Abstract
We characterized the RNA elements involved in the packaging of Rift Valley fever virus RNA genome segments, L, M, and S. The 5′-terminal 25 nucleotides of each RNA segment were equally competent for RNA packaging and carried an RNA packaging signal, which overlapped with the RNA replication signal. Only the deletion mutants of L RNA, but not full-length L RNA, were efficiently packaged, implying the possible requirement of RNA compaction for L RNA packaging.
TEXT
Rift Valley fever virus (RVFV) (the genus Phlebovirus, family Bunyaviridae) carries three single-stranded, negative-sense RNA segments, L, M, and S. The viral RNA-dependent RNA polymerase (L protein), envelope Gn/Gc glycoproteins, and N protein, all of which are essential for virus replication, are encoded in L, M, and S RNAs, respectively; hence, copackaging of the three genomic RNA segments into a virus particle is necessary for the generation of infectious RVFV, yet our understanding of bunyavirus RNA packaging mechanisms is still in its infancy (2, 4, 11, 15, 19, 23, 26). Using RVFV, we address several unexplored questions in bunyavirus genome packaging, including the biological activities of the noncoding regions (NCRs) of each viral RNA segment for RNA packaging, the identification of RNA packaging signals, and a possible role(s) of the coding regions in viral RNA packaging.
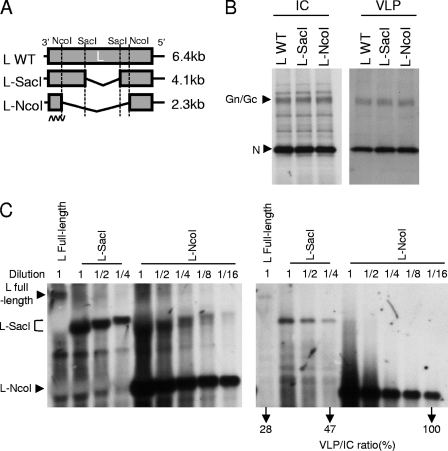
RVFV M RNA as well as S RNA is efficiently packaged, in the absence of any other viral RNA segment, into virus-like particles (VLPs), released from cells expressing the viral structural proteins and harboring the replicating M RNA and S RNA, respectively (26). However, RVFV L RNA is not packaged efficiently into VLPs in the absence of other viral RNA segments; both M and S RNAs are required for efficient L RNA packaging (26). To test the possibility that L RNA lacks a packaging signal, BSR-T7/5 cells stably expressing T7 RNA polymerase (3) were cotransfected with a plasmid expressing T7 polymerase-driven anti-viral-sense L RNA-derived L-SacI RNA or L-NcoI RNA, each carrying a large internal deletion within the L gene (Fig. 1A), along with the plasmids expressing L, Gn/Gc, and N proteins (26). As a control, we used a plasmid expressing the full-length L RNA. At 3 days posttransfection, cell extracts were collected and the VLPs released into the supernatant were purified by ultracentrifugation (10, 26). The intracellular accumulations and the incorporations of Gn/Gc and N proteins into VLPs were similar among all three samples (Fig. 1B), suggesting the production of similar levels of VLPs. The intracellular accumulation of full-length viral-sense L RNA was appreciably lower than that of the two deletion mutants (Fig. 1C, left panel); the L RNA deletion mutants most probably replicate faster than the full-length L RNA due to their shorter lengths, resulting in higher intracellular accumulation of the L RNA deletion mutants. The amount of the full-length L RNA in VLPs was also substantially lower than that of the deletion mutants (Fig. 1C, right panel). Comparing the band intensities of the full-length L RNA in undiluted intracellular and VLP samples and those of mutant RNAs in serially diluted intracellular and VLP samples revealed a trend toward less efficient packaging of longer L RNAs (Fig. 1C). These data indicated the presence of a functional packaging signal(s) in L RNA and also suggested that the large genome size prevented the efficient packaging of the full-length L RNA.
Fig 1.
