Abstract
In recent studies, the nuclear domain 10 (ND10) components PML, Sp100, human Daxx (hDaxx), and ATRX were identified to be cellular restriction factors that are able to inhibit the replication of several herpesviruses. The antiviral function of ND10, however, is antagonized by viral effector proteins by a variety of strategies, including degradation of PML or relocalization of ND10 proteins. In this study, we analyzed the interplay between infection with herpesvirus saimiri (HVS), the prototypic rhadinovirus, and cellular defense by ND10. In contrast to other herpesviruses, we found that HVS specifically degraded the cellular ND10 component Sp100, whereas other factors like PML or hDaxx remained intact. We could further identify the ORF3 tegument protein of HVS, which shares homology with the cellular formylglycinamide ribotide amidotransferase (FGARAT) enzyme, to be the viral factor that induces the proteasomal degradation of Sp100. Interestingly, recent studies showed that the ORF3-homologous proteins ORF75c of murine gammaherpesvirus 68 and BNRF-1 of Epstein-Barr virus modulate the ND10 proteins PML and ATRX, respectively, suggesting that the ND10 targets of viral FGARAT-homologous proteins diversified during evolution. Furthermore, a virus with the ORF3 deletion was efficiently complemented in Sp100-depleted cells, indicating that Sp100 is able to inhibit HVS in the absence of antagonistic mechanisms. In contrast, we observed that PML, which was neither degraded nor redistributed after HVS infection, strongly restricted both wild-type HVS and virus with the ORF3 deletion. Thus, HVS may lack a factor that efficiently counteracts the repressive function of PML, which may foster latency as the outcome of infection.
INTRODUCTION
Mammalian cells have developed complex mechanisms in order to protect themselves from virus replication and spread. Besides the well-characterized innate and adaptive immune response, a rapidly expanding area of research is focusing on a better understanding of an intrinsic cell-based defense which recently emerged as the frontline protection against invading pathogens (3). Unlike the innate and adaptive parts of the immune system, which require virus-induced signaling cascades in order to be turned on, the intrinsic antiviral defense is based on cellular restriction factors that are constitutively expressed and, thus, active even before a pathogen enters the cell. However, during coevolution viruses have evolved strategies to counteract the antiviral potential instituted by such host restriction factors. The finding that herpesviruses are also subjected to intrinsic immune responses mediated by cellular restriction factors like PML, Sp100, human Daxx (hDaxx), or ATRX has substantially contributed to this field. Interestingly, all these factors have been found to be constituents of a cellular, subnuclear structure referred to as PML nuclear bodies (PML-NBs) or nuclear domain 10 (ND10) (reviewed in references 11 and 39).
Herpesvirus saimiri (HVS), the prototypic gamma-2 herpesvirus, is closely related to Kaposi's sarcoma-associated herpesvirus (KSHV). The double-stranded DNA genome has a variable length of 130 to 160 kb and consists of an AT-rich coding region harboring at least 77 open reading frames. The coding region is flanked by a variable number of GC-rich, noncoding repetitive units termed H-DNA (10). HVS was isolated from squirrel monkeys (Saimiri sciureus) and presumably persists in the T lymphocytes of its natural host (26). While no symptoms were described in squirrel monkeys, other susceptible New World monkey species like common marmosets (Callithrix jacchus) and cottontop tamarins (Saguinus oedipus) develop rapidly growing T cell malignancies after experimental infection (13). HVS strains are classified into three subgroups (A, B, C) (25), with subgroup C strains like C488 being the most oncogenic and also able to transform human T cells to antigen-independent growth in vitro (4). Responsible for T cell transformation are the two oncogenes stpC (saimiri transformation-associated protein C) and tip (tyrosine kinase-interacting protein), which are encoded by a bicistronic transcript. Productive lytic replication of HVS is supported by owl monkey kidney (OMK) cells and other cell lines of different nonhuman primate species. However, a variety of human cell types can also be infected very efficiently by HVS, although in most cases the infection is nonproductive, meaning that no new viral particles are produced. The reason for this abortive infection remains unclear. One possible reason may be that humans are not the natural host of HVS and that there is a block in the lytic replication cycle due to cellular intrinsic restriction factors that cannot be counteracted by HVS. Another reason could be the absence of a factor in human cells that is indispensable for gene expression and replication and that is not conserved between human and nonhuman primate species. All herpesviruses and, specifically, the lymphotropic gammaherpesviruses have acquired numerous, usually intronless cellular gene homologs during coevolution with their host. The functions of these homologs have been adapted to specific viral purposes, including immune evasion, modulation of apoptosis, autophagy, or nucleotide metabolism. Specifically, the gammaherpesviruses encode one to three homologs of the cellular gene encoding the enzyme formylglycinamide ribotide amidotransferase (phosphoribosyl-formylglycinamidine synthase; EC 6.3.5.3; PurL, FGARAT, PFAS) (9); this gene family is thus found in organisms ranging from viruses to bacteria and in all eukaryotes. The function of most of these putative viral tegument proteins has not been studied in-depth; however, one of three homologs in murine gammaherpesvirus 68 (MHV-68) was reported to be responsible for degradation of ND10 host restriction factors (23). The HVS genome contains two genes encoding putative tegument proteins with homology to related gammaherpesviral FGARATs, the orf3 gene located at the left end and the orf75 gene located at the right end of the genome; no other function has been assigned so far.
In order to shed more light on the coevolution of herpesviruses and the cellular intrinsic immunity, this study addresses the role of ND10 components in the restriction of HVS infection in cell types of different primate species.
MATERIALS AND METHODS
Cells and viruses.
Primary human foreskin fibroblasts (HFFs) were prepared from human foreskin tissue as described previously (18) and were maintained in Eagle's minimal essential medium (Gibco/BRL, Eggenstein, Germany) supplemented with 5% fetal calf serum. HFFs with a small interfering RNA (siRNA)-mediated knockdown of PML (PML-kd; siPML2 cells), hDaxx (siDaxx1 cells), and Sp100 (siSp100 cells) and the respective control cells (designated vector or siC) were cultured in Dulbecco's minimal essential medium (Gibco/BRL, Eggenstein, Germany) supplemented with 10% fetal calf serum and 5 μg/ml puromycin (2, 36, 37). Primary rhesus fibroblasts (kindly provided by A. Kaur, Harvard Medical School, Southborough, MA), OMK cells, and Vero cells were cultivated in Dulbecco's minimal essential medium (Gibco/BRL, Eggenstein, Germany) supplemented with 10% fetal calf serum and gentamicin. The HVS strain employed in this study was obtained by reconstitution of infectious viruses using Bac43MOD containing the genomic sequence of HVS strain C488 (10) and a cytomegalovirus (CMV) immediate-early promoter-driven enhanced green fluorescent protein (EGFP) expression cassette inserted in the SwaI site at position 5656, analogous to earlier descriptions (42, 47). Stocks of wild-type (wt) and recombinant viruses were prepared, and the amount of viral genomes in the supernatant of infected OMK cells was quantified by quantitative PCR of the MCP locus with primers MCP_for (5′-CCATTTGCCTGTGTTGAGAGTTAA-3′) and MCP_rev (5′-CTCATTACCAGACCCATGTTATGAA-3) and probe MCP_probe (5′/56-FAM/CTCCGAGAG/ZEN/AGCCTATCTGAGATGCCC/3lABkFQ/-3′, where FAM is 6-carboxyfluorescein, ZEN is internal ZEN Quencher, and 3IABKFQ is 3′ Ioua Black FQ Quencher) (Integrated DNA Technologies, Coralville, IA).
