Abstract
Five New World (NW) arenaviruses cause human hemorrhagic fevers. Four of these arenaviruses are known to enter cells by binding human transferrin receptor 1 (hTfR1). Here we show that the fifth arenavirus, Chapare virus, similarly uses hTfR1. We also identify an anti-hTfR1 antibody, ch128.1, which efficiently inhibits entry mediated by the glycoproteins of all five viruses, as well as replication of infectious Junín virus. Our data indicate that all NW hemorrhagic fever arenaviruses utilize a common hTfR1 apical-domain epitope and suggest that therapeutic agents targeting this epitope, including ch128.1 itself, can be broadly effective in treating South American hemorrhagic fevers.
TEXT
New World (NW) arenaviruses are responsible for recurrent hemorrhagic fever outbreaks in South America: Junín virus (JUNV), Machupo virus (MACV), Guanarito virus (GTOV), and Sabiá virus (SABV) are the etiological agents of Argentine, Bolivian, Venezuelan, and Brazilian hemorrhagic fevers, respectively (4, 6, 21), while Chapare virus (CHAV) was isolated from a recent case of hemorrhagic fever in Bolivia (7). These viruses establish persistent infections in their host species, wild rodents, which act as natural reservoirs from which viruses spill over into human populations. Hemorrhagic fever outbreaks are thus confined mostly to these rodents' habitats and are promoted by human-rodent contacts.
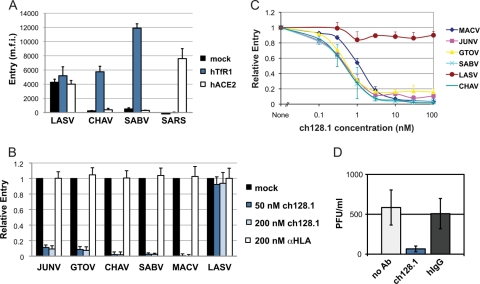
We and others have previously shown that JUNV, MACV, GTOV, and SABV use human transferrin receptor 1 (hTfR1) to enter human cells (11, 22). Here we show that this is also the case for CHAV. CHO-K1 hamster cells expressing hTfR1, but not those expressing a control receptor, became highly permissive to CHAV envelope glycoprotein-bearing murine leukemia virus (MLV) pseudotypes (Fig. 1A), whereas entry of severe acute respiratory syndrome (SARS) coronavirus or Lassa virus (LASV) pseudoviruses was not affected. Consistent with this observation, CHAV pseudovirus entry into human HEK293T cells was efficiently inhibited by an anti-hTfR1 antibody (Fig. 1B). These data establish that all known pathogenic NW arenaviruses use hTfR1 as their major cellular receptor.
Fig 1.
All NW hemorrhagic fever arenaviruses use hTfR1 and their entry is efficiently inhibited by the anti-hTfR1 antibody ch128.1. (A) Like the other NW hemorrhagic fever arenaviruses, CHAV uses hTfR1 as a receptor. CHO-K1 (ATCC CCL-61) cells were transfected with empty vector (mock) or plasmids expressing hTfR1 or human angiotensin converting enzyme 2 (hACE2), the receptor for SARS coronavirus (15). Two days later, cells were infected with MLV pseudoviruses expressing a green fluorescent protein (GFP) reporter and bearing the envelope GP of the indicated viruses, as described previously (2). Infection levels were assessed the following day by flow cytometry. Depicted are means + standard deviations (SD) from two duplicated experiments. m.f.i., mean fluorescence intensity. (B) HEK293T cells were preincubated for 30 min with ch128.1, the control antibody anti-HLA ABC, or medium alone (mock), and then MLV pseudoviruses were added and incubation continued overnight. Infection levels were assessed as described for panel A, and m.f.i. values were normalized to those for mock-treated cells. Shown are means + SD from two duplicated experiments. (C) HEK293T cells were preincubated with ch128.1, the indicated pseudoviruses were added, and incubation continued overnight. Infection levels were assessed as described for panel A and normalized to those in the absence of ch128.1. Data represent means + SD from two duplicated experiments. (D) A549 cells (ATCC CCL-185) were preincubated with 200 nM ch128.1, hIgG, or medium alone (no Ab) and infected with replication-competent JUNV at a multiplicity of infection (MOI) of 1. Viruses were replaced 1 h later with the respective antibody-supplemented medium or medium alone. Total JUNV production over 24 h in A549 cell supernatants was measured using a PFU assay in Vero cells. Depicted are means ± SD from two duplicated experiments.
