Abstract
Epstein-Barr virus (EBV) is a common human herpesvirus. Infection with EBV is associated with several human malignancies in which the virus expresses a set of latent proteins, among which is latent membrane protein 1 (LMP1). LMP1 is able to transform numerous cell types and is considered the main oncogenic protein of EBV. The mechanism of action is based on mimicry of activated members of the tumor necrosis factor (TNF) receptor superfamily, through the ability of LMP1 to bind similar adapters and to activate signaling pathways. We previously generated two unique models: a monocytic cell line and a lymphocytic (NC5) cell line immortalized by EBV that expresses the type II latency program. Here we generated LMP1 dominant negative forms (DNs), based on fusion between green fluorescent protein (GFP) and transformation effector site 1 (TES1) or TES2 of LMP1. Then we generated cell lines conditionally expressing these DNs. These DNs inhibit NF-κB and Akt pathways, resulting in the impairment of survival processes and increased apoptosis in these cell lines. This proapoptotic effect is due to reduced interaction of LMP1 with specific adapters and the recruitment of these adapters to DNs, which enable the generation of an apoptotic complex involving TRADD, FADD, and caspase 8. Similar results were obtained with cell lines displaying a latency III program in which LMP1-DNs decrease cell viability. Finally, we prove that synthetic peptides display similar inhibitory effects in EBV-infected cells. DNs derived from LMP1 could be used to develop therapeutic approaches for malignant diseases associated with EBV.
INTRODUCTION
Epstein–Barr virus (EBV) is a human herpesvirus involved in infectious mononucleosis and the development of several human malignancies, such as B and T lymphomas and several carcinomas (35). EBV can infect B cells and transform them into lymphoblastoid cell lines (LCLs) (19). EBV may also infect T cells and monocytes (27) or epithelial cells (35). In transformed cells, EBV is found in a latent state, and several viral genes are expressed. Latent membrane protein 1 (LMP1) is derived from one of these genes and has been shown to be essential for B-cell immortalization and the proliferation of monocytes transformed by EBV (19, 27). LMP1 can be regarded as an oncogene product per se, since it can transform rodent fibroblasts (46) and sensitize transgenic mice to lymphomas (22). LMP1 is a 63-kDa plasma membrane protein with six transmembrane domains and can mimic a constitutively activated cell surface receptor that belongs to the tumor necrosis factor receptor (TNFR) superfamily (6). In contrast to the mechanism of action of TNFR1, spontaneous oligomerization mediated by the transmembrane and cytoplasmic N-terminal domains of LMP1 induces the activation of several signaling pathways (26). Several signaling domains have been identified so far in the cytoplasmic C-terminal region of the protein (8, 15, 16). These domains bind various adapters and induce specific signaling pathways. The critical residues responsible for adapter binding, as well as for some signaling and transforming properties, have been identified by mutational analysis. Two domains, named transformation effector site 1 (TES1) and TES2, have been implicated in the transforming effect of LMP1 (3, 5, 7, 16). Both TES1 and TES2 bind TNFR-associated factors (TRAFs), either directly in the case of TES1 (4, 5) or indirectly for TES2 through binding of the TNFR-associated death domain (TRADD) (15). TES2 has also been shown to interact with receptor-interacting protein (RIP) (14), another adapter of TNF receptors.
Several studies have shown that LMP1 is essential for B-lymphocyte immortalization, transformation, and survival in latency III LCLs. In our previous studies, we showed that LMP1 is also required for the survival of EBV type II latency monocytes (TE1) and T lymphocytes (NC5) (11, 27). We developed mutants derived from the C-terminal region of LMP1 and demonstrated that their expression inhibits signaling pathways induced both by LMP1 and by TNF in the HEK cell line. These dominant negative forms (DNs) act by squelching adapters required for LMP1 signaling, such as TRAFs, TRADD, and RIP (1, 34). We showed as well that in several cellular contexts, DNs can induce the formation of a complex containing FADD and caspase 8 that can lead to apoptosis (34). In this study, we generated stable EBV-transformed cell lines with inducible vectors carrying the DNs LMP1–TES1S and LMP1–TES2S. We also investigated an alternative approach by designing synthetic peptides containing the TES1 sequence. The results indicate that these DNs and peptides alter cell growth due to inhibition of the constitutive activation of NF-κB by LMP1 and generation of an apoptotic complex involving FADD and caspase 8. These tools can be used to develop therapeutic approaches for EBV-associated malignant diseases.
MATERIALS AND METHODS
Cell culture.
HEK 293, a human embryonic kidney cell line, was obtained from the American Type Culture Collection (ATCC) (CRL 1573) and was grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, and antibiotics. Lymphoblastoid cell lines (LCLs) were obtained after infection of B cells by EBV. NC5 (a T-lymphocytic cell line) and TE1 (a monocytic cell line) are both transformed by EBV after infection of peripheral blood mononuclear cells (PBMC) by B95.8 supernatants (11, 27). LCLs and the NC5 and TE1 cell lines were propagated in RPMI 1640 medium supplemented with 5% fetal calf serum, 2 mM glutamine, 1 mM sodium pyruvate, 1% nonessential amino acids, and antibiotics. All cells were maintained at 37°C under a humidified atmosphere with 5% CO2.
Constructs and antibodies.
The pRT1 expression vectors were a generous gift from G. W. Bornkamm (2) and carry a doxycycline (Dox)-inducible version of LMP1 dominant negative forms (LMP1-TES1S and LMP1-TES2S) and green fluorescent protein (GFP). The pRT1-LMP1-TES1S and pRT1-LMP1-TES2S vectors were derived from pGFP-LMP1-TES1S and pGFP-LMP1-TES2S (34), which expressed transformation effector site 1 (TES1) (DSLPHPQQATDDSGHE) or TES2 (DDDDPHGPVQLSYYD) of the C-terminal region of LMP1 fused to GFP. LMP1-CT has been described previously (1). All constructs were verified by sequencing. Plasmids were purified with NucleoBond EF kits (Macherey-Nagel) according to the manufacturer's instructions. The reporter plasmids used in this study carry the firefly luciferase gene under the control of different regulating sequences. The NF-κB luciferase reporter construct has five NF-κB-responsive elements in tandem and was from Stratagene. The gal4-luc and Gal4-Jun vectors were a generous gift from B. Derijard and M. Ptashne. The normalizing vector pRLnull has no promoter sequence to drive expression of the Renilla luciferase gene and was from Promega.
The monoclonal antibody against LMP1 was obtained from the S12 hybridoma culture supernatant (a generous gift from P. Busson). Antibodies against JNK1 (sc-474), TRAF2 (sc-876), TRAF3 (sc-1828), TRADD (sc-1163), GFP (sc-9996), RIP (sc-7881), FLIP (sc-7111), FADD (sc-5559), P-Akt (sc-52940), Akt (sc-81435), caspase 3 (sc-1225), ICAM1 (sc-8439), phosphorylated c-Jun N-terminal kinase (P-JNK) (sc-6254), P-IκB-α (sc-8404), IκB-α (sc-1643), and β-actin (sc-8432) were purchased from Santa Cruz Biotechnology. Antibodies against caspase 8 (catalog no. 9746) and poly(ADP-ribose) polymerase (PARP) (catalog no. 9542) were purchased from Cell Signaling Technologies.
