels of extracellular HERV-Fc1 gag RNA and HERV-H/F Gag expression in CD4+, CD8+, and CD19+ cells and monocytes in patients with nonactive MS. The x axes represent FI for CD4+, CD8+, and CD19+ cells and monocytes These relationships were evaluated using the Spearman correlation test; r, Spearman rank correlation. *, P ≤ 0.05; **, P ≤ 0.01.
Fig 5.
Correlation between HERV-H/F Gag expression and levels of extracellular HERV-Fc1 gag RNA in patients with active MS. The graphs show a positive correlation between levels of extracellular HERV-Fc1 gag RNA and HERV-H/F Gag expression in CD4+, CD8+, and CD19+ cells and monocytes in patients with active MS. The x axes represent FI for CD4+, CD8+, and CD19+ cells and monocytes. These relationships were evaluated using the Spearman correlation test; r, Spearman rank correlation. The orange dots indicate patients without drug treatment in the course of blood sampling. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
DISCUSSION
In a previous study, we established direct genetic evidence that HERV-Fc1 is associated with multiple sclerosis (42). In 3 analyzed cohorts of MS patients and matching controls, a marker, rs391745, had the lowest combined P value of 0.0003 for association with disease after Bonferroni correction (Fisher's formula). The identified locus on chromosome X harbored the HERV-Fc1 locus and no other coding capacities. No microRNA genes or small nucleolar RNA genes have been reported in the region, and the nearest known genes lie 141 kb upstream and 57 kb downstream, respectively, further substantiating the association.
In this study, anti-HERV-H/F Gag antibodies and flow cytometric analyses were used for the characterization of HERV-H/F Gag expression in PBMCs derived from MS patients. We showed, for the first time, significant differences in the expression of HERV-H/F Gag protein epitopes between PBMCs from healthy controls and those from MS patients. We found higher HERV-H/F Gag expression in monocytes and a tendency toward higher HERV-H/F Gag expression in CD19+ cells from patients with nonactive MS than in cells from healthy controls. In the group of patients with active MS, much greater differences in the HERV-H/F Gag expression relative to healthy controls were detected for CD4+ and CD8+ T cells. This is further evidence that the HERV-H/F family may play a role in the extensive activation of the adaptive immune responses in MS (15, 62). A previous study indicated that transcripts from HERV-H may play a biological role during T-cell activation. Transcription of HERV-H was restricted to the hematopoietic lineage and appeared to be maximal in T cells; therefore, its restriction could imply specificity (29).
Strong cell-mediated immune responses to gammaretroviral HERV antigens (concomitantly with herpesvirus antigens) have been reported in MS (5). Moreover, it has been shown that HERV peptides stimulate proliferation and immune response in MS patients with acute MS, but not in individuals with stable MS (15). HERV peptide-specific proliferation and type 1 cytokine production by PBMCs was observed in individuals with acute MS, but not in those with stable MS, in whom a type 2 cytokine profile dominates (15). Additionally, the authors observed that HERV-stimulated cytokine production is supported by CD4+ T helper cells. Different immune responses in patients with acute or chronic disease suggest that a cell-mediated and HERV-specific immune response is associated with MS (15). In addition, the existence of a T-cell-mediated immune pathogenicity previously confirmed in vivo with cell-free MSRV (HERV-W) virions injected into SCID mice humanized with human lymphoid grafts caused T-lymphocyte-dependent neuropathology (21). This study suggests that MSRV retroviral particles from MS cultures have immunopathogenic properties mediated by T cells, which is compatible with the previously reported superantigen activity in vitro (21).