Bacterial artificial chromosome (BAC) mutagenesis and plasmids.
The HVS Bac43MOD clone was used for recombination-mediated genetic engineering of Δorf3 and Δorf75 recombinants by a two-step markerless bacteriophage λ Red-mediated recombination strategy, using the kanamycin gene as a first selection marker (41). Linear fragments for homologous recombination were generated by PCR using Phusion High Fidelity DNA polymerase (Finnzymes); DpnI was added to digest the methylated plasmid template, and the amplification product was purified from an agarose gel with a Nucleobond gel extraction kit (Macherey & Nagel, Düren, Germany). Primers that were used to generate linear fragments for the manipulations are D3For (GCGACTTCAGTTTTTTTAGAGAATGGCCCACTACAAGTTACGTCTTAAAGTATCTCCATGGAAAACTATGAGGATGACGACGATAAGTAGGG), D3Rev (CATTTATGTAAATCTAAAAACATAGTTTTCCATGGAGATACTTTAAGACGTAACTTGTAGTGGGCCATTCAACCAATTAACCAATTCTGATTAG), D75For (ACTCACCGCACTGTAATTTATTATGCTAAGTGTGACTTTTCTCCATAGAACCAGCCCTTTCTTTTCATACAACCAATTAACCAATTCTGATTAG), and D75Rev (GGTATATGTTGCCATTGCCAAGTATGAAAAGAAAGGGCTGGTTCTATGGAGAAAAGTCACACTTAGCATAGGATGACGACGATAAGTAGGG) (purchased as ultramers; Integrated DNA Technologies).
For homologous recombination, the PCR fragment was then transformed into Escherichia coli strain GS1783 (gift of G. Smith, Northwestern University) (40) harboring Bac43MOD, and bacteriophage λ Red-mediated recombination was performed as described earlier (41, 46). Cells were then plated on agar plates containing 15 μg/ml kanamycin (first recombination) or 15 μg/ml chloramphenicol and 1% arabinose (second recombination) and incubated at 32°C for 1 to 2 days. Bacterial colonies growing on these plates were further analyzed. Reconstitution of recombinant HVS using purified BAC DNA was performed in OMK cells as described previously (42).
The full coding sequence of ORF3 and truncated versions were further amplified by PCR using Phusion polymerase and were subsequently cloned into vector pHM971 under transcriptional control of the CMV immediate-early promoter, resulting in FLAG epitope-tagged expression vectors for ORF3. In addition, the ORF3 coding sequence was inserted into the lentiviral vector pWPI, after including the hemagglutinin (HA) epitope tag at the amino terminus during PCR. Vesicular stomatitis virus G-protein-pseudotyped lentiviral vectors were produced by cotransfection of 293T cells with pWPI or pWPI-HA-ORF3, psPAX2, and pMDG2 (plasmids kindly provided by D. Trono, Geneva, Switzerland).
Viral nucleic acid isolation and analysis.
BACmid DNA was isolated by standard alkaline lysis from 5-ml liquid cultures. Subsequently, the integrity of BACmid DNA was analyzed by digestions with restriction enzyme XhoI and separation in 0.8% pulsed-field gel electrophoresis (PFGE) agarose (Bio-Rad) gels and 0.5× TBE (Tris-borate-EDTA) buffer by pulsed-field gel electrophoresis at 6 V/cm with a 120-degree field angle and a switch time linearly ramped from 1 s to 5 s over 16 h (CHEF DR III; Bio-Rad). For characterization of insertion or deletion of the aphaI selection marker, recombinant BACmids were analyzed by PCR, and the recombined regions within the BACmid DNA were controlled by sequence analysis (ABI 3130XL genetic analyzer; Applied Biosystems, Weiterstadt, Germany) in order to confirm the correct deletion sequences and to exclude accidental mutations.
Antibodies.
Endogenous PML was detected with the following antibodies: (i) a rabbit polyclonal antibody, generously provided by Peter Hemmerich (Leibniz Institute for Age Research, Fritz Lipmann Institute, Jena, Germany), which is directed against amino acids (aa) 460 to 497 of PML, (ii) mouse monoclonal antibody PG-M3 (Santa Cruz Biotechnology, Santa Cruz, CA), or (iii) mouse monoclonal antibody 5E10 (kindly provided by Roel van Driel, University of Amsterdam, Amsterdam, The Netherlands). Human Sp100 was detected by applying one of the following antibodies: (i) polyclonal antibody AB1380 from Chemicon (Schwalbach, Germany), (ii) rabbit polyclonal antiserum SpGH (a kind gift from Hans Will, Heinrich Pette Institute for Experimental Virology and Immunology, University of Hamburg, Hamburg, Germany), or (iii) a rabbit polyclonal antibody raised against aa 209 to 227 of Sp100 (a gift from Peter Hemmerich, Leibniz Institute for Age Research, Fritz Lipmann Institute, Jena, Germany). Primate Sp100 was detected with a rabbit polyclonal antiserum (ProteinTech Group, Chicago, IL). A rabbit monoclonal antibody (Epitomics, Burlingame, CA) or the mouse monoclonal antibody MCA2143 (Serotec, Duesseldorf, Germany) was used for detection of hDaxx. Monoclonal antibody AC-15, which recognizes beta-actin, was purchased from Sigma-Aldrich (Deisenhofen, Germany). Epitope-tagged proteins were detected using either the anti-FLAG tag antibody M2 (Sigma-Aldrich, Taufkirchen, Germany) or the anti-HA tag antibody HA.11 16B12 (Covance, Denver, CO). Horseradish peroxidase-conjugated antimouse and antirabbit secondary antibodies for Western blot analysis were obtained from Dianova (Hamburg, Germany), while Alexa Fluor 488-, Alexa Fluor 555-, and Alexa Fluor 647-conjugated secondary antibodies for indirect immunofluorescence experiments were purchased from Molecular Probes (Karlsruhe, Germany).
Peptide affinity-purified polyclonal rabbit antisera were raised against specific polypeptides derived from HVS proteins (ORF50, ORF3, and ORF75) (GenScript, Piscataway, NJ). Anti-ORF17 rabbit antiserum has been described earlier (34).
Immunofluorescence and Western blot analysis.
For immunofluorescence analysis, 3 × 105 HFFs or rhesus fibroblasts were plated onto coverslips for HVS infection. At the indicated time points postinfection, the cells were washed three times with phosphate-buffered saline (PBS), followed by fixation with 4% paraformaldehyde for 10 min at room temperature. Then, the cells were permeabilized with PBS–0.2% Triton X-100 on ice for 20 min, followed by incubation with the respective primary or secondary antibodies for 30 min at 37°C. Finally, the cells were mounted by using Vectashield mounting medium plus 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). The samples were examined by using a Leica TCS SP5 confocal microscope, with 488-nm, 543-nm, or 633-nm laser lines scanning each channel separately under image-capture conditions that eliminated channel overlap. The images were exported as tagged-image file format (TIFF) files and were then minimally processed using Photoshop software.
For Western blotting, extracts from infected cells were prepared in a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer, separated on sodium dodecyl sulfate-containing 8% or 12.5% polyacrylamide gels, and transferred to nitrocellulose or polyvinylidene difluoride membranes. Enhanced chemiluminescence was detected according to the manufacturer's protocol (GE Healthcare).