While the total number of cases is low, South American hemorrhagic fevers typically have a high case-fatality rate (10 to 35%) when untreated (8, 16). Except for a clinically well-supported live attenuated JUNV vaccine (18), the options to combat South American hemorrhagic fevers are limited. Passive immunotherapy with convalescent patient sera is currently the only treatment with demonstrated success (10, 17) but has practical disadvantages, including limited supply and the possible transmission of blood-borne pathogens. Another option may be the nucleoside analog ribavirin (9), although this drug was only moderately effective when tested against JUNV in primate models (19, 26). Finally, the recently developed small molecules (3, 13, 14) remain unproven in vivo.
As all pathogenic NW arenaviruses use hTfR1, targeting hTfR1 may provide a common treatment against all South American hemorrhagic fevers. A number of anti-hTfR1 antibodies have been developed for cancer therapy, most of which compete with transferrin for binding to hTfR1, resulting in cell growth inhibition. To avoid this undesirable property, we investigated an antibody, ch128.1 (previously known as anti-hTfR IgG3), that does not compete with transferrin (20). Ch128.1 was constructed by fusing the variable chains of the murine monoclonal anti-hTfR1 antibody 128.1 (27) to the constant chains of human IgG3 (20).
We first assessed ch128.1 for its ability to block the entry of pathogenic NW arenaviruses. As shown in Fig. 1B, the entry of MLV pseudotyped with the envelope glycoproteins (GP) of JUNV, GTOV, CHAV, SABV, and MACV was efficiently inhibited (85 to 98%) by ch128.1 at 50 nM while that of LASV, which uses α-dystroglycan as a receptor (5), was not affected. This inhibition was ch128.1 specific, since a control antibody (anti-HLA) did not inhibit any of the pseudoviruses tested. We then determined the 50% inhibitory concentration (IC50) of ch128.1 for these pseudoviruses in HEK293T cells. Under conditions in which 20 to 35% of the cells were infected in the absence of ch128.1, the IC50 of ch128.1 was subnanomolar for all NW hemorrhagic fever arenaviruses (Fig. 1C). We further investigated whether ch128.1 could antagonize replication-competent JUNV. In an experiment with human A549 lung epithelial cells, where multiple rounds of infection with JUNV (attenuated strain IV445) were allowed, 200 nM ch128.1 strongly inhibited the infection while purified human IgG (hIgG) did not (Fig. 1D). Similar levels of inhibition by ch128.1 were also obtained when NH4Cl was added to cells 4 h postinfection to ensure a single round of infection (data not shown). These data indicate that ch128.1 can efficiently inhibit all five known pathogenic NW arenaviruses and suggest that it may be useful in the treatment of South American hemorrhagic fevers.
