Abstract
ADAR1, the interferon (IFN)-inducible adenosine deaminase acting on RNA, catalyzes the C-6 deamination of adenosine (A) to produce inosine (I) in RNA substrates with a double-stranded character. Because double-stranded RNA is a known inducer of IFN, we tested the role of ADAR1 in IFN induction following virus infection. HeLa cells made stably deficient in ADAR1 (ADAR1kd) were compared to vector control (CONkd) and protein kinase PKR-deficient (PKRkd) cells for IFN-β induction following infection with either parental (wild-type [WT]) recombinant Moraten vaccine strain measles virus (MV) or isogenic knockout mutants deficient for either V (Vko) or C (Cko) protein expression. We observed potent IFN-β transcript induction in ADAR1kd cells by all three viruses; in contrast, in ADAR1-sufficient CONkd cells, only the Cko mutant virus was an effective inducer and the IFN-β RNA induction was amplified by PKR. The enhanced IFN-β transcript-inducing capacity of the WT and Vko viruses seen in ADAR1-deficient cells correlated with the enhanced activation of PKR, IFN regulatory factor IRF3, and activator of transcription ATF2, reaching levels similar to those seen in Cko virus-infected cells. However, the level of IFN-β protein produced was not proportional to the level of IFN-β RNA but rather correlated inversely with the level of activated PKR. These results suggest that ADAR1 functions as an important suppressor of MV-mediated responses, including the activation of PKR and IRF3 and the induction of IFN-β RNA. Our findings further implicate a balanced interplay between PKR and ADAR1 in modulating IFN-β protein production following virus infection.
INTRODUCTION
Measles virus (MV), a member of the Morbillivirus genus of the Paramyxoviridae family, possesses an ∼15.9-kb negative-sense single-stranded RNA (ssRNA) genome that specifies six genes, N, P/V/C, M, F, H, and L, that encode six structural proteins. The P/V/C gene is polycistronic and encodes the V and C nonstructural proteins in addition to the structural protein product P, an essential cofactor for the viral polymerase (20). Studies of isogenic knockout virus mutants defective for the expression of either V (Vko) or C (Cko) have established that these proteins modulate the host response to MV infection (15, 43). The mutants show strong adaptive immune responses, but innate responses are dysregulated (9, 12, 55). Infection with MV causes acute febrile illness. In rare cases, MV infections can progress to a persistent infection of the central nervous system, resulting in the chronic and often fatal disease known as subacute sclerosing panencephalitis (35). Although there is an effective vaccine, MV infections globally continue to cause significant morbidity and mortality. The need for improved MV vaccines and increased adherence to recommendations with existing vaccines (20), together with the potential for using recombinant MV vaccine strains with defined mechanisms of attenuation as oncolytic therapeutic agents (9), has led to further efforts to better understand the host responses to MV infection at the molecular level.
A cornerstone of the antiviral innate immune response is the interferon (IFN) response. IFN production is triggered by pathogen-associated molecular patterns that include viral RNAs detected by multiple sensors, among which are the retinoic-acid-inducible protein (RIG-I)-like cytosolic sensors (RLRs) and the endosomal membrane-associated Toll-like receptors (TLRs) (24, 60). MV is known to activate the RLR pathway that signals through the mitochondrial adaptor protein IPS-1 to activate IFN-β gene transcription (29, 43) through IFN regulatory factor 3 (IRF3), nuclear factor κB (NF-κB), and activating transcription factor 2 (ATF2/c-jun) that form the IFN-β enhanceosome (37).
IFN action involves binding of IFN to cognate receptors and subsequent prototypical JAK-STAT signal transduction leading to transcriptional activation of IFN-stimulated genes (ISGs), some of which encode protein products that alter virus multiplication (7, 47). Among the IFN-inducible genes are two that encode double-stranded RNA (dsRNA) binding proteins with enzymatic activities: the protein kinase regulated by RNA (PKR) and the adenosine deaminase acting on RNA (ADAR1). dsRNA is a regulatory effector of PKR and a substrate of ADAR1 (45, 46, 57). PKR possesses two dsRNA binding domains and a C-terminal catalytic kinase domain (30, 57) and is activated by binding dsRNA or structured ssRNA, leading to autophosphorylation, dimerization, and subsequent substrate phosphorylation (4, 5, 28, 32, 45, 57). The best-characterized substrate remains eukaryotic translation initiation factor 2α (48), in which phosphorylation leads to inactivation, thereby altering the translational pattern in cells and inducing apoptosis. PKR function typically is antiviral and proapoptotic (39, 45, 47).
The adenosine deaminase enzyme ADAR1 catalyzes the C-6 deamination of adenosine (A) to inosine (I) in RNA substrates with a double-stranded character, a reaction known as A-to-I editing (17, 57). This editing can lead to destabilization of RNA structures because I · U mismatch pairs are less stable than A · U pairs, or alternatively, A-to-I editing can cause changes in genetic decoding during translation or viral RNA replication because I base pairs as G with C, instead of A with U (3, 46). Among the RNAs selectively edited by ADARs in a manner that affects their translational decoding are cellular pre-mRNAs that encode two important types of neurotransmitter receptors (GluR-B, 5HT-2cR) and hepatitis delta virus antigenome RNA (17). Nonselective editing at multiple sites can occur when RNA substrates possess extensive duplex character, as has been described for MV and polyomavirus infections (46). Two size isoforms of ADAR1 protein are generated by a mechanism that involves the use of alternative promoters and alternative splicing (18, 38). A long form of ADAR1 (p150) is IFN inducible and localizes to both the nucleus and the cytoplasm, whereas a short form (p110) is constitutively expressed and localizes predominantly, if not exclusively, to the nucleus (17, 18, 38, 41). Both ADAR1 isoforms, p150 and p110, possess three copies of the dsRNA binding domain first discovered in PKR (27, 38). Remarkably, even though ADAR1 is encoded by an ISG, its function is often proviral and antiapoptotic or cell protective in nature (16, 39, 46).
Both IFN production and action are impaired in MV-infected cells (43). Antagonism of IFN action occurs through P, V, and also, to some extent, C protein, which inhibits STAT activation and nuclear translocation, thereby impairing the induction of ISGs (8, 36, 42, 43, 59). The molecular basis of how IFN production is antagonized is less well understood. The V protein inhibits MDA5-mediated RLR signaling leading to IFN production (1, 10, 12); the C protein also inhibits IFN production (12), possibly indirectly by affecting viral RNA synthesis. Virus expressing both C and V is a poor inducer of IFN, but mutants lacking either V or C are inducers of IFN-β transcription (29). PKR functions to amplify IFN-β RNA induction in an IPS-1-dependent signaling response following MV infection (29). The amplification of IFN-β transcript levels by PKR correlates with a PKR-dependent activation of ATF2 and NF-κB, whereas the activation of IRF3 following MV infection is PKR independent (29, 56).
