Summary
A genome-wide association study of serum uric acid levels was performed in a relatively isolated population of European descent from an island of the Adriatic coast of Croatia. The study sample included 532 unrelated and 768 related individuals from 235 pedigrees. Inflation due to relatedness was controlled by using genomic control. Genetic association was assessed with 2,241,249 SNPs in 1300 samples after adjusting for age and gender. Our study replicated four previously reported serum uric acid loci (SLC2A9, ABCG2, RREB1, and SLC22A12). The strongest association was found with a SNP in SLC2A9 (rs13129697, P=2.33×10−19), which exhibited significant gender-specific effects, 35.76μmol/L (P=2.11×10−19) in females and 19.58 μmol/L (P=5.40×10−5) in males. Within this region of high linkage disequilibrium, we also detected a strong association with a non-synonymous SNP, rs16890979 (P=2.24×10−17), a putative causal variant for serum uric acid variation. In addition, we identified several novel loci suggestive of association with uric acid levels (SEMA5A, TMEM18, SLC28A2, and ODZ2), although the P-values (P<5×10−6) did not reach the threshold of genome-wide significance. Together, these findings provide further confirmation of previously reported uric acid-related genetic variants and highlight suggestive new loci for additional investigation.
Keywords: Serum uric acid, genome-wide association, Adriatic island population
Introduction
Genome-wide association studies (GWAS) have uncovered over 30 common sequence variants influencing serum uric acid (SUA) concentration and gout (Hindorff et al. 2011). Among these, the most significant findings are the single nucleotide polymorphisms (SNPs) located within the solute carrier family 2 member 9 (SLC2A9) gene on chromosome 4, which have been consistently replicated across multiple populations (Li et al. 2007; Dehghan et al. 2008; Doring et al. 2008; McArdle et al. 2008; Vitart et al. 2008; Wallace et al. 2008; Kolz et al. 2009; Yang et al. 2010; Charles et al. 2011). Additional GWAS and meta-analyses have identified variants in several other genes including PDZK1, GCKR, ABCG2, RREB1, LRRC16A, SLC17A1, SLC17A3, SLC22A11, SLC22A12 that have reached genome-wide significance levels (Dehgan et al. 2008; Kolz et al. 2009; Yang et al. 2010). We conducted a GWAS of metabolic traits, including SUA concentration, in a relatively isolated population from the Adriatic coast of Croatia. This study strongly replicated the SLC2A9 findings and identified several suggestive novel loci that may represent genuine effects. In addition, our study also replicated associations of SNPs in ABCG2, RREB1 and SLC22A12, although the signals did not reach genome-wide significance.
Materials and Methods
Subjects
The study population has been described previously (Zhang et al. 2010; Karns et al. 2011). Briefly, participants were derived from the middle Dalmatian island of Hvar on the eastern Adriatic coast of Croatia. The population is primarily of Slavic descent, which had emigrated from the mainland before the 18th century and remained relatively isolated since that time (Rudan et al. 1992). Phenotypic measures and blood samples were collected in two field surveys conducted in May 2007 and May 2008, with no consideration of disease status or medication. Blood samples were collected following an overnight fast, and SUA levels were measured using the enzymatic color method. In total, 1,395 related and unrelated subjects aged >20 years with SUA measures were included in the current study. Descriptive statistics of quantitative traits (age, body mass index, fasting plasma glucose, blood pressure) and prevalence of four metabolic disorders (type 2 diabetes, hypertension, gout and metabolic syndrome) are provided in the Supplementary Table. Data on type 2 diabetes, gout and hypertension were collected through self-reports, medical review and clinical diagnostic measures. The study was approved by the Ethics Committee of the Institute for Anthropological Research in Zagreb, Croatia and the Institutional Review Board of the University of Cincinnati.
Genotyping
Genome-wide SNP genotyping was performed using the Affymetrix Human SNP Array 5.0 following the manufacture's protocol. Genotype calls were determined using the CRLMM algorithm (Carvalho et al. 2007, 2010) among chips that passed the prescribed Dynamic Model genotyping QC call rate (> 0.86). Following further QC filtering of the genotype data (MAF >0.02, HWE P >0.0001, call rate >95%) using the check.marker function implemented in GenABEL (Aulchenko et al. 2007), we obtained a cleaned data set of 344,512 SNPs in 1300 samples (563 males and 737 females). From this cleaned data set, we performed genotype imputation using MACH (Li et al. 2009) and the reference haplotype data from the Phase II CEU HapMap (International HapMap Consortium 2007). The same QC procedures were performed on the imputed data, yielding a final genotype data set of 2,241,249 SNPs in 1300 samples.