We also found that in patients in the active phase of MS, the expression of HERV-H/F Gag by CD4+ T cells is significantly higher than that in CD8+ T cells. The importance of T cells in disease induction and further progression has been well documented (19). Studies reported that brain-infiltrating CD8+ T cells persist as clonal expansions in the CSF and blood of MS patients (60). Furthermore, selective enrichment of memory CD8+ T cells in the CSF of multiple sclerosis patients has been documented, suggesting a role for these CD8+ T cells in the pathogenesis of disease (26). These data imply that many different T-cell populations can, in principle, be involved in the induction, propagation, and modulation of the disease. Both CD4+ and CD8+ T cells have been demonstrated in MS lesions, with CD4+ T cells predominating in acute lesions and CD8+ T cells being observed more frequently in chronic lesions (52). Additionally, activated antigen-specific CD4+ T cells are found in all four described histopathological subtypes of MS (36). CD4+ cells are the main effector T cells in experimental autoimmune encephalomyelitis (EAE), an animal model of MS. Furthermore, activated CD4+ Th1 cells contribute to inflammation in the CNS and to increased permeability of the blood brain barrier (4). Recent data have established that interleukin 17 (IL-17)-producing CD4+ T cells, driven by IL-23 and referred to as TH17 cells, play a pivotal role in the pathogenesis of EAE (2). In chronic TMEV disease (Theiler's murine encephalomyelitis virus [TMEV]-induced demyelinating disease), which is similar to the progressive forms of MS, recruitment of macrophages, TMEV-specific T cells, and antibodies induces apoptosis, inflammation, and demyelination in the CNS (46). While early T-cell responses are directed to TMEV, the chronic T-cell-mediated disease involves the activation of myelin-reactive CD4+ cells (46).
The observed differences in HERV-H/F Gag expression for CD4+ and CD8+ T cells potentially substantiate the role of this HERV in regulating the activation of the adaptive immune responses in MS. The etiopathogenic mechanism and the importance of our findings are not yet known. The relatively normal levels of CD4+ and CD8+ T cells in patients with MS and the low CD4/CD8 ratio in active MS are in keeping with the apparently contradictory reports of the role of T helper cells in MS (34) but are consistent with some earlier reports (41).
A recently published flow cytometric study reported increased surface expression of specific gammaretroviral HERV-H and HERV-W Env epitopes on the surfaces of B cells and monocytes in patients with active MS. Furthermore, the higher expression of the HERV-H/W Env correlated with increased antibody reactivities to epitopes in sera from patients with active MS (6). Together, the current and previous studies demonstrate that expression of these major structural components of the HERV H/F family is consistently increased in MS.
These findings are in accordance with previous reports indicating significantly higher antibody reactivity to HERV-H-derived Env and Gag antigens in sera (10, 51) and CSF (7, 13) from MS patients than in healthy controls. The increased expression of gammaretroviral HERV Env and Gag epitopes in PBMCs and increased anti-HERV antibody reactivities in the active phase of MS also substantiate a role for HERVs as potential biomarkers in the disease.
In the present study, the expression of HERV-H/F Gag protein epitopes in PBMCs was positively correlated with the levels of extracellular HERV-Fc1 RNA molecules detected in plasma, suggesting that the detected Gag proteins are Fc1 related. However, further studies with HERV-Fc1-specific antibodies are necessary to assess the levels of specific HERV-Fc1-encoded proteins in MS patients. Finally, flow cytometry analyses of HERV-H/F Gag expression in PBMCs could be useful in follow-up studies of MS patients to assess disease activity and response to the treatment. The importance of the observed expression has yet to be elucidated.
Extracellular HERV-Fc1 RNAs were detected in plasma samples from both healthy controls and MS patients. A 4-fold increase in the amount of extracellular HERV-Fc1 RNA was found in plasma samples from patients with active MS compared with healthy controls. The above data, obtained by HERV-Fc1 gag-specific absolute Q-PCR assays, show that HERV-Fc1-like RNA sequences are expressed and released to a greater extent by cells from MS patients and that the HERV-Fc1 gag gene is at least partially transcribed. Most importantly, our results show a statistically significant association between the extracellular HERV-Fc1 RNA titer in plasma and disease activity as assessed by comparing the clinically defined active/nonactive disease. The association may reflect the increased cellular activation observed during active disease periods, which may augment the activation and perhaps replication of HERV-Fc1. We are aware that the detected HERV-Fc1 RNA transcripts, containing only gag sequences, do not comprise full-length proviral sequences but may represent, e.g., terminated transcripts. However, we have observed a linear relationship between extracellular RNA for HERV-Fc1 gag transcript and HERV-H/F Gag protein levels, which suggests that most of the detected transcripts were full length. As observed, a 4-fold increase in levels of extracellular HERV-Fc1 gag RNA in patients with active MS strengthens the link between HERV-Fc1 and MS and at least indicates that HERV-Fc1 expression in plasma can be used as a marker of disease activity in MS.