Because ADAR1 has been shown to suppress MV-induced activation of both PKR and IRF3 (56), we hypothesized that ADAR1 might also suppress IFN-β induction by MV. To test the possible role of ADAR1 in IFN-β induction following MV infection, we used HeLa cells stably deficient in ADAR1 (ADAR1kd). Results obtained with the wild-type (WT) parental Moraten vaccine strain were compared to those obtained with isogenic Vko and Cko mutants. We found that the WT and Vko viruses were poor inducers of IFN-β RNA in ADAR1-sufficient cells compared to the Cko virus, as anticipated from prior results (29). However, all three viruses remarkably became robust inducers of IFN-β RNA in ADAR1kd cells. Comparably high levels of IFN-β transcripts were found in WT, Vko, and Cko virus-infected, ADAR1-deficient cells in the presence of PKR, but the level of IFN-β protein produced did not correlate with the steady-state level of IFN-β RNA. Suppression of IFN-β RNA induction in the presence of ADAR1 correlated with suppression of IRF3 phosphorylation and dimerization and ATF2 phosphorylation, whereas the efficiency of IFN-β protein production correlated inversely with PKR activation. Our results establish ADAR1 as an important modulator of the host IFN response triggered by MV infection.
MATERIALS AND METHODS
Cells and viruses.
HeLa cells were maintained in Dulbecco's modified Eagle's medium supplemented with 5% (vol/vol) fetal bovine serum (HyClone), 100 μg/ml of penicillin, and 100 U/ml streptomycin (GIBCO/Invitrogen) as previously described (63). HeLa cells made stably deficient in either ADAR1 (ADAR1kd) (25, 56) or PKR (PKRkd) (61, 63) by an integrated short hairpin silencing RNA interference strategy utilizing the pSUPER.retro.puro vector were as previously described, as were the drug-resistant knockdown vector control cells (CONkd). Knockdown cells were maintained in medium containing 1 μg/ml puromycin (Sigma). The recombinant parental Moraten MV vaccine strain, herein designated the WT, includes the gene encoding green fluorescent protein inserted downstream of the viral H gene. The WT virus and isogenic V-deficient (Vko) and C-deficient (Cko) mutants were as described previously (13, 55). Infections were carried out as previously described (29, 55), at a multiplicity of infection of 3 50% tissue culture infective doses per cell unless otherwise noted.
Quantitative real-time PCR (qPCR).
IFN-β and IκBα transcripts were measured by qPCR as previously described (29). Cells seeded into six-well plates were infected with WT, Vko, or Cko MV or left uninfected. Total RNA was isolated from cells at 24 h after infection and from uninfected control cells with TRIzol (Invitrogen) according to the manufacturer's instructions. Random-primed reverse transcription was carried out using ∼2 μg of RNA and SuperScript II (Invitrogen) according to the manufacturer's protocol. For PCR analyses, the following primer pairs were used: GAPDH (glyceraldehyde 3-phosphate dehydrogenase), forward primer GCCTTCCGTGTCCCCACTG and reverse primer CGCCTGCTTCACCACCTTC; IFN-β, forward primer AAACTCATGAGCAGTCTGCA and reverse primer AGGAGATCTTCAGTTTCGGAGG; IκBα, forward primer AATTGCTGAGGCACTTCTGG and reverse primer TAGCCTTCAGGATGGAGTGG. qPCRs were performed in duplicate with each reverse transcription template by using IQ SYBR green Supermix (Bio-Rad) and a Bio-Rad MyIQ qPCR instrument. For IFN-β, the cycle program was a 3-min hot start, followed by 30 s at 95°C, 45 s at 58°C, and 45 s at 72°C, repeated 40 times. For IκBα, the cycle program was a 3-min hot start, followed by 30 s at 95°C, 30 s at 55°C to 65°C (gradient set as follows: A, 65.0°C; B, 64.3°C; C, 63.1°C; D, 61.2°C; E, 58.7°C; F, 57.0°C; G, 55.8°C; H, 55.0°C), and 30 s at 72°C, repeated 40 times. The IFN-β and IκBα values were normalized to GAPDH values.
Enzyme-linked immunosorbent assay (ELISA) for IFN-β.
Cells seeded into six-well plates were infected with WT, Vko, or Cko MV or left uninfected. Cell culture supernatant fractions (1.5 ml) were harvested 24 h postinfection and assayed for IFN-β protein using a VeriKine Human IFN Beta ELISA kit (Pestka Biomedical Laboratories) in accordance with the manufacturer's protocol. IFN-β protein levels (pg/ml) were calculated based on a standard curve generated at the time of the assay.
Western immunoblot analysis.
Cells were harvested 24 h postinfection, and whole-cell lysates were prepared as previously described (55). Protein concentration was determined by the Bradford assay method (Bio-Rad). Protein (20 to 30 μg) was fractionated by either 7% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred to nitrocellulose membranes, and the membranes then were blocked with 5% milk (wt/vol) in phosphate-buffered saline. For detection of phosphoproteins, membranes were blocked with 5% (wt/vol) bovine serum albumin and 0.1% Tween 20 (vol/vol) in Tris-buffered saline. Antibodies used to detect specific proteins were as previously described for ADAR1 (38), PKR (Santa Cruz Biotechnology), phospho-Thr446 PKR (Epitomics), IRF3 (Santa Cruz Biotechnology), phospho-Ser396 IRF3 (Cell Signaling), ATF2 (Santa Cruz Biotechnology), phospho-Thr71 ATF2 (Cell Signaling), green fluorescent protein (GFP; Santa Cruz Biotechnology), β-actin (Sigma), and α-tubulin (Sigma). Antibody against MV H protein was generously provided by R. Cattaneo (Mayo Clinic, Rochester, MN) as previously described (55). Detection of immunoblots was performed as previously described (29), using the Odyssey infrared imaging system (Li-COR).
IRF3 dimerization assay.
IRF3 dimer formation was measured by native PAGE essentially as described by Sankar et al. (49). Extracts were prepared from cells, either infected or uninfected, using extract buffer (20 mM HEPES [pH 7.9], 400 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 1% NP-40, 1× complete protease inhibitor cocktail [Sigma], 1× phosphatase inhibitor cocktail [Sigma]). Protein fractionation was on 8% acrylamide gels using 25 mM Tris (pH 8.3)–192 mM glycine running buffer in the anode chamber and running buffer supplemented with 1% sodium deoxycholate in the cathode chamber. Gels were prerun at 40 mA for 30 min prior to sample loading; electrophoresis was at 20 mA until the dye reached near the bottom of the gel. Proteins were transferred to a nitrocellulose membrane using ice-cold Tris-glycine buffer at 150 V for 90 min; monomer and dimer IRF3 detection was then done by Western immunoblot assay with IRF3 antibody or phospho-Ser396 IRF3.
RESULTS
ADAR1 suppresses PKR activation and the induction of IFN-β RNA by MV.