Synthetic fused peptides.
Peptides were designed using the corresponding sequences of TES1 (DSLPHPQQATDDSHGE) and its mutated version TES1mut (DSLPHAQAAADDSGHE) fused with the polyarginine HIV Tat sequence (GRKKRRQRRR) to enable and facilitate their entry into cells. These peptides were biotinylated to allow their detection by immunofluorescence, immunoprecipitation, and Western blotting. In addition, these peptides were synthesized in retro-inverso form (using d-enantiomer amino acids and reversed peptide bonds) to increase their stability. Peptides were ordered at JPT Peptide Technologies GmbH, Berlin, Germany. Purity (>95%) was analyzed at a 220-nm wavelength by high-performance liquid chromatography (HPLC) (on a C18 column with a linear gradient) and mass spectrometry.
Generation of inducible cell lines.
According to the manufacturer's instructions, 1.5 million NC5 and TE1 cells were nucleofected with 1 μg of pRT1-LMP1-TES1S, pRT1-LMP1-TES2S, or pRT1-GFP in Amaxa's solution T with the T-15 setting (NC5), and in solution V with the V-01 setting (TE1), in an Amaxa Nucleofector (Amaxa Biosystems). After 3 days, hygromycin B was added at 50 μg/ml. The hygromycin B concentration was progressively increased to 150 μg/ml during the first 2 weeks of selection. Stable cell clones were cultured in the presence of 150 μg of hygromycin B (Euromedex)/ml and 5% FCS (tetracycline free). After 4 weeks, DN expression was induced with doxycycline (Sigma). Cells were washed once in RPMI 1640 medium and were resuspended in a standard medium without hygromycin; doxycycline was then added at the dose indicated in the corresponding figures.
Immunoprecipitation and Western blotting.
Immunoprecipitations were performed using 107 cells that were incubated in a medium with 2 μg doxycycline/ml. At the indicated time, cells were washed twice with phosphate-buffered saline (PBS) and were then lysed for 15 min on ice in 500 μl of lysis buffer PY (20 mM Tris-HCl [pH 7.4], 50 mM NaCl, 5 mM EDTA, 1% Triton X-100, 1 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM sodium orthovanadate, and 5 IU/ml aprotinin). Lysates (500 μg) were clarified by centrifugation (20,000 × g for 15 min), and supernatants were incubated under gentle agitation with appropriate antibodies for 1 h at 4°C. Protein A-Sepharose beads (Amersham Pharmacia) were added, and the mixtures were gently rotated for 1 h at 4°C. Beads were centrifuged for 1 min at 20,000 × g, washed four times in lysis buffer PY (with 0.1% Triton), and eluted by boiling (94°C) in 30 μl of Laemmli buffer. Eluted samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were subjected to Western blotting. For Western blotting, cells were incubated with or without doxycycline at the dose and time indicated. Next, cells were washed twice with PBS and were then lysed for 15 min on ice in 500 μl of lysis buffer PY. The lysates were clarified by centrifugation, and 100 μg of total proteins was separated by SDS-PAGE. Proteins were transferred to membranes (Immobilon-P; Millipore) by electroblotting. Membranes were saturated with 5% milk in Tris-buffered saline (TBS)–0.1% Tween and were incubated with appropriate primary antibodies, described above. After several washes in TBS–0.2% Tween, membranes were incubated with specific horseradish peroxidase (HRP)-conjugated secondary antibodies (Jackson ImmunoResearch). HRP was detected with the ECL Plus reagent (Amersham), and the light emitted was detected by autoradiographic film (Hyperfilm; Amersham) and a charge-coupled device (CCD) camera (LAS3000; Fujifilm). The digital images obtained were directly analyzed with Multi-Gauge software, version 3.0 (Fujifilm), and fold induction is shown in the corresponding figures.
Measurement of cell survival or cell death. (i) Trypan blue and growth assays.
To assess cell viability after the induction of LMP1 DN expression, 5 × 104 cells were seeded in a 6-well plate and were incubated at 37°C in a standard medium for 24 h with or without 2 μg/ml doxycycline. Cells were then counted after the addition of trypan blue. For growth assays, 2 × 105 cells were incubated as described above and were counted every 24 h for 3 days.
(ii) Thymidine incorporation.
Cell proliferation was measured by thymidine incorporation. In a 96-well plate, 5 × 104 cells/well of the wild-type (wt) NC5 cell line or NC5 stably transfected with pRT1-LMP1-TES1S, pRT1-LMP1-TES2S, or pRT1-GFP were incubated in their standard medium with or without doxycycline (2 μg/ml) for 48 h. Radioactive thymidine was incorporated as described previously (27). Each experiment was repeated at least twice and was carried out in triplicate. Proliferation was calculated in comparison with that for the wt NC5 cell line. A similar protocol was performed for LCLs. BAY 11-7082 (Calbiochem), an inhibitor of NF-κB, was used as a control in LCLs.
(iii) Flow cytometry analysis.
Cell death was measured by flow cytometry analysis. Total cell death was measured using 50 μg/ml propidium iodide (PI) (Sigma) labeling, and apoptotic cells were assessed by Annexin V-phycoerythrin (PE) (BD Pharmingen) and TOPRO-3 iodide (642/661) (Invitrogen) labeling. Briefly, 24 h after induction of an LMP1 DN or GFP control vector, cells were washed and stained with Annexin V-PE according to the manufacturer's instructions. Cell viability was assessed by addition of 1 mM TOPRO-3 or 50 μg/ml PI to samples just before flow cytometry analysis. For Annexin V-labeled cells, analyses were performed on GFP-positive cells.
RESULTS
Inducible expression of LMP1 dominant negative forms has a functional effect on EBV-transformed lymphocytes and monocytes.