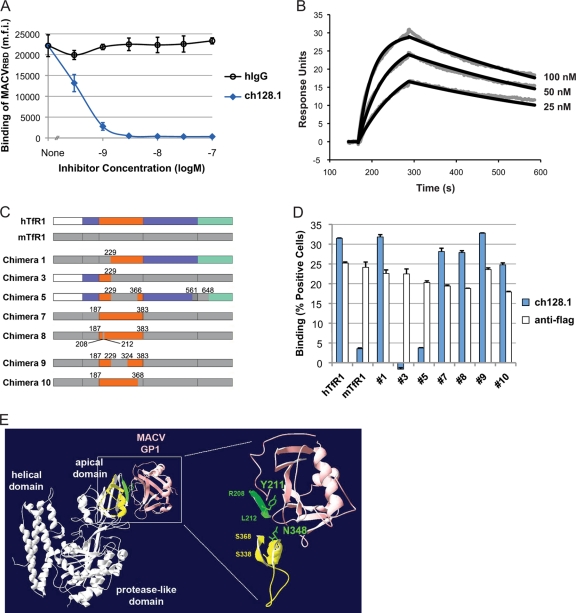
Next, we investigated the mechanism of inhibition by ch128.1 and determined its affinity and binding site. As shown in Fig. 2A, the association of MACVRBD-mIgG (MACV GP receptor binding domain [RBD] residues E87 to E258 fused to murine IgG2a [mIgG]) with HEK293T cells was potently inhibited by ch128.1 but not by hIgG, suggesting that ch128.1 inhibits entry by blocking the viruses' hTfR1 binding site. Surface plasmon resonance assays confirmed that ch128.1 has a high affinity for hTfR1, since the equilibrium dissociation constant (KD) for ch128.1 binding to the soluble ectodomain of hTfR1 was 5.7 nM (Fig. 2B). To map the ch128.1 binding region of hTfR1, CHO-K1 cells were transfected with previously characterized human-mouse TfR1 chimeric constructs (23, 25) (Fig. 2C), as well as with wild-type hTfR1, which supports NW hemorrhagic fever arenavirus entry, and with mouse TfR1 (mTfR1), which does not (23). Ch128.1 bound TfR1 chimeras 1, 7, 8, 9, and 10 comparably to wild-type hTfR1. In contrast, like mTfR1, chimeras 3 and 5 did not bind ch128.1 (Fig. 2D). These results narrow the ch128.1 binding site to the hTfR1 apical domain between Ser 324 and Ser 368, a region that overlaps with regions known to be essential for MACV GP1 binding and pseudovirus entry (1, 23) (Fig. 2E).
Fig 2.
Ch128.1 competes with arenavirus GP for overlapping binding sites on the hTfR1 apical domain. (A) HEK293T cells were preincubated on ice with increasing concentrations of ch128.1 or hIgG, MACVRBD-mIgG was added to obtain a concentration of 5 μg/ml, and binding was detected by flow cytometry with a phycoerythrin (PE)-conjugated anti-mouse Fc antibody. Data are means ± SD from two duplicated experiments. (B) Surface plasmon resonance assay results. Increasing concentrations of soluble hTfR1 (residues 117 to 760) were added to ch128.1 immobilized on a CM5 chip (Biacore) by amine coupling. Rate constants were calculated by fitting the measured data (gray) to a 1:1 Langmuir binding model (black); the association constant (Ka) was 2.85 × 105 M−1s−1, the dissociation constant (Kd) was 1.61 × 10−3 s−1, and the resulting KD was 5.7 nM. Depicted is one of two sets of values from duplicated measurements with similar results. (C) Schematic diagram of the human-mouse TfR1 chimeras used as described in the legend to panel D. The protease-like domain is shown in blue, the apical domain in orange, and the helical domain in green. (D) Ch128.1 binding of TfR1 chimeras. Flag-tagged chimeric TfR1, hTfR1, or mTfR1 (23, 25) was expressed on CHO-K1 cells. TfR1 expression levels were measured by flow cytometry using an anti-Flag tag antibody. In parallel, aliquots of the same cells were assessed for ch128.1 binding at 5 μg/ml by flow cytometry followed by anti-hIgG-PE. Depicted are means + SD from a representative experiment performed in duplicate. (E) Ribbon diagram of TfR1 in complex with MACV GP1 (Protein Data Bank identification no. 3KAS), in pink (1). Previously established hTfR1 determinants for MACV GP1 binding (23) are in green. Surface-exposed residues (S338 to S368) of the putative ch128.1 binding site (S324 to S368) are indicated in yellow. Note that N348 is part of both the ch128.1 and MACV GP1 binding sites.