Statistical Analysis
All statistical analyses were performed in R v2.11; genome-wide association analysis was performed using the GenABEL package (v.1.6). Single-locus tests adjusted for age and sex were conducted using the qtscore routine. Since our samples included 532 unrelated as well as 768 related individuals from 235 families, genomic control (GC) was applied to correct for inflation due to inclusion of related individuals (Devlin & Roeder 1999; Devlin et al. 2001). The inflation factor (λ) was estimated using the median method (Bacanu et al. 2002), and P-values based on the adjusted test statistics (1 d.f. assuming additive effects) were reported. Association signals of significant regions were plotted using LocusZoom (Pruim et al. 2010).
Results
SUA levels were normally distributed in both males (N=563) and females (N=737) and the mean levels were significant higher in males (361.0±79.19 μmol/L) than females (265.4±77.65 μmol/L), though Bartlett's and Fligner's tests revealed no significant gender-based differences in SUA variance. Regression analysis indicated that SUA levels were significantly associated with age in both genders and the association was more significant in females. SUA change per year in females was 1.797 μmol/L (P=2.2×10−24, r2=13.1%) and in males was 0.786 μmol/L (P=0.00019, r2=2.28%) (Supplementary Figure S1).
As anticipated from the relatedness among the samples, the test statistics were inflated compared to the null distribution with an estimated inflation factor λ=1.20. As shown in the quantile-quantile (QQ) plot (Supplementary Figure S2), after adjustment for this inflation factor and exclusion of significant SNPs in the SLC2A9 region the test statistics fit well with the expected values, indicating appropriate control of false positive rate.
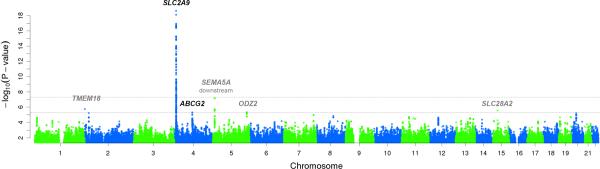
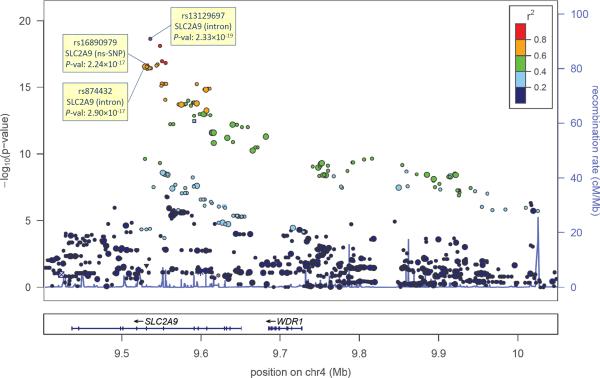
A Manhattan plot of the genome-wide association signals (Figure 1) shows the strongest association around the SLC2A9 gene, a well-established uric acid-associated gene. The significant region spans roughly 650kb and covers the SLC2A9 and the WDR1 genes (Figure 2). This region is delimited by two recombination hot spots with local recombination rate >25cM/Mb. One-hundred and sixteen SNPs with P-value less than the genome-wide significant level (5×10−8) were identified within this region. The strongest signal was found on the imputed SNP rs13129697 (P=2.33×10−19); the minor allele was associated with an average SUA decrease of 28.99μmol/L. Consistent with previous studies, the effect size estimate showed substantial gender difference with 35.76μmol/L (P=2.11×10−19) in females and 19.58 μmol/L (P=5.40×10−5) in males. This pattern was similar across all of the 116 SNPs that reached genome-wide significance. Of interest is a non-synonymous SNP, rs16890979 (P=2.24×10−17), also reported previously with genome-wide significance (Dehghan et al. 2008; McArdle et al. 2008). Re-analysis of the region, conditional on either rs13129697 or rs16890979 failed to completely abolish the signals of the other SNPs with the smallest conditional P~1.7×10−3 (data not shown) suggesting the possibility of multiple functional variants within the region.
Figure 1.