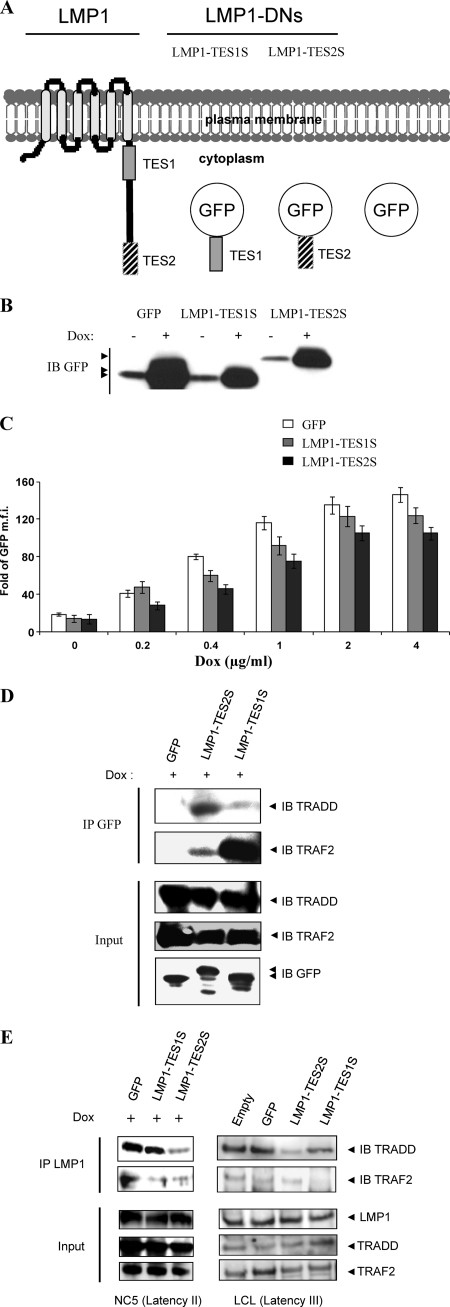
In our previous study, we designed different versions of LMP1 C terminus-derived dominant negative forms that were able to recruit the whole set of adapters shared by LMP1 and the TNFR superfamily (34). We showed their ability to impair both specific signaling pathways and phenotypes induced by TNF and LMP1 in epithelial and endothelial cells. Here, to investigate the potential role of our DNs in EBV-infected cell lines, we analyzed lymphocytic (NC5) (11) and monocytic (TE1) (27) cell lines infected and transformed by EBV. We used doxycycline (Dox)-inducible vectors (pRT1) (2) to conditionally express the short versions of the dominant negative LMP1-TES1S and LMP1-TES2S. The resulting constructs, pRT1-LMP1-TES1S and pRT1-LMP1-TES2S, and the control vector pRT1-GFP (Fig. 1A) express proteins that are, in contrast to wt LMP1, monomeric and cytoplasmic. These constructs were stably transfected into the TE1 and NC5 cell lines. To check the inducibility of LMP1-DN constructs with Dox, stable NC5 cells were incubated with or without 2 μg/ml Dox for 24 h, and then cell lysates were analyzed by Western blotting as shown in Fig. 1B. To evaluate the dose responsiveness of LMP1-DN expression, we incubated NC5 cells containing LMP1-DNs or GFP with increasing doses of Dox (0, 0.2, 0.4, 1, 2, and 4 μg/ml). Twenty-four hours after Dox administration, cells were analyzed by flow cytometry. The results (expressed as the fold increase in the mean fluorescence intensity [MFI] for each condition over the MFI for control nontransfected cells) are reported in histograms (Fig. 1C). Except at 0.2 μg/ml Dox, the inducibilities of LMP1-TES1 and LMP1-TES2 were similar. To check the ability of LMP1-DNs to bind to LMP1 adapters in our models (NC5 and TE1), we performed DN immunoprecipitation (using an anti-GFP antibody) followed by immunoblot analysis of adapters. The data indicate that LMP1-DNs bind adapters in both TE1 and NC5 cells, in which LMP1 is constitutively expressed and activated (11, 27). LMP1-TES1S binds TRAF2, whereas LMP1-TES2 binds TRADD and, to a lesser extent, TRAF2 in NC5 and TE1 cells (Fig. 1D; also data not shown). In addition, immunoprecipitation of wt LMP1 in the presence of LMP1-DNs showed that wt LMP1 binds TRAF2 and TRADD (Fig. 1E, left, lane GFP) and that LMP1-DNs are able to impair these interactions. LMP1-TES1S reduces the binding of TRAF2 to wt LMP1, whereas LMP1-TES2S reduces TRADD and TRAF2 binding (Fig. 1E for NC5 cells; data not shown for TE1 cells). Similar results were obtained in LCLs. In these cells, immunoprecipitation of wt LMP1 in the presence of LMP1-DNs showed that wt LMP1 binds TRAF2 and TRADD (Fig. 1E, right, lanes GFP and Empty) and that LMP1-DNs are able to impair these interactions. LMP1-TES1S and LMP1-TES2S reduce the binding of TRAF2 and TRADD, respectively, to wt LMP1 (Fig. 1E). These data demonstrate that LMP1-DNs can bind LMP1 adapters and impair their recruitment by LMP1 in cell lines expressing the virus latency II or III program.
Fig 1.
Expression of LMP1 dominant negative forms in latency II EBV-transformed T cells. (A) Diagram of LMP1 and LMP1 dominant negative forms. GFP alone or fused with TES1 or TES2 is cloned into a vector inducible by doxycycline. (B) Expression of LMP1 dominant negative forms in latency II EBV-transformed T cells. Whole-cell lysates from NC5-GFP, NC5-LMP1-TES1S, and NC5-LMP1-TES2S cells that had been mock treated or treated with 2 μg/ml doxycycline were examined by Western blotting with an anti-GFP antibody. (C) Dox-induced expression of DNs in NC5 cell lines. NC5-GFP, NC5-LMP1-TES1S, and NC5-LMP1-TES2S cells were incubated in a medium with doxycycline at 0, 0.2, 0.4, 1, 2, and 4 μg/ml for 24 h. Then cells were analyzed by flow cytometry. Mean fluorescence intensity (MFI) values were compared with those for the NC5 parental cell line to obtain the fold increase in expression. Three experiments were carried out in triplicate, and results are shown as means ± standard deviations. (D and E) LMP1-DNs recruit LMP1 adapters (D) and impair their binding to wt LMP1 (E). Infected NC5 lymphocytes expressing inducible LMP1-DNs or GFP were treated with 2 μg/ml doxycycline for 20 h. Then immunoprecipitations (IP) were performed in parallel with an anti-GFP antibody (D) or an anti-LMP1 antibody (S12) (E). Samples and whole-cell lysates were subjected to Western immunoblot (IB) analysis for LMP1, GFP-DNs, and endogenous TRADD and TRAF2. LCLs displaying a latency III program were used as controls. LCLs transiently transfected with an empty vector, GFP, LMP1-TES1S, or LMP1-TES2S were subjected to IP with an anti-LMP1 antibody (S12) before IB analysis for LMP1 and for endogenous TRADD and TRAF2.
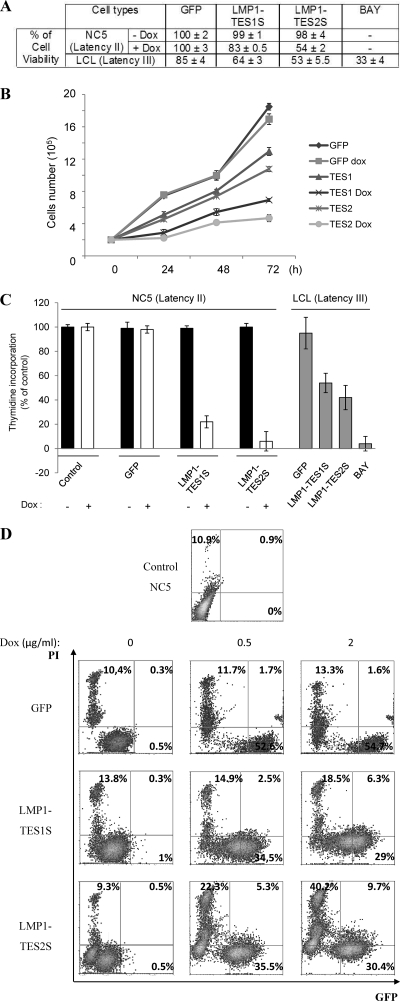
Expression of LMP1 dominant negative forms reduces the proliferation and viability of latency II EBV-infected cells (NC5) by activating cell death.