At the DNA level, we have not seen any change in HERV-Fc1 gag copy numbers in lymphocytes of MS patients. There was no significant change in HERV-Fc1 gag DNA copies in MS patients compared with healthy controls. However, we have to take into account that certain individuals may have HERV-Fc1 copies on their X chromosomes without the in-frame stop codon between the gag pol genes, which could readily express longer HERV-Fc1 viral transcripts following transactivation by cofactors, as yet undefined.
An important caveat for this study is that we have not controlled for possible effects of the treatment of MS patients on the transcriptional activation of HERV-Fc1 elements. However, over half of the patients registered as having active MS in this study were not in treatment at the time of sampling. Nevertheless, a significant decrease in anti-Env antibody reactivity for HERV-H and HERV-W as a consequence of IFN-β therapy, closely linked to the efficacy of therapy, has been reported (51). Furthermore, there are also suggestions that IFN therapy caused prompt and complete inhibition of MSRV circulation (38).
The pathogenic potential of HERV-Fc1 has not yet been fully explored. The proviral DNA contains two stop codons and a frameshift in the pol gene. The reading frames of the gag and env genes appear to be complete (3, 27, 30). Possible HERV-Fc1 infectivity can result from the contributions of other endogenous viruses through recombination or pseudotyping. Furthermore, the production of pathogenic molecules could be related to HERV-Fc1 retroviral expression. We suggest that there may be a pathogenic cascade in MS involving several specific pathogens interacting with particular genetic elements, leading to enhanced HERV-Fc1 protein expression.
In this paper, we show for the first time the quantification of HERV-Fc1 gag transcripts in plasma from patients with active or nonactive MS. We found the highest levels of extracellular HERV-Fc1 RNAs in the patients with active disease. Despite the diversity of findings related to HERV expression, with several conflicting and negative reports (40), numerous studies in the past have reported expression of HERVs in MS (13, 17, 49). This applies to particles, viral proteins, and viral RNA (22, 37, 45). However, causality has been notoriously difficult to establish from these experiments. Even if a significant association was established, the direction of causality was uncertain: did the virus induce disease, or did the disease induce virus? However, this is fundamentally different for HERV-Fc1, where genetic evidence also points to an association. Nevertheless, it remains to be directly demonstrated whether HERV-Fc1 is responsible for pathogenesis in MS.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant J.2008-7306 from Warwara Larsen Fonden and grant R44-A4331 from Lundbeck Fonden.
We have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Published ahead of print 25 January 2012
Supplemental material for this article may be found at http://jvi.asm.org.
REFERENCES
- 1. Alberti A, et al. 2002. Envelope-induced cell transformation by ovine betaretroviruses. J. Virol. 76:5387–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aranami T, Yamamura T. 2008. Th17 cells and autoimmune encephalomyelitis (EAE/MS). Allergol. Int. 57:115–120 [DOI] [PubMed] [Google Scholar]
- 3. Benit L, Calteau A, Heidmann T. 2003. Characterization of the low-copy HERV-Fc family: evidence for recent integrations in primates of elements with coding envelope genes. Virology 312:159–168 [DOI] [PubMed] [Google Scholar]
- 4. Ben-Nun A, Cohen IR. 1982. Experimental autoimmune encephalomyelitis (EAE) mediated by T cell lines: process of selection of lines and characterization of the cells. J. Immunol. 129:303–308 [PubMed] [Google Scholar]
