Abstract
Infectious pancreatic necrosis virus (IPNV) (serotype Sp) was exposed to temperatures between 60 and 90°C in a medium mimicking the water-soluble phase of hydrolyzed fish by-products. D values ranged from 290 to 0.5 min, and the z value was approximately 9.8°C. Addition of formic acid to create a pH 4 medium did not enhance heat inactivation. Predicted inactivation effects at different temperature-time combinations are provided.
TEXT
In 2008, the global capture and aquaculture production of fish were 90 million and 53 million tons, respectively. Of this production, 19% was destined for fishmeal and fish oil production (8, 9). Most known fish pathogens have been detected in wild fish (15). Both wild and farmed fish can represent a risk for transmission. The Animal By-Products Regulation (European Commission [EC]) no. 1069/2009 categorizes fish by-products according to the level of risk they represent to public and animal health (5, 6). Category 3 by-products are whole wild fish caught for the purpose of fishmeal production and fresh by-products from wild and farmed fish caught for human consumption. These products are allowed for manufacturing of feed. Fishmeal must be submitted to a processing method which ensures that the product complies with microbiological standards (5, 6). Category 2 by-products may include dead fish from disease outbreaks. Steam pressure sterilization is required before the material can be disposed as waste or transformed into biogas or organic fertilizers. Infectious pancreatic necrosis virus (IPNV) is regarded as one of the most heat-resistant fish pathogens (3, 4); however, few kinetic data on thermal inactivation are available.
This study presents D values and z values for heat inactivation of IPNV in a relevant matrix. Such data are needed by the industry and authorities in order to decide whether thermal processes provide adequate inactivation of fish pathogens. As thermal inactivation may be affected by the heating medium, natural matrices should be used in experiments. High content of organic matter has been shown to protect viruses (1). However, as salmon by-products exhibited an adverse toxic effect on the BF-2 cells, fish homogenate was replaced by a peptone-salt medium (PSM) containing peptone (121 g/liter) and NaCl (2 g/liter). The pH was adjusted to 7.0 with NaOH or to 4.0 with formic acid. The PSM contained 10.2% protein and 0.3% salt, percentages which are similar to those of the water-soluble phase of fish silage and therefore more relevant than cell culture media used in previous studies.
IPNV (serotype Sp) was grown on BF-2 cells at 15°C using Eagle's minimal essential medium (EMEM) with 10% fetal bovine serum (FBS), 4 mM l-glutamine, and 50 μg/ml gentamicin. After 7 days, virus was harvested by freeze-thawing and kept at 4°C. IPNV was titrated on cells (without FBS) in 96-well plates with 6 parallels. Virus suspensions were left on the cells for 2 h, medium was added, and after 7 days, the cells were screened for cytopathogenic effects. The virus titer was determined by the Kärber method (11). The detection limit of the assay was 2.8 log10 of the 50% tissue culture infective dose (TCID50)/ml. An IPNV suspension with titers at 6.6 ± 0.2 log10 TCID50/ml was diluted 1:10 in PSM (pH 7 and 4) and submerged (2.2 ml in 14- by 100-mm glass tubes) in water baths at temperatures between 60 and 90°C. The heat treatment was terminated by cooling in ice-water. The temperature in the PSM during heating was less than 1°C below target temperatures after 60 s.
The heat resistance of microorganisms is frequently described by D values (time needed to kill 90% of a population at a certain temperature) and the z value (temperature change needed to alter the D value by a factor of 10). Plotting the logarithmic number of survivors versus time provides inactivation curves for which the D value is the negative reciprocal of its slope. Similarly, the z values are calculated from a plot of log D values versus exposure temperatures. The relationship between D values and temperature is expressed by Bigelow's equation (2), log Dx = (Ty − Tx)/z + log Dy, where Dx and Dy are decimal reduction times at temperatures Tx and Ty, respectively. With Bigelow's equation, D values can be estimated for temperatures other than those experimentally examined.
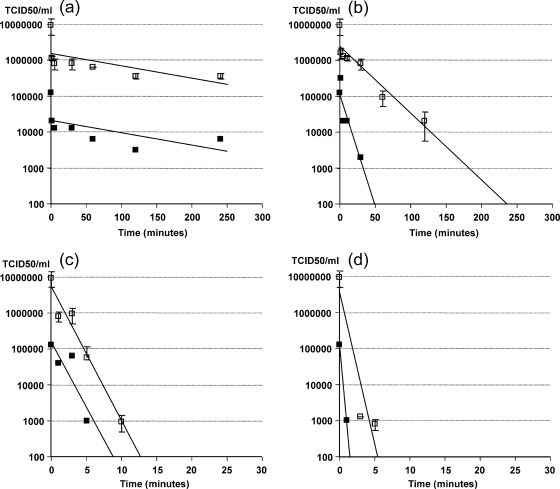
A prestudy determined the appropriate combinations of exposure times and temperatures and indicated that IPNV was more stable at pH 4 than at pH 7 (Table 1). The inactivation curves at 60, 70, 80, and 90°C fit first-order kinetics except at 60°C, when approximately 80% of infectivity was lost during the first minute (Fig. 1). D values obtained at pH 4 and 7 are quite similar (Table 2). However, the amount of infective IPNV is at least 1 log10 higher at pH 4 than at pH 7, even before heat treatment (Fig. 1). The effect of pH could be due to conformational change of viral proteins at a higher pH which reduces virus infectivity. As IPNV seems to be less stable at pH 7, studies should be performed on the effect of alkaline storage.