Packaging of L RNA and its internal deletion mutants into VLPs. (A) Schematic diagrams of L RNA (L WT) and its internal deletion mutants, L-SacI and L-NcoI. L-SacI and L-NcoI were constructed from L RNA expression plasmids by digestion with SacI, to remove the internal fragment from nt 2542 to 4802 of L RNA, and with NcoI, to remove the internal fragment from nt 686 to 5320 of L RNA, respectively. The sizes of L RNA and its deletion mutants are shown on the right. The wavy line represents the RNA probe-binding site. (B) The plasmid expressing anti-viral-sense L RNA or one of the mutant RNAs shown in panel A was cotransfected with plasmids expressing L, Gn/Gc, and N proteins. Cell extracts and VLPs were collected at 3 days posttransfection, and VLPs were purified by sucrose gradient centrifugation. Western blot analysis using anti-RVFV mouse antibody (10, 26) shows the levels of N and Gn/Gc proteins in the intracellular (IC) samples and in the purified VLPs (VLP). (C) The samples prepared in panel B were subjected to Northern blot analysis. Equal amounts of total IC RNA, from cells supporting the replication of each L-derived RNA (left panel), and RNA samples extracted from the purified VLPs (right panel) were resolved on an agarose-formaldehyde gel. The detection of similar amounts of rRNAs by ethidium bromide staining of the gels confirmed the loading of similar amounts of IC RNAs (data not shown). In addition, 2-fold serially diluted samples containing L-SacI or L-NcoI RNAs were also applied to the gels. The sample dilutions are indicated at the top of each lane; lane 1 represents undiluted samples. The agarose gels were treated with 200 mM sodium hydroxide to partially hydrolyze the RNA for size-independent transfer (25), and the RNAs were subsequently transferred to a nylon membrane. The RNAs were detected using digoxigenin-labeled RNA probes that specifically hybridized with nt 8 to 756 in the 3′ end of the viral-sense L RNA (10, 26). The band intensities of L RNA and its mutants were measured by ImageJ software. The numbers below the lanes of the right panel represent the packaging efficiencies of the respective L RNAs, reported as approximate percentages of the packaging efficiency of the L-NcoI RNA sample with the highest dilution. The packaging efficiency for any given L RNA was calculated as the ratio of the band intensities of VLP and IC RNA. Representative data from three independent experiments are shown.
To know whether the viral sequences in the intergenic region and the coding regions of S RNA contribute to S RNA packaging efficiency, we prepared S-N/rLuc RNA, by replacing the NSs gene with the Renilla luciferase (rLuc) gene (8), and SNCR-rLucR RNA, in which the regions encompassing the N gene, intergenic region, and the NSs gene were replaced with the rLuc gene (Fig. 2A). Cotransfection of plasmids expressing N, L, and Gn/Gc proteins along with the plasmid expressing either S-N/rLuc RNA, SNCR-rLucR RNA, or S RNA resulted in the production of similar levels of VLPs (Fig. 2B). The intracellular accumulation levels and the packaging efficiencies of the three RNAs into VLPs were similar (Fig. 2C). The lack of incorporation of rLuc mRNA, which is transcribed from S-N/rLuc RNA, into purified VLPs demonstrated the selectivity in minigenome RNA packaging (Fig. 2C). Because SNCR-rLucR RNA lacked the intergenic region, which includes the transcriptional termination signal (1, 9, 12), the production of an mRNA that is structurally identical to rLuc mRNA of S-N/rLuc RNA in cells supporting SNCR-rLucR RNA replication was unlikely. These data suggested that sequences in the intergenic region and the coding regions of S RNA had little to no effect on the packaging efficiency of RVFV S RNA into VLPs.
Fig 2.