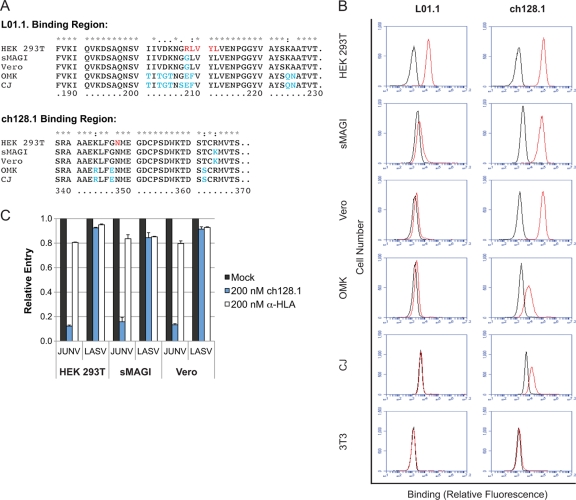
Because the preclinical evaluation of therapeutic candidates requires animal studies, we further assessed whether nonhuman primates could be used to evaluate the efficacy of ch128.1. Established primate models for South American hemorrhagic fevers include rhesus macaques (Macaca mulatta), African green monkeys (Cercopithecus aethiops), and common marmosets (Callithrix jacchus) (12). Another primate model commonly used in research is the owl monkey, Aotus trivirgatus. We first cloned the TfR1 orthologs from these four species by using the cell lines sMAGI, Vero, CJ, and OMK, respectively. As the sequences in Fig. 3A indicate, the ch128.1 binding region shows an overall higher degree of conservation than the binding region of another anti-hTfR1 antibody (clone L01.1; BD Biosciences) that also blocks NW arenavirus entry into human cells (22). As expected based on the sequence data, ch128.1 was able to bind to all nonhuman primate cells (Fig. 3B) while L01.1 was not. Nevertheless, 200 nM ch128.1 was able to block JUNV pseudovirus entry only in sMAGI and Vero cells (Fig. 3C), i.e., the cells derived from the Old World primates M. mulatta and C. aethiops, but not in the New World primate cells OMK and CJ (data not shown). Together these data suggest that the in vivo evaluation of ch128.1-type antibodies is feasible in M. mulatta and C. aethiops.
Fig 3.
Ch128.1 binds nonhuman primate TfR1 orthologs and blocks JUNV pseudovirus entry into Old World monkey cells. (A) TfR1 coding sequences corresponding to the binding region of ch128.1 and that of another anti-hTfR1 antibody (clone L01.1; BD Biosciences) (unpublished data). TfR1 sequences from M. mulatta, C. aethiops, A. trivirgatus, and C. jacchus were obtained by reverse transcription-PCR using sMAGI, Vero, OMK, and CJ cell cDNAs, respectively (GenBank accession numbers JQ014203 to JQ014208). Residues R208 through L212 and N348, which are critical for GP1 binding to hTfR1 and pseudovirus entry (23), are colored in red. Nonconserved residues are highlighted in blue. (B) Binding of ch128.1 or L01.1 to various cells is indicated in red. Purified hIgG or mouse IgG, respectively, was used as a negative control (black). HEK293T cells are human embryonic kidney cells (ATCC CRL-11268), sMAGI cells are rhesus macaque mammary tumor cells (NIBSC ARP287), Vero cells are African green monkey kidney cells (ATCC CCL-81), OMK are owl monkey kidney cells (ATCC CRL-1556), CJ cells are common marmoset fibroblasts (New England Primate Research Center, Southborough, MA), and 3T3 cells are mouse embryonic fibroblasts (ATCC CCL-92). (C) Ch128.1 inhibits JUNV pseudovirus entry into Old World primate cells. HEK293T, sMAGI, and Vero cells were preincubated with ch128.1, the control antibody anti-HLA, or medium alone (mock), and then pseudoviruses were added and incubation continued overnight. The percentage of positive cells was assessed 48 h later by flow cytometry and was normalized to that for mock-treated cells. Shown are means + SD from two duplicated experiments.
