Abstract
Objective
To present a global update of drug-resistant tuberculosis (TB) and explore trends in 1994–2010.
Methods
Data on drug resistance among new and previously treated TB patients, as reported by countries to the World Health Organization, were analysed. Such data are collected through surveys of a representative sample of TB patients or surveillance systems based on routine drug susceptibility testing. Associations between multidrug-resistant TB (MDR-TB) and human immunodeficiency virus (HIV) infection and sex were explored through logistic regression.
Findings
In 2007–2010, 80 countries and 8 territories reported surveillance data. MDR-TB among new and previously treated cases was highest in the Russian Federation (Murmansk oblast, 28.9%) and the Republic of Moldova (65.1%), respectively. In three former Soviet Union countries and South Africa, more than 10% of the cases of MDR-TB were extensively drug-resistant. Globally, in 1994 to 2010 multidrug resistance was observed in 3.4% (95% confidence interval, CI: 1.9–5.0) of all new TB cases and in 19.8% (95% CI: 14.4–25.1) of previously treated TB cases. No overall associations between MDR-TB and HIV infection (odds ratio, OR: 1.4; 95% CI: 0.7–3.0) or sex (OR: 1.1; 95% CI: 0.8–1.4) were found. Between 1994 and 2010, MDR-TB rates in the general population increased in Botswana, Peru, the Republic of Korea and declined in Estonia, Latvia and the United States of America.
Conclusion
The highest global rates of MDR-TB ever reported were documented in 2009 and 2010. Trends in MDR-TB are still unclear in most settings. Better surveillance or survey data are required, especially from Africa and India.
Résumé
Objectif
Présenter une mise à jour globale de la tuberculose (TB) pharmacorésistante et explorer les tendances de 1994 à 2010.
Méthodes
Les données relatives à la résistance aux médicaments des nouveaux patients de TB et de ceux traités antérieurement, telles que rapportées par les pays à l'Organisation mondiale de la Santé, ont été analysées. Ces données sont recueillies par des enquêtes représentatives auprès des patients ou des systèmes de surveillance basés sur des tests systématiques de sensibilité. Les associations entre la tuberculose ultra-résistante (TB-UR), le virus de l'immunodéficience humaine (VIH) et le sexe ont été explorées par régression logistique.
Résultats
En 2007-2010, 80 pays et 8 territoires ont fourni des données de surveillance. Parmi les cas nouveaux et traités antérieurement, la TB-UR était la plus élevée dans la Fédération de Russie (oblast de Mourmansk, 28,9%) et la République de Moldavie (65,1%), respectivement. Dans trois pays de l'ex-Union soviétique et en Afrique du Sud, plus de 10% des cas de TB-UR étaient ultra-résistants. Globalement, en 1994-2010, la multirésistance aux médicaments a été observée chez 3,4% (intervalle de confiance 95%, IC: 1,9 à 5,0) de tous les nouveaux cas de TB et 19,8% (IC 95%: 14,4 à 25,1) des cas de TB traités antérieurement. Aucune association générale entre la TB-UR et l'infection au VIH (rapports de cotes, OR: 1,4, IC 95%: 0,7 à 3,0) ou le sexe (OR: 1,1, IC 95%: 0,8 à 1,4) n’a été trouvée. Entre 1994 et 2010, les taux de TB-UR dans la population générale ont augmenté au Botswana, en République de Corée et au Pérou et diminué en Estonie, Lettonie et aux Etats-Unis d'Amérique.
Conclusion
Les taux mondiaux les plus élevés jamais signalés de TB-UR ont été documentés en 2009 et 2010. Les tendances de la TB-UR sont encore peu claires dans la plupart des paramètres. De meilleures données de surveillance ou d'enquête sont nécessaires, surtout en provenance d'Afrique et d'Inde.
Resumen
Objetivo
Presentar una actualización global de la situación de la tuberculosis (TB) resistente a los medicamentos y analizar las tendencias entre 1994 y 2010.
Método
Se analizaron los datos sobre la resistencia a los medicamentos entre los pacientes recién diagnosticados con TB y los que habían recibido tratamiento con anterioridad, en base a los informes que los países remitieron a la Organización Mundial de la Salud. Dichos datos se recopilaron a través de encuestas representativas a pacientes o mediante sistemas de vigilancia basados en pruebas rutinarias de sensibilidad a los medicamentos. A través de una regresión logística se analizaron las asociaciones existentes entre la tuberculosis multirresistente (TB-MDR), el virus de la inmunodeficiencia humana y el sexo.
Resultados
Entre 2007 y 2010, 80 países y 8 territorios facilitaron sus datos de vigilancia. Los niveles más elevados de TB-MDR entre los casos recién diagnosticados y previamente tratados se registraron en la Federación de Rusia (Murmansk oblast, 28,9%) y la República de Moldova (65,1%), respectivamente. En tres de los países de la antigua Unión Soviética y en Sudáfrica, más de un 10% de los casos de TB-MDR fueron extremadamente resistentes. Entre 1994 y 2010 se observó en todo el mundo una multirresistencia de un 3,4% (95% de intervalo de confianza, IC: 1,9–5,0) en todos los casos nuevos de TB y de un 19,8% (95% IC: 14,4–25,1) en los casos de TB previamente tratados. No se observaron asociaciones globales entre la TB-MDR y la infección por el VIH (cociente de posibilidades, OR: 1,4; IC del 95%: 0,7–3,0) ni con el sexo (OR: 1,1; IC del 95%: 0,8–1,4). Entre los años 1994 y 2010, las tasas de TB-MDR en la población general aumentaron en Botswana, la República de Corea y Perú y descendieron en Estonia, Letonia y Estados Unidos de América.
Conclusión
En 2009 y 2010 se registraron las tasas globales de MDR-TB más altas de la historia. Las tendencias de TB-MDR siguen sin quedar claras en la mayoría de los entornos. Es necesaria una mejor vigilancia o más datos procedentes de encuestas, especialmente en los casos de África e India.
ملخص
الغرض
تقديم تحديث عالمي للسل المقاوم للأدوية واستكشاف الاتجاهات فيما بين 1994 و2010.
الطريقة
تم تحليل البيانات الخاصة بمقاومة الأدوية بين مرضى السل الجدد والذين سبق علاجهم وفق التقارير المرفوعة من البلدان إلى منظمة الصحة العالمية. وتم تجميع هذه البيانات من خلال مسوح تمثيلية للمرضى أو نظم المراقبة بناءً على الاختبار الروتيني للحساسية الدوائية. وتم استكشاف الارتباطات بين السل المقاوم للأدوية المتعددة (MDR-TB) وعدوى فيروس نقص المناعة البشرية (HIV) والجنس من خلال الارتداد اللوجستي.
النتائج
فيما بين عامي 2007 و2010، أبلغت 80 بلدًا و8 أقاليم عن بيانات المراقبة. وكانت معدلات السل المقاوم للأدوية المتعددة بين الحالات الجديدة، والتي سبق علاجها أعلى في الاتحاد الروسي (مورمانسك أوبلاست، 28.9%) وجمهورية مولدوفا (65.1%)، على التوالي. وكانت نسبة أكثر من 10% من حالات السل المقاوم للأدوية المتعددة شديدة المقاومة للأدوية في ثلاثة من بلدان الاتحاد السوفيتي السابق وجنوب أفريقيا. وعلى الصعيد العالمي، لوحظت مقاومة للأدوية المتعددة في الفترة ما بين عامي 1994 و2010 في 3.4% (معامل الثقة 95%، معامل الثقة: 1.9-5.0) من جميع حالات السل الجديدة وفي 19.8% (معامل الثقة 95%، معامل الثقة: 14.4-25.1) من حالات السل التي سبق علاجها. ولم يتم العثور على ارتباطات عامة بين السل المقاوم للأدوية المتعددة (MDR-TB) وعدوى فيروس نقص المناعة البشرية (HIV) (نسبة الاحتمال، نسبة الاحتمال: 1.4، معامل الثقة 95%، معامل الثقة: 0.7-3.0) أو الجنس (نسبة الاحتمال، نسبة الاحتمال: 1.1، معامل الثقة 95%، معامل الثقة: 0.8-1.4). وفيما بين عامي 1994 و2010، زادت معدلات السل المقاوم للأدوية المتعددة (MDR-TB) في عامة السكان في بوتسوانا وجمهورية كوريا وبيرو وانخفضت في إستونيا ولاتفيا والولايات المتحدة الأمريكية.