Packaging efficiencies of S RNA, M RNA, and their mutants into VLPs. (A) Schematic diagrams of S RNA and S RNA-derived mutants. The NSs gene in S RNA was replaced with the rLuc gene to generate S-N/rLuc, while the N gene, the intergenic region, and the NSs gene in S RNA were replaced with the rLuc gene to generate SNCR-rLucR. The wavy lines represent the N probe, a riboprobe that hybridizes with nt 1 to 738 of the N gene and detects the viral-sense S RNA, and the double lines represent rLuc probe 1, a riboprobe that hybridizes with nt 1 to 702 of the rLuc gene and detects the rLuc mRNA and viral-sense SNCR-rLucR and S-N/rLuc RNAs. (B) Cell extracts and VLPs were prepared using the methods described for Fig. 1B. Western blot analysis using anti-RVFV mouse antibody shows the accumulation of Gn/Gc and N proteins in cells (IC) and in purified VLP (VLP) samples. (C) Intracellular RNAs (IC) and RNAs in VLPs (VLP) were subjected to Northern blot analysis. N probe was used for lanes 1 to 6, and rLuc probe 1 was used for lanes 7 to 12. The asterisk represents rLuc mRNA, transcribed from S-N/rLuc. The numbers below the lanes represent the packaging efficiencies of S-N/rLuc and SNCR-rLucR, reported as approximate percentages of the packaging efficiencies of S RNA and S-N/rLuc RNA, respectively. The packaging efficiency for a given S RNA was calculated as the ratio of the band intensities of VLP RNA and IC RNA. (D) Schematic diagram of M RNA and M RNA-derived mutant. The M gene ORF was replaced with the rLuc gene ORF in MNCR-rLuc. (E) Cell extracts and VLPs were prepared using the methods described in Fig. 1B. Western blot analysis using anti-RVFV mouse antibody shows the accumulation of Gn/Gc and N proteins in cells (IC) and in VLP (VLP) samples. (F) Intracellular RNAs (IC) and RNAs in purified VLPs (VLP) were subjected to Northern blot analysis using M NCR probe (wavy lines in panel D) that hybridizes with nt 3672 to 3874 of the 5′ NCR in M virus-sense RNA. The aberrant migration of intracellular M RNA in the gel was caused by the presence of large amounts of 28S rRNA, which is similar in size to the M RNA. The numbers below the lanes of the right panel represent the packaging efficiency of MNCR-rLuc reported as approximate percentages of the packaging efficiency of M RNA. The packaging efficiency of M RNA and its mutant was calculated as the ratio of the band intensities of VLP RNA and IC RNA. Representative data from three independent experiments are shown.
We performed similar experiments to examine whether the sequences in the coding region of M RNA contribute to the efficiency of M RNA packaging by using full-length M RNA and MNCR-rLuc RNA, in which the M gene open reading frame (ORF) was replaced with the rLuc gene ORF (Fig. 2D). Due to the expression of Gn/Gc proteins from the replicating M RNA, the levels of Gn/Gc proteins in cells harboring the replicating M RNA were slightly higher than the levels in those harboring the replicating MNCR-rLuc RNA (Fig. 2E). Similarly, the amount of incorporated Gn/Gc proteins in VLPs obtained from cells harboring the replicating M RNA was also slightly higher, implying slightly higher VLP production from these cells (Fig. 2E). The amounts of MNCR-rLuc RNA in cells and in VLPs were substantially higher than those of M RNA (Fig. 2F). However, the packaging efficiencies of M RNA and MNCR-rLuc RNA were found to be comparable, after taking into consideration the small differences in the production of VLPs between the two samples (Fig. 2F). These data imply that the coding region of M RNA did not play a major role in determining M RNA packaging efficiency.
To directly compare the RNA packaging competencies of the NCRs in each RNA segment, we examined the packaging efficiencies of the minigenome RNAs, LNCR-rLuc, MNCR-rLuc, and SNCR-rLuc, derived from L, M, and S RNAs, respectively; the minigenome RNAs carried only the rLuc gene flanked by the 3′- and 5′-terminal NCRs from the respective RNA segments (Fig. 3A). Similar amounts of VLPs were produced from cells supporting the replication of each mutant RNA (Fig. 3B). All the mutant RNAs replicated to similar levels and were packaged into VLPs with similar efficiencies (Fig. 3C), demonstrating that the NCRs of each RVFV RNA segment had similar RNA packaging competencies.
Fig 3.
