Abstract
Objective
To determine whether cotrimoxazole reduces mortality in adults receiving antiretroviral therapy (ART) for human immunodeficiency virus (HIV) infection in low- and middle-income countries through a systematic review and meta-analysis.
Methods
PubMed and Embase were searched for randomized controlled trials and prospective and retrospective cohort studies that compared mortality or morbidity in HIV-infected individuals aged ≥ 13 years on cotrimoxazole and ART and on ART alone. The Newcastle–Ottawa Quality Assessment Scale was used to assess selection, confounding and measurement bias. Publication bias was assessed using Egger’s and Begg’s tests. Sensitivity analysis was performed because the I-squared statistic indicated substantial heterogeneity in study results. A random-effects model was used for meta-analysis.
Findings
Nine studies were included. Begg and Egger P-values for the seven that reported the effect of cotrimoxazole on mortality were 0.29 and 0.49, respectively, suggesting no publication bias. The I-squared statistic was 93.2%, indicating high heterogeneity in study results. The sensitivity analysis showed that neither the follow-up duration nor the percentage of individuals with World Health Organization stage 3 or 4 HIV disease at baseline explained the heterogeneity. The summary estimate of the effect of cotrimoxazole on the incidence rate of death was 0.42 (95% confidence interval: 0.29–0.61). Since most studies followed participants for less than 1 year, it was not possible to determine whether cotrimoxazole can be stopped safely after ART-induced immune reconstitution.
Conclusion
Cotrimoxazole significantly increased survival in HIV-infected adults on ART. Further research is needed to determine the optimum duration of cotrimoxazole treatment in these patients.
Résumé
Objectif
Déterminer si le cotrimoxazole réduit la mortalité des adultes recevant une thérapie antirétrovirale (TAR) contre l’infection par le virus de l'immunodéficience humaine (VIH) dans les pays à revenu faible et moyen, par un examen systématique et une méta-analyse.
Méthodes
On a cherché dans PubMed et Embase des essais contrôlés randomisés et des études de cohorte prospectives et rétrospectives comparant la mortalité ou la morbidité chez des individus âgés de 13 ans et plus, infectés par le VIH, sous cotrimoxazole et TAR, et uniquement sous TAR. L'échelle d’évaluation de qualité Newcastle-Ottawa a été utilisée pour évaluer les biais de sélection, de confusion et de mesure. Le biais de publication a été évalué à l'aide des tests d’Egger et de Begg. Une analyse de sensibilité a été effectuée, car la statistique I-carré indiquait une hétérogénéité importante des résultats de l'étude. Un modèle à effets aléatoires a été utilisé pour la méta-analyse.
Résultats
Neuf études ont été incluses. Les valeurs P de Begg et Egger pour les sept études rapportant l'effet du cotrimoxazole sur la mortalité étaient de 0,29 et de 0,49, respectivement, suggérant l’absence de biais de publication. La statistique I-carré était de 93,2%, indiquant une forte hétérogénéité des résultats de l'étude. L'analyse de sensibilité a montré que ni la durée du suivi ni le pourcentage de personnes atteintes par le VIH au stade 3 et 4 de l'Organisation mondiale de la Santé, au moment de référence, n’expliquaient l'hétérogénéité. L'estimation sommaire de l'effet du cotrimoxazole sur le taux d'incidence des décès était de 0,42 (intervalle de confiance de 95%: 0,29–0,61). Comme la plupart des études avaient suivi les participants pendant moins d’un an, il n'était pas possible de déterminer si l’administration de cotrimoxazole pouvait être interrompue en toute sécurité après reconstitution immunitaire induite par le TAR.
Conclusion
Le cotrimoxazole augmentait significativement la survie des adultes infectés par le VIH sous traitement antirétroviral. Des recherches supplémentaires sont nécessaires pour définir la durée optimale du traitement par cotrimoxazole chez ces patients.
Resumen
Objetivo
Determinar si cotrimoxazol reduce la mortalidad en adultos que reciben tratamiento antirretrovírico (TAR) para el virus de la inmunodeficiencia humana (VIH) en países de ingresos bajos y medios través de un examen sistemático y un metaanálisis.
Métodos
Se realizó una búsqueda en PubMed y Embase para evaluar los ensayos aleatorizados controlados y los estudios de cohortes prospectivos y retrospectivos en los que se comparaban la mortalidad o la morbilidad en personas infectadas por el VIH con edades ≥13 años en tratamiento con cotrimoxazol y TAR, y solo con TAR. Se utilizó la Escala de Evaluación de la Calidad de Newcastle-Ottawa para evaluar el sesgo de selección, de confusión y de medición. El sesgo de publicación se evaluó mediante los tests de Egger y Begg. Se llevó a cabo un análisis de sensibilidad debido a que la estadística I-cuadrado indicó una heterogeneidad significativa en los resultados del estudio. El metanálisis siguió un modelo de efectos aleatorios.
Resultados
Se incluyeron nueve estudios. Los valores de P de Begg y Egger para los siete estudios que recogieron el efecto de cotrimoxazol sobre la mortalidad fueron de 0,29 y 0,49, respectivamente, lo que sugiere que no existe sesgo de publicación. La estadística I-cuadrado fue del 93,2%, lo que indica una alta heterogeneidad en los resultados de los estudios. El análisis de sensibilidad mostró que dicha heterogeneidad no se debe ni a la duración de seguimiento ni al porcentaje de individuos con enfermedad por el VIH en los estadios 3 o 4 según la Organización Mundial de la Salud al inicio del estudio. La estimación global de los efectos del cotrimoxazol sobre la tasa de incidencia de la muerte fue de 0,42 (intervalo de confianza del 95%: 0,29–0,61). Dado que la mayoría de los estudios realizaron un seguimiento de los participantes durante menos de 1 año, no fue posible determinar la posibilidad de suspender la administración de cotrimoxazol sin peligro después de la restauración inmunitaria inducida por la TAR.
Conclusión
Cotrimoxazol aumentó significativamente la supervivencia en adultos infectados por el VIH que recibieron TAR. Es preciso llevar a cabo investigaciones adicionales con el objetivo de determinar la duración óptima del tratamiento con cotrimoxazol en estos pacientes.
الملخص
الغرض
تحديد ما إذا كان كوتريموكسازول يحد من الوفيات في البالغين الذين يخضعون لعلاج مضاد للفيروسات (ART) لعلاج عدوى الإصابة بفيروس العوز المناعي البشري (HIV) في البلدان منخفضة ومتوسطة الدخل عن طريق استعراض منهجي وتحليل تلوي.
الطرق
تم البحث في قواعد بيانات PubMed وEmbase عن التجارب الخاضعة للضبط والتي تستخدم عينات عشوائية وعن الدراسات الأترابية الاستباقية والاسترجاعية التي قارنت بين الوفيات أو الاعتلال لدى الأشخاص المصابين بفيروس العوز المناعي البشري في أعمار 13 عاماً أو أكبر وأثره على كوتريموكسازول والعلاج المضاد للفيروسات وأثره على العلاج المضاد للفيروسات وحده. وتم استخدم مقياس نيوكاسل أوتاوا لتقييم الجودة من أجل تقييم المجموعة المختارة، والعوامل المربكة وتحيز القياس. وتم تقييم عامل التحيز في البحوث العلمية المنشورة باستخدام اختبارات إيغر وبيغ. وتم إجراء تحليل الحساسية لأن إحصاء التغاير التربيعي أشار إلى تغايرية كبيرة في نتائج الدراسة. وتم استخدام نموذج للآثار العشوائية للتحليل التلوي.