الاستنتاج
فيما بين عامي 2009 و2010 تم توثيق أعلى المعدلات العالمية للسل المقاوم للأدوية المتعددة (MDR-TB) التي تم الإبلاغ عنها. ولا تزال الاتجاهات في السل المقاوم للأدوية المتعددة (MDR-TB) غير واضحة في معظم البيئات. ويلزم وجود مراقبة أو بيانات مسوح أفضل، خاصة من أفريقيا والهند.
摘要
目的
介绍抗药性结核病(TB)的全球更新并探索 1994 年至 2010 年的趋势。
方法
分析由各个国家向世界卫生组织报告的初诊和复诊 TB 病人的抗药性数据。这些数据是通过基于例行药物敏感性测试的典型病人调查或监察系统收集而来。使用逻辑回归分析研究耐多药 TB(MDR-TB)与人类免疫缺陷病毒(HIV)感染和性行为之间的关联。
结果
在 2007 年至 2010 年期间有 80 个国家及 8 个地区报告监察数据。初诊和复诊病例中 MDR-TB 比率最高的分别是俄罗斯联邦(摩尔曼斯克州,28.9%)和摩尔多瓦共和国(65.1%)。三个前苏联国家和南非有超过 10% 的 MDR-TB 病例是广泛抗药性结核病。1994 年至 2010 年期间全球所有初诊 TB 病例中有 3.4%(95% 置信区间,CI:1.9–5.0)的病例观察到耐多药结核病,在复诊 TB 病例中有 19.8%(95% CI:14.4–25.1)的病例观察到耐多药结核病。未发现 MDR-TB 与 HIV 感染(优势比,OR:1.4;95% CI:0.7–3.0)或性行为(OR:1.1;95% CI:0.8–1.4)之间的整体性关联。在 1994 年至 2010 年期间,博茨瓦纳、韩国和秘鲁一般大众的 MDR-TB 率升高,而爱沙尼亚、拉脱维亚和美国的 MDR-TB 率则降低。
结论
2009 年和 2010 年出现了有报告以来的全球最高MDR-TB 率。在多数环境中 MDR-TB 趋势依旧不明。需要更好的监察或调查数据,尤其是非洲和印度的数据。
Резюме
Цель
Представить новейший глобальный анализ лекарственно-устойчивого туберкулеза (TB) и исследовать тенденции в период с 1994 по 2010 гг.
Методы
Были проанализированы данные об устойчивости к лекарственным препаратам среди новых и уже проходивших лечение от туберкулеза пациентов, полученные на основе докладов стран, направленных во Всемирную Организацию Здравоохранения. Эти данные были собраны посредством репрезентативных обследований пациентов или же с использованием систем мониторинга, основанных на обычном тестировании лекарственной устойчивости. Посредством логистической регрессии были исследованы связи между полирезистентным туберкулезом (MDR-TB), вирусом иммунодефицита человека (HIV) и полом.
Результаты
В 2007–2010 гг. 80 стран и 8 территорий предоставили данные, полученные на основе проведенного мониторинга. Количество случаев полирезистентного туберкулеза среди новых или уже проходивших лечение от туберкулеза пациентов было самым высоким в Российской Федерации (Мурманская область, 28,9%) и Республике Молдова (65,1%), соответственно. В трех бывших республиках Советского Союза и Южной Африке более 10% случаев полирезистентного туберкулеза в основном являлись устойчивыми к воздействию медицинских препаратов. В мире, в период с 1994 по 2010 гг., лекарственная устойчивость наблюдалась в 3,4% (95% доверительный интервал, ДИ: 1,9–5,0) всех новых случаев заболевания туберкулезом и у 19,8% (95% доверительный интервал, ДИ: 14.4–25.1) пациентов, уже проходивших лечение от туберкулеза. Не было обнаружено каких-либо общих связей между полирезистентным туберкулезом, вирусом иммунодефицита человека (отношение рисков, ОР: 1,4; 95% ДИ: 0,7–3,0) или полом (ОР: 1,1; 95% ДИ: 0,8–1,4). В период с 1994 по 2010 гг. темпы заболеваемости полирезистентным туберкулезом среди населения в целом увеличились в Ботсване, Республике Корея и Перу и снизились в Эстонии, Латвии и Соединенных Штатах Америки
Вывод
Самый высокий глобальный уровень заболеваемости полирезистентным туберкулезом из когда-либо зарегистрированных был отмечен в 2009 и 2010 гг. По большинству параметров тенденции в отношении полирезистентного туберкулеза остаются неясными. Необходимо улучшить систему мониторинга и сбора данных, в особенности в Африке и Индии.
Introduction
Surveillance of resistance to drugs against tuberculosis (TB) is a cornerstone of any TB control programme. Surveillance data on drug resistance are needed to track the effectiveness of TB prevention and control activities; accurately forecast the need for patient treatments and plan accordingly; design standardized regimens for the treatment of drug-resistant TB; assess epidemiological trends; and promptly identify and respond to outbreaks of drug-resistant TB.1 Since 1994 the Global Project on Anti-Tuberculosis Drug Resistance Surveillance of the World Health Organization (WHO) has supported national TB control programmes worldwide in implementing drug resistance surveillance activities. Country data are routinely collected, analysed and disseminated to describe the global problem of drug-resistant TB.2–11
Patients whose mycobacteria are resistant to rifampicin, isoniazid and other anti-TB drugs require longer, expensive and more toxic treatment regimens and are less likely to be cured. This presents a formidable challenge to programmes, particularly in low-resource settings.12 Policy guidance on the programmatic management of drug-resistant TB13–15 and on how to control the transmission of resistant strains16 has been developed by WHO, and access to quality-assured second-line anti-TB drugs for the treatment of multidrug-resistant TB (MDR-TB) is facilitated through the Green Light Committee mechanism.17 The number of TB patients diagnosed and treated for MDR-TB, which is defined as TB caused by strains of Mycobacterium tuberculosis that are resistant to at least isoniazid and rifampicin,13 is increasing worldwide, but much remains to be done. In 2010, only 16% of the TB patients estimated to have MDR-TB were diagnosed and given appropriate treatment.11,12, Routine surveillance of drug resistance must be linked to patient care.
Over the past three years, WHO has been actively encouraging countries to establish continuous TB drug resistance surveillance systems based on routine drug susceptibility testing of all patients, with priority given to patients previously treated, who are at highest risk of developing drug resistance.18–20 Although limited laboratory capacity for drug susceptibility testing still represents a major obstacle to the establishment of surveillance systems in low-resource settings, new diagnostic tools such as line probe assays21 and Xpert MTB/RIF,22 combined with greater resources for laboratory strengthening, offer an unprecedented opportunity to scale up surveillance systems worldwide.