Table 1.
Log10 IPNV titers after heat treatment in PSM
| pH of medium | Log10 IPNV titer (TCID50/ml) for each heat treatment |
||
|---|---|---|---|
| No heatinga | 70°C for 25 min | 85°C for 25 min | |
| 4.0 | 6.1 | 5.1 | <2.8 |
| 7.0 | 4.5 | 3.8 | <2.8 |
20 to 25°C for 25 min.
Fig 1.
Inactivation curves for IPNV in PSM at pH 4.0 (open squares) and pH 7.0 (filled squares) at 60°C (a), 70°C (b), 80°C (c), and 90°C (d). The straight lines are regression exponential curves. The data points for pH 4 are the averages of two parallel analyses. Error bars show the standard deviations.
Table 2.
D values for IPNV in PSM
| Temp (°C) | Value for indicated pHa |
|||
|---|---|---|---|---|
| 7.0 |
4.0 |
|||
| D value (min) | R2 | D value (min) | R2 | |
| 60 | 287.82 | 0.35 | 291.47 | 0.39 |
| 70 | 15.86 | 0.83 | 53.92 | 0.88 |
| 80 | 2.74 | 0.74 | 2.65 | 0.96 |
| 90 | 0.48 | 1.00 | 1.17 | 0.88 |
R2, squared correlation coefficient.
The basis for z value calculation was the D values at 60, 70, and 80°C. At 90°C, IPNV was reduced to below the detection limit before the target temperature was reached. The z values for pH 4 and 7 were 9.8°C (R2 = 0.97) and 9.9°C (R2 = 0.98), respectively. Plots of log D values versus exposure temperatures showed that the temperature must be approximately 1.6°C higher at pH 4 than at pH 7 to achieve equal inactivation.
Previous studies have demonstrated that thermal inactivation of IPNV is biphasic. A 3 log10 reduction was found at 60°C during the first 30 min and then there was a 1 log10 reduction every 80 min (12). Similarly, 60°C gave a 1.5 log10 reduction after 20 min and then a 1 log10 reduction every 160 min (10). The Department for Environment, Food and Rural Affairs (Defra) (3) studied inactivation of IPNV at 60°C and found that serotype Sp was reduced by 5.1 log10 in 24 h. Smail et al. (14) found that 5 h at 60°C gave a 2.58 log10 reduction of strain Sp in fish silage. The titer decreased by 2 log10 in the first 2 h. With an initial concentration of 2 to 2.5 log10 PFU/ml, which was claimed to be typical for native silages, no infective virus was detected after 1 h. The conclusion was that 2 h of treatment of fish silage at 60°C is sufficient for virus inactivation, allowing the silage to be used for feedstuff. The inactivation curve for 60°C at pH 4 in our study seems to correspond well to the results of Smail et al. For 70, 80, and 90°C, there is limited information on D values. In a study on IPNV (VR-299), 8 h, 2 h, and 10 min were needed to reduce infectivity to below the detection limit at 60, 70, and 80°C, respectively (16). This is consistent with our results. In the same investigation, it was found that IPNV decreased more rapidly when heated to 65°C at neutral pH than at pH 4.
A Norwegian regulation laid down in 2007 required a minimum of a 3 log10 reduction of infectious IPNV in materials from aquaculture fish used as feed for aquaculture fish. The regulation was repealed after the former Animal By-Products Regulation (EC) no. 1774/2002 came into force but is still a relevant reference for sufficient inactivation of fish pathogens.
In a traditional fishmeal process, category 3 by-products are heated to above 90°C and kept above 80°C for 20 min. According to our estimates, 3-log10 reductions of IPNV will be achieved after 0.7 min at 90°C, 7.1 min at 80°C, or 23 min at 75°C (Table 3). Category 2 by-products have to be heat treated with, e.g., steam pressure sterilization at 133°C and 3 × 105 Pa of pressure for 20 min (5, 6). Our estimated effect of this method was 106 log10 reductions of IPNV. Therefore, a traditional fishmeal process (7) provides adequate IPNV inactivation, while steam pressure sterilization is excessive in relation to inactivation of IPNV.
Table 3.