The NC5 cell line is an EBV-infected T-lymphocyte line. We have demonstrated that this cell line expresses the virus latency II program (expression of LMP1, LMP2, and EBNA1) and that it is dependent on LMP1 for survival (11). Using the inducible DNs LMP1-TES1S and LMP1-TES2S, we examined the survival of NC5 cells 24 h after DN induction. Increased expression of LMP1-DNs reduced the viability of NC5 cells from that of the control (Fig. 2A) when 2 μg/ml of Dox was added. In LCLs exhibiting a latency III program, transient expression of LMP1-DNs decreases cell viability (Fig. 2A). These results confirmed those obtained with the dominant negative LMP1-CT (10), which contains the whole C-terminal region of LMP1 fused with GFP. We then examined the effects of the DNs on cell growth. To that end, we seeded 2 × 105 cells in 6-well plates and counted cells every day for 3 days (Fig. 2B). The results show that the exponential growth of NC5 control cells was drastically reduced with expression of the LMP1-DN TES1S or TES2S. This experiment was repeated three times, and cells were counted in triplicate at each time point. Similar results (growth curves and viability) were obtained with the TE1 cell line (data not shown). In order to decipher these phenotypes induced by LMP1-DNs, we performed a thymidine incorporation assay to assess the effect of DNs on cell proliferation in latency II (NC5) or latency III (LCL) cells. A total of 5 × 104 transiently transfected LCLs or NC5 cells/well, either left untreated or treated with Dox for 48 h, were seeded in 96-well plates, and thymidine was added during the last 6 h, after which thymidine incorporation was analyzed. LMP1-TES1S and LMP1-TES2S induced significant reductions in cell proliferation (Fig. 2C) in NC5 cells as well as LCLs. This effect was dose dependent and increased with time (data not shown). Interestingly, the dominant negative LMP1-TES2S is more potent than LMP1-TES1S at reducing cell growth and inducing cell death. The induction of cell death by expression of LMP1-DNs in the TE1 (data not shown) and NC5 cell lines was assessed by flow cytometry (Fig. 2D). Cells were incubated for 24 h with increasing doses of Dox, and propidium iodide (PI) was added just before flow cytometry analysis. The data indicate that for NC5 cells expressing DN LMP1-TES1S or LMP1-TES2S, the numbers of dead (PI-positive) cells increase in a dose-dependent manner. These results demonstrate that LMP1-DN expression decreases cell proliferation and increases apoptosis, resulting in growth inhibition.
Fig 2.
The LMP1-DNs LMP1-TES1S and LMP1-TES2S display an antiproliferative effect on latency II EBV-transformed T-cell lines (NC5). (A) Effects of LMP1-DNs on the viability of NC5 cells or LCLs. Wild-type NC5 T cells and those with inducible GFP or with dominant negative LMP1-TES1S or LMP1-TES2S were counted. Then 2 × 105 cells for each assay were seeded twice in a medium with or without 2 μg/ml doxycycline for 24 h. LCLs transiently transfected with GFP, LMP1-TES1S, or LMP1-TES2S, or treated with BAY 11-7082, were counted. Then 2 × 105 cells for each assay were seeded. For each assay, cells were counted twice using trypan blue to analyze viability. (B) Effects of LMP1-DNs on cell growth. NC5 T cells with inducible GFP or with the DN LMP1-TES1S or LMP1-TES2S were counted and seeded as described above with or without 2 μg/ml doxycycline for 3 days. For each assay, cells were counted every 24 h for growth analysis. Results are shown as means ± standard deviations for 3 experiments carried out in triplicate. We compared induced and noninduced conditions for each cell line, and statistical analysis indicated that the growth of the NC5 T-cell line with inducible GFP was the same under both conditions, whereas the growth retardation of cells expressing LMP1-TES1S or LMP1-TES2S after doxycycline induction was statistically significant. (C) Effects of LMP1-DNs on cell proliferation. Wild-type NC5 T cells and those with inducible GFP or dominant negative LMP1 with (open bars) or without (filled bars) doxycycline (2 μg/ml), and LCLs transiently transfected with GFP, LMP1-TES1S, or LMP1-TES2S, or treated with BAY 11-7082 (shaded bars), were counted. Then 5 × 104 cells for each assay were seeded twice in 96-well plates with 1 μCi/well of [methyl-3H]thymidine. The incorporation of thymidine was analyzed after 48 h. The results are shown as means ± standard deviations for triplicate experiments. (D) Cell death due to LMP1-DN induction is dose dependent. NC5 T cells with inducible LMP1-DNs or a control vector were incubated with the indicated dose of doxycycline (0, 0.5, or 2 μg/ml) for 24 h; then cell death was assessed by addition of 50 μg/ml propidium iodide (PI) to samples just before flow cytometric analysis. Percentages indicate the proportion of cells in each quadrant.
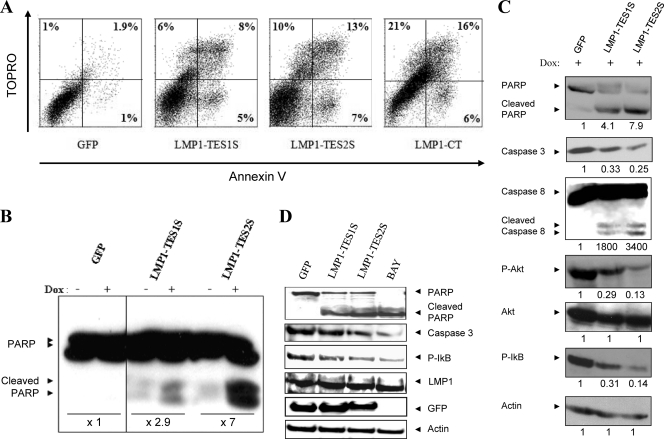
Inhibition of LMP1 in a latency II cell line (NC5) induces apoptosis and impairs survival.
The mechanism of action by which LMP1-DNs hamper LMP1 signaling consists of squelching of its adapters in the cytoplasm (34). The consequent massive induction of cell death is shown in Fig. 2. To characterize this phenotype, we performed Annexin V/TOPRO labeling after LMP1-DN induction. The results of flow cytometry analysis indicated increases in the proportions of Annexin V- and TOPRO-positive cells induced by the DNs LMP1-TES1S and LMP1-TES2S (Fig. 3A). These data showed the induction of early and late apoptosis by LMP1-DNs. The induction of apoptosis was confirmed by Western blotting for the proteolysis of poly(ADP ribose) polymerase (PARP) (Fig. 3B), a caspase 3, -6, and -7 substrate. In addition, Western blotting of whole-cell lysates after Dox addition for 24 h indicated PARP cleavage, reductions in procaspase 3 levels, and caspase 8 cleavage (Fig. 3C). At the same time, the LMP1-DNs displayed a negative effect on survival signaling induced by LMP1. Indeed, expression of LMP1-DNs resulted in reduced phosphorylation of Akt and IκB (Fig. 3C), survival pathways induced by LMP1. In LCLs, the decrease in cell viability is due to an increase in the rate of apoptosis and a reduction in the survival signaling pathway (NF-κB), as indicated in Fig. 3D. LMP1-DNs and BAY 11-7082 favor the cleavage of caspase 3 and PARP and reduce IκB phosphorylation.