In this paper we evaluate the existing information on anti-TB drug resistance surveillance, with an emphasis on data reported in 2007–2010, after the publication of WHO’s fourth global report on anti-TB drug resistance surveillance.8,9 We present a global overview of the extent of the problem of MDR-TB, explore associations between MDR-TB and human immunodeficiency virus (HIV) infection and sex, discuss time trends in drug resistance, and present available data on extensively drug-resistant TB (XDR-TB) – the latter defined as MDR-TB plus resistance to a fluoroquinolone and at least one second-line injectable agent (amikacin, kanamycin or capreomycin).13
Methods
Definitions and data collection
Drug resistance surveillance data are gathered following three main principles: (i) the data are representative of TB cases in the country or geographical setting under study; (ii) drug resistance among new TB cases is examined separately from drug resistance among previously treated TB cases; and (iii) laboratory methods for drug susceptibility testing are selected from among those that are recommended by WHO, with quality assurance for all laboratory processes conducted in cooperation with a partner supranational reference laboratory from the global network of 29 such laboratories.18,19,23,24
Drug resistance surveillance data are collected separately for new (previously untreated) and previously treated TB cases25 via special surveys or continuous surveillance. Special surveys measure drug resistance among a representative sample of notified cases of smear-positive pulmonary TB; continuous surveillance systems are based on routine diagnostic drug susceptibility testing in all bacteriologically-confirmed TB patients. Aggregated data from special surveys are collected through a standard data collection form, whereas continuous surveillance data are captured through "WHO[’s ] global TB data collection system”.26 WHO ascertains whether survey and continuous surveillance data meet quality and representativeness standards through criteria detailed elsewhere.10 The main indicator reported to estimate the frequency of MDR-TB is the proportion of confirmed TB cases with resistance to rifampicin and isoniazid. Data on resistance to any fluoroquinolone and second-line injectable agent among confirmed cases of MDR-TB are used to estimate the frequency of XDR-TB.
Data description, analysis and trends
The proportions of new and previously treated TB cases with MDR-TB and the proportion of MDR-TB cases with XDR-TB were calculated using the latest available national and subnational data. To derive global estimates for these indicators and to investigate the association between MDR-TB and HIV infection and sex, individual-level analyses were conducted using random-effects or robust standard errors logistic regression models to account for the clustering effect at the level of a country or territory. We used the I2 index27 to assess heterogeneity in country-level odds ratios (OR) before we combined these to obtain a pooled estimate. STATA version 11 (StataCorp. LP, College Station, United States of America) was used for all analyses.
Time trends in MDR-TB rates (annual number of new cases per 100 000 population)28 between 1994 and 2010 were calculated by multiplying the new TB case notification rates reported annually to WHO11 by the reported frequency of MDR-TB among new TB cases in the same setting and year. Exponential lines were fitted and the annual percentage change in the rate of MDR-TB was calculated for settings where anti-TB drug resistance had been measured in at least three different years.
Results
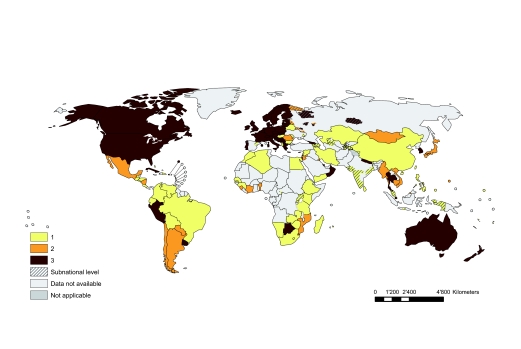
Since the launch of the Global Project on Anti-tuberculosis Drug Resistance Surveillance in 1994, drug resistance data have been systematically collected and analysed from 127 countries, or 66% of WHO’s 193 Member States. This includes 64 countries that have continuous surveillance systems based on routine diagnostic drug susceptibility testing of all patients. The remaining 63 countries have relied on special surveys of representative samples of patients. Of the 127 countries with surveillance information, 56 have data from one year only, 20 from two years, and 51 from three or more years (Fig. 1).
Fig. 1.
Number of country–year data points for drug resistance surveillance, 1994–2010
Most recent data, 2007–2010
Between 2007 and 2010, resistance to first-line anti-TB drugs was reported from 80 countries and 8 territories, 72 of which provided data from continuous surveillance and 16 from special surveys (Table 1, available at: http://www.who.int/bulletin/volumes/90/2/11-092585). Almost all countries (82/88, or 93%) reported nationwide data. Bangladesh (14 districts covering a population of 30 million), the Plurinational State of Bolivia, Chile, Colombia, El Salvador, Fiji, Kazakhstan, Lebanon, Mongolia, the Republic of Moldova, Rwanda and Sri Lanka provided continuous surveillance data on previously treated but not new TB cases. Subnational data were reported from Bangladesh, the Central African Republic, Indonesia, the Russian Federation (12 oblasts [administrative regions] and republics), Tajikistan and Uganda.
Table 1. Countries and territories reporting dataa on multidrug resistant tuberculosis (MDR-TB), 2007–2010.
| Country or setting | Source | Year | New cases |