To compare the effects of ADAR1 and PKR on IFN-β induction by MV, we utilized HeLa cell clonal lines in which either ADAR1 or PKR was stably knocked down by a short hairpin RNA interference strategy. We used the recombinant Moraten vaccine strain of MV because entry by MV is through the CD46 receptor that is present on HeLa cells and because isogenic mutants of the parental (WT) Moraten virus lacking either V or C protein expression (Vko, Cko) are available (33, 55). ADAR1kd cells have less than 15% of the ADAR1 p110 and p150 proteins found in drug-treated CONkd or PKRkd cells, and the PKR expression level in PKRkd cells is less than 5% of that in PKR-sufficient CONkd or ADAR1kd cells (Fig. 1A), as previously described (25, 56, 61).
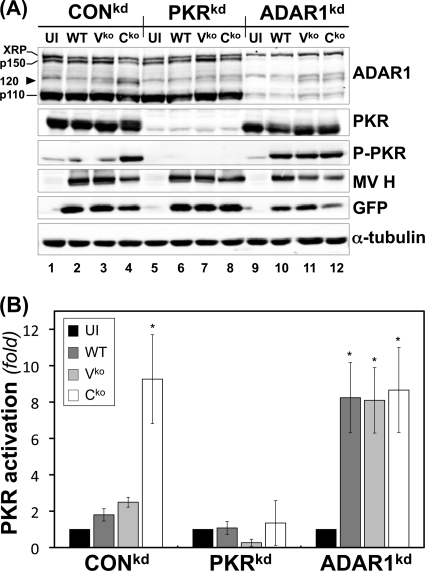
Fig 1.
ADAR1 deficiency enhances PKR activation following infection with WT, Vko, or Cko MV. (A) CONkd, PKRkd, and ADAR1kd cells were either left uninfected (UI) or infected with WT, Vko, or Cko MV as indicated. At 24 h postinfection, whole-cell extracts were prepared and analyzed by Western immunoblot assay with antibodies against ADAR1, PKR, phospho-Thr446-PKR, MV H, GFP, and α-tubulin. XRP marks the position of ADAR1 antibody-cross-reacting protein (mobility slower than that of p150); the arrowhead marks the postulated caspase-mediated p120 ADAR1 cleavage product. (B) Quantification of n-fold activation of PKR, as measured by the level of phospho-Thr446-PKR to total PKR, determined by Western immunoblot analysis as shown in panel A. *, P < 0.05 by Student t test for comparison of PKR activation in uninfected cells and cells infected with WT, Vko, or Cko MV. The results shown are the means and standard errors of three independent experiments.
In ADAR1-sufficient CONkd cells, only the Cko mutant efficiently activated PKR, as measured by phosphorylation of Thr446 (Fig. 1A, lane 4); the level of phospho-PKR in WT and Vko virus-infected cells remained low and similar to that in uninfected (UI) cells (Fig. 1A and B). In contrast, in ADAR1-deficient (ADAR1kd) cells (Fig. 1A, lanes 9 to 12), both WT and Vko virus infections increased PKR Thr446 phosphorylation to a level comparable to that seen in Cko virus-infected cells (Fig. 1B). The PKRkd cells did not show detectable virus-mediated phosphorylation due to the low level of PKR present in the cells (Fig. 1A, lanes 5 to 8). Growth of MV was assessed by measuring viral H protein and GFP expression. Consistent with earlier findings (55, 56), both H and GFP were reduced in ADAR1kd cells, most notably in Cko virus-infected cells, whereas Cko virus-infected PKRkd cells showed increased protein expression, reaching levels comparable to those observed with the WT and Vko viruses (Fig. 1A).
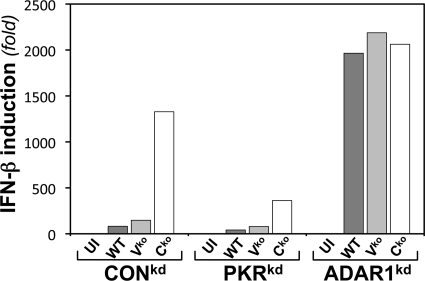
Because we had observed that PKR acts to amplify the induction of IFN-β via IPS-1-dependent signaling following either infection with Vko or Cko MV or transfection with dsRNA (28, 29) and because ADAR1 deficiency results in substantially enhanced activation of PKR following MV infection, as illustrated by the results shown in Fig. 1A, we tested the effect of ADAR1 deficiency on the induction of IFN-β transcripts by MV. The results of a representative qPCR data set are shown in Fig. 2. Consistent with prior observations (29), WT and Vko mutant MVs were both poor inducers of IFN-β RNA compared to Cko virus in CONkd (ADAR-sufficient, PKR-sufficient) cells; the induction by Cko virus was amplified by PKR, as the IFN-β transcript level in CONkd cells was typically 3- to 5-fold greater in CONkd cells than in PKRkd cells with the Cko mutant (Fig. 2). In striking contrast to ADAR1-sufficient cells, in ADAR1-deficient cells, the inducing capacity of the WT and Vko viruses was not severely impaired. The WT and Vko viruses were both potent inducers of IFN-β RNA in ADAR1kd cells, with the induction increased by ∼100-fold in ADAR1kd cells compared to either CONkd or PKRkd cells. The induction of IFN-β transcripts in ADAR1kd cells by the WT and Vko viruses was comparable to that seen with the Cko virus (Fig. 2). Analysis of IFN-β transcript levels in three independent experiments by the Student t test gave a P value of >0.7, for Cko virus-infected ADAR1kd cells versus WT or Vko virus-infected ADAR1kd cells. In contrast, comparison of IFN-β transcript levels in WT and Vko virus-infected CONkd (or PKRkd) versus WT and Vko virus-infected ADAR1kd cells gave a P value of <0.0001.
Fig 2.
ADAR1 deficiency results in enhanced induction of IFN-β transcripts following infection with MV. CONkd, PKRkd, and ADAR1kd cells were left uninfected (UI) or infected with WT or mutant (Vko or Cko) MV, as indicated. Total RNA was isolated at 24 h after infection, and IFN-β mRNA levels normalized to GAPDH were determined by qPCR.
IRF3 activation is enhanced in ADAR1-deficient cells following MV infection.
Because virus-induced activation of IRF3 is an important contributor to the transactivation of IFN-β expression, including in MV-infected cells through IPS-1 dependent signaling (26, 29, 37), we next examined IRF3 activation levels in ADAR1kd cells infected with the WT, Vko, and Cko viruses compared to CONkd or PKRkd virus-infected cells or uninfected cells as controls (Fig. 3). Activation was assessed by measuring both virus-induced phosphorylation of IRF3 at Ser396 (Fig. 3A and B) and virus-induced IRF3 dimerization (Fig. 3C and D).