النتائج
تم تضمين تسع دراسات. وكانت القيم الاحتمالية لاختبارات بيغ وإيغر للدراسات السبع التي أشارت إلى تأثير كوتريموكسازول على الوفيات هي 0.29 و0.49 على التوالي، مما يشير إلى عدم وجود عامل تحيز في البحوث العلمية المنشورة. وكان إحصاء التغاير التربيعي 93.2٪، مما يشير إلى التغايرية المرتفعة في نتائج الدراسة. وأظهر تحليل الحساسية أن مدة المتابعة ونسبة الأفراد المصابين بفيروس العوز المناعي البشري من المرحلة الثالثة أو الرابعة وفق تصنيف منظمة الصحة العالمية عند خط الأساس لم توضحا التغايرية. وكان ملخص تقييم تأثير كوتريموكسازول على معدل حدوث الوفيات هو 0.42 (حدود ثقة 95٪: 0.29-0.61). ولأن معظم الدراسات تابعت المشاركين لأقل من عام واحد، لم يكن من الممكن تحديد ما إذا كان من الممكن وقف كوتريموكسازول بأمان بعد الاستنشاء المناعي المستحث بواسطة العلاج المضاد للفيروسات (ART).
الاستنتاج
نجح كوتريموكسازول في تحقيق زيادة كبيرة في البقاء على قيد الحياة لدى البالغين المصابين بفيروس العوز المناعي البشري مقارنة بغيره من العلاج المضاد للفيروسات. وتوجد حاجة لإجراء المزيد من الأبحاث لتحديد مدة العلاج المثلى باستخدام كوتريموكسازول بالنسبة لهؤلاء المرضى.
摘要
目的
通过系统评价和meta分析,确定复方新诺明是否降低中低收入国家中接受抗逆转录病毒疗法(ART)的成年人类免疫缺陷病毒(HIV)感染者的死亡率
方法
检索PubMed和Embase,寻找对比单独使用ART和配合使用复方新诺明和ART 的13岁以上艾滋病毒感染个体的死亡率或发病率的随机对照试验以及前瞻性和回顾性队列研究。使用纽卡斯尔– 渥太华质量评价量表(Newcastle–Ottawa Quality Assessment Scale)对选择、混杂以及测量偏倚进行评估。使用Egger’s 和 Begg’s检验评估发表偏倚。由于I方统计在研究结果中显示出显著的异质性,因此进行了敏感性分析。meta分析使用了随机效应模型。
结果
包括了九项研究。第七项报告复方新诺明对死亡率影响的Begg 和 Egger P值分别为 0.29 和 0.49,说明无发表偏倚。I方统计为93.2%,表明研究结果中的高异质性。敏感性分析表明,随访期和世界卫生组织的基线第3或者4阶段HIV疾病对异质性都不能解释。复方新诺明对死亡的发病率影响的总结的估算值为 0.42 (95%置信区间:0.29–0.61)。由于大多数的随访参与者不到1年,还无法确定在ART诱导免疫功能重建后是否可以安全停止复方新诺明。
结论
复方新诺明显著地提高了采用ART的成年HIV感染者的存活率。确定这些患者复方新诺明治疗的最佳时间还需要进一步研究。
Резюме
Цель
Посредством систематического обзора и мета-анализа определить влияние котримоксазола на снижение уровня смертности у взрослых при проведении антиретровирусной терапии (АРТ) вируса иммунодефицита человека (ВИЧ) в странах с низким и средним уровнем дохода.
Методы
В базах данных публикаций PubMed и Embase был произведен поиск рандомизированных контролируемых испытаний, а также перспективных и ретроспективных обследований групп пациентов, в которых производилось сравнение уровня смертности и критических осложнений у ВИЧ-инфицированных лиц в возрасте ≥ 13 лет при применении котримоксазола в сочетании с АРТ или при проведении только АРТ. Для оценки выборки, дополнительных факторов и систематических ошибок измерений была использована шкала оценки качества Ньюкасл-Оттава. Систематические ошибки публикаций были оценены с помощью тестов Эггера и Бегга. Был проведен анализ чувствительности, поскольку метод сбалансированной оценки статистических данных показал значительную неоднородность результатов исследований. Для проведения мета-анализа была использована модель со случайными уровнями факторов.
Результаты
Были учтены результаты девяти исследований. P-показатели в тестах Бегга и Эггера по семи исследованиям, в которых были приведены данные о влиянии котримоксазола на уровень смертности, составляли 0,29 и 0,49 соответственно, подтверждая тем самым отсутствие систематических ошибок в публикациях. Метод сбалансированной оценки статистических данных показал 93,2%, тем самым указывая на высокую неоднородность результатов исследований. Анализ чувствительности показал, что ни продолжительность последующего наблюдения, ни процентное соотношение лиц, инфицированных ВИЧ 3-ей или 4-ой стадии по шкале Всемирной Организации Здравоохранения, не объясняли данную неоднородность. Суммарная оценка влияния котримоксазола на коэффициент заболеваемости со смертельным исходом была равна 0,42 (95% доверительный интервал: 0,29–0,61). Поскольку в большей части исследований наблюдение за участниками осуществлялось не более одного года, было невозможно определить, можно ли без риска прекратить прием котримоксазола после иммунного восстановления, индуцированного АРТ.
Вывод
Котримоксазол значительно повысил уровень выживаемости ВИЧ-инфицированных взрослых при проведении АРТ. Необходимы дальнейшие исследования для определения оптимальной продолжительности терапии с применением котримоксазола у данных пациентов.
Introduction
In 2010, there were 1.8 million deaths among the 34 million people infected with the human immunodeficiency virus (HIV).1 Of these deaths, 1.2 million occurred in the 22.9 million HIV-infected Africans.1 Antiretroviral therapy (ART) has transformed HIV infection into a manageable chronic condition and the World Health Organization (WHO) currently recommends ART for individuals with a CD4+ T lymphocyte (CD4 cell) count ≤ 350 cells/µL.2 In 2010, 42% of the 9 million individuals in need of treatment globally were receiving ART.3 Unfortunately, in low-income countries patients are started on ART at lower CD4 cell counts than in high-income countries: in 2006, the median count was reported to be 108 cells/µL and 234 cells/µL in these two types of countries, respectively.4 Moreover, after adjusting for immunodeficiency at baseline, mortality during the first months of ART was higher in low-income countries than in high-income countries.4,5 High mortality rates early in ART have been documented in Africa,6 the Caribbean,7 Latin America7 and south-eastern Asia.8,9 Consequently, additional interventions are needed to decrease early mortality during ART in low- and middle-income countries.