RESULTS
HVS selectively targets the ND10 component Sp100 for degradation.
Modulation of ND10 protein abundance appears to be a general feature of herpesviruses in order to counteract this antiviral host defense. MHV-68 (murid herpesvirus 4), for instance, has been shown to target PML for degradation in order to compromise ND10 integrity (23). Thus, we set out to elucidate whether the related primate rhadinovirus HVS is likewise capable of influencing ND10 composition. To address this issue, primary HFFs were infected in parallel with recombinant GFP-expressing HVS and MHV-68 using high-multiplicity-of-infection (high-MOI) conditions, as verified by monitoring of GFP-positive cells (data not shown). Importantly, HFF cells were chosen for this purpose since ND10 bodies have already been extensively characterized in these cells in prior studies of our group (36, 37, 38). Then, 24 h after virus inoculation, cell extracts were harvested and analyzed for PML, Sp100, and hDaxx protein levels (Fig. 1A to C). Interestingly, although it has been claimed that the MHV-68-induced degradation of PML is limited to murine fibroblasts and cannot be observed with human PML (23), we could detect a complete elimination of human PML variants upon infection of HFF cells with MHV-68 (Fig. 1A, lane 2). Furthermore, this complete loss of PML as a consequence of MHV-68 infection presumably also accounted for the simultaneously observed disappearance of the low-mobility isoforms of Sp100 (Fig. 1B, lane 2), comparable to the situation in herpes simplex virus type 1-infected cells (7) or after depletion of the defining ND10 factor in HFFs by short hairpin RNA-mediated knockdown of PML (12, 36). Intriguingly, PML protein levels remained completely unaltered following infection with HVS (Fig. 1A, lane 3). Instead, HVS induced a total loss of all Sp100 isoforms compared to MHV-68- or mock-infected cells (Fig. 1B, lane 3), while hDaxx protein levels were unaltered after infection with both rhadinoviruses (Fig. 1C). The selective targeting of Sp100 by HVS could additionally be confirmed by immunofluorescence analysis, showing that in HVS-infected cells, which were identified by GFP expression (Fig. 1D, panels b, f, and k), PML and hDaxx were still detectable in numerous foci, being characteristic for their assembly in ND10 structures (Fig. 1D, panels c and g, respectively). Sp100 accumulations, on the contrary, could be found only in uninfected HFFs but were absent from neighboring HVS-positive cells (Fig. 1D, panel m). Thus, HVS seems to have evolved a unique strategy to cope with the antiviral aspects of ND10 since it specifically attacks the host factor Sp100 and leaves PML and other ND10 components unaffected.
Fig 1.
Examination of the relative abundance of the major ND10 factors PML, Sp100, and hDaxx during infection with HVS and MHV-68, respectively. (A to C) Immunoblots of primary HFFs infected with MHV-68/GFP or HVS-GFP at a high MOI. Cell lysates were harvested at 24 h postinfection and subjected to SDS-PAGE. The blots were probed with antibodies against the three major ND10 constituents, PML (A), Sp100 (B), and hDaxx (C), as indicated on the right of the panels. Detection of beta-actin was included as an internal loading control. Molecular masses are indicated to the left of each panel. (D) Immunofluorescence analysis of HVS-GFP-infected HFF cells. At 24 h postinfection, cells were fixed and stained for the ND10 factors PML, hDaxx, and Sp100. HVS-positive cells were identified by GFP expression. Cell nuclei were visualized by costaining with DAPI.
Downregulation of Sp100 by HVS in cells of nonhuman primates.
Experimental data for the retroviral restriction factor TRIM5alpha (33), which is, like PML, a member of the tripartite motif family of proteins (29), suggest a close coevolution of viruses and cellular restriction factors. Since HVS infects various primate species, we wanted to determine the influence of HVS on PML, Sp100, and Daxx of nonhuman primate origin. To this end, fibroblast cells derived from rhesus macaques (Macaca mulatta) were infected with wt HVS-GFP for immunofluorescence analyses. In this context, it is of note that some of the antibodies which readily detected human-derived PML and Sp100 failed to visualize the respective primate counterparts (anti-PML-mouse PG-M3 from Santa Cruz; anti-Sp100-rabbit generated by Peter Hemmerich from Jena, Germany) (Fig. 2A). This further reflects the existence of species-specific sequence differences of these host restriction factors that may be of importance for their recognition by virus-encoded effector proteins. Nevertheless, we were able to identify antibodies that could be used for the detection of PML, Sp100, and Daxx in rhesus fibroblasts (Fig. 2B). Comparable to the results obtained for ND10 factors of human origin, we found that PML and Daxx remained unaffected, while Sp100 signals vanished completely after infection of rhesus fibroblasts with HVS (Fig. 2C).
Fig 2.
Sp100 downregulation of nonhuman primate origin by HVS. (A to C) Fibroblast cells (Fibs) derived from rhesus macaques (Macaca mulatta) were either mock infected (A and B) or infected with HVS-GFP (C). Twenty-four hours later, cells were fixed and stained for the major ND10 components PML (anti-PML rabbit antiserum [PML-r] generated by Peter Hemmerich, Jena, Germany,), hDaxx (Daxx-m; MCA2143), and Sp100 (ProteinTech Group). No signals were obtained with the monoclonal antibody PG-M3 against PML (A, panel c, PML-m) and the anti-Sp100 rabbit antibody (Sp100-r; generated by Peter Hemmerich, Jena, Germany) (A, panel f). HVS-positive cells were identified by GFP expression. Cell nuclei were visualized by costaining with DAPI.
Proteasomal activity is required for HVS-mediated degradation of Sp100.
In order to clarify the mechanism by which HVS is able to induce the destruction of Sp100 upon infection, we next explored the role of the proteasome in this context. Results shown in Fig. 3A and B indicate that treatment of cells with the proteasome inhibitor MG132 significantly diminished HVS-promoted Sp100 degradation since considerable amounts of Sp100 became detectable again (Fig. 3A, lane 3, and B, panels i and k to m). In this context, it is of note that stabilization of Sp100 in the presence of MG132, in addition, resulted in a clear reduction in HVS-based GFP expression compared to that by MG132-untreated cells (Fig. 3A, third panel). This finding further supports the notion that Sp100 exerts a repressive function on HVS replication which has to be antagonized by the virus. To determine whether loss of Sp100 following HVS infection was dependent on synthesis of viral or cellular proteins, we treated HFF cells with cycloheximide (CHX) to block protein production. However, as evident from Fig. 3A and B, Sp100 was still efficiently downregulated by HVS in the presence of CHX, whereas de novo viral GFP expression was clearly inhibited (Fig. 3A, lane 4, and B, panels n to q). This suggests that a tegument protein of HVS is responsible for depleting Sp100 immediately upon infection. Intriguingly, treatment of cells with CHX resulted in an aberrant, asymmetric PML distribution in HVS-infected cells which could not be observed in uninfected HFFs treated with CHX (Fig. 3B, panel p, and data not shown). Taken together, our data indicate that Sp100 turnover in HVS-infected cells is regulated in a proteasome-dependent manner by a component of the virus particle.
Fig 3.