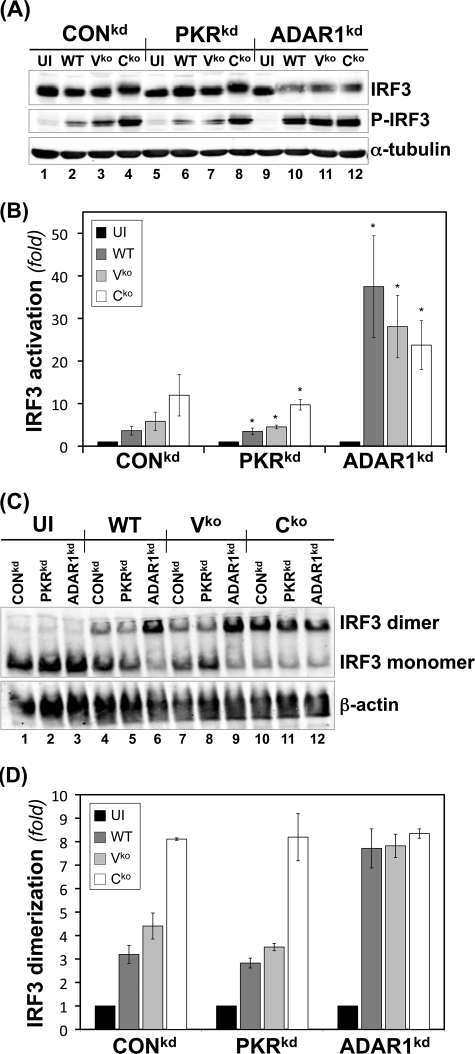
Fig 3.
IRF3 activation is enhanced in ADAR1-deficient cells following infection with WT, Vko, or Cko MV. CONkd, PKRkd, and ADAR1kd cells were either left uninfected (UI) or infected with WT, Vko, or Cko MV as indicated. (A) At 24 h postinfection, whole-cell extracts were prepared and analyzed by Western immunoblot assay with antibodies against IRF3, phospho-Ser396-IRF3, and α-tubulin. (B) Quantification by Western immunoblot analysis of the n-fold activation of IRF3 expressed as the ratio of phospho-Ser396-IRF3 to total IRF3. *, P < 0.05 by Student t test for comparison of IRF3 activation in uninfected cells versus cells infected with the WT, Vko, or Cko virus. The results shown are means and standard errors (n = 3). (C) IRF3 activation as measured by dimer formation. Extracts were prepared and analyzed for IRF3 dimer formation by native PAGE as described in Materials and Methods. The blot was probed with antibody against IRF3 to detect dimer complexes fractionated from monomer protein. (D) Quantification of dimerization expressed as the ratio of IRF3 dimer to total IRF3 (monomer plus dimer). The results shown are means and standard deviations (n = 3).
While Cko virus induced Ser396 phosphorylation in both CONkd and PKRkd cells, as measured by Western analysis, only low levels of IRF3 phosphorylation were seen in these cells infected with either the WT or the Vko virus (Fig. 3A and B). In contrast, in ADAR1kd cells following infection with the WT and Vko viruses, levels of phospho-IRF3 comparable to those seen with the Cko virus were found (Fig. 3A and B). Similar results were seen when IRF3 dimerization was measured by PAGE using native gels. Cko virus induced comparably high levels of IRF3 dimerization in all three types of cells, CONkd, PKRkd, and ADAR1kd, whereas the WT and Vko viruses induced efficient IRF3 dimerization only in ADAR1-deficient ADAR1kd cells and not in CONkd or PKRkd cells (Fig. 3C and D). The slightly reduced mobility of total IRF3 on SDS-PAGE seen in Cko virus-infected cells correlated with C-terminal Ser396 phosphorylation (Fig. 3A), as previously described (26, 29, 61). IRF3 protein levels were comparable in uninfected CONkd, PKRkd, or ADAR1kd cells, and neither phosphorylation at Ser396 (Fig. 3A) nor dimerization (Fig. 3C) of IRF3 was detected to any significant extent in uninfected cells. Finally, the finding that the IRF3 phosphorylation at Ser396 (Fig. 3A and B) and the extent of IRF3 dimerization (Fig. 3C and D) in Cko virus-infected CONkd and PKRkd cells are comparable suggests that IRF3 phosphorylation is PKR independent. The enhanced activation of IRF3 seen in ADAR1-deficient cells (Fig. 3) is consistent with the enhanced induction of IFN-β seen in ADAR1kd cells (Fig. 2).
ATF2 phosphorylation is enhanced in ADAR1-deficient cells following infection with WT and Vko MVs.
In ADAR1-sufficient and -deficient cells, the different levels of IFN-β induction by the Cko virus correlated with the phosphorylation of PKR on Thr446, as shown in Fig. 1 and 2. Because activation of mitogen-activated protein kinase signaling and ATF2 phosphorylation shows PKR dependency (29, 62) and because PKR activation is enhanced in ADAR1-deficient cells (Fig. 1), we examined the effect of ADAR1 deficiency on the phosphorylation level of ATF2 directly by Western immunoblot analysis with a monoclonal antibody specific for ATF2 phospho-Thr71 and indirectly by reduction of ATF2 protein mobility on SDS-PAGE (Fig. 4).
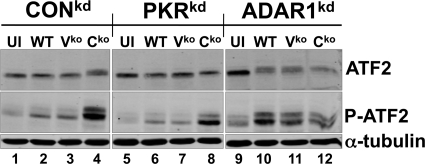
Fig 4.
Effect of ADAR1 or PKR deficiency on MV-induced activation of ATF2. CONkd, PKRkd, and ADAR1kd cells were either left uninfected (UI) or infected with WT, Vko, or Cko MV, as indicated. At 24 h postinfection, whole-cell extracts were prepared and analyzed by Western immunoblotting with antibodies against ATF2, phospho-Thr71-ATF2, and α-tubulin.
The phosphorylation of ATF2 was increased in CONkd and PKRkd cells, both ADAR1 sufficient, following infection with Cko virus but not following infection with either WT or Vko virus (Fig. 4). However, in ADAR1-deficient cells, infection with all three viruses (WT, Vko, and Cko) gave rise to reduced gel mobility of the ATF2 protein and increased phospho-Thr71 levels compared to those in uninfected cells (Fig. 4). Uninfected CONkd, PKRkd, and ADAR1kd cells show similar levels of total ATF2 protein.
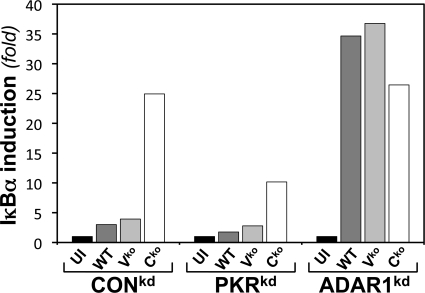
Induction of IκBα is higher in ADAR1-deficient cells than in ADAR1-sufficient cells.