Cotrimoxazole contains two antibiotics: sulfamethoxazole and trimethoprim. Cotrimoxazole provides good coverage against gram-positive bacteria (e.g. Streptococcus pneumoniae), gram-negative bacteria (e.g. Escherichia coli and non-typhoid Salmonella), protozoa (e.g. Isospora belli, Toxoplasma gondii and Plasmodium falciparum) and fungi (e.g. Pneumocystis jirovecii). The patent for cotrimoxazole has expired and it costs around 7 United States dollars for 1 year of daily therapy.10 In high-income countries, cotrimoxazole is used in adults with HIV infection as chemoprophylaxis against P. jirovecii pneumonia and T. gondii infection.11 Randomized trials that included ART-naïve Africans found that cotrimoxazole improved survival while reducing the risk of malaria, pneumonia, sepsis, isosporiasis, T. gondii encephalitis, wasting and Kaposi’s sarcoma.12–14 However, a Senegalese trial that used half the recommended adult cotrimoxazole dose reported no mortality benefit in ART-naïve adults.15 A study in Uganda showed that treating HIV-infected adults with cotrimoxazole and ART reduced mortality in their uninfected children and the number of orphans.16
In settings where the health-care infrastructure is limited, WHO recommends cotrimoxazole for adults with WHO clinical stage 2, 3 or 4 HIV infection.17 If the prevalence of HIV infection is high, however, WHO recommends that all infected adults be treated because cotrimoxazole reduces morbidity irrespective of clinical disease stage or CD4 cell count and because it simplifies cotrimoxazole distribution.17
Common causes of mortality in adults receiving ART in low- and middle-income countries include sepsis, tuberculosis, Crytptococcus neoformans meningitis, T. gondii encephalitis, P. jirovecii pneumonia, Kaposi’s sarcoma and chronic diarrhoea.6,18–20 Given the results of earlier trials,12–14 cotrimoxazole may decrease both mortality and morbidity in adults with HIV infection, regardless of ART status. The aim of this study was to carry out a systematic review of the effect of cotrimoxazole on mortality and morbidity in individuals aged 13 years or more who were receiving ART for an HIV infection.
Methods
This systematic review was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.21 The PubMed and Embase databases were searched systematically in December 2010 for randomized controlled trials and prospective and retrospective cohort studies on the effect of daily cotrimoxazole in HIV-infected individuals aged 13 years or more who were receiving ART. The search strategies (Table 1, available at: http://www.who.int/bulletin/volumes/90/2/11-093260) were designed with a specialist librarian and there were no language, publication or date restrictions. The primary outcome of interest was death. Secondary outcomes were hospitalization, incident events synonymous with WHO clinical stage 3 or 4 HIV disease, incident malaria events and adverse events leading to hospitalization or cotrimoxazole cessation. The Cochrane Central Register of Controlled Trials and the International Standard Randomized Controlled Trial Number Register were searched using the terms “cotrimoxazole” and “antiretroviral therapy”.
Table 1. Search strategies for publications on cotrimoxazole’s effect on mortality and morbidity in adults with human immunodeficiency virus (HIV) infection receiving antiretroviral therapy (ART).
| Search number | PubMed search terms | Search number | Embase search terms |
|---|---|---|---|
| 1 | HIV infections (MeSH term) | 1 | HIV |
| 2 | Acquired immunodeficiency syndrome (MeSH term) | 2 | AIDS |
| 3 | AIDS-related opportunistic infections (MeSH term) | 3 | Terms used in search 1 or 2 |
| 4 | AIDS | 4 | Antiretroviral |
| 5 | HIV | 5 | Anti-retroviral |
| 6 | Terms used in search 1 or 2 or 3 or 4 or 5 | 6 | HAART |
| 7 | Antiretroviral therapy, highly active (MeSH term) | 7 | cART |
| 8 | Anti-infective agents (MeSH term) | 8 | ART |
| 9 | Antiretroviral | 9 | Terms used in search 4 or 5 or 6 or 7 or 8 |
| 10 | Anti-retroviral | 10 | Cotrimoxazole |
| 11 | HAART | 11 | Co-trimoxazole |
| 12 | ART | 12 | CPT |
| 13 | cART | 13 | Trimethoprim-sulfamethoxazole |
| 14 | Terms used in search 7 or 8 or 9 or 10 or 11 or 12 or 13 | 14 | Sulfamethoxazole-trimethoprim |
| 15 | Trimethoprim-sulfamethoxazole combination (MeSH term) | 15 | Terms used in search 10 or 11 or 12 or 13 or 14 |
| 16 | Cotrimoxazole | 16 | Mortality |
| 17 | Co-trimoxazole | 17 | Death |
| 18 | CPT | 18 | Hospitalization |
| 19 | Terms used in search 15 or 16 or 17 or 18 | 19 | Adverse event |
| 20 | Mortality (MeSH term) | 20 | Terms used in search 16 or 17 or 18 or 19 |
| 21 | Death (MeSH term) | 21 | Terms used in searches 3 and 9 and 15 and 20 |
| 22 | Hospitalization (MeSH term) | ||
| 23 | Mortality | ||
| 24 | Death | ||
| 25 | Hospitalization | ||
| 26 | Adverse event | ||
| 27 | Terms used in search 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 | ||
| 28 | Terms used in searches 6 and 14 and 19 and 27 |
AIDS, acquired immunodeficiency syndrome; cART, combination antiretroviral therapy; CPT, cotrimoxazole preventive therapy; HAART, highly active antiretroviral therapy; MeSH, medical subject heading.
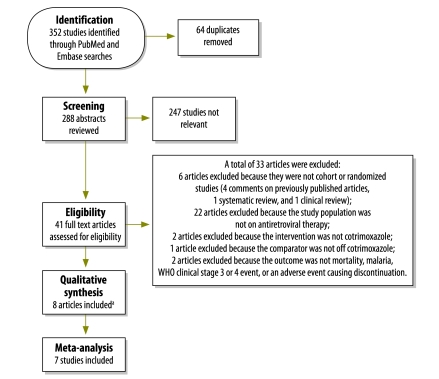
The abstracts of all publications identified were reviewed independently by two of the authors (ABS and AVR). The full texts of all articles selected by one or both reviewers were then matched against the inclusion criteria and articles satisfying these criteria were included in the review (Fig. 1, available at: http://www.who.int/bulletin/volumes/90/2/11-093260). The references of all articles that met the inclusion criteria were also considered for incorporation into the systematic review.
Fig. 1.
Flow diagram of article selection for systematic review of cotrimoxazole’s effect on mortality and morbidity in adults with human immunodeficiency virus (HIV) infection receiving antireotroviral therapy (ART)
WHO, World Health Organization.
a One additional study was identified during the review of references for the eight studies meeting eligibility criteria.
Data were extracted from the articles included in the review using a standardized spreadsheet. The information collected included the name of the article’s first author, the year of publication, the study’s methods and design, the study population, the study intervention and control intervention, the duration of follow-up, inclusion and exclusion criteria, primary and secondary outcomes, and losses to follow-up.
In accordance with the recommendations of the Cochrane Collaboration,22 the Newcastle–Ottawa Quality Assessment Scale was used to identify bias in the cohort studies.23 This scale rates studies from 0 to 9 using eight criteria that cover three sources of bias: selection, confounding and measurement bias. Each criterion is worth one point except confounding bias, which is worth two points. Selection bias was assessed using four criteria: (i) the patient cohort receiving cotrimoxazole and ART was representative of adults receiving this treatment in the community from which the cohort was drawn; (ii) the cohort receiving ART alone was representative of the cohort on both cotrimoxazole and ART; (iii) cotrimoxazole use had been ascertained; and (iv) the majority of study participants were known to be asymptomatic (i.e. had WHO clinical stage 1 or 2 disease) at study baseline. A study was regarded as having addressed confounding due to the baseline level of immunodeficiency if the results had been adjusted for the baseline CD4 cell counts. Measurement bias was assessed using three criteria: (i) all deaths were validated; (ii) there was adequate follow-up to detect cotrimoxazole’s preventive effect on death (i.e. a median or mean follow-up period of at least 3 months), and (iii) ≤ 20% of study participants were lost during follow-up. A score of 7 to 9 indicated high methodological quality, a score of 4 to 6 indicated moderate quality, and a score of 0 to 3 indicated low quality.
