Abstract
Endocytosis has recently been implicated in rotavirus (RV) entry. We examined the role of Rabs, which regulate endosomal trafficking, during RV entry. Several structural proteins of neuraminidase-sensitive and -insensitive RVs colocalized with Rab5, an early endosome marker, but not Rab7, a late endosome marker. Dominant-negative and constitutively active mutants demonstrated that Rab5 but not Rab4 or Rab7 affects rhesus RV (RRV) infectivity. These data suggest that early RRV trafficking is confined to the early endosome compartment and requires Rab5.
TEXT
Rotavirus (RV), a nonenveloped member of the Reoviridae family, is the single most important cause of severe diarrhea globally and is a leading cause of death in children under the age of 5 (17). The virus particle encloses 11 double-stranded RNA segments within a triple-layered icosahedral capsid. The outermost layer is composed of the glycoprotein VP7 and protruding spikes of trimeric VP4. Two major domains comprise VP4. VP5* forms the foot of the spike, whereas VP8* is the head (5, 6). While the mechanisms governing RV cellular entry are not fully understood, both VP7 and VP4 are required for binding and penetration (15, 21). Like fusion mechanisms in enveloped viruses, VP5* is believed to rearrange upon uncoating and membrane interaction, resulting in a folded-back trimeric structure which is proposed to mediate membrane penetration (4, 5, 26, 28, 29). Although RV entry was initially thought to occur via direct plasma membrane penetration (7, 13), recent studies indicate that RV traffics through the endosomal pathway (1, 10, 22, 27). As different RV strains, which vary in neuraminidase (NA) sensitivity of cell binding, may use distinct endocytic pathways, a definitive model of RV entry does not currently exist (10, 22, 27). We recently demonstrated that rhesus RV (RRV) enters a polarized epithelium using the endosomal route, as evidenced by colocalization of trimeric VP7 and VP5* with endosomal markers Rab4 and Rab5 (27). Furthermore, specific pharmacological interventions reduced RRV infectivity (27). Rabs, small cellular GTPases important in endosomal trafficking, are powerful tools for studying endocytosis due to their unique expression in specific endocytic compartments and fine regulation of vesicular trafficking (24). Dominant-negative (DN) and constitutively active (CA) Rab mutants have been widely used to identify the entry trafficking steps required by various viral infections (11, 14, 16, 19, 23). Rab5, which is present at the plasma membrane but is preferentially associated with early endosome (EE) vacuoles (23), functions in internalization, transport of newly formed vesicles to the EE, fusion, and trafficking to the late endosome (LE). Rab4 localizes primarily to the EE and regulates recycling to the plasma membrane. Rab7, present on the LE, governs transfer to lysosomes. Rab5 and Rab7 are proposed to be present on the maturing endosome, an intermediary vesicle between the EE and LE (16).
Here, we examined the roles of Rab proteins during RRV entry. Rab5 has previously been demonstrated to colocalize with trimeric VP7 and VP5* in polarized MDCK cells (27). The present study used MA104 cells, which are derived from rhesus monkey kidneys and are reasonably transfectable and highly permissive for many RV strains. Furthermore, RRV entry into MA104 cells has been widely studied (2, 9, 10, 12, 22). To verify colocalization of RRV structural proteins with Rab5 during MA104 entry, cells were transfected with Rab5-enhanced green fluorescent protein (EGFP) for 16 h (Lipofectamine LTX; Invitrogen). A total of 25 to 50% of the cells subsequently expressed detectable levels of Rab5, as assessed by EGFP expression. Western blot analysis using an anti-GFP antibody confirmed that EGFP expression was due to Rab5-EGFP expression and not EGFP expression alone (data not shown). Transfected cells were then infected with RRV (multiplicity of infection [MOI], 100; grown and activated as previously described [27]) for 15 min at 37°C. Cells were washed 3 times with phosphate-buffered saline (PBS) and fixed with 3% paraformaldehyde in 100 mM phosphate buffer (pH 7.4) for 10 min. Immunostaining was performed with monoclonal antibodies (MAb) to the RV structural proteins VP6 (1E11), VP7 (159), and VP5* (2G4) and anti-mouse Alexa 594 secondary antibody (Invitrogen). Mounting and imaging by confocal microscopy (LSM710; Zeiss, Jena, Germany) were performed as described previously (27). Each experiment was performed at least 5 times, and an average of 5 fields was acquired for each experiment under each condition.