Fig 3.
Induction of LMP1-DNs triggers an apoptosis cascade and inhibits survival pathways. (A) Annexin V-TOPRO staining and flow cytometric analysis were performed to study the induction of apoptosis after the expression of LMP1-DNs TES1 and TES2. NC5-LMP1-DN and NC5-GFP cells were incubated in a medium with doxycycline at 2 μg/ml for 24 h. Then each sample was stained with Annexin V and 1 mM TOPRO. Cells were analyzed by flow cytometry. The percentage of Annexin V- and TOPRO-positive or -negative cells is given in each quadrant. Total percentages of dead cells were 3.9%, 19%, and 30% for NC5-GFP, NC5-LMP1-TES1S, and NC5-LMP1-TES2S cells, respectively. Cells expressing LMP1-CT were used as a control (total cell death, 43%). (B) Apoptosis resulting from LMP1-DN expression is correlated with caspase activation. NC5 T-cell lines (NC5-LMP1-TES1S, NC5-LMP1-TES2S, and NC5-GFP) were incubated for 24 h with or without 2 μg/ml doxycycline. Cells were lysed with PY buffer supplemented with a phosphatase inhibitor (PhoSTOP; Sigma-Aldrich), and each sample was analyzed by Western blotting to evaluate caspase activation through the cleavage of poly(ADP) ribose polymerase (cleaved PARP). The intensities of bands on the Western blot were quantified, and the fold induction relative to the control due to 2 μg/ml Dox is given for each cell line. (C) Effects of LMP1-DNs on apoptosis and survival signals. LMP1-DN expression was induced in NC5 T-cell lines as described above. Then total-cell lysates were subjected to electrophoresis. Western blotting was performed using antibodies against PARP, caspase 8, caspase 3, P-Akt, Akt, and P-IκB. β-Actin detection was used as a control for equal protein loading. The intensities of bands on the Western blot were quantified, and the results obtained for cells expressing LMP1-DNs were compared to those for cells expressing GFP as a control after normalization to actin expression (or, for P-Akt, to Akt expression). (D) Effects of LMP1-DNs on apoptosis and survival signals in LCLs. LCLs were transiently transfected with GFP, LMP1-TES1S, or LMP1-TES2S or were treated with BAY 11-7082. Total-cell lysates (100 μg) were subjected to electrophoresis. Western blotting was performed using antibodies against PARP, caspase 3, P-IκB, LMP1, or GFP. β-Actin detection was used as a control for equal protein loading.
We then investigated the role of LMP1-DNs in the two main signaling pathways induced by LMP1: the NF-κB and c-Jun N-terminal kinase (JNK) pathways. We performed a Western blot experiment to detect IκB phosphorylation after LMP1-DN expression for 24, 48, and 72 h. The results show an impairment of LMP1-mediated IκB phosphorylation in LMP1-TES1S and LMP1-TES2S samples (Fig. 4A), indicating reduced activation of the NF-κB pathway. Moreover, with regard to the JNK pathway, a weak decrease in phosphorylated JNK (P-JNK) detection was observed for LMP1-TES1 and a stronger decrease for LMP1-TES2 (Fig. 4B). Collectively, these data reveal a decrease in the level of LMP1-activated NF-κB and, to a lesser extent, in JNK signaling after LMP1-DN expression.
Fig 4.
LMP1-DNs impair NF-κB survival signaling but not the c-Jun N-terminal kinase (JNK) signaling pathway in EBV-transformed lymphocyte (NC5) lines. LMP1-DN expression was induced by doxycycline for 0, 24, and 48 h. Then the cells were lysed as described for Fig. 3. Shown are the effects of LMP1-DNs on the NF-κB (A) and JNK (B) pathways. Total-cell lysates were analyzed by Western blotting using antibodies to phosphorylated IκB, phosphorylated JNK, JNK, and β-actin. The intensities of bands on the Western blot were quantified, and the results obtained for cells expressing LMP1-DNs or GFP were compared to those for the control (without Dox) after normalization to actin expression (A) or JNK expression (B).
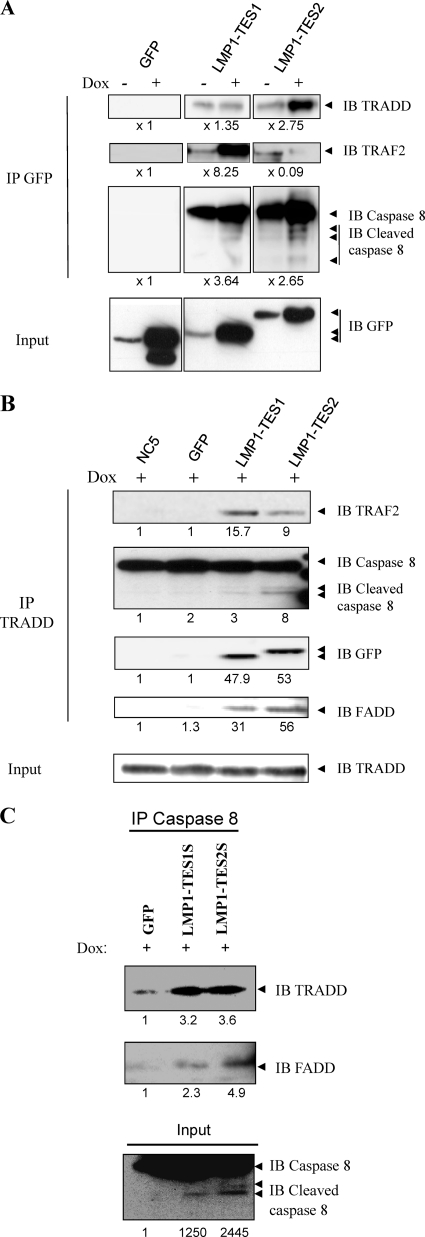
LMP1-DNs form an apoptotic complex involving TRADD, FADD, and caspase 8 in an EBV-transformed cell line (NC5) expressing a latency II program.
To clarify the mechanism of action of these DNs inducing cell death in latency II cell lines, we performed coimmunoprecipitation experiments to detect proteins that are able to bind to LMP-DNs. We show that after immunoprecipitation of LMP1-DNs with an anti-GFP antibody, TRADD, a proximal adapter that binds to the TES2 domain of LMP1, and TRAF2, a proximal adapter that binds to the TES1 domain, are recruited by the LMP1-TES2S and LMP1-TES1S dominant negative forms of LMP1 (Fig. 5A).
Fig 5.