Manhattan plot of GWA single-locus P-values. The two horizontal dash lines indicate significant thresholds at 5×10−8 and 5×10−6. Six regions that reach suggestive genome-wide significance (P<5×10−6) are highlighted with names of nearby genes. Gene names in black are previously reported uric acid associated genes.
Figure 2.
LocusZoom plot of the SLC2A9 region. GC adjusted single-locus P-values are plotted against SNP physical positions (NCBI build 36). Pairwise linkage disequilibrium (r2) from the most significant SNP (rs13129697) is color-coded. Size of each dot indicates whether the SNP is a genotyped (large) or imputed (small). The light blue curve shows the local recombination rate based on HapMap Phase II data. rs16890979 is the non-synonymous SNP and rs874432 is the most significant genotyped SNP.
In addition to the well-established SLC2A9 region, five other potentially significant regions (Table 1) were identified with at least one SNP above a threshold of P<5×10−6. The most salient of these is a suggestive novel locus in an intergenic region on chromosome 5; the most significant variant, rs200113 (P=7.02×10−8), is located ~400 kb downstream of the SEMA5A gene. The remaining four regions are located within or near the genes TMEM18, SLC28A2, ODZ2 and ABCG2, respectively. ABCG2 is a confirmed uric acid associated gene (Dehghan et al. 2008; Kolz et al. 2009; Yang et al. 2010) and the variant showing the highest signal (P=5.14×10−6) is a non-synonymous SNP (rs2231142, NP_004818.2, Gln141Lys) and the mutant allele associated with an average increase of 27.40 μmol/L (Supplementary Figure S3). This SNP showed significant gender-specific effects, with 31.11μmol/L in males compared to 22.97μmol/L in females. Reanalysis conditional on this SNP explained all the association across the region, which is highly suggestive of this missense variant being the functional SNP at the ABCG2 locus.
Table 1.
Summary of the significant SNPs and their regions
| Significant Region | Most significant (index) SNP of the region | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chr | Start | End | Gene(s) | rs ID | Position | A1 | A2 | MAF | CEU MAF | P-value | Eff-All | Eff-M | Eff-F |
| 4 | 9401384 | 10050458 | SLC2A9, WDR1 | rs13129697 | 9536065 | T | G | 0.36 | 0.29 | 2.33×10−19 | −28.99 | −19.58 | −35.76 |
| 5 | 8654931 | 8780372 | SEMA5A (downstream) | rs200113 | 8705870 | T | C | 0.1 | 0.17 | 7.02×10−08 | 28.59 | 33.34 | 23.59 |
| 2 | 628483 | 730659 | TMEM18 | rs12999373 | 653483 | G | A | 0.27 | 0.22 | 1.79×10−06 | −17.65 | −11.67 | −21.26 |
| 15 | 43262339 | 43621220 | SLC28A2 | rs765787 | 43287339 | A | G | 0.15 | 0.16 | 2.81×10−06 | 20.9 | 26.93 | 14.45 |
| 5 | 1.67E+08 | 1.67E+08 | ODZ2 | rs13358864 | 1.67E+08 | T | A | 0.1 | 0.13 | 4.68×10−06 | −24.09 | −24.25 | −22.8 |
| 4 | 89151747 | 89313430 | ABCG2 | rs2231142 | 89271347 | G | T | 0.08 | 0.11 | 5.14×10−06 | 27.4 | 31.11 | 22.97 |
Note: Position is based on NCBI Genome Build 36; A1 and A2 are the major and minor alleles respectively; Eff-All, Eff-M, Eff-F are effect sizes (μmol/L) of the minor alleles in total, male and female samples. A significant region was selected starting with an “index” SNP (P < 5×10−6) and was progressively extended to adjacent (<50kb) significant SNPs (P < 5×10−3). The ABCG2 region was included in the table although the P-value of the index SNP (rs2231142, 5.14×10−06), a non-synonymous variant, did not strictly reach the standard threshold (P < 5×10−6).
To compare our findings with previous GWA studies, we analyzed 30 serum urate- or uric acid-associated SNPs listed in the GWA catalog (six of the 36 reported SNPs were missing from our imputed and cleaned data set) (Table 2). In addition to the aforementioned SLC2A9 and ABCG2 loci, we replicated associations with nominal significance (P<0.05) at two additional loci, RREB1 (rs675209, P=0.0032) and SLC22A12 (rs17300741, P=0.0034). The effects of all significant SNPs were in the same direction as those reported in previous GWA studies.