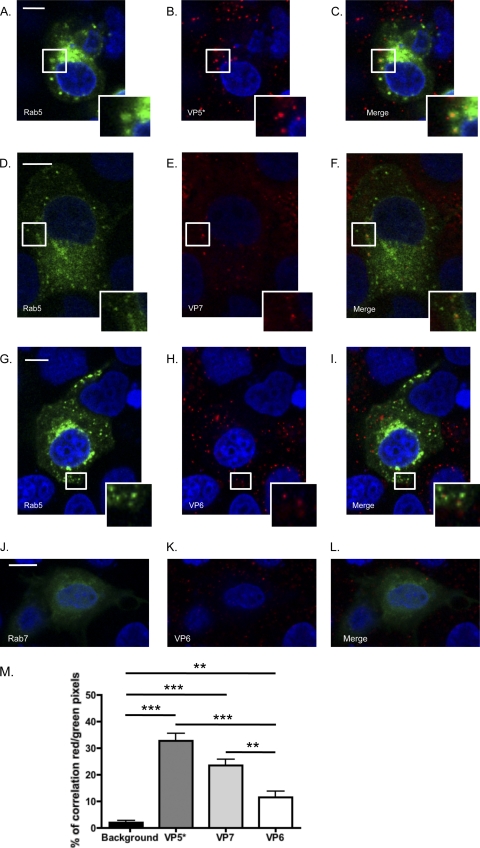
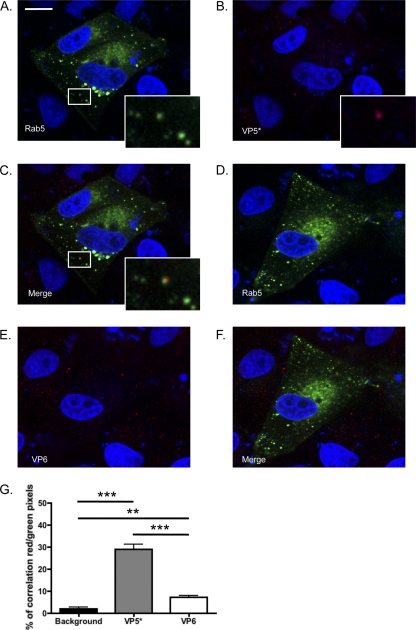
We detected VP5* (Fig. 1A to C and M), trimeric VP7 (Fig. 1D to F and M), and a significant but smaller amount of VP6 (Fig. 1G to I and M) in Rab5-EGFP+ vesicles, indicating that RRV enters MA104 cells via endocytosis and traffics through the EE pathway. Colocalization correlation (Fig. 1M) was calculated using the Volocity Colocalization module (PerkinElmer). Consistent with previous findings for MDCK cells (27), colocalization was not observed between Rab7 and VP6 (Fig. 1G to L) or VP7 or VP5*, and colocalization levels were not significantly different from background levels under these conditions (data not shown). We performed the same experiment using UK, a bovine NA-insensitive RV strain with cellular receptor requirements different from those of NA-sensitive RRV (8, 10). As observed for RRV, Rab5 colocalized with UK VP5* (Fig. 2A to C and G) and significantly less with UK VP6 (Fig. 2D to F and G), indicating that Rab5 is important for RV entry independent of NA sensitivity and receptor use. These findings demonstrate that RV is present in the EE pathway during the early stages of entry but do not exclude a later transfer of RV through the LE that is too transitory to be detected.
Fig 1.
RRV VP5* (A to C) and VP7 (D to F) associated with the early endosomal marker Rab5-EGFP in MA104 cells at 15 min postinfection. Colocalization events are highlighted by white boxes that are shown at higher magnification at the bottom right of the pictures. VP6 associated with Rab5 to a significant but lesser degree (G to I and M) but not with Rab7-EGFP (J to L). Bar, 10 μm. (M) Average percentage of red pixels (RRV+) that are also green (Rab5+) under each condition for each field. Fields in which cells were both transfected but not infected and infected but not transfected were used to measure background levels. ***, P < 0.001; **, P < 0.01 (one-way analysis of variance [ANOVA] test followed by the Newman-Keuls multiple-comparison posttest).
Fig 2.
(A to C) UK VP5* associated with the early endosome marker Rab5-EGFP in MA104 cells at 15 min postinfection. Colocalization events are highlighted by white boxes that are shown at higher magnification at the bottom right of the pictures. (D to F and G) UK VP6 was significantly less associated with Rab5 than VP5*. Bar, 10 μm. (G) Average percentage of red pixels (UK+) that are also green (Rab5+) under each condition for each field. ***, P < 0.001; **, P < 0.01 (one-way ANOVA test followed by the Newman-Keuls multiple-comparison posttest).