In addition to the five known NW hemorrhagic fever viruses, there are a number of related viruses that have the potential to emerge as human pathogens. Because outbreaks are in most cases limited, it is not feasible to develop therapeutics specific to each virus. Here we show that all five NW hemorrhagic fever arenaviruses can be inhibited by a single antibody. This observation suggests that even infections with uncharacterized and newly emerging viruses could be effectively treated with such an antibody. Although recently identified small-molecule inhibitors, which bind the conserved GP2 or stable signal peptide domains of NW arenavirus GP (3, 13, 14, 28), may similarly block a range of viruses, antibodies like ch128.1 likely have longer half-lives in serum, lower toxicity, and greater specificity and afford the viruses little means of escape.
The therapeutic efficacy of ch128.1 in animal models and humans will critically depend on whether TfR1 is the sole or primary entry gateway into cells that support NW hemorrhagic fever arenavirus replication in vivo. This remains unproven, although it is indicated by circumstantial evidence: for example, the GP of NW hemorrhagic fever arenaviruses appear to be specifically adapted to the TfR1 orthologs in their respective host species, and the ability of the viruses to cause human diseases strictly correlates with their ability to bind human TfR1 (2, 23). A second concern is the risk associated with targeting an important host protein. However, this risk is mitigated by the fact that ch128.1 does not compete with the two physiological ligands of TfR1, transferrin and hereditary hemochromatosis protein (20, 24), and thus should interfere only marginally with known roles of TfR1. This risk is further reduced by the short duration of treatment required for these acute, fast-progressing hemorrhagic fevers. Thus, our data suggest that further development of ch128.1-type antibodies as therapeutics against South American hemorrhagic fevers is warranted.
ACKNOWLEDGMENTS
This project was supported by the following grants: ANPCyT-FONARSEC PICT-PRH 2008-00315 to G.H., NIH AI074879 to H.C. and AI057159 to H.C. and M.F., NIH CA107023 to M.L.P., and ANPCyT 2007-761 and UBA 2008-035 to N.A.C. G.H., S.M.C., and M.G.M. are supported by the Consejo Nacional de Investigaciones Científicas y Tecnológicas. S.J. is supported by a fellowship from the Swiss National Science Foundation. J.A. and J.A.R. are Howard Hughes Medical Institute Gilliam fellows. J.A.R. is further funded by the UCLA MBI Whitcome Fellowship.
Footnotes
Published ahead of print 25 January 2012
REFERENCES
- 1. Abraham J, Corbett KD, Farzan M, Choe H, Harrison SC. 2010. Structural basis for receptor recognition by New World hemorrhagic fever arenaviruses. Nat. Struct. Mol. Biol. 17:8–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abraham J, et al. 2009. Host-species transferrin receptor 1 orthologs are cellular receptors for nonpathogenic New World clade B arenaviruses. PLoS Pathog. 5:e1000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolken TC, et al. 2006. Identification and characterization of potent small molecule inhibitor of hemorrhagic fever New World arenaviruses. Antiviral Res. 69:86–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buchmeier MJ, de la Torre JC, Peters CJ. 2007. Arenaviridae: the viruses and their replication, p 1791–1819 In Knipe DM, et al. (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 5. Cao W, et al. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:79–2081 [DOI] [PubMed] [Google Scholar]
- 6. Charrel RN, de Lamballerie X. 2003. Arenaviruses other than Lassa virus. Antiviral Res. 57:89–100 [DOI] [PubMed] [Google Scholar]
- 7. Delgado S, et al. 2008. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS Pathog. 4:e1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Manzione N, et al. 1998. Venezuelan hemorrhagic fever: clinical and epidemiological studies of 165 cases. Clin. Infect. Dis. 26:8–313 [DOI] [PubMed] [Google Scholar]