Among the transcripts dependent upon NF-κB for transcriptional activation, in addition to IFN-β, is IκBα (22, 37). As a beacon for activation of NF-κB (54), we compared the IκBα transcript levels in ADAR1-sufficient and ADAR1-deficient cells by qPCR following MV infection (Fig. 5). Infection of ADAR1kd cells with either the WT or the Vko mutant resulted in an IκBα RNA level ∼10- to 20-fold higher than the low level seen in ADAR1-sufficient (CONkd or PKRkd) cells infected with either WT or Vko virus or in uninfected cells. However, infection with Cko mutant MV showed similarly high IκBα transcript levels in ADAR1-sufficient (CONkd) and ADAR1-deficient (ADAR1kd) cells, both of which are PKR sufficient, whereas the transcript level was reduced in PKR-deficient, ADAR1-sufficient (PKRkd) cells (Fig. 5).
Fig 5.
ADAR1 deficiency results in enhanced induction of IκBα following MV infection. CONkd, PKRkd, and ADAR1kd cells were either left uninfected (UI) or infected with WT, Vko, or Cko MV, as indicated. At 24 h postinfection, total RNA was isolated and IκBα mRNA normalized to GAPDH was measured by qPCR.
ADAR1 deficiency leads to decreased efficiency of IFN-β protein production.
Because the IFN-β transcript level is increased in ADAR1-deficient cells following MV infection (Fig. 2) but the activation of PKR is also increased in ADAR1kd cells (Fig. 1B), and because Cko virus infection gave rise to both increased IFN-β RNA transcription and increased PKR activation in ADAR1kd cells (Fig. 1 and 2), we tested whether IFN-β protein was differentially produced and secreted in the infected cells in an ADAR1-dependent manner. We performed ELISAs, and as shown in Table 1, the Cko virus was a better inducer of IFN-β protein than the WT and Vko viruses, as anticipated for all three cell types, CONkd, PKRkd, and ADAR1kd. However, the level of the IFN-β protein was only about 2-fold higher in Cko virus-infected cells than in WT or Vko virus-infected, PKR-sufficient (CONkd, ADAR1kd) cells and about 7-fold higher in PKR-deficient cells. While the Cko virus was the best inducer of IFN-β protein, as expected, the level of IFN-β protein produced was unexpectedly less than anticipated from the level of IFN-β RNA transcript (Table 2). When the relative amount of IFN-β protein produced per amount of IFN-β RNA expressed was considered, as an index of the relative efficiency of IFN-β production, the efficiency was highest in PKR-deficient cells and lowest in ADAR1-deficient cells that display high levels of PKR activation. The relative efficiencies in PKRkd cells (8.63, 3.92, and 7.85) were about 10- to 30-fold higher than in ADAR1kd cells (0.27, 0.34, and 0.57) for all three viruses (Table 2).
Table 1.
Effect of deficiency of ADAR1 or PKR on IFN-β protein production in response to MV infectiona
| Cell type | Virus infection | IFN-β (pg/ml) |
|---|---|---|
| CONkd | UI | <10 |
| WT | 464 ± 23 | |
| Vko | 406 ± 46 | |
| Cko | 985 ± 68 | |
| PKRkd | UI | <10 |
| WT | 354 ± 21 | |
| Vko | 310 ± 9.1 | |
| Cko | 2,558 ± 371 | |
| ADAR1kd | UI | <10 |
| WT | 533 ± 39 | |
| Vko | 743 ± 66 | |
| Cko | 1,178 ± 32 |
CONkd, PKRkd, or ADAR1kd cells in six-well plates were infected with either WT, Vko, or Cko virus or left uninfected (UI). IFN-β accumulation in cell culture supernatant fractions (1.5 ml) after 24 h was measured by ELISA as described in Materials and Methods. Results are means and standard errors (n = 4).
Table 2.
Effect of ADAR1 or PKR deficiency on efficiency of IFN-β expressiona
| Cell type and virus | IFN-β RNAb | IFN-β proteinc | Relative expression efficiency |
|---|---|---|---|
| CONkd | |||
| WT | 82 | 464 | 5.66 |
| Vko | 147 | 406 | 2.76 |
| Cko | 1,328 | 985 | 0.74 |
| PKRkd | |||
| WT | 41 | 354 | 8.63 |
| Vko | 79 | 310 | 3.92 |
| Cko | 326 | 2,558 | 7.85 |
| ADAR1kd | |||
| WT | 1,965 | 533 | 0.27 |
| Vko | 2,187 | 743 | 0.34 |
| Cko | 2,062 | 1,178 | 0.57 |
Relative expression efficiency was calculated from the amount of IFN-β protein per amount of IFN-β RNA expressed as follows: relative expression efficiency = IFN-β protein/IFN-β RNA.
IFN-β RNA transcript levels were determined by qPCR (Fig. 2).
IFN-β protein production was determined by ELISA (Table 1).
DISCUSSION
The objectives of our study were to test whether ADAR1 plays a role in virus-induced IFN-β expression using MV and whether any of the observed ADAR1-dependent responses were modulated by the viral V or C accessory protein. We found, using cells stably deficient in ADAR1, that IFN-β RNA expression was increased ∼100-fold or more following virus infection. Most strikingly, WT and mutant viruses defective for either V or C protein expression all induced comparably high levels of IFN-β RNA in ADAR1-deficient cells, but in ADAR1-sufficient cells, only the Cko mutant was a strong inducer. ADAR1 deficiency was accompanied by enhanced activation of signaling factors known to amplify IFN-β RNA expression, including IRF3, ATF2, and PKR. While the Cko mutant also induced higher IFN-β protein levels than either the WT or Vko virus, the efficiency of IFN-β protein production was markedly decreased in ADAR1-deficient cells, which showed enhanced activation of PKR compared to that in ADAR1-sufficient cells.
Our studies were undertaken because ADAR1 is known to destabilize dsRNA structure and dsRNA is a potent inducer of IFN and activator of PKR (46, 47), PKR amplifies IFN-β transcript production in ADAR1-sufficient cells infected with either Vko or Cko mutant virus but not in those infected with the WT virus (29), and enhanced activation of PKR is observed in ADAR1-deficient HeLa cells (25, 55). Therefore, we anticipated that if PKR activation is central to the amplification of IFN-β RNA expression and if ADAR1 deficiency leads to increased virus-induced activation of PKR, then ADAR1 deficiency might result in increased expression of IFN-β transcripts. This indeed was observed. But unexpectedly, all three viruses, the WT and the isogenic mutants defective for either V and C protein expression, were robust inducers of IFN-β RNA in ADAR1-deficient cells, whereas in ADAR1-sufficient cells, only the C mutant was a robust inducer. Furthermore, the efficiency of IFN-β protein production per steady-state amount of IFN-β RNA was substantially reduced in ADAR1-deficient cells compared to that in ADAR1-sufficient cells in a manner that correlated with increased PKR activation, indicating a balanced interplay between ADAR1 and PKR in modulating IFN-β production following infection.