- 5. Brudek T, Christensen T, Hansen HJ, Bobecka J, Moller-Larsen A. 2004. Simultaneous presence of endogenous retrovirus and herpes virus antigens has profound effect on cell-mediated immune responses: implications for multiple sclerosis. AIDS Res. Hum. Retrovir. 20:415–423 [DOI] [PubMed] [Google Scholar]
- 6. Brudek T, et al. 2009. B cells and monocytes from patients with active multiple sclerosis exhibit increased surface expression of both HERV-H Env and HERV-W Env, accompanied by increased seroreactivity. Retrovirology 6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christensen T. 2005. Association of human endogenous retroviruses with multiple sclerosis and possible interactions with herpes viruses. Rev. Med. Virol. 15:179–211 [DOI] [PubMed] [Google Scholar]
- 8. Christensen T. 2010. HERVs in neuropathogenesis. J. Neuroimmune Pharmacol. 5:326–335 [DOI] [PubMed] [Google Scholar]
- 9. Christensen T, Dissing Sorensen P, Riemann H, Hansen HJ, Moller-Larsen A. 1998. Expression of sequence variants of endogenous retrovirus RGH in particle form in multiple sclerosis. Lancet 352:1033. [DOI] [PubMed] [Google Scholar]
- 10. Christensen T, et al. 2000. Molecular characterization of HERV-H variants associated with multiple sclerosis. Acta Neurol. Scand. 101:229–238 [DOI] [PubMed] [Google Scholar]
- 11. Christensen T, et al. 1997. Characterization of retroviruses from patients with multiple sclerosis. Acta Neurolog. Scand. Suppl. 169:49–58 [DOI] [PubMed] [Google Scholar]
- 12. Christensen T, et al. 2007. Gene-environment interactions in multiple sclerosis: innate and adaptive immune responses to human endogenous retrovirus and herpesvirus antigens and the lectin complement activation pathway. J. Neuroimmunol. 183:175–188 [DOI] [PubMed] [Google Scholar]
- 13. Christensen T, Sorensen PD, Hansen HJ, Moller-Larsen A. 2003. Antibodies against a human endogenous retrovirus and the preponderance of env splice variants in multiple sclerosis patients. Multiple Sclerosis 9:6–15 [DOI] [PubMed] [Google Scholar]
- 14. Christensen T, Tonjes RR, zur Megede J, Boller K, Moller-Larsen A. 1999. Reverse transcriptase activity and particle production in B lymphoblastoid cell lines established from lymphocytes of patients with multiple sclerosis. AIDS Res. Hum. Retrovir. 15:285–291 [DOI] [PubMed] [Google Scholar]
- 15. Clerici M, et al. 1999. Immune responses to antigens of human endogenous retroviruses in patients with acute or stable multiple sclerosis. J. Neuroimmunol. 99:173–182 [DOI] [PubMed] [Google Scholar]
- 16. de Parseval N, Casella J, Gressin L, Heidmann T. 2001. Characterization of the three HERV-H proviruses with an open envelope reading frame encompassing the immunosuppressive domain and evolutionary history in primates. Virology 279:558–569 [DOI] [PubMed] [Google Scholar]
- 17. Dolei A, et al. 2002. Multiple sclerosis-associated retrovirus (MSRV) in Sardinian MS patients. Neurology 58:471–473 [DOI] [PubMed] [Google Scholar]
- 18. Ebers GC, et al. 1986. A population-based study of multiple sclerosis in twins. N. Engl. J. Med. 315:1638–1642 [DOI] [PubMed] [Google Scholar]
- 19. Ercolini AM, Miller SD. 2006. Mechanisms of immunopathology in murine models of central nervous system demyelinating disease. J. Immunol. 176:3293–3298 [DOI] [PubMed] [Google Scholar]
- 20. Fazakerley JK, Walker R. 2003. Virus demyelination. J. Neurovirol. 9:148–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Firouzi R, et al. 2003. Multiple sclerosis-associated retrovirus particles cause T lymphocyte-dependent death with brain hemorrhage in humanized SCID mice model. J. Neurovirol. 9:79–93 [DOI] [PubMed] [Google Scholar]
- 22. Garson JA, Tuke PW, Giraud P, Paranhos-Baccala G, Perron H. 1998. Detection of virion-associated MSRV-RNA in serum of patients with multiple sclerosis. Lancet 351:33. [DOI] [PubMed] [Google Scholar]
- 23. Gilden DH. 2005. Infectious causes of multiple sclerosis. Lancet Neurol. 4:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giovannoni G, et al. 2006. Infectious causes of multiple sclerosis. Lancet Neurol. 5:887–894 [DOI] [PubMed] [Google Scholar]