Previously treated cases |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases with DST results (for H & R) | MDR-TB (%) | Resistance (%) |
Cases with DST results (for H & R) | MDR-TB (%) | Resistance (%) |
||||||
| H | R | H | R | ||||||||
| Albania | Surveillance | 2010 | 186 | 0.5 | 3.2 | 0.5 | 19 | 5.3 | 31.6 | – | |
| Andorra | Surveillance | 2010 | 4 | 0.0 | 25.0 | 0.0 | 0 | – | – | – | |
| Australia | Surveillance | 2010 | 868 | 2.4 | 8.9 | 2.6 | 48 | 22.9 | 29.2 | – | |
| Austria | Surveillance | 2010 | 240 | 2.1 | 8.8 | 2.1 | 16 | 18.8 | 18.8 | – | |
| Bahamas | Surveillance | 2010 | 21 | 0.0 | 0.0 | 23.8 | 2 | 0.0 | 0.0 | – | |
| Bahrain | Surveillance | 2010 | 162 | 0.0 | 0.0 | 0.0 | 0 | – | – | – | |
| Bangladesh (14 districts covering 30 million population) | Surveillance | 2008 | – | – | – | – | 599 | 28.0 | 37.6 | – | |
| Belarus | Surveillance | 2010 | 1972 | 25.7 | 33.2 | 27.2 | 1697 | 60.2 | 66.7 | – | |
| Belgium | Surveillance | 2009 | 621 | 0.6 | 3.9 | 0.8 | 56 | 5.4 | 10.7 | – | |
| Benin | Survey | 2010 | 403 | 0.5 | 7.9 | 1.2 | 45 | 13.3 | 31.1 | – | |
| Bermuda | Surveillance | 2010 | 1 | 0.0 | 0.0 | 0.0 | 0 | – | – | – | |
| Bolivia (Plurinational State of) | Surveillance | 2010 | – | – | – | – | 664 | 16.0 | 24.4 | 16.6 | |
| Bosnia and Herzegovina | Surveillance | 2009 | 854 | 0.0 | 0.6 | 0.0 | 66 | 3.0 | 12.1 | 3.0 | |
| Botswana | Survey | 2008 | 924 | 2.5 | 7.6 | 3.6 | 137 | 6.6 | 10.3 | 13.2 | |
| Brunei Darussalam | Surveillance | 2010 | 181 | 0.0 | 2.8 | 0.0 | 5 | 0.0 | 0.0 | 0.0 | |
| Bulgaria | Surveillance | 2010 | 801 | 2.0 | 6.6 | 2.9 | 165 | 24.2 | 35.1 | 28.4 | |
| Cambodia | Survey | 2007 | 781 | 1.4 | 7.2 | 1.8 | 56 | 10.7 | 17.8 | 10.7 | |
| Canada | Surveillance | 2010 | 987 | 1.5 | 8.2 | 1.5 | 51 | 0.0 | 9.8 | 0.0 | |
| Central African Republic, Bangui and Bimbo | Survey | 2009 | 225 | 0.4 | 9.3 | 0.4 | – | – | – | – | |
| Chile | Surveillance | 2010 | – | – | – | – | 276 | 2.9 | 13.4 | 7.2 | |
| China | Survey | 2007 | 3037 | 5.7 | 15.9 | 6.6 | 892 | 25.6 | 38.8 | 29.7 | |
| China, Hong Kong Special Administrative Region | Surveillance | 2009 | 2056 | 0.7 | 4.6 | 1.0 | 234 | 2.6 | 7.7 | 2.6 | |
| China, Macao Special Administrative Region | Surveillance | 2010 | 221 | 1.8 | 6.3 | 2.2 | 39 | 5.1 | 10.2 | 5.1 | |
| Colombia | Surveillance | 2010 | – | – | – | – | 495 | 15.8 | 25.5 | 17.0 | |
| Curaçao | Surveillance | 2010 | 5 | 0.0 | 40.0 | 0.0 | 0 | – | – | – | |
| Cyprus | Surveillance | 2009 | 27 | 14.8 | 22.2 | 14.8 | 4 | 0.0 | – | – | |
| Czech Republic | Surveillance | 2009 | 413 | 1.2 | 2.9 | 1.9 | 39 | 7.7 | 10.3 | 7.7 | |
| Denmark | Surveillance | 2009 | 209 | 0.5 | 6.7 | 0.5 | 33 | 3.0 | 12.1 | 3.0 | |
| El Salvador | Surveillance | 2009 | – | – | – | – | 85 | 1.2 | 5.9 | 1.2 | |
| Estonia | Surveillance | 2010 | 197 | 18.3 | 25.9 | 18.8 | 61 | 44.3 | 50.9 | 44.3 | |
| Fiji | Surveillance | 2009 | – | – | – | – | 2 | 0.0 | 0.0 | 0.0 | |
| Finland | Surveillance | 2010 | 239 | 2.1 | 6.7 | 2.5 | 3 | 12.5 | 12.5 | 12.5 | |
| France | Surveillance | 2009 | 1304 | 1.0 | 2.7 | 1.1 | 106 | 13.2 | 17.0 | 14.2 | |
| French Polynesia | Surveillance | 2009 | 42 | 0.0 | 0.0 | 0.0 | 4 | 0.0 | 0.0 | 0.0 | |
| Georgia | Surveillance | 2010 | 1987 | 9.5 | 23.1 | 9.9 | 558 | 30.6 | 44.0 | 31.1 | |
| Germany | Surveillance | 2010 | 2138 | 1.3 | 7.1 | 1.6 | 130 | 6.2 | 15.4 | 7.7 | |
| Greece | Surveillance | 2009 | 140 | 6.4 | 11.4 | 6.4 | 14 | 28.6 | 50.0 | 35.7 | |
| Guam | Surveillance | 2010 | 56 | 3.6 | 10.7 | 3.6 | 2 | 0.0 | 0.0 | 0.0 | |
| Hungary | Surveillance | 2009 | 486 | 3.3 | 8.4 | 3.7 | 55 | 7.3 | 18.2 | 7.3 | |
| Iceland | Surveillance | 2010 | 19 | 0.0 | 31.6 | 0.0 | 0 | – | – | – | |
| Indonesia, Central Java province | Survey | 2006 | 1126 | 1.8 | 11.4 | 2.1 | 70 | 17.1 | 24.2 | 21.7 | |
| Ireland | Surveillance | 2010 | 176 | 1.1 | 5.1 | 2.2 | 21 | 0.0 | 4.8 | 0.0 | |
| Israel | Surveillance | 2010 | 245 | 4.9 | 11.4 | 7.3 | 2 | 0.0 | 50.0 | 0.0 | |
| Italy | Surveillance | 2009 | 1051 | 3.2 | 8.4 | 3.8 | 264 | 12.5 | 21.2 | 13.7 | |
| Jordan | Surveillance | 2009 | 95 | 6.3 | 9.5 | 6.3 | 7 | 28.6 | 28.6 | 28.6 | |
| Kazakhstan | Surveillance | 2010 | – | – | – | – | 4655 | 45.1 | 58.8 | 48.3 | |
| Kuwait | Surveillance | 2010 | 437 | 1.1 | 10.0 | 1.3 | 0 | – | – | – | |
| Latvia | Surveillance | 2010 | 613 | 10.3 | 25.8 | 10.3 | 102 | 23.5 | 39.2 | 23.5 | |
| Lebanon | Surveillance | 2010 | – | – | – | – | 14 | 35.7 | 35.7 | 35.7 | |
| Lithuania | Surveillance | 2009 | 1074 | 10.6 | 21.4 | 11.1 | 404 | 51.5 | 61.6 | 52.2 | |
| Luxembourg | Surveillance | 2009 | 27 | 0.0 | 11.1 | 0.0 | – | – | – | – | |
| Madagascar | Survey | 2007 | 810 | 0.5 | 4.6 | 0.5 | 51 | 3.9 | 9.8 | 5.9 | |
| Malta | Surveillance | 2009 | 17 | 0.0 | 5.9 | 0.0 | 0 | – | – | – | |
| Marshall Islands | Surveillance | 2010 | 68 | 1.5 | 3.0 | 1.5 | 3 | 0.0 | 0.0 | 0.0 | |
| Mauritius | Surveillance | 2010 | 105 | 1.0 | 2.9 | 1.0 | 7 | 14.3 | 14.3 | 14.3 | |
| Mexico | Survey | 2009 | 1584 | 2.4 | 8.5 | 2.6 | 191 | 6.5 | 15.5 | 10.9 | |
| Mongolia | Surveillance | 2010 | – | – | – | – | 561 | 30.1 | 36.5 | 30.8 | |
| Mongolia | Survey | 2007 | 650 | 1.4 | 12.6 | 2.2 | – | – | – | – | |
| Montenegro | Surveillance | 2010 | 61 | 0.0 | 4.9 | 0.0 | 12 | 0.0 | 0.0 | 0.0 | |
| Mozambique | Survey | 2007 | 1102 | 3.5 | 7.8 | 3.7 | 25 | 11.2 | 15.2 | 19.6 | |