HVS-induced Sp100 degradation occurs by the proteasome and is associated with a virion component. (A) Immunoblots of HFFs infected with wt HVS-GFP at a high MOI that were either untreated or treated with 5 μM of the proteasome inhibitor MG132 or with 150 μg/ml of cycloheximide (CHX). The cells were harvested at 8 h postinfection (8 hpi), and the relative protein levels of Sp100, PML, and GFP were analyzed. Beta-actin served as a loading control. (B) HFF cells were mock infected or infected with HVS-GFP for 10 h. Some cells were simultaneously treated with MG132 or CHX at the time of virus inoculation, as indicated at the left of the respective panels. The cells were stained with antibodies directed against PML and Sp100. Cell nuclei were detected by DAPI staining.
Herpesvirus saimiri FGARATs are incorporated into virions.
MHV-68 ORF75c, one of three viral FGARAT (vFGARAT) homologs, leads to the degradation of PML and, consequently, other ND10 components (14, 23). In a candidate gene approach, we therefore addressed the question whether FGARATs of HVS play a role in the degradation of Sp100; first, we analyzed the expression of ORF3 and ORF75 in infected OMK cells. As shown in Fig. 4, both proteins are expressed late during the lytic infection cycle, and both ORF3 and ORF75 show similar expression kinetics. Moreover, by immunoblotting of virions purified by ultracentrifugation, we were able to demonstrate that both proteins are incorporated into viral particles. This is consistent with their description as putative tegument proteins and also as FGARAT proteins of related rhadinoviruses like KSHV and MHV-68 (14, 31).
Fig 4.
HVS ORF3 and ORF75 proteins are incorporated into viral particles. Expression of viral ORF3 and ORF75 in infected OMK cells detected at the indicated times postinfection and in viral particles prepared from supernatants of infected cells. Immunoblotting was done with ORF3- and ORF75-specific rabbit polyclonal antibodies and was detected by enhanced chemiluminescence.
Deletion of orf3 and orf75 genes from the HVS genome.
For further elucidation of the involvement of HVS ORF3 and ORF75 in Sp100 degradation, we decided to delete the genes for both proteins separately from the HVS genome. The orf3 and orf75 genes are located close to the left end and at the right end of the coding-rich, low-density L-DNA, respectively (Fig. 5A). We generated recombinant HVS deletion mutants by using recombination-mediated, site-directed mutagenesis of cloned HVS BACmid Bac43MOD. Recombinant BACmid technology has significantly advanced genetic studies in large DNA viruses (1, 27); the internal repeat (IR) and the multiple terminal repetitive (TR) sequences present in the rhadinoviruses mandate extra caution when applying the techniques of homologous recombination in E. coli in multiple rounds of mutagenesis. In particular, the possibility of the loss of large parts of these TR sequences cannot be reliably excluded by conventional electrophoresis or by DNA sequencing, which might be useful for the detection of other genome rearrangements (45). Significant loss of TR can lead to genome sizes below the packaging limit of the viral particle and thereby inefficient reconstitution or selection of aberrant recombination events that replenish the missing repeats. This underlines the necessity to verify recombinant gammaherpesvirus BACmids also by techniques such as PFGE. All mutants were validated using PCR analysis, XhoI restriction enzyme digestion followed by PFGE, and direct sequencing of the recombination junctions. Pulsed-field gel electrophoresis shows expected restriction patterns of wild-type (Fig. 5C, third lane) and deletion mutant (Fig. 5C, fourth and fifth lanes) BACs, including the full maintenance of the terminal repeat sequences. Since the orf75 gene harbors a restriction site for XhoI, deletion of the respective open reading frame (ORF) leads to an altered XhoI cleavage pattern (Fig. 5B and C). All of these experiments proved that the recombinant genomes contained the correct deletion with no other detectable rearrangements, and they were therefore used for further analysis.
Fig 5.
Construction and replication of ORF3 and ORF75 deletion viruses. (A) Representation of the BACmid Bac43MOD encompassing the HVS strain C488 genome. The locations of the mini-F plasmid, the EGFP expression cassette, and orf3 and orf75 are indicated. (B) Schematic details of the deletions introduced into the recombinant viruses. (C) Pulsed-field gel electrophoresis of XhoI-digested BACmids. (D) Expression of viral early ORF50/RTA, late ORF17/protease, and ORF3 and ORF75 in infected OMK cells detected by immunoblotting using specific rabbit polyclonal antibodies and swine antirabbit horseradish peroxidase conjugate. (E) Delayed lytic replication of BACmid-derived HVS with orf3 and orf75 deletions compared to replication of the wild-type virus. Viral genome copy numbers in the supernatant of infected cells were quantified by a quantitative PCR detecting the viral orf25 gene at the indicated time points after infection of OMK cells at a low MOI (MOI, 0.05).
Recombinant viruses were generated by transfection of BACmid DNA into permissive OMK cells, which resulted in the reconstitution of both orf3 and orf75 deletion mutants. In order to characterize the replication of these mutants, OMK cells were infected at a high MOI (MOI, 1) and cells were harvested at 12, 24, 36, and 48 h postinfection. Subsequent Western blot analyses revealed that, as expected, deletion of the orf3 and orf75 genes from the viral genomes resulted in the absence of the respective proteins compared to infection with BACmid-derived wild-type virus (Fig. 5D). We then tested the expression of two other proteins, ORF50 (RTA), the early R transactivator protein, and ORF17, the late lytic viral protease/minor capsid scaffold protein; both showed expression kinetics in deletion viruses similar to those in the wild type (Fig. 5D). Moreover, we assessed whether the deletion mutants showed similar replication kinetics as the wild-type virus. To this end, OMK cells were infected with a low MOI (MOI, 0.05), and the viral genome copy number from supernatants of infected cells was quantified at various time points. As depicted in Fig. 5E, both deletion viruses showed a replication defect compared to Bac43wt, with the Bac43 Δorf3 virus showing the defect even more than the Bac43 Δorf75 virus. This replication defect could not be observed in single-step growth curves after infection with a high MOI, which is consistent with observations in other herpesviruses and the hypothesis that cellular intrinsic immunity can be overwhelmed with large numbers of viral particles infecting a cell.
ORF3 but not ORF75 is necessary for the selective degradation of Sp100.
To examine whether one of the two HVS-encoded FGARAT homologs, ORF3 or ORF75, is involved in Sp100 degradation, we infected HFFs with mutant viruses lacking the respective ORFs. As indicated in Fig. 6A and B, deletion of ORF75 did not abrogate the Sp100-degrading activity of HVS, since HVS ΔORF75-infected HFFs exhibited a complete loss of Sp100 (Fig. 6A, lane 3, and B, panels i and k to m), comparable to wt HVS-infected cells (Fig. 6A, lane 2, and B, panels e to h). While the ORF75 mutant virus was still able to downregulate Sp100, an ORF3-deficient virus, on the contrary, failed to target Sp100 (Fig. 6A, lane 4, and B, panels n to q). The overall Sp100 protein abundance in HVS ΔORF3-infected cells was similar to that in mock-treated HFFs (Fig. 6A, lane 4). By immunofluorescence analysis, we could demonstrate that Sp100 was still detectable in GFP-expressing HVS ΔORF3-infected cells in dotted structures that colocalized with PML in ND10 (Fig. 6B, panels n to q). Hence, the viral tegument protein ORF3 could be identified to be responsible for degradation of Sp100 following HVS infection. Finally, we could again show that PML and hDaxx protein levels as well as their subnuclear distribution pattern remain unaltered upon HVS infection (Fig. 6A, second and third panels, and C). This further underlines the assumption that Sp100 is the only major ND10 factor which is modulated by HVS upon infection and that this occurs in an ORF3-dependent manner.