Table 2.
Genome-wide replication of previously reported GWA SNPs
| SNP | Chr | Position | A1 | A2 | MAF | P-value | Eff | Gene |
|---|---|---|---|---|---|---|---|---|
| rs1967017 | 1 | 144435002 | C | T | 0.48 | 0.451555 | 2.46 | PDZK1 |
| rs12129861 | 1 | 144437046 | A | G | 0.49 | 0.405643 | 2.75 | |
| rs780094 | 2 | 27594741 | C | T | 0.49 | 0.492119 | −2.27 | GCKR |
| rs780093 | 2 | 27596107 | C | T | 0.49 | 0.492119 | −2.27 | |
| rs6442522 | 3 | 15415560 | C | T | 0.47 | 0.462695 | −2.38 | NR |
| rs16890979 | 4 | 9531265 | C | T | 0.32 | 2.24E-17 | −28.29 | SLC2A9, WDR1 |
| rs734553 | 4 | 9532102 | T | G | 0.31 | 4.10E-17 | −28.21 | |
| rs13129697 | 4 | 9536065 | T | G | 0.36 | 2.33E-19 | −28.99 | |
| rs737267 | 4 | 9543842 | G | T | 0.32 | 2.42E-17 | −28.21 | |
| rs6855911 | 4 | 9545008 | A | G | 0.32 | 1.95E-17 | −28.25 | |
| rs7442295 | 4 | 9575478 | A | G | 0.28 | 1.98E-14 | −27.05 | |
| rs3775948 | 4 | 9604280 | C | G | 0.30 | 1.46E-15 | −27.48 | |
| rs717615 | 4 | 9713768 | A | G | 0.44 | 0.003137 | −9.57 | |
| rs2199936 | 4 | 89264355 | G | A | 0.08 | 9.68E-06 | 26.87 | ABCG2 |
| rs2231142 | 4 | 89271347 | G | T | 0.08 | 5.14E-06 | 27.40 | |
| rs3776331 | 5 | 142421987 | C | T | 0.29 | 0.699763 | −1.35 | NR |
| rs675209 | 6 | 7047083 | C | T | 0.20 | 0.003237 | 11.88 | RREB1 |
| rs742132 | 6 | 25715550 | A | G | 0.32 | 0.837566 | 0.71 | LRRC16A SLC17A3 |
| rs1165196 | 6 | 25921129 | A | G | 0.46 | 0.154939 | −4.55 | |
| rs1183201 | 6 | 25931423 | T | A | 0.47 | 0.138613 | −4.73 | |
| rs1165205 | 6 | 25978521 | A | T | 0.47 | 0.136142 | −4.74 | |
| rs9478751 | 6 | 157482742 | A | G | 0.08 | 0.6207 | −3.17 | ARID1B |
| rs2244967 | 10 | 49894772 | T | C | 0.22 | 0.895854 | 0.50 | C10orf72 |
| rs17300741 | 11 | 64088038 | A | G | 0.48 | 0.420491 | −2.59 | SLC22A12 |
| rs2078267 | 11 | 64090690 | C | T | 0.49 | 0.490532 | −2.22 | |
| rs505802 | 11 | 64113648 | T | C | 0.34 | 0.003435 | 10.03 | |
| rs1106766 | 12 | 56095723 | C | T | 0.14 | 0.082384 | −8.31 | R3HDM2, INHBC |
| rs4771450 | 13 | 102767492 | A | G | 0.32 | 0.50946 | 2.22 | NR |
| rs6085920 | 20 | 7128056 | A | T | 0.10 | 0.701849 | 2.10 | NR |
| rs8139900 | 22 | 24724724 | A | G | 0.46 | 0.959965 | −0.16 | MYO18B |
Note: Replicated regions reaching nominal significance are shown in bold
Discussion
We present the results of a genome-wide association study of SUA in an isolated island population based on 2,241,249 imputed and genotyped SNPs in 1300 samples. Our purpose was to replicate previously reported loci and uncover novel SUA-related loci, taking advantage of population attributes of limited admixture and homogeneous environmental exposures. We have used GC to provide correction for inflation due to relatedness while maximizing power to detect associations by including all samples. Previous study indicated that GC is a valid and powerful method for the analysis of pedigree based quantitative trait loci (Amin et al. 2007).