Recruitment of an apoptotic complex by LMP1-DNs in infected NC5 cell lines. (A) LMP1-DNs bind proximal and distal proteins involved in apoptosis. LMP1-DN expression was induced for 16 h by doxycycline at 2 μg/ml, and coimmunoprecipitation was performed using an anti-GFP antibody followed by Western blot analysis to detect the binding of proteins that can form an apoptotic complex (TRADD, TRAF2, caspase 8) to LMP1-DNs. Whole-cell lysates were used to detect LMP1-DN expression with an anti-GFP antibody. The intensities of bands on the Western blot were quantified, and the results obtained for cells expressing LMP1-DNs or GFP were compared to those for the control (without Dox). (B and C) Role of LMP1-DNs in apoptotic complex generation. To assess the formation of an apoptotic complex following LMP1-DN induction, coimmunoprecipitation experiments were performed using antibodies against TRADD (B) or caspase 8 (C), followed by Western blotting to analyze the increases in the levels of protein partners such as cleaved caspase 8, FADD, and LMP1-DNs in this complex. The intensities of bands on the Western blot were quantified, and the results obtained for cells expressing LMP1-DNs or GFP were compared to those for the parental NC5 cell line (B) or for GFP-expressing cells (C).
Surprisingly, TRADD is shown to be detected with the LMP1-TES1S dominant negative form, probably through TRAF2 binding. Interestingly, caspase 8 and activated caspase 8, proteins that are not able to be precipitated by LMP1, are coimmunoprecipitated with each LMP1-DN after Dox induction, and caspase 8 is coimmunoprecipitated even without Dox induction. This result suggested the formation of an apoptotic complex involving activated caspase 8. To confirm these data and decipher this complex, we performed another coimmunoprecipitation. The results indicated that in the presence of LMP1-DNs, TRADD binds to LMP1-DNs, FADD, TRAF2, and activated caspase 8 (Fig. 5B). When caspase 8 is immunoprecipitated, TRADD and FADD are also found in the same complex in the presence of LMP1-DNs (Fig. 5C). The specificity of these coimmunoprecipitations was confirmed by the absence of these proteins when immunoprecipitations were performed with NC5 or NC5-GFP cell lysates.
Synthetic peptides derived from LMP1 C-terminal sequences display phenotypes similar to those of LMP1 dominant negative vectors.
To confirm the effects of LMP1-DNs, we designed two small peptides of 9 amino acids (aa) from TES1 sequences (wt or mutated) that are fused with HIV-1 Tat sequences to facilitate entry into cells. These peptides were biotinylated for immunofluorescence detection and immunoprecipitation experiments. Peptides were synthesized by the retro-inverso method (using d-enantiomers and reversed peptide bonds) to increase their stability.
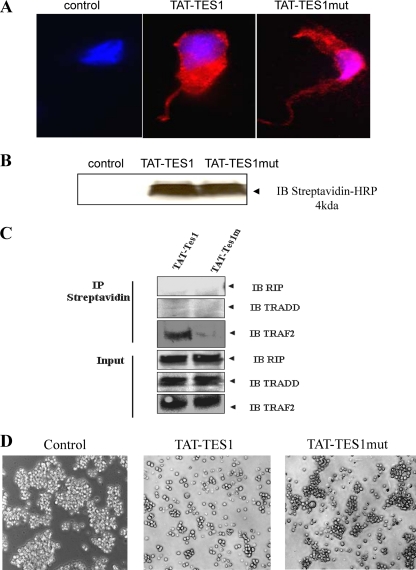
After 5 min of incubation (5 μM peptides) at 37°C in HEK cells, the TAT-TES1 peptide and its mutated version, TAT-TES1mut, penetrate cells and are distributed in all intracellular compartments (Fig. 6A and B). Immunoprecipitation experiments performed using streptavidin beads show that the TAT-TES1 peptide interacts with TRAF2 but TAT-TES1mut does not. Moreover, TAT-TES1 does not recruit the adapters TRADD and RIP (Fig. 6C). Finally, the TAT-TES1 peptide, but not TAT-TES1mut, is able to inhibit LMP1 phenotypes in EBV-infected and -transformed cells and leads to the induction of NC5 cell death and dissociation in culture (Fig. 6D). After 48 h of peptide treatment, the number of cells decreased by as much as 35% with TAT-TES1 and by as much as 88% with TAT-TES1mut.
Fig 6.
Effects of synthetic peptides derived from LMP1 C-terminal sequences. (A) Monitoring of cellular uptake of peptides in HEK cells and subcellular localization. A total of 2 × 105 HEK cells were first plated and then incubated with 5 μM biotinylated TAT-TES1, TAT-TES1mut, or the vehicle only for 5 min. Then cells were labeled with Hoechst dye for nucleus detection, and phycoerythrin-labeled streptavidin was used for peptide detection. Cells were then analyzed by fluorescence microscopy. (B) HEK cells were incubated with each peptide as described above. Then the cells were lysed and analyzed by Western blotting using a streptavidin-horseradish conjugate for peptide detection. (C) HEK cells were incubated with peptides for 10 min; then cells were lysed. Peptides were immunoprecipitated with streptavidin coupled to Sepharose beads. Western blotting was then performed using antibodies against TRAF2, TRADD, and RIP to verify the abilities of peptides to interact with LMP1 signaling adaptors. (D) Effects of peptides on EBV-infected cell lines. NC5 cells were incubated with 15 μM each peptide for 10 h; then the mortality rates and induced phenotypes of cells were analyzed by photomicrography.
DISCUSSION
Latent membrane protein 1 (LMP1) is the major oncogenic product of EBV, and its role in malignant disease is well documented (43). Here we provide new tools for the inhibition of LMP1 and its associated phenotypes. In most cases of EBV-associated pathology, the latency II program of EBV, expressing LMP1, LMP2, and EBNA1, is observed (35). In this work, we used our models of EBV-infected and -transformed cells displaying this latency II program. These models are investigated in order to study the effects of our dominant negative forms (DNs) and peptides derived from the LMP1 C-terminal domain.
We have developed mutants derived from two LMP1 signaling domains, and by using doxycycline-inducible pRT1 vectors (2), we stably transfected these mutants into NC5 and TE1 cells. By immunoprecipitation analysis, we have shown that LMP1-TES1S and LMP1-TES2S bind to TRAF2, TRADD, and RIP and affect the recruitment of these adapters to wt LMP1.
The consequences of the squelching of LMP1 adapters can be measured through impairment of signal transduction, reduced cell viability and proliferation, and increased apoptosis after expression of LMP1-DNs. Interestingly, similar results were obtained in LCLs displaying a latency III program (squelching of adapters; diminution of signal transduction, leading to apoptosis and growth arrest). LMP1 is constitutively active (9) and induces the activation of NF-κB, which regulates the main biological process in cells (18, 45). NF-κB-induced genes involved in survival include antiapoptotic proteins, such as cellular FLIP, Bcl-2, A20, c-IAPs, and TRAFs (28, 30, 36, 42, 44, 45). Constitutive NF-κB activation contributes to the transformation of cell lines by LMP1 (19). When LMP1-DN expression is induced, NF-κB activation is impaired, and survival signals disappear. Interestingly, LMP1-DNs impair the other pathways involved in survival, such as phosphatidylinositol 3-kinase (PI3K)/Akt and p38 (data not shown), but their effects on the JNK pathway are less important.