Fig 6.
The HVS tegument protein ORF3 acts as an antagonist of Sp100 and induces its degradation. (A to C) HFFs were infected in parallel for Western blotting (A) as well as immunofluorescence analyses (B and C) with wt HVS-GFP or mutant viruses deficient in either ORF75 (ΔORF75) or ORF3 (ΔORF3) using high-MOI conditions (as verified by GFP expression). (A) Cell extracts for immunoblotting were harvested at 24 h postinfection and analyzed for expression of Sp100, PML, hDaxx, and beta-actin. (B and C) For indirect immunofluorescence analysis, the cells were fixed at 24 h postinfection and stained with antibodies against PML, Sp100, and hDaxx, as well as costained with DAPI. Infected cells were identified by GFP fluorescence.
ND10 integrity is not required for the ORF3-induced loss of Sp100.
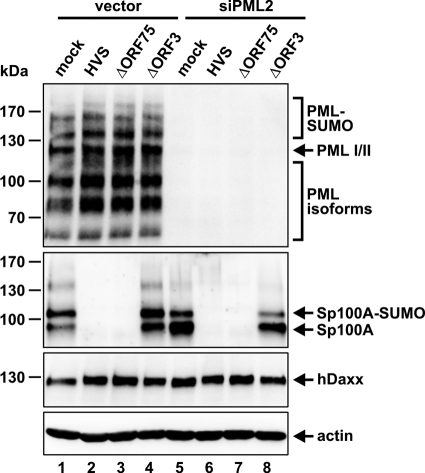
The PML protein has been shown to play a critical role in the formation of ND10 bodies as it recruits Sp100 and hDaxx to these subnuclear structures (17). Next, we set out to elucidate whether intact ND10 domains are a prerequisite for ORF3 to target Sp100 for degradation. To this end, we infected PML-kd HFFs (siPML2) devoid of ND10 structures, as well as corresponding control cells, with either wt HVS or the aforementioned viruses with ORF75 and ORF3 deletions. Interestingly, even in the absence of PML (Fig. 7, first panel, lanes 5 to 8), wt HVS and the ORF75-knockout virus were still capable of diminishing Sp100 protein abundance (Fig. 7, second panel, lanes 6 and 7), while the ORF3-deficient virus again lost the ability to do so (Fig. 7, second panel, lane 8). On the basis of this finding, we conclude that the Sp100-degrading activity of ORF3 is not dependent on a localization of Sp100 in ND10.
Fig 7.
ORF3-mediated Sp100 degradation occurs in an ND10-independent manner. PML-kd (siPML2) or control HFFs (vector) were mock infected or infected with either wt HVS, HVS ΔORF75 (ΔORF75), or HVS ΔORF3 (ΔORF3). Lysates were harvested at 24 h postinfection and analyzed by Western blotting for PML, Sp100, and hDaxx protein levels. Beta-actin was included as an internal loading control.
HVS ORF3 mediates the degradation of cellular Sp100.
Next, we generated FLAG-tagged ORF3 expression constructs as well as a lentiviral vector encoding an HA-tagged full-length ORF3. In order to explore the effect of isolated ORF3 expression on endogenous Sp100 levels, HeLa cells were either transduced with lentiviral particles or transfected with an expression construct encoding aa 562 to 959 of ORF3. After expression of full-length ORF3, we detected an accumulation of ORF3 within the nuclei of transduced HeLa cells (Fig. 8a and c). When Sp100 and ORF3 were costained, we observed that ORF3-expressing cells were either totally devoid of Sp100 or Sp100 protein levels were significantly reduced, indicating that ORF3 expression alone is sufficient to induce Sp100 degradation (Fig. 1A to D). Moreover, these experiments revealed that a truncated ORF3 encoding the central domain (aa 562 to 959) of the protein was no longer able to degrade Sp100 (Fig. 8e to i and k to m). Interestingly, this protein exhibited a strong colocalization with Sp100 both in HeLa cells and in rhesus fibroblasts (Fig. 8e to i and k to m), suggesting that the central part of ORF3 is sufficient to mediate an interaction with ND10.
Fig 8.
ORF3 expression alone is sufficient to modulate Sp100 protein. HeLa cells (a to h) and primary rhesus fibroblasts (i and k to m) either were transduced with a lentiviral vector encoding an epitope-tagged full-length HA-ORF3 (a to d) or were transfected with an expression plasmid for FLAG-ORF3-aa 562 to 959 (panels e to i and k to m). Cells showing full-length ORF3 protein in the nucleus are either devoid of detectable Sp100 or exhibit a strongly reduced and microdispersed staining pattern (panels b to d); the central region of ORF3 colocalizes with Sp100 but is not sufficient to mediate Sp100 dispersal (panels e to i and k to m). Fixed cells were stained with rabbit Sp100/antirabbit Alexa Fluor 488 and anti-HA/antimouse Alexa Fluor 555 (for detection of full-length HA-ORF3) (a to c) or FLAG M2/antimouse Alexa Fluor 555 (for detection of FLAG-ORF3-aa 562 to 959) (e to i and k to m), counterstained with DAPI, and detected by confocal microscopy.
Knockdown of Sp100 results in derepression of the ORF3 mutant virus.
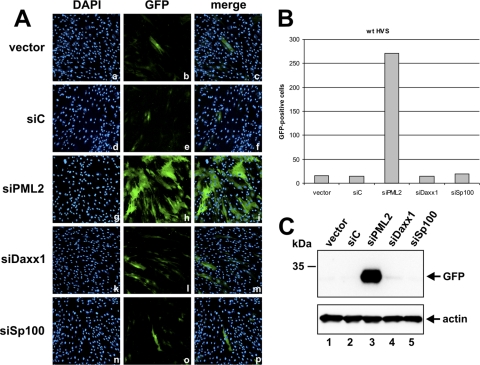
Consistent with the idea that PML carries out an intrinsic cellular defense against MHV-68, murid herpesvirus 4 replicates with higher efficiency in cells depleted for PML (23). To investigate whether Sp100 exerts a similar effect on HVS, we tested whether knockdown of Sp100 could complement the defect of the ORF3 deletion virus. In accordance with this assumption, we detected significantly more GFP-positive cells following HVS ΔORF3 infection in case Sp100 was absent, as determined by immunofluorescence analyses (Fig. 9A and B; compare control cells designated vector and siC to the Sp100-kd cells, siSp100). In addition, loss of Sp100 resulted in expression of elevated amounts of GFP from the viral genome (Fig. 9C, first panel; compare lanes 1 and 2 with lane 5). Consequently, these findings further strengthen the concept that Sp100 mediates an antiviral response against HVS which is counteracted by ORF3. Intriguingly, however, the positive effect on viral gene expression was even more pronounced when the ND10-defining component PML was missing (Fig. 9A, panels g to i; B, siPML2; and C, lane 3). Knockdown of hDaxx, on the contrary, did not affect HVS infection (Fig. 9A, panels k to m; B, siDaxx1; and C, lane 4). Collectively, these results give rise to the idea that HVS replication is compromised not only by Sp100 but also by the ND10 key component PML.
Fig 9.