| Myanmar | Survey | 2008 | 1071 | 4.2 | 5.2 | 4.9 | 299 | 10.0 | 11.7 | 10.7 | |
| Namibia | Survey | 2008 | 1054 | 3.8 | 13.5 | 4.6 | 354 | 16.4 | 38.4 | 22.0 | |
| Netherlands | Surveillance | 2010 | 741 | 1.3 | 8.5 | 1.4 | 29 | 3.4 | 13.7 | 3.4 | |
| New Zealand | Surveillance | 2009 | 237 | 2.5 | 9.3 | 2.5 | 8 | 12.5 | 12.5 | 12.5 | |
| Northern Mariana Islands | Surveillance | 2010 | 17 | 0.0 | 5.9 | 0.0 | 0 | – | – | – | |
| Norway | Surveillance | 2009 | 210 | 3.8 | 9.0 | 4.3 | 20 | 0.0 | 10.0 | 0.0 | |
| Oman | Surveillance | 2010 | 185 | 0.0 | 8.1 | 0.5 | 8 | 12.5 | 12.5 | 12.5 | |
| Palau | Surveillance | 2010 | 11 | 0.0 | 0.0 | 0.0 | 0 | – | – | – | |
| Paraguay | Survey | 2008 | 319 | 0.3 | 1.9 | 0.9 | 48 | 14.6 | 16.7 | 14.6 | |
| Poland | Surveillance | 2008 | 3758 | 0.5 | 3.1 | 0.5 | 607 | 5.6 | 9.7 | 5.9 | |
| Portugal | Surveillance | 2009 | 1391 | 0.9 | 6.8 | 1.0 | 148 | 6.1 | 8.8 | 6.1 | |
| Puerto Rico | Surveillance | 2010 | 69 | 0.0 | 10.1 | 0.0 | 4 | 0.0 | 0.0 | 0.0 | |
| Qatar | Surveillance | 2010 | 324 | 1.2 | 5.8 | 1.2 | 0 | – | – | – | |
| Republic of Moldova | Surveillance | 2010 | – | – | – | – | 1077 | 65.1 | 74.0 | 66.4 | |
| Russian Federation, Arkhangelsk oblast | Surveillance | 2009b | 292 | 25.7 | – | – | 68 | 58.8 | – | – | |
| Russian Federation, Belgorod oblast | Surveillance | 2009b | 358 | 19.8 | – | – | 91 | 51.6 | – | – | |
| Russian Federation, Bryansk oblast | Surveillance | 2009b | 560 | 11.1 | – | – | 54 | 27.8 | – | – | |
| Russian Federation, Ivanovo oblast | Surveillance | 2009b | 276 | 20.3 | – | – | 52 | 57.7 | – | – | |
| Russian Federation, Kaliningrad oblast | Surveillance | 2009b | 354 | 22.3 | – | – | 51 | 43.1 | – | – | |
| Russian Federation, Mary El Republic | Surveillance | 2009b | 366 | 15.6 | – | – | 53 | 37.7 | – | – | |
| Russian Federation, Murmansk oblast | Surveillance | 2009b | 190 | 28.9 | – | – | 14 | 35.7 | – | – | |
| Russian Federation, Orel oblast | Surveillance | 2009b | 256 | 6.3 | – | – | 29 | 48.3 | – | – | |
| Russian Federation, Pskov oblast | Surveillance | 2009b | 304 | 24.3 | – | – | 44 | 50.0 | – | – | |
| Russian Federation, Republic of Chuvashia | Surveillance | 2009b | 579 | 15.2 | – | – | 92 | 45.7 | – | – | |
| Russian Federation, Tomsk oblast | Surveillance | 2009b | 435 | 11.3 | – | – | 80 | 53.8 | – | – | |
| Russian Federation, Vladimir oblast | Surveillance | 2009b | 422 | 20.9 | – | – | 55 | 32.7 | – | – | |
| Rwanda | Surveillance | 2010 | – | – | – | – | 431 | 19.0 | 20.2 | 21.3 | |
| Serbia | Surveillance | 2008 | 923 | 0.7 | 2.0 | 0.7 | 130 | 7.7 | 12.3 | 7.7 | |
| Singapore | Surveillance | 2010 | 923 | 0.2 | 2.3 | 0.3 | 79 | 1.3 | 6.4 | 2.6 | |
| Slovakia | Surveillance | 2010 | 185 | 0.0 | 2.7 | 0.0 | 32 | 3.1 | 9.4 | 3.1 | |
| Slovenia | Surveillance | 2009 | 167 | 0.5 | 2.4 | 0.6 | 8 | 0.0 | 0.0 | 0.0 | |
| Sri Lanka | Surveillance | 2010 | – | – | – | – | 378 | 1.6 | 5.3 | 2.6 | |
| Swaziland | Survey | 2009 | 352 | 7.7 | 13.4 | 8.0 | 231 | 33.9 | 45.3 | 36.4 | |
| Sweden | Surveillance | 2010 | 440 | 2.5 | 9.1 | 3.0 | 30 | 23.3 | 40.0 | 23.3 | |
| Switzerland | Surveillance | 2010 | 270 | 0.4 | 2 | 0.4 | 33 | 9.1 | 33.3 | 12.1 | |
| Tajikistan, Dushanbe city and Rudaki district | Survey | 2009 | 139 | 16.5 | 26.6 | 16.6 | 125 | 61.6 | 74.4 | 64.8 | |
| Former Yugoslav Republic of Macedonia | Surveillance | 2010 | 153 | 1.3 | 3.9 | 1.3 | 28 | 17.9 | 17.9 | 17.9 | |
| Uganda, Kampala | Survey | 2008 | 473 | 1.1 | 5.8 | 1.5 | 60 | 11.7 | 20.0 | 13.4 | |
| United Kingdom of Great Britain and Northern Ireland | Surveillance | 2009 | 3957 | 0.9 | 6.6 | 1.1 | 364 | 3.3 | 7.7 | 4.1 | |
| United States of America | Surveillance | 2010 | 6514 | 1.1 | 7.5 | 1.5 | 293 | 4.4 | 16.0 | 5.8 | |
The proportion of new TB cases reported as showing multidrug resistance in these years ranged from 0% to 28.9%. Proportions exceeding 12% (in countries reporting more than 10 MDR-TB cases) were documented in Belarus (25.7%), Estonia (18.3%), several oblasts of the Russian Federation (with Murmansk having the highest level, 28.9%) and Tajikistan (Dushanbe city and Rudaki district, 16.5%).
The proportion of previously treated cases having MDR-TB ranged from 0% to 65.1%. Countries or subnational areas with proportions exceeding 50% included Belarus (60.2%), Lithuania (51.5%), the Republic of Moldova (65.1%), five oblasts of the Russian Federation, and Tajikistan (Dushanbe city and Rudaki district, 61.6%) (Table 1).
The largest country that conducted a nationwide survey in the reporting period was China, where 5.7% of new TB cases and 25.6% of previously treated cases were found to have multidrug resistance (Table 1).
Surveillance data on XDR-TB were reported from 38 countries and 3 territories, 34 of which routinely test all patients with MDR-TB for second-line anti-TB drug resistance. Only 6 out of 41 (15%) countries and territories reported more than 10 cases of XDR-TB; the proportion of MDR-TB cases that were extensively drug-resistant exceeded 10% in Estonia (19.7%), Latvia (15.1%), South Africa (10.5%) and Tajikistan (Dushanbe city and Rudaki district, 21.0%) (Table 2, available at: http://www.who.int/bulletin/volumes/90/2/11-092585).
Table 2. Countries and territories reporting dataa on extensively drug-resistant tuberculosis (XDR-TB), 2007–2010.