- 25. Hansen T, et al. 2005. Risk for multiple sclerosis in dizygotic and monozygotic twins. Multiple Sclerosis 11:500–503 [DOI] [PubMed] [Google Scholar]
- 26. Jacobsen M, et al. 2002. Oligoclonal expansion of memory CD8+ T cells in cerebrospinal fluid from multiple sclerosis patients. Brain 125:538–550 [DOI] [PubMed] [Google Scholar]
- 27. Jern P, Sperber GO, Blomberg J. 2004. Definition and variation of human endogenous retrovirus H. Virology 327:93–110 [DOI] [PubMed] [Google Scholar]
- 28. Johnston JB, et al. 2001. Monocyte activation and differentiation augment human endogenous retrovirus expression: implications for inflammatory brain diseases. Ann. Neurol. 50:434–442 [DOI] [PubMed] [Google Scholar]
- 29. Kelleher CA, Wilkinson DA, Freeman JD, Mager DL, Gelfand EW. 1996. Expression of novel-transposon-containing mRNAs in human T cells. J. Gen. Virol. 77:1101–1110 [DOI] [PubMed] [Google Scholar]
- 30. Kjellman C, Sjogren HO, Widegren B. 1999. HERV-F, a new group of human endogenous retrovirus sequences. J. Gen. Virol. 80:2383–2392 [DOI] [PubMed] [Google Scholar]
- 31. Koch-Henriksen N. 1999. The Danish Multiple Sclerosis Registry: a 50-year follow-up. Multiple Sclerosis 5:293–296 [DOI] [PubMed] [Google Scholar]
- 32. Krone B, Grange JM. 2010. Multiple sclerosis: are protective immune mechanisms compromised by a complex infectious background? Autoimmune Dis. 2011:708750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kurtzke JF. 1993. Epidemiologic evidence for multiple sclerosis as an infection. Clin. Microbiol. Rev. 6:382–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lafaille JJ. 1998. The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev. 9:139–151 [DOI] [PubMed] [Google Scholar]
- 35. Li Y, Cardona SM, Traister RS, Lynch WP. 2011. Retrovirus-induced spongiform neurodegeneration is mediated by unique central nervous system viral targeting and expression of env alone. J. Virol. 85:2060–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lucchinetti CF, Bruck W, Rodriguez M, Lassmann H. 1996. Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol. 6:259–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mameli G, et al. 2007. Brains and peripheral blood mononuclear cells of multiple sclerosis (MS) patients hyperexpress MS-associated retrovirus/HERV-W endogenous retrovirus, but not Human herpesvirus 6. J. Gen. Virol. 88:264–274 [DOI] [PubMed] [Google Scholar]
- 38. Mameli G, et al. 2008. Inhibition of multiple-sclerosis-associated retrovirus as biomarker of interferon therapy. J. Neurovirol. 14:73–77 [DOI] [PubMed] [Google Scholar]
- 39. Moller-Larsen A, Christensen T. 1998. Isolation of a retrovirus from multiple sclerosis patients in self-generated Iodixanol gradients. J. Virol. Methods 73:151–161 [DOI] [PubMed] [Google Scholar]
- 40. Moyes DL, et al. 2008. HERV-K113 is not associated with multiple sclerosis in a large family-based study. AIDS Res. Hum. Retrovir. 24:363–365 [DOI] [PubMed] [Google Scholar]
- 41. Munschauer FE, Hartrich LA, Stewart CC, Jacobs L. 1995. Circulating natural killer cells but not cytotoxic T lymphocytes are reduced in patients with active relapsing multiple sclerosis and little clinical disability as compared to controls. J. Neuroimmunol. 62:177–181 [DOI] [PubMed] [Google Scholar]
- 42. Nexo BA, et al. 2011. The etiology of multiple sclerosis: genetic evidence for the involvement of the human endogenous retrovirus HERV-Fc1. PLoS One 6:e16652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nissen K, Pedersen F, Nexo B. 2011. Expression of Gag and Pol from reconstructed HERV-Fc1, associated with multiple sclerosis. Retrovirology 8:P56 [Google Scholar]
- 44. Norrby E. 1978. Viral antibodies in multiple sclerosis. Prog. Med. Virol. 24:1–39 [PubMed] [Google Scholar]