Induction of IFN-β gene transcription by virus involves activation of IRF3 and NF-κB by RLR or TLR signaling, and these factors function together with ATF2/c-jun to constitute the IFN-β enhanceosome that drives IFN-β transcription (37, 43). In the case of MV Moraten infection via CD46, as occurs in HeLa cells (33, 55), activation of IRF3, NF-κB, and ATF2 is maximal in the absence of the C protein and is IPS-1 dependent, whereas in the absence of the V protein or with the WT virus, activation is low and IFN-β induction is poor (29). Our results are consistent with and extend these observations. We found that IRF3 activation was maximal in Cko virus-infected cells but that IRF3 activation was PKR independent. We also found that IRF3 activation was low in WT and Vko virus-infected, ADAR1-sufficient cells, as reported earlier (29), but in ADAR1kd cells, the activation of IRF3 and ATF2 was robust and similar to that seen with the Cko virus (Fig. 3 and 4). The same was observed when IκBα transcript levels were measured as a beacon of NF-κB activity, raising the interesting possibility that NF-κB-dependent expression of cytokines in addition to IFN-β may be enhanced in ADAR1-deficient cells following infection. The IκBα RNA expression pattern (Fig. 4) was similar to that of IFN-β RNA expression (Fig. 2), where WT and Vko viruses induced IFN-β as robustly as the Cko virus did in ADAR1kd cells but induction was not seen in either of the ADAR1-sufficient cell lines (CONkd, PKRkd). For ATF2 and NF-κB activation, our results are in agreement with earlier observations that found that virus-induced activation was enhanced by PKR (29, 62). The fact that IFN-β RNA induction and the activation of PKR, IRF3, and ATF2 all were enhanced in ADAR1-deficient, MV-infected cells suggests the accumulation of RNA structures that trigger IFN induction signaling at an upstream point, although the existence of different RNA structures that trigger the activation of different signaling proteins is an alternative possibility. Our finding that the knockdown of ADAR1 in HeLa cells reveals enhanced IFN-β transcript expression is consistent with a recent mouse knockout study that also implicates ADAR1 as a suppressor of the IFN response. Genetic disruption of ADAR1 causes embryonic lethality in mice (21, 58) and results in the upregulation of ISG expression in hematopoietic stem cells in the absence of infection (21).
Dimerization of IRF3 was typically detectable at a low level in WT and Vko virus-infected cells under conditions where Ser396 phosphorylation of IRF3 was not readily increased. This disparity may reflect different sensitivities of the Western immunoblotting and native gel electrophoresis assays used or the alternative, and perhaps more likely, possibility that phosphorylation at a site other than Ser396 also contributes to the dimerization of IRF3. The phosphorylation of IRF3 has been described to occur at multiple sites in a two-step process, initially at C-terminal serine/threonine sites between amino acids 396 and 405 and then at Ser385 and Ser386 (23, 37, 61).
Among our most striking results are that, in addition to the fact that ADAR1 deficiency led to increased IFN-β transcript levels, particularly in WT and Vko virus-infected ADAR1kd cells compared to ADAR1-sufficient cells (Fig. 1), and also increased IFN-β protein (Table 1), the efficiency of IFN-β protein production was notably reduced in ADAR1kd cells (Table 2). What, then, is the relationship of ADAR1 as an RNA sensor that destabilizes RNA structures (3, 46) relative to PKR as an established regulator of translation (45, 47, 48)?
The efficient IFN-β RNA induction seen in ADAR1kd cells (Fig. 2) was accompanied by increased activation of PKR (Fig. 1), a known inhibitor of translation (45, 48). Decreased MV replication and increased virus-induced apoptosis also have been described for ADAR1kd cells (55, 56). We assessed the growth of the WT, Cko, and Vko viruses by measurement of H protein and GFPs in ADAR1kd, PKRkd, and CONkd cells, and the results (Fig. 1A) are in good agreement with those of previously published studies (55, 56). While viral protein expression was reduced most extensively in Cko virus-infected, as well as Vko virus-infected, ADAR1kd cells, the extent of the reduction depended upon the protein examined, with the reduction of H generally more pronounced than that of GFP. The inhibition seen with the WT virus was more variable, possibly suggesting that PKR activation is not the sole RNA-dependent response modulated by ADAR1 that contributes to the observed decrease in viral gene expression. The replication of the WT, Vko, and Cko viruses was reduced in ADAR1kd cells compared to that in ADAR1-sufficient cells, as previously reported (56), whereas IFN-β RNA induction was greatly enhanced in ADAR1kd cells, as shown herein (Fig. 2). While the WT and Vko viruses replicate efficiently in cells sufficient in both ADAR1 and PKR, the Cko mutant displays a growth restriction phenotype that is partially rescued in PKRkd cells (55) but not ADAR1kd cells (56). The WT and Vko viruses were both poor inducers of IFN-β transcripts in ADAR1- and PKR-sufficient cells, where they replicate to high levels, but the Cko virus, which replicates relatively poorly, was an efficient IFN-β RNA inducer. Our finding that PKR, as well as ADAR1, contributes to IFN-β transcriptional induction in a pronounced manner, particularly with the Cko MV mutant, is consistent with recent observations of human parainfluenza virus type 1 C mutants that produced increased IFN-β and increased dsRNA that activated MDA5 and PKR (6). However, our finding of enhanced activation of PKR in Cko virus-infected control cells, and importantly also in ADAR1kd cells infected with the WT or V or C mutant virus, is consistent with translational control as a likely explanation for the decreased efficiency of IFN-β protein production seen in these cells (Table 2). Whether translational control mediated by PKR also accounts for the enhanced IFN-β transcript levels seen in the cells has yet to be determined.
The MV V protein inhibits RLR signaling likely directly, and the C protein potentially does so indirectly by modulating viral RNA synthesis (1, 2, 10, 31, 44, 51). Our results are consistent with the notions that in the absence of C protein, aberrant viral RNAs with sufficient structure to activate PKR and RLR signaling are produced, and that even in the presence of ADAR1, which would destabilize dsRNA (3, 46), viral dsRNA of sufficient concentration and structure accumulates to trigger dsRNA sensors, including PKR and IPS-1-mediated RLR signaling. According to this possible explanation, in the presence of C protein, as would be the case with the WT and Vko viruses, the level of activating RNAs is low for two reasons: reduced activating RNA synthesis and decreased accumulation because of the action of ADAR1. In the absence of ADAR1, as in ADAR1kd cells, however, the concentration of activator dsRNA is sufficiently high to trigger the signaling pathways. The extent to which viral dsRNA structural features overlap for recognition by ADAR1, PKR, and the RLRs as foreign dsRNA sensors is unknown. This question is under investigation by others and us. dsRNA length and the presence of 5′-triphosphate on ssRNA are among the parameters important for IPS-1-dependent RLR signaling and PKR activation (32, 45, 60). The potential importance of the differential activation of dsRNA sensors, for example, IPS-1-dependent signaling involved in the transcriptional induction of IFN-β gene expression (60) and PKR, which downregulates translation (45, 47), is illustrated by the increased expression of IFN-β transcripts but decreased efficiency of IFN-β protein production observed in ADAR1-deficient, PKR-sufficient cells (Table 2).