| Country or territory | Source | Year | No. of cases of MDR-TB | MDR-TB cases with DST results for 2nd-line drugs | No. of cases of XDR-TB (%) |
|---|---|---|---|---|---|
| Albania | Surveillance | 2010 | 2 | 2 | 0 (0.0) |
| Australia | Surveillance | 2010 | 32 | 32 | 1 (3.1) |
| Austria | Surveillance | 2010 | 15 | 15 | 1 (6.7) |
| Bangladesh (14 districts covering 30 million population)b | Surveillance | 2008 | 168 | 168 | 1 (0.6) |
| Belgium | Surveillance | 2009 | 10 | 10 | 3 (30.0) |
| Botswana | Survey | 2008 | 32 | 24 | 0 (0.0) |
| Bulgaria | Surveillance | 2008 | 32 | 28 | 0 (0.0) |
| Canada | Surveillance | 2010 | 15 | 14 | 1 (7.1) |
| China | Survey | 2008 | 401 | 401 | 29 (7.2) |
| China, Hong Kong Special Administrative Region | Surveillance | 2009 | 3 | 3 | 0 (0.0) |
| China, Macao Special Administrative Region | Surveillance | 2010 | 6 | 6 | 0 (0.0) |
| Cyprus | Surveillance | 2008 | 1 | 1 | 0 (0.0) |
| Czech Republic | Surveillance | 2008 | 11 | 10 | 1 (10.0) |
| Denmark | Surveillance | 2007 | 2 | 2 | 0 (0.0) |
| Estonia | Surveillance | 2010 | 63 | 61 | 12 (19.7) |
| Georgia | Surveillance | 2010 | 359 | 313 | 30 (9.6) |
| Greece | Surveillance | 2009 | 14 | 9 | 3 (33.3) |
| Guam | Surveillance | 2010 | 2 | 2 | 0 (0.0) |
| Iceland | Surveillance | 2008 | 1 | 1 | 0 (0.0) |
| India, Gujarat state | Survey | 2006 | 216 | 216 | 7 (3.2) |
| Israel | Surveillance | 2010 | 12 | 12 | 1 (8.3) |
| Italy | Surveillance | 2009 | 82 | 32 | 1 (3.1) |
| Latvia | Surveillance | 2010 | 87 | 86 | 13 (15.1) |
| Marshall Islands | Surveillance | 2010 | 1 | 1 | 0 (0.0) |
| Montenegro | Surveillance | 2009 | 1 | 1 | 0 (0.0) |
| Namibia | Survey | 2008 | 100 | 100 | 0 (0.0) |
| Norway | Surveillance | 2008 | 4 | 4 | 0 (0.0) |
| Oman | Surveillance | 2010 | 1 | 1 | 0 (0.0) |
| Paraguay | Survey | 2008 | 8 | 8 | 0 (0.0) |
| Poland | Surveillance | 2008 | 52 | 52 | 5 (9.6) |
| Qatar | Surveillance | 2010 | 4 | 4 | 0 (0.0) |
| Singapore | Surveillance | 2010 | 3 | 3 | 0 (0.0) |
| Slovakia | Surveillance | 2010 | 1 | 1 | 0 (0.0) |
| South Africa | Surveillance | 2008 | 8026 | 5451 | 572 (10.5) |
| Swaziland | Survey | 2009 | 122 | 122 | 1 (0.8) |
| Sweden | Surveillance | 2009 | 13 | 9 | 0 (0.0) |
| Switzerland | Surveillance | 2010 | 8 | 8 | 0 (0.0) |
| Tajikistan, Dushanbe city and Rudaki district | Survey | 2009 | 100 | 100 | 21 (21.0) |
| Former Yugoslav Republic of Macedonia | Surveillance | 2010 | 7 | 5 | 1 (20.0) |
| United Kingdom of Great Britain and Northern Ireland | Surveillance | 2009 | 58 | 40 | 2 (5.0) |
| United States of America | Surveillance | 2010 | 92 | 59 | 1 (1.7) |
Overall data, 1994–2010
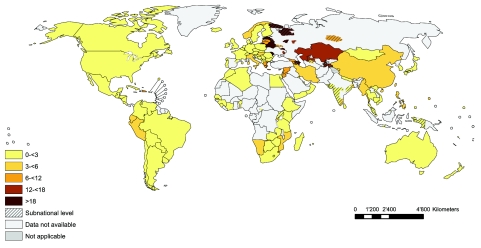
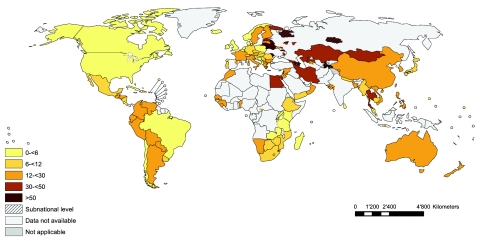
The proportions of new and previously treated TB cases in the world that were multidrug resistant are shown in Fig. 2 and Fig. 3, respectively. Overall, when data from all countries and territories were combined, the global proportions of new and previously treated TB cases showing multidrug resistance were 3.4% (95% CI: 1.9–5.0) and 19.8% (95% CI: 14.4–25.1), respectively. Regional level estimates of the proportion of cases with MDR-TB are shown in Table 3.
Fig. 2.
Distribution of percentage of new tuberculosis cases with multidrug-resistant tuberculosis (MDR-TB)
Note: Showing latest available data, 1994–2010. Reported data for the Democratic Republic of the Congo, Luxembourg, New Caledonia and the Solomon Islands are not disaggregated by new and previously treated tuberculosis cases and are therefore not shown.
Fig. 3.
Percentage of previously treated tuberculosis patients with multidrug-resistant tuberculosis (MDR-TB)
Note: Showing latest available data, 1994–2010. Reported data for the Democratic Republic of the Congo, Luxembourg, New Caledonia and the Solomon Islands are not disaggregated by new and previously treated tuberculosis cases and are therefore not shown.
Table 3. Average proportions of cases of tuberculosis, new or previously treated, that are multidrug-resistant in regions of the World Health Organization (WHO) and the world, 1994–2000.
| WHO region | New cases |
Previously treated cases |
|||
|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | ||
| African region | 1.9 | 0.6–3.3 | 9.4 | 3.0–15.8 | |
| Region of the Americas | 2.1 | 0.7–3.4 | 11.5 | 3.8–19.2 | |
| Eastern Mediterranean region | 3.4 | 0.9–5.9 | 20.6 | 7.5–33.7 | |
| European regiona | 12.1 | 8.6–15.6 | 36.5 | 32.5–40.6 | |
| South-East Asia region | 2.1 | 1.7–2.5 | 17.2 | 16.5–17.7 | |
| Western Pacific region | 4.9 | 3.6–6.1 | 23.2 | 19.6–26.9 | |
| World | 3.4 | 1.9–5.0 | 19.8 | 14.4–25.1 | |
CI, confidence interval.
a The values for Europe reflect the high levels of multidrug resistance documented in eastern European countries: 14.3% (95% CI: 12–16.7) among new cases and 39% (95% CI: 35.1–42.9) among previously treated cases of tuberculosis.
Note: The data were obtained from the World Health Organization.10,11
XDR-TB has been identified in 77 countries globally, and 57 countries and 3 territories were able to report representative data from continuous surveillance or special surveys on the proportion of XDR-TB cases among MDR-TB cases. Combined data from all countries and territories showed that the proportion of MDR-TB cases with extensive drug resistance was 9.4% (95% CI: 7.4–11.6).
Risk factors
When data from 17 countries and 1 territory that reported drug resistance data stratified by HIV status were combined, the odds of having MDR-TB among HIV-positive cases were found to be 40% higher than among HIV-negative cases (pooled odds ratio, OR: 1.4; 95% CI: 0.7–3.0; OR consistent across countries, I2 = 23.2%; P-value = 0.19), but the difference was not significant. Thus, no association was noted between the presence of MDR-TB and HIV status.
A total of 58 countries and 2 special territories reported drug resistance surveillance data disaggregated by sex. Overall, when data from these settings were combined, the odds of having MDR-TB were found to be 10% higher among females than males (OR: 1.1; 95% CI: 0.8–1.4; OR heterogeneous across countries, I2 = 32.9%; P-value = 0.009), but the difference was not significant. Thus, no association was noted between the presence of MDR-TB and the sex of the patient.