The most significant associations emerged from multiple SNPs in and around SLC2A9 on chromosome 4, a widely replicated SUA-associated region. The SNP with the strongest signal, rs13129697, is located in intron 7 of the gene. Of particular interest, however, was the association of rs16890979 (Val253Ile), a non-synonymous imputed SNP that has been reported in previous GWAS (Dehghan et al. 2008; McArdle et al. 2008).
We performed a comparative analysis of previously reported per-allele effect sizes of the significant SNPs in SLC2A9 and found that, in general, our effect sizes are somewhat higher than those reported in previous GWAS (Dehghan et al. 2008; Doring et al. 2008; McArdle et al. 2008; Kolz et al. 2009; Yang et al. 2010; Zemunik et al. 2009) (Supplementary Figure S4). Across studies, SLC2A9 variant effect sizes in females are markedly elevated compared to males. In our population we found males had significantly higher mean SUA concentrations, though female SUA concentration was more strongly associated with age. Sex-specific effects of SLC2A9 variants were more extensively examined by Doring et al. (2008), who showed that in addition to genotypic effects, SLC2A9 expression levels were stronger in females. Together these observations suggest that the SLC2A9 variants may play a more significant role influencing uric acid concentrations in females which could be due to physiological and vascular differences between males and females and due to decreased uricosuric-related estrogen action following menopause (Adamopoulos et al. 1977; Puig et al. 1991). In addition, they suggest a potential gene-environment interaction that may be related to the gender-specific effects of the SLC2A9 variants.
In addition to reconfirming the SLC2A9 locus, we provide replications for three previously reported GWAS loci (ABCG2, RREB1, and SLC22A12), though the P-values do not reach strict GWAS significance. We report significant gender-specific effects of a non-synonymous variant in ABCG2, similar to those previously reported by Kolz et al. (2009). In addition to the replicated regions, we observed suggestive association signals (P<5×10−6) at several novel loci. The most significant was a SNP (rs200116) located downstream of SEMA5A, which encodes the semaphorin-5A protein. SNPs in its vicinity were significantly associated with Parkinson's disease and autism in separate GWAS (Maraganore et al. 2005; Weiss et al. 2009). While elevated uric acid is correlated with lower risk of developing Parkinson's disease (Davis et al. 1996; de Lau et al. 2005; Weisskopf et al. 2007; Alonso et al 2007), apart from a hyperuricosuric subtype of autism (Page et al. 2000) no link between autism and uric acid has been reported.
In summary, our study replicated four previously reported SUA associated loci (SLC2A9, ABCG2, RREB1 and SLC22A12) with different levels of significance, and detected suggestive associations at several novel loci (SEMA5A, TMEM18, SLC28A2, ODZ2) that did not reach the threshold of genome-wide significance. However, due to the moderate sample size and the lack of a replication cohort the observed associations at these novel loci are preliminary and require further exploration and confirmation in other populations.
Supplementary Material
Acknowledgements
The study was supported by grants from the National Institutes of Health, USA (R01 DK069845 and P30 ES006096) and from the Croatian Ministry of Science, Education and Sports (196-1962766-2751, 196-1962766-2747 and 196-0342282-0291). RK was supported by a training grant fellowship from the National Institutes of Environmental Health Sciences, USA (T32 ES010957).
Footnotes
Authors' Contributions The study was conceived and designed by RD, PR and RC. Recruitment of subjects, data and sample collection, data cleaning was conducted by DH-A, NN, DR, SM, ZD and PR. Genotyping was performed by GS, SRI and HC. Statistical analysis was conducted by GZ and RK. The draft manuscript was prepared by RK, GZ, PR, RC and RD. RK and GZ have contributed equally. All authors read and approved the manuscript.
Conflict of Interest Statement The authors declare no conflict of interest.
Supplementary information is available at the AHG's website.