Influence of the NCRs of L, M, and S RNAs on RNA packaging. (A) Schematic diagrams of LNCR-rLuc, MNCR-rLuc, and SNCR-rLuc, which carried the rLuc gene inserted between the 3′ and 5′ NCRs of L, M, and S RNAs, respectively. (B) Cell extracts and purified VLPs were prepared using the methods described for Fig. 1B. Western blot analysis using anti-RVFV mouse antibody shows the accumulation of Gn/Gc and N proteins in cells (IC) and in VLP (VLP) samples. (C) Intracellular RNAs (IC) and RNAs in VLPs (VLP) were subjected to Northern blot analysis using rLuc probe 2 that hybridizes with nt 1 to 702 of the rLuc gene and detects viral-sense RNAs of LNCR-rLuc, MNCR-rLuc, and SNCR-rLuc. The packaging efficiencies of the three RNAs were determined as described for Fig. 2 and reported as approximate percentages of the packaging efficiency of LNCR-rLuc RNA. Representative data from three independent experiments are shown.
Next, we tested whether the entire NCR in each RVFV RNA segment is required for efficient packaging of the minigenome RNAs into VLPs. We constructed a series of 5′ NCR deletion mutants using the parental minigenomes, LNCR-rLuc, MNCR-rLuc, and SNCR-rLucR. The rLuc gene was inserted in the opposite orientation in SNCR-rLucR. Each mutant had a deletion of different length in the 5′-terminal NCR (Fig. 4A, D, and G). Because the 3′- and 5′-terminal 16 to 19 nucleotides (nt) in the NCR of each RNA segment are important for the formation of a panhandle structure, which is most likely essential for genome replication (7), the terminal 15 nt of the 5′ NCR were kept intact in each deletion mutant. In experiments comparing the deletion mutants and their respective parental minigenomes, similar amounts of VLPs were produced and the amounts of packaged minigenome RNAs were roughly proportional to their intracellular levels of accumulation (Fig. 4B, C, E, F, H, and I). Also, the minigenomes carrying only the terminal 15 nt of the 5′ NCR replicated less efficiently than did the other mutants. These data demonstrated that the entire 5′ NCR and the terminal 15 to 25 nt of the 5′ NCR in each RVFV RNA segment were equally competent for minigenome RNA packaging and that the RNA replication signals in the 5′ NCR of each RVFV RNA segment overlapped with the viral RNA packaging signal.
Fig 4.
Effects of deletions within the 5′ NCR of RVFV minigenomes on RNA replication and packaging. (A, D, G, and J) Schematic diagrams of mutant RNAs. All the RNAs carried the rLuc gene, and the orientation of the rLuc gene is illustrated. Each mutant had a deletion of a different length in the 5′-terminal NCR, and the nucleotide lengths of the remaining intact 5′ NCR in the RNAs are shown. (B, E, H, and K) Cell extracts and VLPs were prepared using the methods described for Fig. 1B. Western blot analysis using anti-RVFV mouse antibody shows the accumulation of Gn/Gc and N proteins in cells (IC) and in VLP (VLP) samples. (C, F, I, and L) Intracellular RNAs (IC) and RNAs in VLPs (VLP) were subjected to Northern blot analysis using rLuc probe 2 (A, D, and J) or rLuc probe 1 (G). The packaging efficiencies of the RNAs were determined as described in the Fig. 2 legend and reported in panels C, F, I, and L as approximate percentages of the packaging efficiency of LNCR-rLuc, MNCR-rLuc, SNCR-rLucR, and LNCR-25, respectively. Representative data from three independent experiments are shown.
To determine the contribution of the terminal 25 nt in the 5′ NCR of each RNA segment toward RNA packaging, we compared the replication and packaging competencies of LNCR-25 RNA, MNCR-25 RNA, and a new construct, S RNA-derived SNCR-25 RNA, all of which carried the rLuc gene flanked by the 3′ NCR and the terminal 25 nt of the 5′ NCR of the respective RNA segments. Similar levels of RNA replication and packaging of these RNAs into VLPs (Fig. 4K and L) demonstrated that the terminal 25-nt sequences in the 5′ NCR of each RNA segment have similar competencies for minigenome RNA replication and packaging.