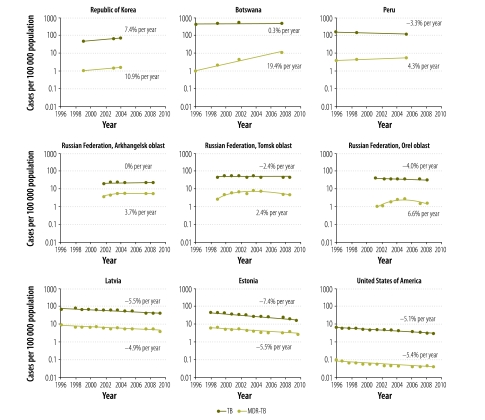
Time trends
Data on time trends in drug resistance were available from 71 countries and 751 country-year data points. Selected data to illustrate the diversity of trends in TB and MDR-TB worldwide are presented in Fig. 4. In a first group of countries, composed of Botswana, Peru and the Republic of Korea, the estimated notification rate of MDR-TB is increasing (+10.9%, +19.4% and +4.3% per year, respectively). In these countries, trends in notifications of new TB cases vary, with a clear increase in the Republic of Korea (+7.4% per year), a rather stable trend in Botswana (+0.3% per year) and a clear decline in Peru (−3.3% per year). A second group is composed of three Russian oblasts where TB notification rates are stable or decreasing. Although in these oblasts MDR-TB rates were on the rise until around 2005–2006, they have subsequently been falling in all three settings. In a third group of countries, composed of Estonia, Latvia and the United States, surveillance data suggest that both TB and MDR-TB rates have been falling for more than a decade. In the United States the rate of MDR-TB is falling even more quickly than the TB case notification rate.
Fig. 4.
Time trends in population rates of new cases of tuberculosis (TB) and new cases of multidrug-resistant tuberculosis (MDR-TB)
Note: Data are for new TB cases, except for those of the United States of America, whose data are for combined new and previously treated TB cases.
Discussion
In 2007–2010, several countries provided drug resistance surveillance data generated from continuous surveillance systems rather than special surveys, a change from previous reports.8,9 Of particular interest is a group of 12 countries that have succeeded in establishing continuous surveillance systems for previously treated TB cases: Bangladesh (14 districts covering a population of 30 million), the Plurinational State of Bolivia, Chile, Colombia, El Salvador, Fiji, Kazakhstan, Lebanon, Mongolia, the Republic of Moldova, Rwanda and Sri Lanka. This is the first step towards routine drug susceptibility testing for all TB cases and allows early identification of drug resistance in the population at greatest risk.18–20
Available data confirm that eastern Europe and central Asia continue to have the world’s highest proportion of MDR-TB among TB cases. In 2007–2010 the highest proportions ever reported globally were documented in areas of the former Soviet Union; MDR-TB was reported among nearly 30% of new TB cases in the oblast of Murmansk in the Russian Federation and among 65% of previously treated TB cases in the Republic of Moldova. In a few other oblasts of the Russian Federation in the same region, levels of MDR-TB appear to be stabilizing or even decreasing, which confirms that addressing MDR-TB is feasible even in high-burden areas. Unfortunately, large parts of eastern Europe and central Asia still lack representative data. This applies to the whole of Kyrgyzstan and most of Azerbaijan, the Russian Federation, Tajikistan, Turkmenistan, Ukraine and Uzbekistan. With planned and ongoing surveys and improvements in continuous surveillance in these countries, major strides towards improving our understanding of the true burden of drug-resistant TB are expected in the near future.
China conducted its first nationwide survey in 2007. The survey, which confirmed previously published estimates8,9 based on extrapolation from subnational level data, represents a critical step towards addressing MDR-TB in one of the largest TB control programmes in the world. Whereas China has been able to conduct a nationwide survey, India and the Russian Federation – the other two large countries that, with China, contribute to more than 50% of the estimated global burden of MDR-TB – have only produced reliable subnational level data to date. To understand the magnitude of the MDR-TB problem and address it, nationwide surveillance systems should be established in all countries, with greater urgency in the highest burden settings.
Only 34 countries and settings have a system in place to routinely test all patients with MDR-TB for second-line anti-TB drug resistance. These are generally countries with established or emerging economies, as laboratory capacity for second-line drug susceptibility testing in resource-limited settings is still scarce.
The average proportions of MDR-TB cases among diagnosed TB cases detected in this study are consistent with previous reports.8,9 The lack of data on drug-resistant TB in most African countries is still a matter of major concern (Fig. 2 and Fig. 3). This situation should be urgently addressed, especially since the African Region accounts for over 80% of the TB cases among people living with HIV and since higher mortality from MDR-TB and XDR-TB has been documented in HIV-positive patients.29 The availability of new molecular technologies for diagnosing TB and detecting rifampicin resistance, including line probe assays and Xpert MTB/RIF, represent an unprecedented opportunity for countries with severely limited laboratory infrastructure to diagnose drug resistance more easily. Line probe assays permit safer transportation of specimens, require a lower workload than conventional culture and drug susceptibility tests, and reduce to two days the time needed for the diagnosis of MDR-TB.30,31 Xpert MTB/RIF is an automated nucleic acid amplification technology that detects rifampicin resistance in less than two hours. It is very simple to use and requires limited training and biosafety measures.22 A few countries have piloted the use of molecular technologies in drug resistance surveys,30,31 but data from surveys using exclusively those techniques are not yet available. Molecular technologies are expected to contribute substantially to surveillance of drug-resistant TB in low-resource settings in the future.
The analysis of risk factors for MDR-TB showed that the overall risk of harbouring MDR-TB strains is not influenced by sex. The sex distribution of patients with MDR-TB does not differ from that of patients with drug-susceptible TB. This finding is not surprising, since MDR-TB is a form of TB and has similar risk factors. Countries where an association is documented should be investigating the possible reasons.
Although an association between HIV infection and MDR-TB has been widely documented in hospital outbreaks of drug-resistant TB among people living with HIV,32–34 the population-based data gathered to date suggest that the relationship between multidrug resistance and HIV infection is not consistent across settings (although the available data are limited to a few countries). In addition, HIV status is unknown for large proportions of patients in these cohorts. Countries are still experiencing great difficulties in incorporating HIV testing in drug resistance surveys, as this requires strong collaboration between HIV and TB control programmes. Understanding the relationship between HIV infection and drug-resistant TB at the population level is critical to identify high-risk groups in need of additional support.
Trend analysis suggests that MDR-TB can be controlled once bold policy decisions are put into practice and the correct prevention and control measures are implemented. This is illustrated by recent findings reported from selected oblasts in the Russian Federation, where MDR-TB has been recognized as a serious problem since the time of the dissolution of the Soviet Union. In Arkhangelsk, Tomsk and Orel oblasts, TB case notifications are stable or decreasing, and although MDR-TB rates were increasing until 2005–2006, more recent data show a stabilizing (Arkhangelsk) or even declining trend (Tomsk and Orel). These settings, which have been treating many cases with MDR-TB in recent years, show that MDR-TB can be controlled even in places heavily affected by drug resistance. The same can be said for Estonia and Latvia, where TB and MDR-TB have been declining for more than a decade. In the United States, rates of MDR-TB are falling even more quickly than rates of TB. These last three countries have strong control programmes that have succeeded in reducing both susceptible and resistant forms of TB. In contrast, in the Republic of Korea, TB and MDR-TB notifications are both increasing, the latter more rapidly than the former. The diversity of treatment options and case management in the country, particularly in the large private health sector, may be facilitating the development of drug resistance.35 In Botswana TB notification rates have stabilized, whereas in Peru they have declined, in line with previous assessments.36 However, in both countries MDR-TB notification rates are showing a very marked increase.
Conclusion
Following 15 years of intensive effort, high-quality surveillance data on anti-TB drug resistance are available for two thirds of all countries in the world. These data show where MDR-TB rates are highest and demonstrate that in selected settings a proper response can alleviate the problem. At the same time, global trends in rates of MDR-TB remain unclear, largely because national representative data are lacking in many large countries, including India and several African countries. A better understanding of epidemiological trends in drug resistance at the global and national levels can be achieved only through repeated surveys and, ultimately, by establishing continuous surveillance based on routine drug susceptibility testing of all confirmed TB cases, with priority given to previously treated patients. Special studies are also needed to help us better understand the factors conducive to the development and spread of MDR-TB. If properly and intensively implemented and followed by appropriate treatment of all TB patients, new technologies can accelerate the response to the threat of drug resistance, save lives and reduce the burden TB imposes on individuals, households and communities.