References
- Adamopoulos D, Vlassopoulos C, Seitanides B, Contoyiannis P, Vassilopoulos P. The relationship of sex steroids to uric acid levels in plasma and urine. Acta Endocrinol (Copenh) 1977;85:198–208. doi: 10.1530/acta.0.0850198. [DOI] [PubMed] [Google Scholar]
- Alonso A, Rodriguez LA, Logroscino G, Hernan MA. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007;69:1696–1700. doi: 10.1212/01.wnl.0000279518.10072.df. [DOI] [PubMed] [Google Scholar]
- Amin N, van Duijn CM, Aulchenko YS. A genomic background based method for association analysis in related individuals. PLoS One. 2007;2:e1274. doi: 10.1371/journal.pone.0001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23:1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- Bacanu SA, Devlin B, Roeder K. Association studies for quantitative traits in structured populations. Genet Epidemiol. 2002;22:78–93. doi: 10.1002/gepi.1045. [DOI] [PubMed] [Google Scholar]
- Carvalho BS, Louis TA, Irizarry RA. Quantifying uncertainty in genotype calls. Bioinformatics. 2010;26:242–249. doi: 10.1093/bioinformatics/btp624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho B, Bengtsson H, Speed TP, Irizarry RA. Exploration, normalization, and genotype calls of high-density oligonucleotide SNP array data. Biostatistics. 2007;8:485–499. doi: 10.1093/biostatistics/kxl042. [DOI] [PubMed] [Google Scholar]
- Charles BA, Shriner D, Doumatey A, Chen G, Zhou J, Huang H, Herbert A, Gerry NP, Christman MF, Adeyemo A, Rotimi CN. A genome-wide association study of serum uric acid in African Americans. BMC Med Genomics. 2011;4:17. doi: 10.1186/1755-8794-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid levels and the risk of idiopathic Parkinson's disease. Am J Epidemiol. 1996;144:480–484. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- Dehghan A, Köttgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, Boerwinkle E, Levy D, Hofman A, Astor BC, Benjamin EJ, van Duijn CM, Witteman JC, Coresh J, Fox CS. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–1961. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- Devlin B, Roeder K, Bacanu SA. Unbiased methods for population-based association studies. Genet Epidemiol. 2001;21:273–284. doi: 10.1002/gepi.1034. [DOI] [PubMed] [Google Scholar]
- Döring A, Gieger C, Mehta D, Gohlke H, Prokisch H, Coassin S, Fischer G, Henke K, Klopp N, Kronenberg F, Paulweber B, Pfeufer A, Rosskopf D, Völzke H, Illig T, Meitinger T, Wichmann HE, Meisinger C. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet. 2008;40:430–436. doi: 10.1038/ng.107. [DOI] [PubMed] [Google Scholar]
- Hindorff LA, Junkins HA, Hall PN, Mehta JP, Manolio TA. A Catalog of Published Genome-Wide Association Studies. 2011 www.genome.gov/gwastudies.
- International HapMap Consortium A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karns R, Zhang G, Jeran N, Havas-Augustin D, Missoni S, Niu W, Indugula SR, Sun G, Durakovic Z, Narancic NS, Rudan P, Chakraborty R, Deka R. Replication of genetic variants from genome-wide association studies with metabolic traits in an island population of the Adriatic coast of Croatia. Eur J Hum Genet. 2011;19:341–346. doi: 10.1038/ejhg.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, Mangino M, Albrecht E, Wallace C, Farrall M, Johansson A, Nyholt DR, Aulchenko Y, Beckmann JS, Bergmann S, Bochud M, Brown M, Campbell H, EUROSPAN Consortium. Connell J, Dominiczak A, Homuth G, Lamina C, McCarthy MI, ENGAGE Consortium. Meitinger T, Mooser V, Munroe P, Nauck M, Peden J, Prokisch H, Salo P, Salomaa V, Samani NJ, Schlessinger D, Uda M, Völker U, Waeber G, Waterworth D, Wang-Sattler R, Wright AF, Adamski J, Whitfield JB, Gyllensten U, Wilson JF, Rudan I, Pramstaller P, Watkins H, PROCARDIS Consortium. Doering A, Wichmann HE, KORA Study. Spector TD, Peltonen L, Völzke H, Nagaraja R, Vollenweider P, Caulfield M, WTCCC. Illig T, Gieger C. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Sanna S, Maschio A, Busonero F, Usala G, Mulas A, Lai S, Dei M, Orrù M, Albai G, Bandinelli S, Schlessinger D, Lakatta E, Scuteri A, Najjar SS, Guralnik J, Naitza S, Crisponi L, Cao A, Abecasis G, Ferrucci L, Uda M, Chen WM, Nagaraja R. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 2007;3:e194. doi: 10.1371/journal.pgen.