Publication bias was evaluated using a funnel plot, with the treatment effect measure on the x-axis and the standard error of the log of the effect measure on the y-axis (details are available from the corresponding author on request). Egger’s and Begg’s tests were used to assess the symmetry of the funnel plot. Since the studies were similar enough to combine, a meta-analysis was performed and statistical heterogeneity was assessed. Effect measures were quantified as the natural log of the effect measure and the standard error24 as:
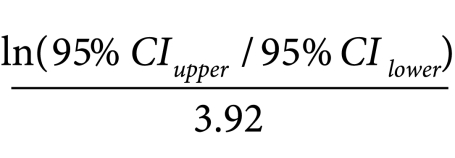 |
Since both χ2 and tau statistical tests of the heterogeneity of the magnitude of a treatment effect across studies depend on knowledge of the number of events in each study arm and this information was not available for all studies that met the inclusion criteria, an I-squared statistic was used. In practice, the I-squared statistic is calculated by subtracting the number of degrees of freedom from the Q-statistic and then dividing the result by the Q-statistic.25 Values of I-squared less than 25% indicate low heterogeneity, values near 50% indicate moderate heterogeneity and values above 75% indicate high heterogeneity.26 A random-effects statistical model is preferred when I-squared is ≥ 25%.27
Since the value of I-squared obtained for the studies included in this analysis was greater than 25%, a random-effects model was used to derive a summary estimate of the effect of cotrimoxazole on mortality. The possible causes of the observed heterogeneity, including the duration of follow-up and the percentage of participants who had WHO stage 3 or 4 disease at baseline, were explored by a sensitivity analysis. Unfortunately, neither the baseline viral load nor the baseline CD4 cell count could be included in the sensitivity analysis because data on the baseline viral load were not collected in most studies and data on the median or mean baseline CD4 cell count were collected in only four of the seven eligible studies reporting on the primary outcome. All analyses were performed using Stata version 10.0 (StataCorp. LP, College Station, United States of America).
Results
Details of the study participants and study designs described in the nine articles included in the systematic review are shown in Table 2 and Table 3, respectively. Of the nine, seven reported an estimate for the effect of cotrimoxazole on mortality,28–34 whereas three reported an estimate for the effect on morbidity.28,35,36 Two studies also reported the preventive effect on malaria.28,36 Assessment of publication bias indicated that three studies28–30 were of high methodological quality, while the other six31–36 were of moderate methodological quality (Table 4, available at: http://www.who.int/bulletin/volumes/90/2/11-093260). In addition, one on-going trial investigating when to stop cotrimoxazole during ART was identified.37 That trial involves 2000 individuals in Uganda and has a randomized, double-blind, placebo-controlled non-inferiority design. Its aim is to test the hypothesis that stopping cotrimoxazole in adults on ART will not increase morbidity or mortality and will decrease haematological adverse events. The results are due in 2014.
Table 2. Participants in studies included in systematic review of cotrimoxazole’s effect on mortality and morbidity in adults with human immunodeficiency virus (HIV) infection receiving antiretroviral therapy (ART).
| First author, year | Study country | Inclusion and exclusion criteria for study participants | Percentage of participants with HIV disease at baseline |
Baseline CD4 cell count | BMI or body weight | |
|---|---|---|---|---|---|---|
| WHO stage 3 | WHO stage 4 | |||||
| Walker, 201028 | Uganda and Zimbabwe | HIV-infected individuals aged ≥ 18 years with WHO stage 2 to 4 disease and a CD4 cell count ≤ 200 cells/µL but with no previous ART were included. Follow-up data from participants whose ART was interrupted were excluded | 56 | 23 | Median CD4 cell count: 83 cells/µL (IQR: 29–137) | Median BMI: 21 kg/m2 (IQR: 19–24) |
| Hoffmann, 201029 | South Africa | Participants were ≥ 18 years old and ART-naïve at ART initiation. Those with a CD4 cell count < 250 cells/µL, WHO stage 4 disease regardless of CD4 cell count, or WHO stage 3 disease with a CD4 cell count < 350 cells/µL were eligible for ART at workplace clinics. Community clinics required participants to have a CD4 cell count < 200 cells/µL or WHO stage 4 disease for ART | 27 | 35 | Median CD4 cell count at ART initiation: 132 cells/µL (IQR: 60–206) | 16%, 79% and 6% of participants weighed ≤ 60 kg, 61–75 kg and > 75 kg, respectively |
| Lowrance,200730 | Malawi | Individuals aged ≥ 15 years with WHO stage 3 or 4 disease or a CD4 cell count ≤ 200 cells/µL were included | 63 | 24 | 13% of participants had a CD4 cell count < 200 cells/µL at ART initiation | Not reported |
| Madec, 200731 | Cambodia | Individuals aged ≥ 13 years with WHO stage 4 disease or a CD4 cell count < 200 cells/µL were included | 45 | 46 | Median CD4cell count at ART initiation: 20 cells/µL (IQR: 6–78) | Median BMI: 18.5 kg/m2 (IQR: 16.6–20.4) in men and 18.7 kg/m2 (IQR: 16.4–21.0) in women |
| Fairall, 200832 | South Africa | HIV-infected individuals aged ≥ 16 years who had been in contact with the programme at least twice were included | 53 | 11 | 48.4% of participants had a baseline CD4 cell count < 200 cells/µL | Median weight: 55 kg (IQR: 48–64) |
| van Oosterhout, 201033 | Malawi | ART-naïve individuals aged ≥ 18 years who started ART with a BMI < 18.5 kg/m2 were included. Those who were unable to follow instructions for ART, had active liver disease or were pregnant were excluded | 64 | 28 | Mean CD4 cell count for all participants initiating ART: 132 cells/µL | Mean BMI: 16.5 kg/m2 for all participants initiating ART |
| Alemu, 201034 | Ethiopia | Participants were ≥ 15 years of age and received ART on at least two clinic visits. Those who started treatment at another location, who had previously received ART, whose baseline CD4 cell count was unknown, or whose personal information was missing were excluded | 54 | 17 | 85% of participants had a CD4 cell count < 200 cells/µL at initiation of ART | 15%, 45%, 30% and 10% of participants weighed ≤ 40 kg, 40–50 kg, 50–60 kg and ≥ 60 kg, respectively |
| Miiro, 200935 | Uganda | Adults aged ≥ 15 years who had had a WHO stage 4 disease event, who had a recurrent WHO stage 3 disease condition, who had tuberculosis and a CD4 cell count < 350 cells/µL, or who were asymptomatic with a CD4 cell count < 200 cells/µL were included. Those who started ART before joining the cohort or who enrolled in the DART trial were excluded | 34 | 13 | Median CD4 cell count for all participants on ART: 131 cells/µL | Not reported |
| Campbell, 200936 | Uganda | Ugandans initiated ART if they had a CD4 cell count ≤ 250 cells/µL or WHO stage 3 or 4 disease, excluding pulmonary TB. Ugandans on ART for a mean 3.7 years were randomly assigned, by household, to continue or discontinue cotrimoxazole. For Ugandans randomized to discontinue cotrimoxazole, it was required that their two most recent CD4 cell counts be > 200 cells/µL. | Not reported | Not reported | Median CD4 cell count for all participants: 489 cells/µL. | Not reported |
BMI, body mass index; CD4 cell, CD4+ T lymphocyte; DART, Development of Anti-Retroviral Therapy in Africa; IQR, interquartile range; TB, tuberculosis; WHO, World Health Organization.