We observed an increase in the packaging efficiency of L RNA, but not M or S RNAs, with decreasing lengths of viral RNA. Although the mechanism that drives the length-dependent packaging of L RNA into VLPs requires further investigation, the efficient packaging of the 6.4-kb-long L RNA genome into RVFV particles may require proper L RNA compaction; the importance of compactly folded tertiary structure for viral RNA genome packaging has been suggested previously (28). Also, studies on the RNA packaging mechanism of bacteriophage MS2 have suggested that the binding of coat protein to single-stranded viral RNA genome leads to viral RNA compaction and conformational changes of the coat protein, both of which contribute to efficient RNA packaging (24). It is possible that a putative interaction(s) of M and/or S RNAs with L RNA (26) facilitates L RNA compaction that results in the efficient packaging of L RNA. In contrast to L RNA, the 3.9-kb-long M RNA and 1.7-kb-long S RNA may be able to form packaging-competent RNA structures by themselves, resulting in their efficient packaging. Alternatively, the shorter lengths of M and S RNAs may obviate RNA compaction to drive the efficient packaging of these RNAs into RVFV particles.
RVFV minigenomes that replicated well in the cells were also efficiently packaged into VLPs; we were unable to identify discrete cis-acting RNA replication signals and RNA packaging signals as these signals overlapped in RVFV. Our data are consistent with a model in which a panhandle structure formed by the 3′ and 5′ termini of each RNA segment serves as a core RNA replication signal as well as the viral RNA packaging signal, the latter of which interacts with the cytoplasmic tail of Gn protein (23) to facilitate selective viral RNA packaging. The packaging of nonreplicating viral RNA into RVFV VLPs can occur (23); probably, some of the nonreplicating viral RNAs are able to form an appropriate RNA structure suitable for RNA packaging into RVFV VLPs.
In contrast to RVFV, the terminal ∼20-nt sequences of influenza A virus (FLUAV) RNA genome segments serve as minimal RNA replication signals (22), but the NCRs and the coding regions are both required for efficient genome packaging (5, 6, 13, 14, 16, 17, 20, 21, 27). Studies have shown that all eight RNA segments are required for efficient FLUAV RNA packaging (6, 18), which is similar, in principle, to the observations that efficient RVFV L RNA packaging occurs in the presence of M and S RNAs (26) and that a region within the 5′ NCR of M RNA is necessary for the copackaging of M and S RNAs as well as L RNA packaging into VLPs (26). Because the mechanisms of FLUAV RNA genome copackaging are largely unknown and the number of RNA genome segments packaged into RVFV is much lower than that packaged into FLUAV, these studies using RVFV could serve as a more feasible model system to explore the mechanism of genome copackaging in segmented RNA viruses.
ACKNOWLEDGMENTS
This work was supported by a grant from the Department of Homeland Security. S. Murakami and K. Terasaki were supported by the James W. McLaughlin fellowship fund. S. Murakami was also supported by a research fellowship from the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print 25 January 2012
REFERENCES
- 1. Albarino CG, Bird BH, Nichol ST. 2007. A shared transcription termination signal on negative and ambisense RNA genome segments of Rift Valley fever, sandfly fever Sicilian, and Toscana viruses. J. Virol. 81:5246–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brennan B, Welch SR, McLees A, Elliott RM. 2011. Creation of a recombinant Rift Valley Fever virus with a two-segmented genome. J. Virol. 85:10310–10318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buchholz UJ, Finke S, Conzelmann KK. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flick K, et al. 2004. Functional analysis of the noncoding regions of the Uukuniemi virus (Bunyaviridae) RNA segments. J. Virol. 78:11726–11738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fujii K, et al. 2005. Importance of both the coding and the segment-specific noncoding regions of the influenza A virus NS segment for its efficient incorporation into virions. J. Virol. 79:3766–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fujii Y, Goto H, Watanabe T, Yoshida T, Kawaoka Y. 2003. Selective incorporation of influenza virus RNA segments into virions. Proc. Natl. Acad. Sci. U. S. A. 100:2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gauliard N, Billecocq A, Flick R, Bouloy M. 2006. Rift Valley fever virus noncoding regions of L, M and S segments regulate RNA synthesis. Virology 351:170–179 [DOI] [PubMed] [Google Scholar]