PKR is encoded by a well-established ISG of central importance in the antiviral actions of IFNs (47). More recent evidence implicates PKR as a key player in the induction of IFN-β, not only by MV (29) but also by other RNA viruses (6, 19, 52, 53). In contrast, we found that ADAR1, which is also encoded by an ISG, displayed proviral activity with MV in HeLa cells (56). Similarly, with vesicular stomatitis virus, ADAR1 was found to be proviral in both mouse embryonic fibroblasts and HeLa cells by suppressing the activation of PKR phosphorylation (25, 34). Several independent reports concluded that ADAR1 was proviral and increased HIV replication (11, 14, 40). Furthermore, in a screening of ∼380 ISGs by overexpression, ADAR1 was identified as one of a few ISG products that significantly enhanced the replication of multiple viruses, including HIV, West Nile virus, Venezuelan equine encephalitis virus, and yellow fever virus (50). While antagonism of PKR activation is emerging as one mechanism by which ADAR1 may enhance virus replication (16, 46), another potentially more broadly operative mechanism relates to the suppression of virus-induced production of IFN-β RNA in ADAR1-sufficient cells, as observed herein (Fig. 2).
ACKNOWLEDGMENTS
This work was supported in part by research grants AI-12520 and AI-20611 to C.E.S. from the National Institute of Allergy and Infectious Diseases, NIH, U.S. Public Health Service, and by a postdoctoral fellowship award to Z.L. from the Santa Barbara Foundation.
Footnotes
Published ahead of print 25 January 2012
REFERENCES
- 1. Andrejeva J, et al. 2004. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. U. S. A. 101:17264–17269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bankamp B, Wilson J, Bellini WJ, Rota PA. 2005. Identification of naturally occurring amino acid variations that affect the ability of the measles virus C protein to regulate genome replication and transcription. Virology 336:120–129 [DOI] [PubMed] [Google Scholar]
- 3. Bass BL. 2002. RNA editing by adenosine deaminases that act on RNA. Annu. Rev. Biochem. 71:817–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bevilacqua PC, George CX, Samuel CE, Cech TR. 1998. Binding of the protein kinase PKR to RNAs with secondary structure defects: role of the tandem A-G mismatch and noncontiguous helixes. Biochemistry 37:6303–6316 [DOI] [PubMed] [Google Scholar]
- 5. Bischoff JR, Samuel CE. 1989. Mechanism of interferon action. Activation of the human P1/eIF-2 alpha protein kinase by individual reovirus s-class mRNAs: s1 mRNA is a potent activator relative to s4 mRNA. Virology 172:106–115 [DOI] [PubMed] [Google Scholar]
- 6. Boonyaratanakornkit J, et al. 2011. The C proteins of human parainfluenza virus type 1 limit double-stranded RNA accumulation that would otherwise trigger activation of MDA5 and protein kinase R. J. Virol. 85:1495–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borden EC, et al. 2007. Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 6:975–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caignard G, et al. 2007. Measles virus V protein blocks Jak1-mediated phosphorylation of STAT1 to escape IFN-alpha/beta signaling. Virology 368:351–362 [DOI] [PubMed] [Google Scholar]
- 9. Cattaneo R, Miest T, Shashkova EV, Barry MA. 2008. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat. Rev. Microbiol. 6:529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Childs K, et al. 2007. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359:190–200 [DOI] [PubMed] [Google Scholar]
- 11. Clerzius G, et al. 2009. ADAR1 interacts with PKR during human immunodeficiency virus infection of lymphocytes and contributes to viral replication. J. Virol. 83:10119–10128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Devaux P, Hodge G, McChesney MB, Cattaneo R. 2008. Attenuation of V- or C-defective measles viruses: infection control by the inflammatory and interferon responses of rhesus monkeys. J. Virol. 82:5359–5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devaux P, von Messling V, Songsungthong W, Springfeld C, Cattaneo R. 2007. Tyrosine 110 in the measles virus phosphoprotein is required to block STAT1 phosphorylation. Virology 360:72–83 [DOI] [PubMed] [Google Scholar]
- 14. Doria M, Neri F, Gallo A, Farace MG, Michienzi A. 2009. Editing of HIV-1 RNA by the double-stranded RNA deaminase ADAR1 stimulates viral infection. Nucleic Acids Res. 37:5848–5858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fontana JM, Bankamp B, Rota PA. 2008. Inhibition of interferon induction and signaling by paramyxoviruses. Immunol. Rev. 225:46–67 [DOI] [PubMed] [Google Scholar]
- 16. Gélinas JF, Clerzius G, Shaw E, Gatignol A. 2011. Enhancement of replication of RNA viruses by ADAR1 via RNA editing and inhibition of RNA-activated protein kinase. J. Virol. 85:8460–8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. George CX, Gan Z, Liu Y, Samuel CE. 2011. Adenosine deaminases acting on RNA, RNA editing, and interferon action. J. Interferon Cytokine Res. 31:99–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. George CX, Samuel CE. 1999. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible. Proc. Natl. Acad. Sci. U. S. A. 96:4621–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilfoy FD, Mason PW. 2007. West Nile virus-induced interferon production is mediated by the double-stranded RNA-dependent protein kinase PKR. J. Virol. 81:11148–11158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Griffin DE. 2007. Measles virus, p 1551–1585 In Howley P, et al. (ed). Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 21. Hartner JC, Walkley CR, Lu J, Orkin SH. 2009. ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat. Immunol. 10:109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hayden MS, Ghosh S. 2008. Shared principles in NF-kappaB signaling. Cell 132:344–362 [DOI] [PubMed] [Google Scholar]
- 23. Hiscott J. 2007. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 282:15325–15329 [DOI] [PubMed] [Google Scholar]
- 24. Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11:373–384 [DOI] [PubMed] [Google Scholar]
- 25. Li Z, Wolff KC, Samuel CE. 2010. RNA adenosine deaminase ADAR1 deficiency leads to increased activation of protein kinase PKR and reduced vesicular stomatitis virus growth following interferon treatment. Virology 396:316–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin R, Heylbroeck C, Pitha PM, Hiscott J. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu Y, Samuel CE. 1996. Mechanism of interferon action: functionally distinct RNA-binding and catalytic domains in the interferon-inducible, double-stranded RNA-specific adenosine deaminase. J. Virol. 70:1961–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McAllister CS, Samuel CE. 2009. The RNA-activated protein kinase enhances the induction of interferon-beta and apoptosis mediated by cytoplasmic RNA sensors. J. Biol. Chem. 284:1644–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McAllister CS, et al. 2010. Mechanisms of protein kinase PKR-mediated amplification of beta interferon induction by C protein-deficient measles virus. J. Virol. 84:380–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCormack SJ, Thomis DC, Samuel CE. 1992. Mechanism of interferon action: identification of a RNA binding domain within the N-terminal region of the human RNA-dependent P1/eIF-2 alpha protein kinase. Virology 188:47–56 [DOI] [PubMed] [Google Scholar]