Table 3. Studies in systematic review of cotrimoxazole’s effect on mortality and morbidity in adults with human immunodeficiency virus (HIV) infection receiving antiretroviral therapy (ART) .
| First author, year | Study design | Follow-up duration | Cotrimoxazole |
Timing of cotrimoxazole initiation relative to ART initiation | Analytical method used | Variables included in the statistical model | Losses to follow-up | How losses to follow-up were addressed | Risk of death or morbidity in individuals taking cotrimoxazole compared with those not taking cotrimoxazole | |
|---|---|---|---|---|---|---|---|---|---|---|
| Received (No.) | Not received (No.) | |||||||||
| Walker, 201028 | Prospective cohort study | Median duration: 4.9 years (IQR: 4.5–5.3) | 1959 | 1220 | 62% of participants initiated cotrimoxazole before ART initiation, 28% initiated cotrimoxazole after ART initiation, and 10% never initiated cotrimoxazole | Marginal structural model | Baseline variables: CD4 cell count, haemoglobin level, body mass index, history of a WHO stage 3 or 4 HIV disease event, age, sex, randomization year, WHO disease stage, monitoring group and study site. Time-dependent variables: CD4 cell count, haemoglobin level, time on ART and body mass index | 198 of 3 179 participants (6%) were last seen alive more than 4 months before the end of follow-up | Data from participants lost to follow-up was censored, although losses were infrequent and the effect of this censoring by additional weighting was small | Adjusted OR for death on cotrimoxazole: 0.65 (95% CI: 0.50–0.85). There were 83 deaths during 8 128 person–years of follow-up on cotrimoxazole and 105 deaths during 6 086 person–years of follow-up off cotrimoxazole. Adjusted OR for malaria on cotrimoxazole: 0.74 (95% CI: 0.63–0.88). Adjusted OR for new or recurrent WHO clinical stage 3 or 4 disease events on cotrimoxazole: 0.85 (95% CI: 0.74–0.98) |
| Hoffmann, 201029 | Prospective cohort study | Mean duration: 0.77 years | 7508 | 6589 | Cotrimoxazole was initiated between 30 days before and 7 days after the initiation of ART | Cox proportional hazards model | Baseline variables: CD4 cell count, history of tuberculosis, WHO disease stage, sex and age. Time-updated variables: HIV RNA suppression | 1186 participants (18%) not on cotrimoxazole and 901 on cotrimoxazole (12%) discontinued ART, left care or were lost to follow-up | Data from participants who discontinued ART, left care or were lost to follow-up were censored | Adjusted HR for death on cotrimoxazole: 0.64 (95% CI: 0.57–0.72). There were 558 deaths during 6 133 person–years of follow-up on cotrimoxazole and 732 deaths during 4 617 person–years of follow-up off cotrimoxazole |
| Lowrance, 200730 | Retrospective cohort study | 0.5 years | 574 | 478 | Cotrimoxazole was initiated after ART initiation | Cox proportional hazards model | Baseline variables: CD4 cell count, WHO disease stage, tuberculosis history, gender and age | 22 of 574 participants who received cotrimoxazole, were lost to follow-up and 17 transferred out of the study. 51 of 478 patients not on cotrimoxazole, were lost to follow-up and 21 transferred out | Data from participants lost to follow-up were excluded from the analysis | Adjusted RR of death on cotrimoxazole: 0.59 (95% CI: 0.43–0.82). There were 57 deaths in 535 participants on cotrimoxazole and 73 deaths in 406 participants off cotrimoxazole |
| Madec, 200731 | Prospective cohort study | Median duration: 1.08 years (IQR: 5–21 months) | Not reported | Not reported | Not reported | Cox proportional hazards model | Age, sex, WHO disease stage, CD4 cell count, haemoglobin level, body mass index, year of ART initiation, ART regimen and previous exposure to ART | 37 participants (2.13%) were lost to follow-up | Participants lost to follow-up were regarded as having died | Adjusted HR for death on cotrimoxazole: 0.15 (95% CI: 0.11–0.21) |
| Fairall, 200832 | Prospective cohort study | Median duration: 0.33 years (IQR: 1–9 months) | Not reported | Not reported | Not reported | Marginal structural model | Baseline variables: age, sex, district of residence, clinic, weight, CD4 cell count, tuberculosis status and ART status. Time-dependent variables: CD4 cell count, weight and ART status | Not reported | Not reported | Adjusted HR for death on cotrimoxazole: 0.37 (95% CI: 0.32–0.42) |
| van Oosterhout, 201033 | Retrospective cohort study | 0.5 years | 319 | 274 | Cotrimoxazole was initiated before ART initiation | Logistic regression model | Age, sex, WHO disease stage, body mass index, haemoglobin level and baseline CD4 cell count | 7 participants were lost to follow-up and 13 stopped ART after 14 weeks. 16 were lost to follow-up and 15 stopped ART after 26 weeks | Not reported | After 14 weeks, the adjusted OR for death on cotrimoxazole was 0.61 (95% CI: 0.38–0.96). After 26 weeks, it was 0.71 (95% CI: 0.46–1.11) |
| Alemu, 201034 | Retrospective cohort study | Mean duration: 2.01 years | 240 | 31 | Cotrimoxazole was initiated at or before ART initiation in 88.6% of participants | Cox proportional hazards model | Baseline variables: WHO disease stage and haemoglobin level | 48 participants (18%) were lost to follow-up and 19 (7%) transferred to another facility | Data from participants who were lost to follow-up or transferred to another facility were censored | Adjusted HR for death on cotrimoxazole: 0.14 (95% CI: 0.05–0.37) |
| Miiro, 200935 | Retrospective cohort study | 1 year | 179 | 32 | Cotrimoxazole was initiated before ART initiation | Logistic regression model | ART, age, sex, baseline WHO disease stage, haemoglobin level and baseline CD4 cell count | Data from participants followed up for less than 1 year were censored | Data from 157 of the 219 participants (71.7%) were censored at the completion of follow-up | RR for all-cause morbidity on cotrimoxazole: 0.66 (95% CI: 0.41–1.06), although all-cause morbidity was undefined |
| Campbell, 200936 | Randomized trial | 0.32 years | 452 | 384 | Not reported | Not reported | Not reported | Not reported | Not reported | RR for malaria on cotrimoxazole 0.04 (95% CI: 0.01–0.17). RR for diarrhoea on cotrimoxazole 0.56 (95% CI: 0.43–0.77) |
CD4 cell, CD4+ T lymphocyte; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; OR, odds ratio, RNA, ribonucleic acid; RR, relative risk; WHO, World Health Organization.