For several oncogene products, inhibition of their activity leads to a rapid decrease in survival pathway signals without affecting apoptosis pathways (40, 41). In our model, survival signals decline progressively within 72 h, but JNK pathways remain activated. It was not possible to monitor a relevant change in JNK activity after this time, because all cells expressing high levels of DNs had died, and the only cells that remained were nonresponsive to doxycycline induction.
Several studies have shown the transforming properties of LMP1 in EBV-infected cell lines. LMP1 is sufficient to transform many cell types, such as rodent fibroblasts (46), lymphocytes, and monocytes (19, 27). It can induce cell phenotypes such as invasive growth in MDCK epithelial cells (21). We previously showed that LMP1 is able to induce apoptosis in some cases (23, 24). Our study demonstrates for the first time that in EBV latency II type cell lines, inhibition of LMP1 by an inappropriately localized monomeric C-terminal domain (LMP1-DNs) is sufficient to induce apoptosis and to reverse LMP1-induced phenotypes.
The LMP1-DNs characterized in this article are able to inhibit signaling pathways as well as phenotypes linked to LMP1 and can be interesting for the investigation and setup of therapeutic tools for malignant diseases such as nasopharyngeal carcinoma (NPC), in which LMP1 plays an important role (20, 32, 35, 43). Interestingly, we confirm an in vivo role for LMP1-DNs, since the rate of mouse death induced by xenografted EBV latency II cells can be reduced by inhibition of LMP1.
The use of inhibiting peptides synthesized in retro-inverso form, which increased their stability (12, 13), showed the same effects as LMP-DNs in vitro. Studies using inhibitory peptide approaches have yielded promising results. Inhibitory peptides against the NF-κB essential modulator (NEMO), used to block the NF-κB pathway, induce the death of transplanted tumor cells and tumor regression. The results of recent approaches using inhibitors of LMP1 or its downstream signaling adapters appear to be encouraging. Recent data show that the use of dehydroxymethylepoxyquinomicin (DHMEQ), an inhibitor of NF-κB, induces the apoptosis of LCLs and EBV-infected cells expressing LMP1 and prevents tumor growth in NOG mice (31). In our model, further experiments will be performed to monitor the evolution of tumor manifestation or regression after peptide administration. If no adverse effects are observed, our inhibiting peptides will appear to be serious candidates for use in EBV-associated diseases.
We show that inhibition of LMP1 signaling by dominant negative forms or synthetic peptides leads not only to the impairment of survival signals but also to proapoptotic processes. Cell death could be clearly observed around 16 to 18 h, and this coincides with the time required for the expression of DNs and for the interaction of LMP1-DNs with LMP1 signaling adapters. By immunoprecipitation of LMP1-DNs, we discovered that TRAF2 and TRADD, the proximal LMP1 signaling adapters, are coprecipitated, and surprisingly, FADD and caspase 8 are coprecipitated. This interaction resembles TNFR1-induced apoptosis through complex II (17, 29, 33, 47). To exclude the presence of secreted TNF in the culture medium, we performed enzyme-linked immunosorbent assays (ELISAs) on the supernatant, because activated T-lymphocytes produce TNF. We found no significant production of TNF compared to that by human peripheral blood mononuclear cells or enriched CD4+ cells (data not shown). This mechanism confirms the results of our previous study showing that LMP1-DNs reverse TNF-induced phenotypes (34). The mechanism of action of LMP1-DNs resembles that of TNFR1-induced apoptosis. First, LMP1-DNs inhibit the LMP1-induced NF-κB pathway by squelching required adapters. Second, they take part in the formation of an apoptotic complex that includes TRADD but also FADD and activated caspase 8. Since LMP1-DNs have no effect on noninfected cells, it seems that these two effects are necessary in order to lead to apoptosis. The role of DNs in the early stage of this complex generation is unknown. We think that the generation of this complex is probably due to the inhibition of NF-κB, and this is similar to the mechanism of TNF receptor-induced apoptosis. Recruitment of FADD or caspase 8 by DNs facilitates complex II formation. A recent study has shown that when the death domain of TNFR1 was replaced with LMP1 CTAR2, cells previously responsive to TNF-induced cell death became resistant. The authors concluded that recruitment of TRADD by CTAR2 masks the apoptotic properties of TRADD (37). In contrast to our model, their proteins are localized to the membrane, and NF-κB is activated. Moreover, complex II has been shown to be localized to the cytoplasm after the internalization of TNFR1 (25, 29, 38, 39), and our DNs are localized mainly to the cytoplasm.
In the case of TNFR1-induced apoptosis, TRADD has been shown to be the adapter leading to the recruitment of FADD and caspase 8 to the signaling complex (29, 33, 47). However, another laboratory has demonstrated that RIP plays a major role in activating apoptosis (17), but the authors admitted that this phenomenon may be cell type dependent. In our study, we demonstrate interaction between TRADD, FADD, and caspase 8 but not RIP. We could not detect RIP because of its low level of expression in the NC5 and TE1 cell lines.
Our data demonstrate that inhibition of LMP1 reduces the growth of latency II cell lines in vitro and in vivo. These constructs will allow further elucidation of the biochemical events constituting the signaling pathways of LMP1. Inhibition of adapter recruitment may contribute to the clarification of signaling pathways as well as the genes targeted by LMP1, and the dominant negative forms of LMP1 or their derivatives may have useful therapeutic applications.
ACKNOWLEDGMENTS
We thank Pierre Busson for the generous gift of the S12 antibody. We thank Annick Blanpain and Hervé Drobecq for skillful technical assistance.
This study was supported by grants from the Association de la Recherche contre le Cancer, the Ligue Nationale contre le Cancer (Comité du Pas-de-Calais, Comité de l'Aisne), and the Réseau Herpès et Cancer. P. A. Ndour was supported by grants from the Ministère délégué à l'Enseignement Supérieur et à la Recherche and the Association de la Recherche contre le Cancer.