- 9. Enria DA, et al. 1987. Tolerance and antiviral effect of ribavirin in patients with Argentine hemorrhagic fever. Antiviral Res. 7:353–359 [DOI] [PubMed] [Google Scholar]
- 10. Enria DA, Briggiler AM, Sanchez Z. 2008. Treatment of Argentine hemorrhagic fever. Antiviral Res. 78:2–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flanagan ML, et al. 2008. New world clade B arenaviruses can use transferrin receptor 1 (TfR1)-dependent and -independent entry pathways, and glycoproteins from human pathogenic strains are associated with the use of TfR1. J. Virol. 82:938–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gowen BB, Holbrook MR. 2008. Animal models of highly pathogenic RNA viral infections: hemorrhagic fever viruses. Antiviral Res. 78:79–90 [DOI] [PubMed] [Google Scholar]
- 13. Larson RA, et al. 2008. Identification of a broad-spectrum arenavirus entry inhibitor. J. Virol. 82:768–10775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee AM, et al. 2008. Unique small molecule entry inhibitors of hemorrhagic fever arenaviruses. J. Biol. Chem. 283:734–18742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li W, et al. 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426:450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maiztegui JI. 1975. Clinical and epidemiological patterns of Argentine haemorrhagic fever. Bull. World Health Organ. 52:567–575 [PMC free article] [PubMed] [Google Scholar]
- 17. Maiztegui JI, Fernandez NJ, de Damilano AJ. 1979. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet ii:1216–1217 [DOI] [PubMed] [Google Scholar]
- 18. Maiztegui JI, et al. 1998. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. J. Infect. Dis. 177:277–283 [DOI] [PubMed] [Google Scholar]
- 19. McKee KT, Jr, Huggins JW, Trahan CJ, Mahlandt BG. 1988. Ribavirin prophylaxis and therapy for experimental Argentine hemorrhagic fever. Antimicrob. Agents Chemother. 32:04–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ng PP, et al. 2006. Molecular events contributing to cell death in malignant human hematopoietic cells elicited by an IgG3-avidin fusion protein targeting the transferrin receptor. Blood 108:2745–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peters CJ. 2002. Human infection with arenaviruses in the Americas. Curr. Top. Microbiol. Immunol. 262:65–74 [DOI] [PubMed] [Google Scholar]
- 22. Radoshitzky SR, et al. 2007. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature 446:-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radoshitzky SR, et al. 2008. Receptor determinants of zoonotic transmission of New World hemorrhagic fever arenaviruses. Proc. Natl. Acad. Sci. U. S. A. 105:64–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rodriguez JA, et al. 2007. Binding specificity and internalization properties of an antibody-avidin fusion protein targeting the human transferrin receptor. J. Control. Release 124:35–42 [DOI] [PubMed] [Google Scholar]
- 25. Wang E, Albritton L, Ross SR. 2006. Identification of the segments of the mouse transferrin receptor 1 required for mouse mammary tumor virus infection. J. Biol. Chem. 281:10243–10249 [DOI] [PubMed] [Google Scholar]
- 26. Weissenbacher MC, Calello MA, Merani MS, McCormick JB, Rodriguez M. 1986. Therapeutic effect of the antiviral agent ribavirin in Junin virus infection of primates. J. Med. Virol. 20:261–267 [DOI] [PubMed] [Google Scholar]
- 27. White S, Taetle R, Seligman PA, Rutherford M, Trowbridge IS. 1990. Combinations of anti-transferrin receptor monoclonal antibodies inhibit human tumor cell growth in vitro and in vivo: evidence for synergistic antiproliferative effects. Cancer Res. 50:6295–6301 [PubMed] [Google Scholar]
- 28. York J, Dai D, Amberg SM, Nunberg JH. 2008. pH-induced activation of arenavirus membrane fusion is antagonized by small-molecule inhibitors. J. Virol. 82:10932–10939 [DOI] [PMC free article] [PubMed] [Google Scholar]