Analysis of HVS ΔORF3-GFP replication in the absence of the three major ND10 factors PML, hDaxx, and Sp100. Retrovirally transduced HFFs expressing the respective siRNAs, as indicated (vector, siC, siPML2, siDaxx1, and siSp100), were infected with recombinant HVS ΔORF3-GFP. (A) GFP fluorescence 6 days after infection of the indicated HFFs; (B) direct quantification of the number of GFP-positive cells at day 6 postinfection; (C) Western blot analyses of HVS ΔORF3-GFP gene expression in the retrovirally transduced HFFs (vector, siC, siPML2, siDaxx1, and siSp100) using an antibody directed against GFP. The detection of beta-actin served as a loading control.
HVS infection is strongly restricted by PML.
Given the observation that depletion of PML from primary human fibroblasts facilitates HVS ΔORF3 gene expression, thus leading to a higher number of infected cells (see Fig. 9A to C), we intended to dissect the role of PML during wild-type HVS infection. Consistent with the results obtained for HVS ΔORF3, we detected an approximately 10-fold higher number of GFP-expressing PML-kd cells than control cells upon infection with wt HVS (Fig. 10A and B; compare control cells designated vector and siC to the PML-kd cells, siPML2). Interestingly, however, the effect of more cells initiating the viral gene expression program was this time limited to PML-deficient HFFs (Fig. 10C, first and second panels, lane 3), since siSp100 cells behaved like siDaxx1 or the corresponding control cell vector and siC when infected with the wild-type virus (Fig. 10C, first and second panels, lanes 1, 2, 4, and 5). Nonetheless, the outcome of this experiment is not surprising, given the fact that wt HVS expresses ORF3, which efficiently controls the restrictive potential of Sp100. In light of these findings, it is tempting to speculate that the strongly enhanced replication of the wild-type virus in the absence of PML is due to a lack of efficient countermeasures of incoming HVS against the antiviral activity of PML. Thus, these data indicate that PML has the potential to negatively influence HVS replication in fibroblasts.
Fig 10.
Analysis of wt HVS-GFP replication in the absence of the three major ND10 factors PML, hDaxx, and Sp100. Retrovirally transduced HFFs expressing the respective siRNAs, as indicated (vector, siC, siPML2, siDaxx1, and siSp100), were infected with recombinant HVS-GFP. (A) GFP fluorescence 6 days after infection of the indicated HFFs; (B) direct quantification of the number of GFP-positive cells at day 6 postinfection; (C) Western blot analyses of HVS-GFP gene expression in the retrovirally transduced HFFs (vector, siC, siPML2, siDaxx1, and siSp100) using an antibody directed against GFP. The detection of beta-actin served as a loading control.
DISCUSSION
In this study, we describe a novel mode of herpesviral interference with intrinsic immunity instituted by the ND10 protein complex, namely, the selective degradation of the ND10 factor Sp100. So far, herpesviruses have mainly been found to target the major ND10 component PML, as exemplified by the herpes simplex virus regulatory protein ICP0, which has recently been shown to induce the proteasomal degradation of PML either via acting as a SUMO-targeted ubiquitin ligase or via SUMO-independent ubiquitination (5). Although ICP0 is also able to deplete the sumoylated, lower-mobility isoforms of Sp100, this is most probably an indirect effect of PML degradation, since an identical alteration of the Sp100 isoform expression could be detected in PML-knockdown cells (8, 12, 30). Similarly, the IE1 protein of human cytomegalovirus targets PML via inducing a desumoylation of this major ND10 component (22, 28). Since sumoylation of PML is essential for the integrity of ND10, this leads to a dispersal of the subnuclear structure. Interestingly, we and others could recently show that IE1 can also directly affect Sp100 (20, 36). In particular, at late times of the human CMV (HCMV) replicative cycle, a proteasomal degradation of Sp100 was observed; however, degradation was dependent on the additional presence of viral true late gene products (20, 36). Furthermore, HCMV encodes a second ND10-antagonistic protein, the tegument protein pp71, which directly interacts with hDaxx and is able to induce the proteasomal degradation of this ND10 protein (16, 32). Thus, HVS appears to be unique insofar as, specifically, Sp100 was completely depleted after infection of both primary human and rhesus fibroblasts, while the other ND10 factors, PML and hDaxx, were apparently not affected. Of note, similar to HCMV-induced hDaxx degradation, HVS-induced Sp100 depletion could be inhibited by the addition of MG132, indicating a proteasome-dependent mechanism, and was still observable after infection in the presence of cycloheximide, suggesting the involvement of a virion component.
As the responsible viral effector protein, we identified a tegument component encoded by one of the viral homologs of the cellular enzyme formylglycinamide ribotide amidotransferase (FGARAT), the gene orf3 of HVS. This is based on two lines of experimental evidence: (i) deletion of orf3 from the HVS genome completely abrogated Sp100 degradation, and (ii) the isolated expression of full-length orf3 was able to induce Sp100 depletion, demonstrating that ORF3 is not only required but also sufficient for this. Surprisingly, while full-length ORF3 could be detected in the nucleus of transduced cells, it is predominantly cytoplasmic in infected cells (data not shown). Furthermore, a central domain, which was defective in Sp100 degradation, exhibited a nuclear localization with a strict targeting to ND10. This indicates that the central region of ORF3 contains a domain for interaction with ND10 or ND10-associated factors. Whether ORF3 is able to interact with Sp100 in a direct manner will require further investigation; however, we could show that ND10 integrity is not a prerequisite for ORF3-mediated Sp100 degradation. The cytoplasmic localization of an ND10-targeting factor is not without precedent, since the tegument protein pp71 of HCMV also translocates to the cytoplasm during late times of infection (15). The cytoplasmic localization of ORF3 late in infection, which is punctate at times, may correspond to virions on their way to egress; however, further studies will be required to identify the determinants of ORF3 for subcellular localization and to elucidate the exact mode of action on Sp100.
Interestingly, two other members of the viral FGARAT homologs, BNRF1 of Epstein-Barr virus (EBV) and ORF75c of MHV-68, have also been found to target ND10 (14, 23, 43). BNRF1 has recently been shown to colocalize with Daxx to PML nuclear bodies and to disperse the chromatin remodeling factor ATRX from nuclear bodies, which occurs in a Daxx interaction-dependent manner (43). ORF75c has been reported to induce the proteasomal degradation of PML (14, 23). For ORF75c, it was described that PML degradation occurs only after MHV-68 infection of murine fibroblasts (23). In contrast, we observed in this study that MHV-68 can also degrade PML in human cells. This was accompanied by an alteration of Sp100 isoform expression, while hDaxx protein levels were not affected (Fig. 1). However, as discussed for ICP0, the effects of ORF75c on Sp100 are most probably indirect and due to the efficient depletion of PML (8, 12). An ORF75c deletion mutant exhibited a severe replication defect which was, however, due to not only a defect in PML degradation but also the lack of an additional ORF75c function in viral capsid transport (14). For the ORF3 deletion mutant generated in this study, we observed a significant delay in the release of viral particles, confirming the functional importance of this protein for HVS replication. Whether ORF3 exerts multiple functions like ORF75c of MHV-68 will require further investigation.