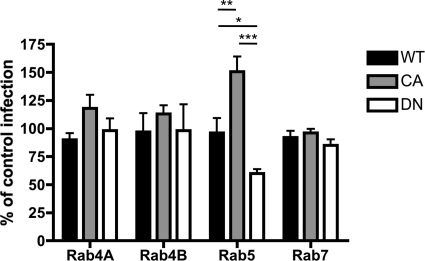
We next used DN and CA mutants of Rab4A, Rab4B, Rab5, and Rab7 to further investigate the functional importance of each Rab during RRV entry. The DN mutants do not get activated and do prevent trafficking to the downstream endosomal compartment (25). CA Rab5 mutants stimulate the rate of endocytosis and homotypic fusion of the EE, whereas DN Rab5 prevents vesicle fusion (25). At 16 h after mutant transfection, MA104 cells were infected with RRV (MOI, 2) for 1 or 6 h. Cells were resuspended, fixed, and permeabilized using Cytofix/Cytoperm (BD Biosciences, San Jose, CA), stained for VP6 (phycoerythrin conjugated by Chromoprobe Inc., Maryland Heights, MO) (3), and examined by flow cytometry. In each experiment, 50,000 events were recorded for each condition. The presence of VP6 staining at 6 hours postinfection (h p.i.) was considered indicative of rotaviral replication, as VP6 staining was not detectable at 1 h p.i. under these low-MOI conditions (data not shown). In each well, infectivity in nontransfected (GFP−) cells, defining 100% of control infections, was compared to infectivity in cells expressing each Rab mutant (GFP+) (Fig. 3). The expression of DN Rab5, but not DN Rab4A/B or DN Rab7, significantly reduced the number of RRV-infected cells. These results indicate that Rab5 activity is required for optimal RRV infectivity, while Rab4A/B and Rab7 activity is dispensable. Interestingly, CA Rab5 expression, which accelerates endocytosis rates and blocks EE-to-LE conversion, inhibiting LE fusion (20), significantly increased RRV infection, suggesting that cells expressing activated Rab5 are more likely to internalize and replicate virus and that trafficking to the LE is dispensable for RRV infectivity. The CA Rab4 mutant slightly but not significantly increased infectivity, whereas CA Rab7 had no effect, further supporting the unique importance of Rab5 for RRV replication.
Fig 3.
Effect of wild-type (WT), DN, and CA Rab4A-, Rab4B-, Rab5-, and Rab7-EGFP expression on RV cell infection. The percentages of cells expressing mutant Rab-GFP proteins that were VP6 positive compared to infection rates of nontransfected cells from the same well are shown. ***, P < 0.001; **, P < 0.01; *, P < 0.05 (one-way ANOVA test followed by the Newman Keuls multiple-comparison posttest).
In addition to increasing the rate of endocytosis, the expression of CA Rab5 stimulates EE homotypic fusion, resulting in a greatly enlarged EE and inhibition of LE fusion (11, 20, 25). Viruses that require trafficking to the LE or lysosome for infectivity accumulate in large amounts in these enlarged vesicles (11, 18, 19). RRV, unlike canine parvovirus, for example, was present only in modest amounts, as evidenced by VP5* (Fig. 4), VP6, or VP7 (data not shown) staining at 1 h p.i. RRV VP5* was present primarily outside EE vesicles, suggesting that RRV exits EEs and does not require trafficking to downstream compartments.
Fig 4.
Distribution of VP5* at 1 h p.i. in MA104 cells expressing CA Rab5-GFP mutants. Individual (A and B) and merged (C) images of Rab-GFP (green) and VP5* (red) are shown. Bar, 10 μm. (D) Average percentage of red pixels (RRV+) that are also green (Rab5-CA+) for each field.
These data support the conclusion that RV enters the cell via an endocytosis pathway that is independent of an NA-dependent component of cell receptor usage. The findings further suggest that RRV entry is substantially restricted to the EE compartment, as virus is detected outside EE vesicles even when trafficking to downstream compartments is blocked. Viral decapsidation and endosomal exit likely occur immediately after the endocytic vesicle fuses with the EE, explaining why relatively few colocalization events between viral proteins and Rab5 are detectable. Together, these results strengthen the current model of RV cell entry in which low Ca2+ concentrations in the EE promote uncoating and VP5* foldback. The resulting formation of a new spike structure penetrates the endosomal membrane, allowing the release of transcriptionally active double-layered rotaviral particles into the cytoplasm.
ACKNOWLEDGMENTS
This study was supported by grants R01 AI-021362 and P30 DK56339-08 from the National Institutes of Health and a VA Merit Review award.
We are grateful to Marci Scidmore for the Rab4 constructs, Walter Atwood for the Rab7, Rab5wt, and Rab5-S34N constructs, and Bernardo Mainou for the Rab5-Q79L plasmid.