- 45. Nowak J, et al. 2003. Multiple sclerosis-associated virus-related pol sequences found both in multiple sclerosis and healthy donors are more frequently expressed in multiple sclerosis patients. J. Neurovirol. 9:112–117 [DOI] [PubMed] [Google Scholar]
- 46. Oleszak EL, Chang JR, Friedman H, Katsetos CD, Platsoucas CD. 2004. Theiler's virus infection: a model for multiple sclerosis. Clin. Microbiol. Rev. 17:174–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pachner AR. 2011. Experimental models of multiple sclerosis. Curr. Opin. Neurol. 24:291–299 [DOI] [PubMed] [Google Scholar]
- 48. Patzke S, Lindeskog M, Munthe E, Aasheim HC. 2002. Characterization of a novel human endogenous retrovirus, HERV-H/F, expressed in human leukemia cell lines. Virology 303:164–173 [DOI] [PubMed] [Google Scholar]
- 49. Perron H, et al. 1997. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. The Collaborative Research Group on Multiple Sclerosis. Proc. Natl. Acad. Sci. U. S. A. 94:7583–7588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perron H, et al. 2005. Human endogenous retrovirus (HERV)-W ENV and GAG proteins: physiological expression in human brain and pathophysiological modulation in multiple sclerosis lesions. J. Neurovirol. 11:23–33 [DOI] [PubMed] [Google Scholar]
- 51. Petersen T, et al. 2009. Effects of interferon-beta therapy on innate and adaptive immune responses to the human endogenous retroviruses HERV-H and HERV-W, cytokine production, and the lectin complement activation pathway in multiple sclerosis. J. Neuroimmunol. 215:108–116 [DOI] [PubMed] [Google Scholar]
- 52. Raine CS. 1994. The Dale E. McFarlin Memorial Lecture: the immunology of the multiple sclerosis lesion. Ann. Neurol. 36(Suppl.):S61–S72 [DOI] [PubMed] [Google Scholar]
- 53. Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. 2010. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol. 9:727–739 [DOI] [PubMed] [Google Scholar]
- 54. Rasmussen HB, Clausen J. 1997. Possible involvement of endogenous retroviruses in the development of autoimmune disorders, especially multiple sclerosis. Acta Neurol. Scand. Suppl. 169:32–37 [DOI] [PubMed] [Google Scholar]
- 55. Reinherz EL, et al. 1980. Loss of suppressor T cells in active multiple sclerosis. Analysis with monoclonal antibodies. N. Engl. J. Med. 303:125–129 [DOI] [PubMed] [Google Scholar]
- 56. Rieger F, et al. 2000. New perspectives in multiple sclerosis: retroviral involvement and glial cell death. Pathol. Biol. 48:15–24 [PubMed] [Google Scholar]
- 57. Rosato MT, et al. 2001. Simultaneous analysis of surface marker expression and cell cycle progression in human peripheral blood mononuclear cells. J. Immunol. Methods 256:35–46 [DOI] [PubMed] [Google Scholar]
- 58. Ryan FP. 2009. An alternative approach to medical genetics based on modern evolutionary biology. Part 3: HERVs in diseases. J. R. Soc. Med. 102:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sibley WA, Bamford CR, Clark K. 1985. Clinical viral infections and multiple sclerosis. Lancet i:1313–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Skulina C, et al. 2004. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc. Natl. Acad. Sci. U. S. A. 101:2428–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Strand M, Lilly F, August JT. 1974. Host control of endogenous murine leukemia virus gene expression: concentrations of viral proteins in high and low leukemia mouse strains. Proc. Natl. Acad. Sci. U. S. A. 71:3682–3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Trabattoni D, et al. 2000. Augmented type 1 cytokines and human endogenous retroviruses specific immune responses in patients with acute multiple sclerosis. J. Neurovirol. 6(Suppl. 2):S38–S41 [PubMed] [Google Scholar]
- 63. Ulrich K, Nexo JA. 1977. Virus protein p30 in blood predicts development of leukaemia in mice injected with MuLV. Nature 267:723–724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.