Thus, although the FGARAT homologs BNRF1, ORF75c, and ORF3 share an ND10-antagonizing function, the specific target proteins have apparently diverged during evolution. Ling et al. (2008) have already provided a first sequence-based comparison between the viral homologs to the human FGARAT (23). All gammaherpesviruses encode at least one FGARAT homolog at the respective position of the HVS ORF75; this is also true for the lymphocrypto- and the rhadinoviruses; since the EBV genome in the GenBank database is in a reverse orientation to HVS, the EBV BNRF1 is found at the left end of the genome. Two vFGARATs are found in the New World primate viruses HVS, herpesvirus ateles (HVA), and the ruminant rhadinoviruses bovine herpesvirus type 4 (BHV4), alcelaphine herpesvirus (AlHV), and ovine herpesvirus type 2 (OvHV2); they are encoded by ORF3 and ORF75 at opposing ends of the respective genomes; as mentioned above, the known Old World primate gammaherpesviruses EBV, rhesus rhadinovirus (RRV), and KSHV encode a single homolog, BNRF1 or ORF75. The murine gammaherpesvirus 68, which was isolated from the bank vole (Myodes glareolus), and the closely related wood mouse (Apodemus sylvaticus) gammaherpesvirus have three adjacent vFGARAT homologs encoded by ORF75a, -75b, and -75c. A Peruvian rodent herpesvirus from the pygmy rice rat (Oligoryzomys microtis) encodes two proteins, ORF75a and -75b, that are homologous to ORF75b and -75c of MHV-68.
The overall homology of these vFGARATs to human or mouse FGARAT is in the range of 23 to 32% amino acid identity; as already noted by Ling and colleagues, the strongest homology is in the carboxy-terminal half of the vFGARAT homologs, corresponding to aa 660 to 1338 of the human or mouse FGARAT, and includes the second PurL repeat, one aminoimidazole ribonucleotide (AIR) synthase C domain, and the carboxy-terminal glutamine amidotransferase 1 domain (GATase1) (23). However, none of the viral proteins seems to have conserved the ATP-binding site or glutamine binding regions of the cellular enzymes. In addition, the amino-terminal half of the vFGARAT shows several domains of homology between the viral genes that are distinct from the cellular FGARAT. When aligned by use of the ClustalW program, the ORF3 and eukaryotic FGARAT homologs, the rhadinoviral ORF75, and the lymphocryptoviral BNRF1 cluster independently, indicating that the viral homologs have evolved in different directions and possibly fulfill distinct functions (Fig. 11). Specifically, this alignment shows that the second vFGARAT encoded by ORF3 of the New World primate rhadinoviruses HVS and HVA and the ruminant viruses BHV4, AlHV1, OvHV2, and equine herpesvirus type 2 (EHV2) has evolved away from the ORF75.
Fig 11.
Phylogenetic tree of cellular and viral FGARAT proteins. The amino acid sequences of the respective FGARAT homologs were aligned by use of the Clustal Omega program (35), and an unrooted phylogenetic tree was calculated with ClustalW2 software (21) using the neighbor-joining algorithm to construct trees from the distance matrix after exclusion of gaps and correction for multiple substitutions. The various ORF3, rodent virus ORF75, and other ORF75 cluster to different branches of the phylogenetic tree. EHV2, equine herpesvirus 2; PoLV, porcine lymphocryptovirus; RoHV, rodent herpesvirus Peru; RhLCV, rhesus lymphocryptovirus; CjLCV, Callithrix jacchus lymphocryptovirus; MfRR V, Macaca fuscata rhadinovirus; MmRRV, Macaca mulatta rhadinovirus; Bac16, KSHV Bac16 strain; KSHV, KSHV BC-1 strain.
Evidence for a functional antagonization of ND10 target proteins by the vFGARAT homologs BNRF1 and ORF75c was derived from complementation studies, demonstrating a more robust replication of mutant viruses in cells that were depleted of the respective ND10 protein (23, 43). Consequently, we investigated whether HVS was affected by a depletion of Sp100: we observed a strong complementation of the ORF3 deletion virus in cells lacking Sp100, whereas, as expected, gene expression of wild-type HVS was not increased. These experiments, which were conducted with cells individually depleted of the major ND10 factors PML, Sp100, and hDaxx, led to another important observation: PML, which was not degraded after HVS infection, strongly restricted gene expression of both wild-type HVS and the virus with the ORF3 deletion. Thus, one may speculate that HVS does not encode a factor that efficiently counteracts the repressive function of PML. Interestingly, this appears to be a common property of primate rhadinoviruses: for instance, PML is highly expressed in KSHV PEL-infected cells (19), which may be in part due to the known proinflammatory effects of KSHV infection. KSHV K10.5/IRF3/LANA2 overexpression is associated with a limited redistribution of PML (24). The KSHV ORF K8 encodes an early lytic protein with a prototypic basic leucine zipper (bZIP) at the C-terminal region that is homologous to the EBV BZLF1 Zta homolog. K8 is a SIM-dependent, SUMO-2/3-specific SUMO E3 ligase (6) that colocalizes with PML (44); it recruits p53 and probably sequesters it to PML bodies but has no function in dispersing PML bodies (19).
Thus, in comparison to the alpha- and betaherpesviruses, PML bodies are inefficiently antagonized by the primate gammaherpesviruses EBV, HVS, and KSHV. It may be conceivable that this virus subgroup did not evolve evasion strategies that fully inactivate the ND10 organizer, and as a consequence, latency is the favored outcome of the typically low multiplicity infection of host cells. In line with this is the observation that pathogenicity by the gammaherpesviruses is exerted by latently infected, transformed cells and less by destructive lytic replication. Furthermore, it may be worthwhile to investigate to which extent the species specificity of gammaherpesvirus replication, e.g., the low efficiency of rodent cell infection by HVS, may be explained by differences in the ability to degrade the rodent Sp100 or other ND10 components.
ACKNOWLEDGMENT
This work was supported by the Deutsche Forschungsgemeinschaft (SFB796, projects B1 and B3).
We thank Peter Hemmerich (Jena, Germany), Roel van Driel (Amsterdam, The Netherlands), and Hans Will (Hamburg, Germany) for the kind gift of antibodies. Furthermore, we thank Brigitte Scholz and Doris Lengenfelder for excellent technical assistance.