Estimated time to inactivate IPNV in PSM
| Amt of inactivation (no. of log10 reductions) | Estimated time to inactivation (min) at indicated temp (°C) |
||||||
|---|---|---|---|---|---|---|---|
| 60 | 65 | 70 | 75 | 80 | 85 | 90 | |
| 1 | 102 | 32 | 10 | 3.0 | 0.9 | 0.3 | 0.1 |
| 2 | 441 | 137 | 42 | 13 | 4.0 | 1.2 | 0.4 |
| 3 | 781 | 242 | 75 | 23 | 7.1 | 2.2 | 0.7 |
| 4 | 1,120 | 347 | 107 | 33 | 10 | 3.1 | 1.0 |
| 5 | 1,460 | 452 | 140 | 43 | 13 | 4.1 | 1.3 |
The Norwegian Seafood Federation has applied for approval of an alternative processing method for category 2 fish by-products which includes ensilage at a pH below 4 for 24 h before heat treatment at 85°C for 25 min. The European Food Safety Authority (EFSA) has concluded that risks related to pathogens present would be adequately reduced by the proposed method. The final approval of the method is still pending.
ACKNOWLEDGMENTS
This work was supported by RUBIN, a foundation working for increased and profitable utilization of by-products from fisheries and fish farming in Norway.
We thank Jørgen Seliussen at Hordafor AS for providing information on the composition of fish silage and Gunn Harriet Knutsen at the Norwegian Seafood Federation for critical review of the manuscript.
Footnotes
Published ahead of print 13 January 2012
REFERENCES
- 1. Alexander DJ, Manvell RJ. 2004. Heat inactivation of Newcastle disease virus (strain Herts 33/56) in artificially infected chicken meat homogenate. Avian Pathol. 33: 222–225 [DOI] [PubMed] [Google Scholar]
- 2. Bigelow WD. 1921. The logarithmic nature of thermal death time curves. J. Infect. Dis. 29: 528–536 [Google Scholar]
- 3. Defra 2005. Inactivation of fish pathogens following ensiling or composting. Research project final report to United Kingdom Department for Environment, Food and Rural Affairs, London, United Kingdom [Google Scholar]
- 4. European Commission 2003. The use of fish by-products in aquaculture. Report of the Scientific Committee on Animal Health and Animal Welfare. Scientific Committee on Animal health and Animal Welfare, European Commission, Brussels, Belgium: http://ec.europa.eu/food/fs/sc/scah/out87_en.pdf [Google Scholar]
- 5. European Commission 2011. Commission Regulation (EU) no. 142/2011 of 25 February 2011 implementing Regulation (EC) no. 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:054:0001:0254:EN:PDF
- 6. European Parliament, Council of the European Union 2009. Regulation (EC) no. 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) no. 1774/2002 (Animal By-Products Regulation). http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:300:0001:0033:EN:PDF
- 7. FAO 1986. The production of fishmeal and oil. Fisheries technical paper no. 142. Fisheries Department, Food and Agriculture Organization of the United Nations, Rome, Italy: http://www.fao.org/docrep/003/x6899e/x6899e00.htm [Google Scholar]
- 8. FAO 2010. The state of world fisheries and aquaculture. Fisheries and Aquaculture Department, Food and Agriculture Organization of the United Nations, Rome, Italy: http://www.fao.org/docrep/013/i1820e/i1820e.pdf [Google Scholar]
- 9. FIN 2010. World food and feed fisheries and aquaculture—key figures. Fishmeal Information Network. http://www.gafta.com/fin/pdfs/Facts&Figures-2.pdf
- 10. Gosting LH, Gould RW. 1981. Thermal inactivation of infectious hematopoietic necrosis and infectious pancreatic necrosis viruses. Appl. Environ. Microbiol. 41: 1081–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kärber G. 1979. Calculation of the LD50 titer by the Kärber method, p 34–35 In Lennette EH, Schmidt NJ. (ed), Diagnostic procedures for viral, rickettsial and chlamydial infections, 5th ed. American Public Health Association, Washington, DC [Google Scholar]
- 12. MacKelvie RM, Desautels D. 1975. Fish viruses—survival and inactivation of infectious pancreatic necrosis virus. J. Fish. Res. Board Can. 32: 1267–1273 [Google Scholar]
- 13. Reference deleted.
- 14. Smail DA, Huntly PJ, Munro ALS. 1993. Fate of four fish pathogens after exposure to fish silage containing fish farm mortalities and conditions for inactivation of infectious pancreatic necrosis virus. Aquaculture 113: 173–181 [Google Scholar]
- 15. Wallace IS, Gregory A, Murray AG, Munro ES, Raynard RS. 2008. Distribution of infectious pancreatic necrosis virus (IPNV) in wild marine fish from Scottish waters with respect to clinically infected aquaculture sites producing Atlantic salmon, Salmo salar L. J. Fish Dis. 31: 177–186 [DOI] [PubMed] [Google Scholar]
- 16. Whipple MJ, Rohovec JS. 1994. The effect of heat and low pH on selected viral and bacterial fish pathogens. Aquaculture 123: 179–189 [Google Scholar]