- 8. Ikegami T, Peters CJ, Makino S. 2005. Rift Valley fever virus nonstructural protein NSs promotes viral RNA replication and transcription in a minigenome system. J. Virol. 79:5606–5615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikegami T, Won S, Peters CJ, Makino S. 2007. Characterization of Rift Valley fever virus transcriptional terminations. J. Virol. 81:8421–8438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ikegami T, Won S, Peters CJ, Makino S. 2005. Rift Valley fever virus NSs mRNA is transcribed from an incoming anti-viral-sense S RNA segment. J. Virol. 79:12106–12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kohl A, Lowen AC, Leonard VH, Elliott RM. 2006. Genetic elements regulating packaging of the Bunyamwera orthobunyavirus genome. J. Gen. Virol. 87:177–187 [DOI] [PubMed] [Google Scholar]
- 12. Lara E, Billecocq A, Leger P, Bouloy M. 2011. Characterization of wild-type and alternate transcription termination signals in the Rift Valley fever virus genome. J. Virol. 85:12134–12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang Y, Hong Y, Parslow TG. 2005. cis-Acting packaging signals in the influenza virus PB1, PB2, and PA genomic RNA segments. J. Virol. 79:10348–10355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang Y, Huang T, Ly H, Parslow TG. 2008. Mutational analyses of packaging signals in influenza virus PA, PB1, and PB2 genomic RNA segments. J. Virol. 82:229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lowen AC, Boyd A, Fazakerley JK, Elliott RM. 2005. Attenuation of bunyavirus replication by rearrangement of viral coding and noncoding sequences. J. Virol. 79:6940–6946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marsh GA, Rabadan R, Levine AJ, Palese P. 2008. Highly conserved regions of influenza a virus polymerase gene segments are critical for efficient viral RNA packaging. J. Virol. 82:2295–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muramoto Y, et al. 2006. Hierarchy among viral RNA (vRNA) segments in their role in vRNA incorporation into influenza A virions. J. Virol. 80:2318–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noda T, et al. 2006. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature 439:490–492 [DOI] [PubMed] [Google Scholar]
- 19. Overby AK, Popov V, Neve EP, Pettersson RF. 2006. Generation and analysis of infectious virus-like particles of Uukuniemi virus (Bunyaviridae): a useful system for studying bunyaviral packaging and budding. J. Virol. 80:10428–10435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ozawa M, et al. 2007. Contributions of two nuclear localization signals of influenza A virus nucleoprotein to viral replication. J. Virol. 81:30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ozawa M, et al. 2009. Nucleotide sequence requirements at the 5′ end of the influenza A virus M RNA segment for efficient virus replication. J. Virol. 83:3384–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parvin JD, Palese P, Honda A, Ishihama A, Krystal M. 1989. Promoter analysis of influenza virus RNA polymerase. J. Virol. 63:5142–5152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piper ME, Sorenson DR, Gerrard SR. 2011. Efficient cellular release of Rift Valley fever virus requires genomic RNA. PLoS One 6:e18070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rolfsson O, Toropova K, Ranson NA, Stockley PG. 2010. Mutually-induced conformational switching of RNA and coat protein underpins efficient assembly of a viral capsid. J. Mol. Biol. 401:309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 26. Terasaki K, Murakami S, Lokugamage KG, Makino S. 2011. Mechanism of tripartite RNA genome packaging in Rift Valley fever virus. Proc. Natl. Acad. Sci. U. S. A. 108:804–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watanabe T, Watanabe S, Noda T, Fujii Y, Kawaoka Y. 2003. Exploitation of nucleic acid packaging signals to generate a novel influenza virus-based vector stably expressing two foreign genes. J. Virol. 77:10575–10583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoffe AM, et al. 2008. Predicting the sizes of large RNA molecules. Proc. Natl. Acad. Sci. U. S. A. 105:16153–16158 [DOI] [PMC free article] [PubMed] [Google Scholar]