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annu Rev Genomics Hum Genet. 2009;10:387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraganore DM, de Andrade M, Lesnick TG, Strain KJ, Farrer MJ, Rocca WA, Pant PV, Frazer KA, Cox DR, Ballinger DG. High-resolution whole-genome association study of Parkinson disease. Am J Hum Genet. 2005;77:685–693. doi: 10.1086/496902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle PF, Parsa A, Chang YP, Weir MR, O'Connell JR, Mitchell BD, Shuldiner AR. Association of a common nonsynonymous variant in GLUT9 with serum uric acid levels in old order Amish. Arthritis Rheum. 2008;58:2874–2881. doi: 10.1002/art.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page T, Coleman M. Purine metabolism abnormalities in a hyperuricosuric subclass of autism. Biochim Biophys Acta. 2000;1500:291–296. doi: 10.1016/s0925-4439(99)00113-1. [DOI] [PubMed] [Google Scholar]
- Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig JG, Michán AD, Jiménez ML, Pérez de Ayala C, Mateos FA, Capitán CF, de Miguel E, Gijón JB. Female gout. Clinical spectrum and uric acid metabolism. Arch Intern Med. 1991;151:726–732. doi: 10.1001/archinte.151.4.726. [DOI] [PubMed] [Google Scholar]
- Rudan P, Sujoldzic A, Simic D, Bennett LA, Roberts DF. Population structure in the eastern Adriatic: the influence of historical processes, migration patterns, isolation and ecological pressures, and their interaction. In: Roberts DF, Fujiki N, Torizuka K, editors. Isolation, Migration and Health. Cambridge University Press; Cambridge (SSHB): 1992. pp. 204–218. 1992. [Google Scholar]
- Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Kolcic I, Polasek O, Graessler J, Wilson JF, Marinaki A, Riches PL, Shu X, Janicijevic B, Smolej-Narancic N, Gorgoni B, Morgan J, Campbell S, Biloglav Z, Barac-Lauc L, Pericic M, Klaric IM, Zgaga L, Skaric-Juric T, Wild SH, Richardson WA, Hohenstein P, Kimber CH, Tenesa A, Donnelly LA, Fairbanks LD, Aringer M, McKeigue PM, Ralston SH, Morris AD, Rudan P, Hastie ND, Campbell H, Wright AF. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40:437–42. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- Wallace C, Newhouse SJ, Braund P, Zhang F, Tobin M, Falchi M, Ahmadi K, Dobson RJ, Marçano AC, Hajat C, Burton P, Deloukas P, Brown M, Connell JM, Dominiczak A, Lathrop GM, Webster J, Farrall M, Spector T, Samani NJ, Caulfield MJ, Munroe PB. Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet. 2008;82:139–49. doi: 10.1016/j.ajhg.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Arking DE, Gene Discovery Project of Johns Hopkins & the Autism Consortium. Daly MJ, Chakravarti A. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461:802–808. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, O'Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol. 2007;166:561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Köttgen A, Dehghan A, Smith AV, Glazer NL, Chen MH, Chasman DI, Aspelund T, Eiriksdottir G, Harris TB, Launer L, Nalls M, Hernandez D, Arking DE, Boerwinkle E, Grove ML, Li M, Linda Kao WH, Chonchol M, Haritunians T, Li G, Lumley T, Psaty BM, Shlipak M, Hwang SJ, Larson MG, O'Donnell CJ, Upadhyay A, van Duijn CM, Hofman A, Rivadeneira F, Stricker B, Uitterlinden AG, Paré G, Parker AN, Ridker PM, Siscovick DS, Gudnason V, Witteman JC, Fox CS, Coresh J. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3:523–530. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemunik T, Boban M, Lauc G, Janković S, Rotim K, Vatavuk Z, Bencić G, Dogas Z, Boraska V, Torlak V, Susac J, Zobić I, Rudan D, Pulanić D, Modun D, Mudnić I, Gunjaca G, Budimir D, Hayward C, Vitart V, Wright AF, Campbell H, Rudan I. Genome-wide association study of biochemical traits in Korcula Island, Croatia. Croat Med J. 2009;50:23–33. doi: 10.3325/cmj.2009.50.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Karns R, Narancic NS, Sun G, Cheng H, Missoni S, Durakovic Z, Rudan P, Chakraborty R, Deka R. Common SNPs in FTO gene are associated with obesity related anthropometric traits in an island population from the eastern Adriatic coast of Croatia. PLoS One. 2010;5:e10375. doi: 10.1371/journal.pone.0010375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.