Table 4. Newcastle–Ottawa Quality Assessment Scale scores for bias in studies included in systematic review of cotrimoxazole’s effect on mortality and morbidity in adults with human immunodeficiency virus (HIV) infection receiving antiretroviral therapy (ART) .
| First author, year | Sources of bias |
Total scorea | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection bias |
Confounding bias |
Measurement bias |
|||||||||
| Cohort on cotrimoxazole and ART was representative of the average adult on this treatment in the communityb | Cohort receiving ART alone was representative of the cohort on both cotrimoxazole and ARTb | Cotrimoxazole use was ascertainedb | Majority of participants were known to be asymptomatic at study baselineb | Mortality estimates were adjusted for baseline CD4 cell countc | Deaths were validatedb | Median or mean follow-up of at least 3 monthsb | ≤ 20% of participants were lost to follow-upb | ||||
| Walker, 201028 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | ||
| Hoffmann, 201029 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 | ||
| Lowrance, 200730 | 1 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 7 | ||
| Madec, 200731 | 0 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 6 | ||
| Fairall, 200832 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 6 | ||
| Van Oosterhout, 201033 | 0 | 1 | 1 | 0 | 2 | 0 | 1 | 1 | 6 | ||
| Alemu, 201034 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 5 | ||
| Miiro, 200935 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 5 | ||
| Campbell, 200936 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 6 | ||
CD4 cell, CD4+ T lymphocyte.
a A score of 7 to 9 indicates high methodological quality, a score of 4 to 6 indicates moderate quality and a score of 0 to 3 indicates low quality.
b Yes = 1 point; No = 0 points.
c Yes = 2 points; No = 0 points.
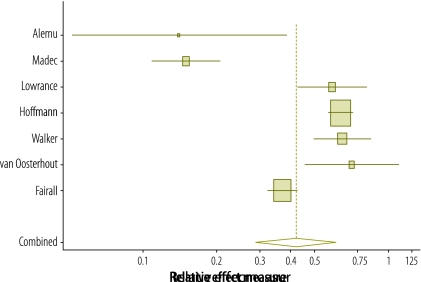
The effect of cotrimoxazole on mortality reported in the seven studies in the meta-analysis is illustrated in Fig. 2. Begg and Egger P-values for publication bias in the studies were 0.29 and 0.49, respectively, which suggests the absence of publication bias. The summary estimate of the effect of cotrimoxazole on the incidence rate of death derived using a random-effects model was 0.42 (95% confidence interval, CI: 0.29–0.61). Survival was increased by either continuing cotrimoxazole at ART initiation,29,33–35 initiating cotrimoxazole at the same time as ART29,34 or initiating cotrimoxazole when the patient was stable on ART.28,30
Fig. 2.
Forest plot of study and summary effect estimates for cotrimoxazole’s effect on death in adults with human immunodeficiency virus (HIV) infection receiving antiretroviral therapy (ART)
Note: The seven studies were included in the meta-analysis. The summary estimate of the treatment effect was calculated using a random-effects model. The risk is reported either as an odds ratio, a hazard ratio or a relative risk in the different studies. The centre of the squares represents the study point estimates while the horizontal lines represent the study 95% confidence intervals (CIs). The centre of the bottom quadrilateral represents the summary effect estimate, whereas its horizontal corners represent its 95% CI.
The Q-statistic and the I-squared statistic for the seven studies reporting on the primary outcome were 102.3 and 93.2%, respectively, indicating high heterogeneity.26 A sensitivity analysis that evaluated the effect of the median or mean number of years of follow-up indicated that the length of the study did not explain the heterogeneity (P = 0.85). Nor was the percentage of participants with symptomatic HIV infection (i.e. WHO stage 3 or 4 disease) at baseline found to provide an explanation on sensitivity analysis (P = 0.91).
Regarding cotrimoxazole’s effect on morbidity, Walker et al. reported that cotrimoxazole resulted in a reduction in the odds of malaria (odds ratio, OR: 0.74; 95% CI: 0.63–0.88).28 This effect was maintained throughout a follow-up period of 5 years. In addition, Walker et al. also reported that cotrimoxazole reduced the odds of new or recurrent WHO clinical stage 3 or 4 disease events (OR: 0.85; 95% CI: 0.74–0.98),28 whereas Miiro et al. reported a trend towards lower all-cause morbidity (relative risk, RR: 0.66; 95% CI: 0.41–1.06), although all-cause morbidity was undefined.35 A trial identified during the review of references of studies that met the inclusion criteria found that the risk of both malaria (RR: 0.04; 95% CI: 0.01–0.17) and diarrhoea (RR: 0.56; 95% CI: 0.43–0.77) was lower among Ugandans on ART who were randomized to cotrimoxazole than among those who were not.36 The patients had been receiving ART for a mean of 3.7 years, their median CD4 cell count was 489 cells/µL and 94% had a viral load < 400 RNA copies/mL.
Two studies gave details of adverse events associated with cotrimoxazole. Walker et al. reported 22 serious cotrimoxazole-related adverse events during 8128 person–years of treatment: all were either haematological adverse events, rash or hypersensitivity.28 Lowrance et al. reported that 10 of 574 patients on cotrimoxazole stopped treatment during 6 months of follow-up but did not state whether or not the cause was cotrimoxazole-related toxicity.30
Since only two of the eight studies followed participants for more than 13 months on average, it was difficult to gauge whether or not the survival benefit of cotrimoxazole waned after ART-induced immune reconstitution. However, Walker et al. did analyse the effect of the duration of cotrimoxazole combined with ART.28 They found that cotrimoxazole was associated with a substantial reduction in the odds of death between weeks 1 and 12 of the administration of cotrimoxazole combined with ART (OR: 0.41; 95% CI: 0.27–0.65) and that the reduction was sustained between weeks 12 and 72 (OR: 0.56; 95% CI: 0.37–0.86) but ceased to be evident after week 72 (OR: 0.96; 95% CI: 0.63–1.45). There was no evidence of variation in this effect resulting from an updated CD4 cell count.
It was also difficult to determine whether the survival benefit of cotrimoxazole was influenced by the baseline CD4 cell count since in six studies most participants initiated ART with a CD4 cell count of less than 200 cells/µL. However, Hoffmann et al. reported that the reduction in the hazard of death was substantial among individuals with a baseline CD4 cell count less than 200 cells/µL (hazard ratio, HR: 0.64; 95% CI: 0.56–0.72) and those with a baseline count between 200 and 350 cells/µL (HR: 0.62; 95% CI: 0.41–0.94) but was not significant among those with a count greater than 350 cells/µL at baseline (HR: 0.80; 95% CI: 0.38–1.70).29
Discussion
This systematic review indicates that cotrimoxazole reduced mortality in individuals aged 13 years or more who were receiving ART. The summary estimate of the effect of cotrimoxazole on the incidence rate of death was 0.42 (95% CI: 0.29–0.61). Although there was no evidence of publication bias in the studies included in the review, there was significant heterogeneity in the findings: the I-squared statistic was 93.2%. Unfortunately, only a limited sensitivity analysis could be performed because of differences in the variables recorded in the various studies. Nonetheless, the sensitivity analysis showed that neither symptomatic HIV infection (i.e. WHO stage 3 or 4 disease) nor the duration of follow-up explained the heterogeneity.