Footnotes
Published ahead of print 25 January 2012
REFERENCES
- 1. Chemello ME, Aristimuno OC, Michelangeli F, Ruiz MC. 2002. Requirement for vacuolar H+-ATPase activity and Ca2+ gradient during entry of rotavirus into MA104 cells. J. Virol. 76:13083–13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ciarlet M, Crawford SE, Estes MK. 2001. Differential infection of polarized epithelial cell lines by sialic acid-dependent and sialic acid-independent rotavirus strains. J. Virol. 75:11834–11850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deal EM, Jaimes MC, Crawford SE, Estes MK, Greenberg HB. 2010. Rotavirus structural proteins and dsRNA are required for the human primary plasmacytoid dendritic cell IFNalpha response. PLoS Pathog. 6:e1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dormitzer PR, Greenberg HB, Harrison SC. 2001. Proteolysis of monomeric recombinant rotavirus VP4 yields an oligomeric VP5* core. J. Virol. 75:7339–7350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dormitzer PR, Nason EB, Prasad BV, Harrison SC. 2004. Structural rearrangements in the membrane penetration protein of a non-enveloped virus. Nature 430:1053–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dormitzer PR, Sun ZY, Wagner G, Harrison SC. 2002. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 21:885–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fukuhara N, Yoshie O, Kitaoka S, Konno T, Ishida N. 1987. Evidence for endocytosis-independent infection by human rotavirus. Arch. Virol. 97:93–99 [DOI] [PubMed] [Google Scholar]
- 8. Graham KL, et al. 2003. Integrin-using rotaviruses bind alpha2beta1 integrin alpha2 I domain via VP4 DGE sequence and recognize alphaXbeta2 and alphaVbeta3 by using VP7 during cell entry. J. Virol. 77:9969–9978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graham KL, Takada Y, Coulson BS. 2006. Rotavirus spike protein VP5* binds alpha2beta1 integrin on the cell surface and competes with virus for cell binding and infectivity. J. Gen. Virol. 87:1275–1283 [DOI] [PubMed] [Google Scholar]
- 10. Gutierrez M, et al. Different rotavirus strains enter MA104 cells through different endocytic pathways: the role of clathrin-mediated endocytosis. J. Virol. 84:9161–9169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harbison CE, Lyi SM, Weichert WS, Parrish CR. 2009. Early steps in cell infection by parvoviruses: host-specific differences in cell receptor binding but similar endosomal trafficking. J. Virol. 83:10504–10514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isa P, Sanchez-Aleman MA, Lopez S, Arias CF. 2009. Dissecting the role of integrin subunits alpha 2 and beta 3 in rotavirus cell entry by RNA silencing. Virus Res. 145:251–259 [DOI] [PubMed] [Google Scholar]
- 13. Kaljot KT, Shaw RD, Rubin DH, Greenberg HB. 1988. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J. Virol. 62:1136–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kolokoltsov AA, Fleming EH, Davey RA. 2006. Venezuelan equine encephalitis virus entry mechanism requires late endosome formation and resists cell membrane cholesterol depletion. Virology 347:333–342 [DOI] [PubMed] [Google Scholar]
- 15. Lopez S, Arias CF. 2004. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol. 12:271–278 [DOI] [PubMed] [Google Scholar]
- 16. Mercer J, Schelhaas M, Helenius A. 2010. Virus entry by endocytosis. Annu. Rev. Biochem. 79:803–833 [DOI] [PubMed] [Google Scholar]
- 17. Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. 2003. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 9:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pelkmans L, Burli T, Zerial M, Helenius A. 2004. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118:767–780 [DOI] [PubMed] [Google Scholar]
- 19. Querbes W, O'Hara BA, Williams G, Atwood WJ. 2006. Invasion of host cells by JC virus identifies a novel role for caveolae in endosomal sorting of noncaveolar ligands. J. Virol. 80:9402–9413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rink J, Ghigo E, Kalaidzidis Y, Zerial M. 2005. Rab conversion as a mechanism of progression from early to late endosomes. Cell 122:735–749 [DOI] [PubMed] [Google Scholar]
- 21. Ruiz MC, Leon T, Diaz Y, Michelangeli F. 2009. Molecular biology of rotavirus entry and replication. ScientificWorldJournal 9:1476–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanchez-San Martin C, Lopez T, Arias CF, Lopez S. 2004. Characterization of rotavirus cell entry. J. Virol. 78:2310–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. 2000. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149:901–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stenmark H. 2009. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10:513–525 [DOI] [PubMed] [Google Scholar]
- 25. Stenmark H, et al. 1994. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 13:1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trask SD, Kim IS, Harrison SC, Dormitzer PR. 2010. A rotavirus spike protein conformational intermediate binds lipid bilayers. J. Virol. 84:1764–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wolf M, Vo PT, Greenberg HB. 2011. Rhesus rotavirus entry into a polarized epithelium is endocytosis dependent and involves sequential VP4 conformational changes. J. Virol. 85:2492–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoder JD, Dormitzer PR. 2006. Alternative intermolecular contacts underlie the rotavirus VP5* two- to three-fold rearrangement. EMBO J. 25:1559–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoder JD, et al. 2009. VP5* rearranges when rotavirus uncoats. J. Virol. 83:11372–11377 [DOI] [PMC free article] [PubMed] [Google Scholar]