Competing interests:
None declared.
References
- 1.Cohn DL, Bustreo F, Raviglione MC. Drug-resistant tuberculosis: review of the worldwide situation and the WHO/IUATLD Global Surveillance Project. International Union Against Tuberculosis and Lung Disease. Clin Infect Dis. 1997;24(Suppl 1):S121–30. doi: 10.1093/clinids/24.Supplement_1.S121. [DOI] [PubMed] [Google Scholar]
- 2.Anti-tuberculosis drug resistance in the world: the WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance Geneva: World Health Organization; 1997 (WHO/TB/97.229). [Google Scholar]
- 3.Pablos-Méndez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, et al. Global surveillance for antituberculosis-drug resistance, 1994-1997. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 1998;338:1641–9. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 4.Anti-tuberculosis drug resistance in the world: the WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Report 2: Prevalence and trends Geneva: World Health Organization; 2000 (WHO/CDS/TB/2000.278). [Google Scholar]
- 5.Espinal MA, Laszlo A, Simonsen L, Boulahbal F, Kim SJ, Reniero A, et al. Global trends in resistance to antituberculosis drugs. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 2001;344:1294–303. doi: 10.1056/NEJM200104263441706. [DOI] [PubMed] [Google Scholar]
- 6.Anti-tuberculosis drug resistance in the world: the WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Third global report Geneva: World Health Organization; 2004 (WHO/HTM/TB/2004.343). [Google Scholar]
- 7.Aziz MA, Wright A, Laszlo A, De Muynck A, Portaels F, Van Deun A, et al. WHO/International Union Against Tuberculosis And Lung Disease Global Project on Anti-tuberculosis Drug Resistance Surveillance Epidemiology of antituberculosis drug resistance (the Global Project on Anti-tuberculosis Drug Resistance Surveillance): an updated analysis. Lancet. 2006;368:2142–54. doi: 10.1016/S0140-6736(06)69863-2. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Anti-tuberculosis drug resistance in the world: the WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Fourth global report Geneva, Switzerland: WHO, 2008 (WHO/HTM/TB/2008.394). [Google Scholar]
- 9.Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, et al. Global Project on Anti-Tuberculosis Drug Resistance Surveillance Epidemiology of antituberculosis drug resistance 2002-07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373:1861–73. doi: 10.1016/S0140-6736(09)60331-7. [DOI] [PubMed] [Google Scholar]
- 10.Multidrug and extensively drug-resistant TB (M/XDR-TB):2010global report on surveillance and response Geneva: World Health Organization; 2010.
- 11.Global tuberculosis control Geneva: World Health Organization; 2011 (WHO/HTM/TB/2011.16). [Google Scholar]
- 12.Towards universal access to diagnosis and treatment of multidrug-resistant and extensively drug-resistant tuberculosis by 2015 Geneva: World Health Organization; 2011 (WHO/HTM/TB/2011.3). [Google Scholar]
- 13.Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: emergency update Geneva: World Health Organization; 2008 (WHO/HTM/TB/2008.402; ISBN 978-92-4-154758-1).
- 14.Guidelines for the programmatic management of drug-resistant tuberculosis. 2011 update Geneva: World Health Organization; 2011 (WHO/HTM/TB/2011.6). [PubMed] [Google Scholar]
- 15.Falzon D, Jaramillo E, Schünemann H, Arentz M, Bayona J, Blanc L, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38:516–28. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 16.WHO policy on TB infection control in health-care facilities, congregate settings and households Geneva: World Health Organization; 2009 (WHO/HTM/TB/2009.419). [PubMed] [Google Scholar]
- 17.Green Light Committee Initiative of the Working Group on MDR-TB of the STOP TB Partnership. Annual report 2009. Geneva: World Health Organization; 2010 (WHO/HTM/TB/2010.14). [Google Scholar]
- 18.Guidelines for surveillance of drug resistance in tuberculosis. Fourth edn. Geneva: World Health Organization; 2009 (WHO/HTM/TB/2009.422).
- 19.Zignol M, van Gemert W, Falzon D, Jaramillo E, Blanc L, Raviglione M. Modernizing surveillance of anti-tuberculosis drug resistance: from special surveys to routine testing. Clin Infect Dis. 2011;52:901–6. doi: 10.1093/cid/cir081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Global Plan to Stop TB 2011–2015 Geneva: Stop TB Partnership & World Health Organization; 2010. [Google Scholar]
- 21.Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR-TB): policy statement Geneva: World Health Organization; 2009. Available from: http://www.who.int/tb/features_archive/policy_statement.pdf [accessed 28 October 2011]
- 22.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aziz MA, Wright A. The World Health Organization/International Union Against Tuberculosis and Lung Disease Global Project on Surveillance for Anti-Tuberculosis Drug Resistance: a model for other infectious diseases. Clin Infect Dis. 2005;41(Suppl 4):S258–62. doi: 10.1086/430786. [DOI] [PubMed] [Google Scholar]
- 24.Van Deun A, Wright A, Zignol M, Weyer K, Rieder HL. Drug susceptibility testing proficiency in the network of supranational tuberculosis reference laboratories. Int J Tuberc Lung Dis. 2011;15:116–24. [PubMed] [Google Scholar]
- 25.Treatment of tuberculosis guidelines Fourth ed. Geneva: World Health Organization; 2009 (WHO/HTM/TB/2009.420). [Google Scholar]
- 26.The WHO global data collection system [Internet]. Geneva: World Health Organization; 2011. Available from: http://www.stoptb.org/tme/ [accessed 28 October 2011]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.United Nations. [database]. Population. New York: Department of Economic and Social Affairs, Population Division. Available from: http://esa.un.org/unpd/wpp/unpp/ [accessed August 2011]
- 29.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 30.Rigouts L, Hoza AS, De Rijk P, Torrea G, Chonde TM, Basra D, et al. Evaluation of the Genotype® MTBDRplus assay as a tool for drug-resistance surveys. Int J Tuberc Lung Dis. 2011;15:959–65. doi: 10.5588/ijtld.10.0515. [DOI] [PubMed] [Google Scholar]
- 31.Barnard M, Albert H, Coetzee G, O'Brien R, Bosman ME. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med. 2008;177:787–92. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 32.Edlin BR, Tokars JI, Grieco MH, Crawford JT, Williams J, Sordillo EM, et al. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514–21. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 33.Moro ML, Gori A, Errante I, Infuso A, Franzetti F, Sodano L, et al. An outbreak of multidrug-resistant tuberculosis involving HIV-infected patients of two hospitals in Milan, Italy. Italian Multidrug-Resistant Tuberculosis Outbreak Study Group. AIDS. 1998;12:1095–102. doi: 10.1097/00002030-199809000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(Suppl 1):S86–107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 35.Seung KJ, Bai GH, Kim SJ, Lew WJ, Park SK, Kim JY. The treatment of tuberculosis in South Korea. Int J Tuberc Lung Dis. 2003;7:912–9. [PubMed] [Google Scholar]
- 36.Suárez PG, Watt CJ, Alarcón E, Portocarrero J, Zavala D, Canales R, et al. The dynamics of tuberculosis in response to 10 years of intensive control effort in Peru. J Infect Dis. 2001;184:473–8. doi: 10.1086/322777. [DOI] [PubMed] [Google Scholar]