Cotrimoxazole is safe, well tolerated, widely available and inexpensive. Although cotrimoxazole can help reduce the high early mortality rate in HIV-infected adults on ART in low- and middle-income countries, it is still not widely used. The slow increase in the uptake of cotrimoxazole has been associated with delays in the dissemination of recommendations on its use (either in stand-alone guidelines or integrated into ART guidelines), problems with drug procurement and supply, poor health-care infrastructure for managing patients before ART and inadequate systems for monitoring and evaluation.30 In addition to resolving these issues, the number of adults receiving cotrimoxazole could also be increased by raising awareness of its benefits and by using indicators to monitor its uptake both globally and at the level of individual treatment programmes.38 Moreover, treatment programmes could also provide forecasts of the future annual demand for cotrimoxazole by using local data on the number of individuals currently receiving or expected to start ART.30
Recently WHO and the Joint United Nations Programme on HIV/AIDS, as part of the Treatment 2.0 initiative,39 prioritized identifying, retaining and caring for people earlier in the course of HIV infection as a way of improving clinical and programmatic outcomes. Indeed, implementing ART earlier within existing guidelines has had a considerable impact: in South Africa, mortality during the first year of ART declined from 8.9 to 5.6% as the median CD4 cell count at ART initiation increased from 68 to 113 cells/µL.40 One way to facilitate earlier access to ART is through expanding HIV testing coverage. For generalized epidemics (i.e. when the prevalence of antenatal HIV infection is over 1%), WHO recommends carrying out provider-initiated HIV testing in all health facilities,41 particularly in patients with tuberculosis.42 Recent evidence suggests that community-based testing can identify individuals earlier in the course of HIV infection43 and increases knowledge of HIV status fourfold relative to health-facility-based testing.44 Reductions in mortality, costs and the rate of new infections will also depend crucially on maintaining good links with personnel monitoring and caring for individuals before they receive ART, on maximizing the retention rate and on initiating ART as early as permitted by national guidelines. These actions will help achieve the Millennium Development Goals on HIV infection.45 Unfortunately, at present over 50% of HIV-infected individuals are lost to care between diagnosis and the start of ART.46–49 Giving free cotrimoxazole can help increase the retention rate,50 provide an opportunity to assess an individual’s adherence to treatment before the start of ART51 and improve survival in those not on ART.52 Efforts to reduce early mortality in HIV-infected individuals and to increase retention rates in treatment programmes could be assisted by implementing intensified tuberculosis case-finding at every health-care visit and by providing free isoniazid to those with tuberculosis who do not have a cough, night sweats, weight loss or fever.53
For settings in which the health-care infrastructure is limited, WHO recommends discontinuing cotrimoxazole in adults who show good adherence to ART, have secure access to treatment and who have not had a new WHO stage 2, 3 or 4 disease event for at least 1 year.17 Data from the one study that reported the estimated effect of cotrimoxazole on mortality after 1 year of combined treatment with ART indicate that there was no reduction in mortality after 72 weeks of ART.28 For settings where the health-care infrastructure is good, WHO recommends discontinuing cotrimoxazole in adults who have received ART for at least 6 months and have a CD4 cell count greater than 350 cells/µL.17 The single study that provided an estimate of the effect of cotrimoxazole on mortality in adults on ART with a baseline CD4 cell count greater than 350 cells/µL suggested that cotrimoxazole had no effect.29
Two limitations of this systematic review were that most studies included had a short follow-up and that they did not include estimates of the effect of cotrimoxazole in adults with a high baseline CD4 cell count. Furthermore, only one randomized trial was identified. Although most studies did attempt to control for bias, prospective and retrospective cohort studies are susceptible to unmeasured confounding. Also, none of the studies assessed adherence to cotrimoxazole and ART. Finally, since the cause of the death was not reported in any study, the precise mechanism of cotrimoxazole’s beneficial survival effect is not clear.
Although the data considered in this review were limited and results from an on-going trial are still awaited, our findings support current WHO recommendations that the use of cotrimoxazole should be scaled up in HIV-infected individuals starting or receiving ART. Further research is needed to determine the optimum duration of cotrimoxazole treatment in adults receiving ART for an HIV infection.
Acknowledgements
Amitabh B Suthar is also affiliated with the Department of HIV/AIDS of the World Health Organization.
Competing interests:
None declared.
References
- 1.UNAIDS report on the global AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2011. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/JC2216_WorldAIDSday_report_2011_en.pdf [accessed 8 December 2011].
- 2.Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach, 2010 revision. Geneva: World Health Organization Department of HIV/AIDS; 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. [accessed 15 September 2011]. [PubMed]
- 3.AIDS at 30: nations at the crossroads Geneva: Joint United Nations Programme on HIV/AIDS; 2011. Available from: http://www.unaids.org/unaids_resources/aidsat30/aids-at-30.pdf. [accessed 15 September 2011].
- 4.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 5.Keiser O, Orrell C, Egger M, Wood R, Brinkhof MWG, Furrer H, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med. 2008;5:e148. doi: 10.1371/journal.pmed.0050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuboi SH, Schechter M, McGowan CC, Cesar C, Krolewiecki A, Cahn P, et al. Mortality during the first year of potent antiretroviral therapy in HIV-1-infected patients in 7 sites throughout Latin America and the Caribbean. J Acquir Immune Defic Syndr. 2009;51:615–23. doi: 10.1097/QAI.0b013e3181a44f0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chasombat S, McConnell MS, Siangphoe U, Yuktanont P, Jirawattanapisal T, Fox K, et al. National expansion of antiretroviral treatment in Thailand, 2000-2007: program scale-up and patient outcomes. J Acquir Immune Defic Syndr. 2009;50:506–12. doi: 10.1097/QAI.0b013e3181967602. [DOI] [PubMed] [Google Scholar]
- 9.Ferradini L, Laureillard D, Prak N, Ngeth C, Fernandez M, Pinoges L, et al. Positive outcomes of HAART at 24 months in HIV-infected patients in Cambodia. AIDS. 2007;21:2293–301. doi: 10.1097/QAD.0b013e32828cc8b7. [DOI] [PubMed] [Google Scholar]
- 10.International drug price indicator guide, 2009 edition Arlington: Management Sciences for Health; 2009. Available from: http://erc.msh.org/dmpguide/pdf/DrugPriceGuide_2009_en.pdf [accessed 15 September 2011].
- 11.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 12.Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d'Ivoire: a randomised trial. Lancet. 1999;353:1463–8. doi: 10.1016/S0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 13.Nunn AJ, Mwaba P, Chintu C, Mwinga A, Darbyshire JH, Zumla A. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ. 2008;337:a257. doi: 10.1136/bmj.a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiktor SZ, Sassan-Morokro M, Grant AD, Abouya L, Karon JM, Maurice C, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d'Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–75. doi: 10.1016/S0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 15.Maynart M, Lievre L, Sow PS, Kony S, Gueye NF, Bassène E, et al. Primary prevention with cotrimoxazole for HIV-1-infected adults: results of the pilot study in Dakar, Senegal. J Acquir Immune Defic Syndr. 2001;26:130–6. doi: 10.1097/00042560-200102010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Mermin J, Were W, Ekwaru JP, Moore D, Downing R, Behumbisze P, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet. 2008;371:752–9. doi: 10.1016/S0140-6736(08)60345-1. [DOI] [PubMed] [Google Scholar]
- 17.Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults: recommendations for a public health approach Geneva: World Health Organization Department of HIV/AIDS; 2006. Available from: http://www.who.int/entity/hiv/pub/guidelines/ctxguidelines.pdf [accessed 15 September 2011].
- 18.Castelnuovo B, Manabe YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis. 2009;49:965–72. doi: 10.1086/605500. [DOI] [PubMed] [Google Scholar]
- 19.Kumarasamy N, Venkatesh KK, Devaleenol B, Poongulali S, Zephthomi T, Pradeep A, et al. Factors associated with mortality among HIV-infected patients in the era of highly active antiretroviral therapy in southern India. Int J Infect Dis. 2010;14:e127–31. doi: 10.1016/j.ijid.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Severe P, Leger P, Charles M, Noel F, Bonhomme G, Bois G, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353:2325–34. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochrane handbook for systematic reviews of interventions, version 5.1.0 [updated March 2011]. London: The Cochrane Collaboration; 2011. Available from: http://www.cochrane-handbook.org/ [accessed 15 September 2011].