Footnotes
Published ahead of print 18 January 2012
REFERENCES
- 1. Adriaenssens E, et al. 2004. A novel dominant-negative mutant form of Epstein-Barr virus latent membrane protein-1 (LMP1) selectively and differentially impairs LMP1 and TNF signaling pathways. Oncogene 23:2681–2693 [DOI] [PubMed] [Google Scholar]
- 2. Bornkamm GW, et al. 2005. Stringent doxycycline-dependent control of gene activities using an episomal one-vector system. Nucleic Acids Res. 33:e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brodeur SR, Cheng G, Baltimore D, Thorley-Lawson DA. 1997. Localization of the major NF-κB-activating site and the sole TRAF3 binding site of LMP-1 defines two distinct signaling motifs. J. Biol. Chem. 272:19777–19784 [DOI] [PubMed] [Google Scholar]
- 4. Devergne O, et al. 1998. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J. Virol. 72:7900–7908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Devergne O, et al. 1996. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol. Cell. Biol. 16:7098–7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eliopoulos AG, Young LS. 2001. LMP1 structure and signal transduction. Semin. Cancer Biol. 11:435–444 [DOI] [PubMed] [Google Scholar]
- 7. Floettmann JE, Rowe M. 1997. Epstein-Barr virus latent membrane protein-1 (LMP1) C-terminus activation region 2 (CTAR2) maps to the far C-terminus and requires oligomerisation for NF-κB activation. Oncogene 15:1851–1858 [DOI] [PubMed] [Google Scholar]
- 8. Gires O, et al. 1999. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 18:3064–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gires O, et al. 1997. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 16:6131–6140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goormachtigh G, et al. 2006. Autoactivation of the Epstein-Barr virus oncogenic protein LMP1 during type II latency through opposite roles of the NF-κB and JNK signaling pathways. J. Virol. 80:7382–7393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Groux H, et al. 1997. Isolation and characterization of transformed human T-cell lines infected by Epstein-Barr virus. Blood 89:4521–4530 [PubMed] [Google Scholar]
- 12. Herve M, et al. 1997. On the immunogenic properties of retro-inverso peptides. Total retro-inversion of T-cell epitopes causes a loss of binding to MHC II molecules. Mol. Immunol. 34:157–163 [DOI] [PubMed] [Google Scholar]
- 13. Hong SS, Gay B, Karayan L, Dabauvalle MC, Boulanger P. 1999. Cellular uptake and nuclear delivery of recombinant adenovirus penton base. Virology 262:163–177 [DOI] [PubMed] [Google Scholar]
- 14. Izumi KM, et al. 1999. The Epstein-Barr virus oncoprotein latent membrane protein 1 engages the tumor necrosis factor receptor-associated proteins TRADD and receptor interacting protein (RIP) but does not induce apoptosis or require RIP for NF-κB activation. Mol. Cell. Biol. 19:5759–5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Izumi KM, Kaye KM, Kieff ED. 1997. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc. Natl. Acad. Sci. U. S. A. 94:1447–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Izumi KM, Kieff ED. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc. Natl. Acad. Sci. U. S. A. 94:12592–12597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin Z, El-Deiry WS. 2006. Distinct signaling pathways in TRAIL- versus tumor necrosis factor-induced apoptosis. Mol. Cell. Biol. 26:8136–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karin M, Lin A. 2002. NF-κB at the crossroads of life and death. Nat. Immunol. 3:221–227 [DOI] [PubMed] [Google Scholar]
- 19. Kaye KM, Izumi KM, Kieff E. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. U. S. A. 90:9150–9154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kieff E. 1996. Epstein-Barr virus and its replication, p 2343–2396 In Fields BN, et al. (ed), Fields virology. vol 2, 3rd ed Lippincott-Raven, Philadelphia, PA [Google Scholar]
- 21. Kim KR, et al. 2000. Transformation of Madin-Darby canine kidney (MDCK) epithelial cells by Epstein-Barr virus latent membrane protein 1 (LMP1) induces expression of Ets1 and invasive growth. Oncogene 19:1764–1771 [DOI] [PubMed] [Google Scholar]
- 22. Kulwichit W, et al. 1998. Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 95:11963–11968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Clorennec C, et al. 2008. Molecular basis of cytotoxicity of Epstein-Barr virus (EBV) latent membrane protein 1 (LMP1) in EBV latency III B cells: LMP1 induces type II ligand independent autoactivation of CD95/Fas with caspase 8-mediated apoptosis. J. Virol. 82:6721–6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Le Clorennec C, et al. 2006. EBV latency III immortalization program sensitizes B cells to induction of CD95-mediated apoptosis via LMP1: role of NF-κB, STAT1, and p53. Blood 107:2070–2078 [DOI] [PubMed] [Google Scholar]
- 25. Legler DF, Micheau O, Doucey MA, Tschopp J, Bron C. 2003. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFα-mediated NF-κB activation. Immunity 18:655–664 [DOI] [PubMed] [Google Scholar]
- 26. Liebowitz D, Wang D, Kieff E. 1986. Orientation and patching of the latent infection membrane protein encoded by Epstein-Barr virus. J. Virol. 58:233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masy E, et al. 2002. Human monocytic cell lines transformed in vitro by Epstein-Barr virus display a type II latency and LMP-1-dependent proliferation. J. Virol. 76:6460–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Micheau O. 2003. Cellular FLICE-inhibitory protein: an attractive therapeutic target? Expert Opin. Ther. Targets 7:559–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Micheau O, Tschopp J. 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114:181–190 [DOI] [PubMed] [Google Scholar]
- 30. Miller WE, Earp HS, Raab-Traub N. 1995. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J. Virol. 69:4390–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyake A, et al. 2008. Induction of apoptosis in Epstein-Barr virus-infected B-lymphocytes by the NF-κB inhibitor DHMEQ. Microbes Infect. 10:748–756 [DOI] [PubMed] [Google Scholar]
- 32. Morris MA, Dawson CW, Young LS. 2009. Role of the Epstein-Barr virus-encoded latent membrane protein-1, LMP1, in the pathogenesis of nasopharyngeal carcinoma. Future Oncol. 5:811–825 [DOI] [PubMed] [Google Scholar]
- 33. Muppidi JR, Tschopp J, Siegel RM. 2004. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity 21:461–465 [DOI] [PubMed] [Google Scholar]
- 34. Ndour PA, et al. 2010. Inhibition of tumor necrosis factor-induced phenotypes by short intracellular versions of latent membrane protein-1. Cell. Signal. 22:303–313 [DOI] [PubMed] [Google Scholar]
- 35. Rickinson A, Kieff E. 2007. Epstein-Barr virus, p 2655–2700 In Knipe DM, et al. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 36. Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. 1997. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 16:6914–6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schneider F, et al. 2008. The viral oncoprotein LMP1 exploits TRADD for signaling by masking its apoptotic activity. PLoS Biol. 6:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schneider-Brachert W, et al. 2004. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity 21:415–428 [DOI] [PubMed] [Google Scholar]
- 39. Schutze S, Schneider-Brachert W. 2009. Impact of TNF-R1 and CD95 internalization on apoptotic and antiapoptotic signaling. Results Probl. Cell Differ. 49:63–85 [DOI] [PubMed] [Google Scholar]
- 40. Sharma SV, et al. 2006. A common signaling cascade may underlie “addiction” to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell 10:425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharma SV, Settleman J. 2007. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 21:3214–3231 [DOI] [PubMed] [Google Scholar]
- 42. Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R. 2001. X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and -7 in distinct modes. J. Biol. Chem. 276:27058–27063 [DOI] [PubMed] [Google Scholar]
- 43. Tsang NM, et al. 2003. Presence of the latent membrane protein 1 gene in nasopharyngeal swabs from patients with mucosal recurrent nasopharyngeal carcinoma. Cancer 98:2385–2392 [DOI] [PubMed] [Google Scholar]
- 44. Uren AG, Pakusch M, Hawkins CJ, Puls KL, Vaux DL. 1996. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc. Natl. Acad. Sci. U. S. A. 93:4974–4978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680–1683 [DOI] [PubMed] [Google Scholar]
- 46. Wang D, Liebowitz D, Kieff E. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43:831–840 [DOI] [PubMed] [Google Scholar]
- 47. Zheng L, et al. 2006. Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1. Mol. Cell. Biol. 26:3505–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]