Footnotes
Published ahead of print 25 January 2012
REFERENCES
- 1. Adler H, Messerle M, Koszinowski UH. 2003. Cloning of herpesviral genomes as bacterial artificial chromosomes. Rev. Med. Virol. 13:111–121 [DOI] [PubMed] [Google Scholar]
- 2. Adler M, Tavalai N, Muller R, Stamminger T. 2011. Human cytomegalovirus immediate-early gene expression is restricted by the nuclear domain 10 component Sp100. J. Gen. Virol. 92:1532–1538 [DOI] [PubMed] [Google Scholar]
- 3. Bieniasz PD. 2004. Intrinsic immunity: a front-line defense against viral attack. Nat. Immunol. 5:1109–1115 [DOI] [PubMed] [Google Scholar]
- 4. Biesinger B, et al. 1992. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc. Natl. Acad. Sci. U. S. A. 89:3116–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boutell C, et al. 2011. A viral ubiquitin ligase has substrate preferential SUMO targeted ubiquitin ligase activity that counteracts intrinsic antiviral defence. PLoS Pathog. 7:e1002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang PC, et al. 2010. Kaposi's sarcoma-associated herpesvirus (KSHV) encodes a SUMO E3 ligase that is SIM-dependent and SUMO-2/3-specific. J. Biol. Chem. 285:5266–5273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chelbi-Alix MK, de The H. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935–941 [DOI] [PubMed] [Google Scholar]
- 8. Cuchet D, et al. 2011. PML isoforms I and II participate in PML-dependent restriction of HSV-1 replication. J. Cell Sci. 124:280–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ensser A, Pflanz R, Fleckenstein B. 1997. Primary structure of the alcelaphine herpesvirus 1 genome. J. Virol. 71:6517–6525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ensser A, Thurau M, Wittmann S, Fickenscher H. 2003. The genome of herpesvirus saimiri C488 which is capable of transforming human T cells. Virology 314:471–487 [DOI] [PubMed] [Google Scholar]
- 11. Everett RD, Chelbi-Alix MK. 2007. PML and PML nuclear bodies: implications in antiviral defence. Biochimie 89:819–830 [DOI] [PubMed] [Google Scholar]
- 12. Everett RD, et al. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 80:7995–8005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fleckenstein B, Desrosiers RC. 1982. Herpesvirus saimiri and herpesvirus ateles, p 253–332 In Roizman B. (ed), The herpesviruses, vol 1 Plenum Press, New York, NY [Google Scholar]
- 14. Gaspar M, Gill MB, Losing JB, May JS, Stevenson PG. 2008. Multiple functions for ORF75c in murid herpesvirus-4 infection. PLoS One 3:e2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hensel GM, et al. 1996. Intracellular localization and expression of the human cytomegalovirus matrix phosphoprotein pp71 (ppUL82): evidence for its translocation into the nucleus. J. Gen. Virol. 77(Pt 12):3087–3097 [DOI] [PubMed] [Google Scholar]
- 16. Hofmann H, Sindre H, Stamminger T. 2002. Functional interaction between the pp71 protein of human cytomegalovirus and the PML-interacting protein human Daxx. J. Virol. 76:5769–5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ishov AM, et al. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein Daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jahn G, et al. 1984. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J. Virol. 49:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katano H, Ogawa-Goto K, Hasegawa H, Kurata T, Sata T. 2001. Human-herpesvirus-8-encoded K8 protein colocalizes with the promyelocytic leukemia protein (PML) bodies and recruits p53 to the PML bodies. Virology 286:446–455 [DOI] [PubMed] [Google Scholar]
- 20. Kim YE, et al. 2011. Human cytomegalovirus infection causes degradation of Sp100 proteins that suppress viral gene expression. J. Virol. 85:11928–11937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larkin MA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 22. Lee HR, et al. 2004. Ability of the human cytomegalovirus IE1 protein to modulate sumoylation of PML correlates with its functional activities in transcriptional regulation and infectivity in cultured fibroblast cells. J. Virol. 78:6527–6542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ling PD, Tan J, Sewatanon J, Peng R. 2008. Murine gammaherpesvirus 68 open reading frame 75c tegument protein induces the degradation of PML and is essential for production of infectious virus. J. Virol. 82:8000–8012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marcos-Villar L, et al. 2009. Kaposi's sarcoma-associated herpesvirus protein LANA2 disrupts PML oncogenic domains and inhibits PML-mediated transcriptional repression of the survivin gene. J. Virol. 83:8849–8858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Medveczky P, Szomolanyi E, Desrosiers RC, Mulder C. 1984. Classification of herpesvirus saimiri into three groups based on extreme variation in a DNA region required for oncogenicity. J. Virol. 52:938–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melendez LV, Daniel MD, Hunt RD, Garcia FG. 1968. An apparently new herpesvirus from primary kidney cultures of the squirrel monkey (Saimiri sciureus). Lab. Anim. Care 18:374–381 [PubMed] [Google Scholar]
- 27. Messerle M, Crnkovic I, Hammerschmidt W, Ziegler H, Koszinowski UH. 1997. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc. Natl. Acad. Sci. U. S. A. 94:14759–14763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muller S, Dejean A. 1999. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 73:5137–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nisole S, Stoye JP, Saib A. 2005. TRIM family proteins: retroviral restriction and antiviral defence. Nat. Rev. Microbiol. 3:799–808 [DOI] [PubMed] [Google Scholar]
- 30. Parkinson J, Everett RD. 2000. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J. Virol. 74:10006–10017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rozen R, Sathish N, Li Y, Yuan Y. 2008. Virion-wide protein interactions of Kaposi's sarcoma-associated herpesvirus. J. Virol. 82:4742–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saffert RT, Kalejta RF. 2006. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J. Virol. 80:3863–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sawyer SL, Wu LI, Emerman M, Malik HS. 2005. Positive selection of primate TRIM5alpha identifies a critical species-specific retroviral restriction domain. Proc. Natl. Acad. Sci. U. S. A. 102:2832–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schäfer A, et al. 2003. The latency-associated nuclear antigen homolog of herpesvirus saimiri inhibits lytic virus replication. J. Virol. 77:5911–5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sievers F, et al. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tavalai N, Adler M, Scherer M, Riedl Y, Stamminger T. 2011. Evidence for a dual antiviral role of the major nuclear domain 10 component Sp100 during the immediate-early and late phases of the human cytomegalovirus replication cycle. J. Virol. 85:9447–9458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tavalai N, Papior P, Rechter S, Leis M, Stamminger T. 2006. Evidence for a role of the cellular ND10 protein PML in mediating intrinsic immunity against human cytomegalovirus infections. J. Virol. 80:8006–8018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tavalai N, Stamminger T. 2008. New insights into the role of the subnuclear structure ND10 for viral infection. Biochim. Biophys. Acta 1783:2207–2221 [DOI] [PubMed] [Google Scholar]
- 39. Tavalai N, Stamminger T. 2009. Interplay between herpesvirus infection and host defense by PML nuclear bodies. Viruses 1:1240–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tischer BK, Smith GA, Osterrieder N. 2010. En passant mutagenesis: a two step markerless red recombination system. Methods Mol. Biol. 634:421–430 [DOI] [PubMed] [Google Scholar]
- 41. Tischer BK, von Einem J, Kaufer B, Osterrieder N. 2006. Two-step red-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. Biotechniques 40:191–197 [DOI] [PubMed] [Google Scholar]
- 42. Toptan T, Ensser A, Fickenscher H. 2010. Rhadinovirus vector-derived human telomerase reverse transcriptase expression in primary T cells. Gene Ther. 17:653–661 [DOI] [PubMed] [Google Scholar]
- 43. Tsai K, Thikmyanova N, Wojcechowskyj JA, Delecluse HJ, Lieberman PM. 2011. EBV tegument protein BNRF1 disrupts DAXX-ATRX to activate viral early gene transcription. PLoS Pathog. 7:e1002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu FY, et al. 2001. Origin-independent assembly of Kaposi's sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J. Virol. 75:1487–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yakushko Y, et al. 2011. Kaposi's sarcoma-associated herpesvirus bacterial artificial chromosome contains a duplication of a long unique-region fragment within the terminal repeat region. J. Virol. 85:4612–4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Y, Buchholz F, Muyrers JP, Stewart AF. 1998. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 20:123–128 [DOI] [PubMed] [Google Scholar]
- 47. Zielke K, et al. 2012. The insulator protein CTCF binding sites in the orf73/LANA promoter region of herpesvirus saimiri (HVS) are involved in conferring episomal stability in latently infected human T-cells. J. Virol. 86:1862–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]