- 31. Nakatsu Y, et al. 2008. Measles virus circumvents the host interferon response by different actions of the C and V proteins. J. Virol. 82:8296–8306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nallagatla SR, et al. 2007. 5′-Triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science 318:1455–1458 [DOI] [PubMed] [Google Scholar]
- 33. Navaratnarajah CK, Leonard VH, Cattaneo R. 2009. Measles virus glycoprotein complex assembly, receptor attachment, and cell entry. Curr. Top. Microbiol. Immunol. 329:59–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nie Y, Hammond GL, Yang JH. 2007. Double-stranded RNA deaminase ADAR1 increases host susceptibility to virus infection. J. Virol. 81:917–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oldstone MB. 2009. Modeling subacute sclerosing panencephalitis in a transgenic mouse system: uncoding pathogenesis of disease and illuminating components of immune control. Curr. Top. Microbiol. Immunol. 330:31–54 [DOI] [PubMed] [Google Scholar]
- 36. Palosaari H, Parisien JP, Rodriguez JJ, Ulane CM, Horvath CM. 2003. STAT protein interference and suppression of cytokine signal transduction by measles virus V protein. J. Virol. 77:7635–7644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Panne D, Maniatis T, Harrison SC. 2007. An atomic model of the interferon-beta enhanceosome. Cell 129:1111–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patterson JB, Samuel CE. 1995. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol. Cell. Biol. 15:5376–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pfaller CK, Li Z, George CX, Samuel CE. 2011. Protein kinase PKR and RNA adenosine deaminase ADAR1: new roles for old players as modulators of the interferon response. Curr. Opin. Immunol. 23:573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phuphuakrat A, et al. 2008. Double-stranded RNA adenosine deaminases enhance expression of human immunodeficiency virus type 1 proteins. J. Virol. 82:10864–10872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poulsen H, Nilsson J, Damgaard CK, Egebjerg J, Kjems J. 2001. CRM1 mediates the export of ADAR1 through a nuclear export signal within the Z-DNA binding domain. Mol. Cell. Biol. 21:7862–7871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramachandran A, Parisien JP, Horvath CM. 2008. STAT2 is a primary target for measles virus V protein-mediated alpha/beta interferon signaling inhibition. J. Virol. 82:8330–8338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Randall RE, Goodbourn S. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1–47 [DOI] [PubMed] [Google Scholar]
- 44. Reutter GL, Cortese-Grogan C, Wilson J, Moyer SA. 2001. Mutations in the measles virus C protein that up regulate viral RNA synthesis. Virology 285:100–109 [DOI] [PubMed] [Google Scholar]
- 45. Sadler AJ, Williams BR. 2007. Structure and function of the protein kinase R. Curr. Top. Microbiol. Immunol. 316:253–292 [DOI] [PubMed] [Google Scholar]
- 46. Samuel CE. 2011. Adenosine deaminases acting on RNA (ADARs) are both antiviral and proviral. Virology 411:180–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Samuel CE. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Samuel CE. 1979. Mechanism of interferon action: phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated kinase processing site specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc. Natl. Acad. Sci. U. S. A. 76:600–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sankar S, Chan H, Romanow WJ, Li J, Bates RJ. 2006. IKK-i signals through IRF3 and NFkappaB to mediate the production of inflammatory cytokines. Cell. Signal. 18:982–993 [DOI] [PubMed] [Google Scholar]
- 50. Schoggins JW, et al. 2011. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472:481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schuhmann KM, Pfaller CK, Conzelmann KK. 2011. The measles virus V protein binds to p65 (RelA) to suppress NF-kappaB activity. J. Virol. 85:3162–3171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schulz O, et al. 2010. Protein kinase R contributes to immunity against specific viruses by regulating interferon mRNA integrity. Cell Host Microbe 7:354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sen A, Pruijssers AJ, Dermody TS, Garcia-Sastre A, Greenberg HB. 2011. The early interferon response to rotavirus is regulated by PKR and depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3. J. Virol. 85:3717–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sun SC, Ganchi PA, Ballard DW, Greene WC. 1993. NF-kappa B controls expression of inhibitor I kappa B alpha: evidence for an inducible autoregulatory pathway. Science 259:1912–1915 [DOI] [PubMed] [Google Scholar]
- 55. Toth AM, Devaux P, Cattaneo R, Samuel CE. 2009. Protein kinase PKR mediates the apoptosis induction and growth restriction phenotypes of C protein-deficient measles virus. J. Virol. 83:961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Toth AM, Li Z, Cattaneo R, Samuel CE. 2009. RNA-specific adenosine deaminase ADAR1 suppresses measles virus-induced apoptosis and activation of protein kinase PKR. J. Biol. Chem. 284:29350–29356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Toth AM, Zhang P, Das S, George CX, Samuel CE. 2006. Interferon action and the double-stranded RNA-dependent enzymes ADAR1 adenosine deaminase and PKR protein kinase. Prog. Nucleic Acid Res. Mol. Biol. 81:369–434 [DOI] [PubMed] [Google Scholar]
- 58. Ward SV, et al. 2011. RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis. Proc. Natl. Acad. Sci. U. S. A. 108:331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yokota S, et al. 2003. Measles virus suppresses interferon-alpha signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-alpha receptor complex. Virology. 306:135–146 [DOI] [PubMed] [Google Scholar]
- 60. Yoneyama M, Fujita T. 2010. Recognition of viral nucleic acids in innate immunity. Rev. Med. Virol. 20:4–22 [DOI] [PubMed] [Google Scholar]
- 61. Zhang P, Jacobs BL, Samuel CE. 2008. Loss of protein kinase PKR expression in human HeLa cells complements the vaccinia virus E3L deletion mutant phenotype by restoration of viral protein synthesis. J. Virol. 82:840–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang P, Langland JO, Jacobs BL, Samuel CE. 2009. Protein kinase PKR-dependent activation of mitogen-activated protein kinases occurs through mitochondrial adapter IPS-1 and is antagonized by vaccinia virus E3L. J. Virol. 83:5718–5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang P, Samuel CE. 2007. Protein kinase PKR plays a stimulus- and virus-dependent role in apoptotic death and virus multiplication in human cells. J. Virol. 81:8192–8200 [DOI] [PMC free article] [PubMed] [Google Scholar]