- 23.The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Ottawa: Ottawa Hospital Research Institute; 2010. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 15 September 2011].
- 24.Cooper H, Hedges L. The handbook of research synthesis New York: Russell Sage Foundation; 1994. [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3:486–504. doi: 10.1037/1082-989X.3.4.486. [DOI] [Google Scholar]
- 28.Walker AS, Ford D, Gilks CF, Munderi P, Ssali F, Reid A, et al. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet. 2010;375:1278–86. doi: 10.1016/S0140-6736(10)60057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann CJ, Fielding KL, Charalambous S, Innes C, Chaisson RE, Grant AD, et al. Reducing mortality with cotrimoxazole preventive therapy at initiation of antiretroviral therapy in South Africa. AIDS. 2010;24:1709–16. doi: 10.1097/QAD.0b013e32833ac6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowrance D, Makombe S, Harries A, Yu J, Aberle-Grasse J, Eiger O, et al. Lower early mortality rates among patients receiving antiretroviral treatment at clinics offering cotrimoxazole prophylaxis in Malawi. J Acquir Immune Defic Syndr. 2007;46:56–61. [PubMed] [Google Scholar]
- 31.Madec Y, Laureillard D, Pinoges L, Fernandez M, Prak N, Ngeth C, et al. Response to highly active antiretroviral therapy among severely immuno-compromised HIV-infected patients in Cambodia. AIDS. 2007;21:351–9. doi: 10.1097/QAD.0b013e328012c54f. [DOI] [PubMed] [Google Scholar]
- 32.Fairall LR, Bachmann MO, Louwagie GM, van Vuuren C, Chikobvu P, Steyn D, et al. Effectiveness of antiretroviral treatment in a South African program: a cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 33.van Oosterhout JJ, Ndekha M, Moore E, Kumwenda JJ, Zijlstra EE, Manary M. The benefit of supplementary feeding for wasted Malawian adults initiating ART. AIDS Care. 2010;22:737–42. doi: 10.1080/09540120903373581. [DOI] [PubMed] [Google Scholar]
- 34.Alemu AW, Sebastián MS. Determinants of survival in adult HIV patients on antiretroviral therapy in Oromiyaa, Ethiopia. Glob Health Action. 2010;3 doi: 10.3402/gha.v3i0.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miiro G, Todd J, Mpendo J, Watera C, Munderi P, Nakubulwa S, et al. Reduced morbidity and mortality in the first year after initiating highly active anti-retroviral therapy (HAART) among Ugandan adults. Trop Med Int Health. 2009;14:556–63. doi: 10.1111/j.1365-3156.2009.02259.x. [DOI] [PubMed] [Google Scholar]
- 36.Campbell J, Moore D, Degerman R, et al. HIV-infected Ugandans on HAART with CD4-cell counts > 200 cells/mm3 who discontinue cotrimoxazole have increased risk of malaria and diarrhea. Proceedings of the: 16th Conference on Retroviruses and Opportunistic Infections; 8–11 February2009,Montreal, Canada [Google Scholar]
- 37.Safety of discontinuing co-trimoxazole prophylaxis among Ugandan adults on antiretroviral therapy (ART) London: Current Controlled Trials Limited; 2011. Available from: http://www.controlled-trials.com/ISRCTN44723643/ [accessed 15 September 2011].
- 38.Date AA, Vitoria M, Granich R, Banda M, Fox MY, Gilks C. Implementation of co-trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull World Health Organ. 2010;88:253–9. doi: 10.2471/BLT.09.066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirnschall G, Schwartlander B. Treatment 2.0: catalysing the next phase of scale-up. Lancet. 2011;378:209–11. doi: 10.1016/S0140-6736(11)60247-X. [DOI] [PubMed] [Google Scholar]
- 40.Cornell M, Grimsrud A, Fairall L, Fox MP, van Cutsem G, Giddy J, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002-2007. AIDS. 2010;24:2263–70. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guidance on provider-initiated HIV testing and counselling in health facilities Geneva: World Health Organization; 2007. Available from: http://whqlibdoc.who.int/publications/2007/9789241595568_eng.pdf [accessed 15 September 2011].
- 42.Interim policy on collaborative TB/HIV activities Geneva: World Health Organization Stop TB Department and Department of HIV/AIDS; 2004. Available from: http://whqlibdoc.who.int/hq/2004/who_htm_tb_2004.330.pdf [accessed 15 September 2011].
- 43.Lugada E, Millar D, Haskew J, Grabowsky M, Garg N, Vestergaard M, et al. Rapid implementation of an integrated large-scale HIV counseling and testing, malaria, and diarrhea prevention campaign in rural Kenya. PLoS ONE. 2010;5:e12435. doi: 10.1371/journal.pone.0012435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sweat M, Morin S, Celentano D, Mulawa M, Singh B, Mbwambo J, et al. Community-based intervention to increase HIV testing and case detection in people aged 16-32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis. 2011;11:525–32. doi: 10.1016/S1473-3099(11)70060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The Millennium Development Goals report 2010 New York: United Nations; 2010. Available from: http://www.un.org/millenniumgoals/pdf/MDG%20Report%202010%20En%20r15%20-low%20res%2020100615%20-.pdf [accessed 15 September 2011].
- 46.Ingle SM, May M, Uebel K, Timmerman V, Kotze E, Bachmann M, et al. Outcomes in patients waiting for antiretroviral treatment in the Free State Province, South Africa: prospective linkage study. AIDS. 2010;24:2717–25. doi: 10.1097/QAD.0b013e32833fb71f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kranzer K, Zeinecker J, Ginsberg P, Orrell C, Kalawe NN, Lawn SD, et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLoS ONE. 2010;5:e13801. doi: 10.1371/journal.pone.0013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tayler-Smith K, Zachariah R, Massaquoi M, Manzi M, Pasulani O, Van Den Akker T, et al. Unacceptable attrition among WHO stages 1 and 2 patients in a hospital-based setting in rural Malawi: can we retain such patients within the general health system? Trans R Soc Trop Med Hyg. 2010;104:313–9. doi: 10.1016/j.trstmh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Rosen S, Fox MP. Retention in HIV care between testing and treatment in Sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohler PK, Chung MH, McGrath CJ, Benki-Nugent SF, Thiga JW, John-Stewart GC. Implementation of free cotrimoxazole prophylaxis improves clinic retention among ART-ineligible clients in Kenya. AIDS. 2011;25:1657–61. doi: 10.1097/QAD.0b013e32834957fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carlucci JG, Kamanga A, Sheneberger R, Shepherd BE, Jenkins CA, Spurrier J, et al. Predictors of adherence to antiretroviral therapy in rural Zambia. J Acquir Immune Defic Syndr. 2008;47:615–22. doi: 10.1097/QAI.0b013e318165dc25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grimwade K, Swingler G. Cotrimoxazole prophylaxis for opportunistic infections in adults with HIV. Cochrane Database Syst Rev. 2003;3:CD003108. doi: 10.1002/14651858.CD003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings Geneva: World Health Organization, Department of HIV/AIDS & Stop TB Department; 2011. Available from: http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf [accessed 15 September 2011].