Abstract
An inulinase-producing Microbulbifer sp. strain, JAM-3301, was isolated from a deep-sea sediment. An inulin operon that contained three open reading frames was cloned and sequenced. Two of the three genes were expressed. One product was an endo-inulinase, and the other was a β-fructofuranosidase. Both enzymes worked together to effectively degrade inulin.
TEXT
Inulinases are produced by 15 genera of fungi, 12 genera of yeasts, and 8 genera of bacteria (5, 7). Although inulinases of a few marine yeasts have been reported (2, 6), there have been no reports on marine bacterial enzymes. We isolated inulinase-producing bacteria from deep-sea sediments collected from Sagami Bay, Japan (35°04.989′N, 139°13.015′E) at a water depth of 900 m. The suspensions were spread on marine agar 2216 (Difco, Detroit, MI) supplemented with 0.2% (wt/vol) inulin (Wako Pure Chemical, Osaka, Japan), which were incubated at 30°C for 2 days. Several colonies were transferred to marine broth 2216 containing 0.2% inulin and propagated at 30°C for 2 days. The inulin-degrading activity in each centrifugal supernatant was measured by the 3,5-dinitrosalicylic acid method (3). Strain JAM-3301 showed the highest inulinase activity. The 16S rRNA gene of strain JAM-3301 was amplified by PCR using the universal primers 27f and 1492r (4), and a colony of strain JAM-3301 was used as the template in a DNA thermal cycler (model 9700; Applied Biosystems, Foster City, CA) with an LA Taq DNA polymerase (Takara Bio, Shiga, Japan). Nucleotide sequencing was performed using a DNA sequencer (model 377; Applied Biosystems) with an ABI Prism BigDye terminator sequencing kit (Applied Biosystems). Of the 1,465-bp nucleotide sequence determined, a 1,458-bp sequence was completely identical to the 1,458-bp nucleotide sequence of Microbulbifer chitinolyticus ABABA212 (1).
Strain JAM-3301 was propagated aerobically at 30°C for 20 h in the optimized medium. The culture broth was concentrated using a hollow-fiber ultrafilter (AIP0013; Mr cutoff, 6,000; Asahi Kasei, Tokyo, Japan). The concentrate was applied to a DEAE Toyopearl column (Tosoh, Tokyo, Japan) equilibrated with 25 mM Tris-HCl buffer (pH 7) plus 1 mM CaCl2. Proteins were eluted with a gradient of 0 to 0.2 M NaCl. To the active fractions was added 0.75 M ammonium sulfate, and the solution was applied to a phenyl Toyopearl column (Tosoh) equilibrated with 10 mM Tris-HCl buffer (pH 7) plus 1 mM CaCl2 and 0.75 M ammonium sulfate. Proteins were eluted with a gradient of 0.75 to 0 M ammonium sulfate. The active fractions were applied to another hydrophobic-interaction column, a butyl Toyopearl column (Tosoh). The enzyme was eluted by the procedures used with the phenyl Toyopearl column. After desalting of the active fractions by gel filtration, the solution was applied to a DEAE Toyopearl column equilibrated with 10 mM MOPS (morpholinepropanesulfonic acid) buffer (pH 6) plus 1 mM CaCl2. The enzyme was purified by elution with a gradient of 0 to 0.25 M NaCl. The molecular mass of the purified enzyme was approximately 80 kDa (Fig. 1). The purified inulinase and the fragments obtained by lysylendopeptidase digestion were electroblotted onto a methanol-wetted Immobilon membrane (Millipore, Billerica, MA). The N-terminal amino acid sequences of the purified enzyme and its lysylendopeptidase-digested fragment were AEEPAYVNSFNRN and NLDLLPGHLAPAV, which were analyzed by a protein sequencer (model 497HT; Applied Biosystems).
Fig 1.
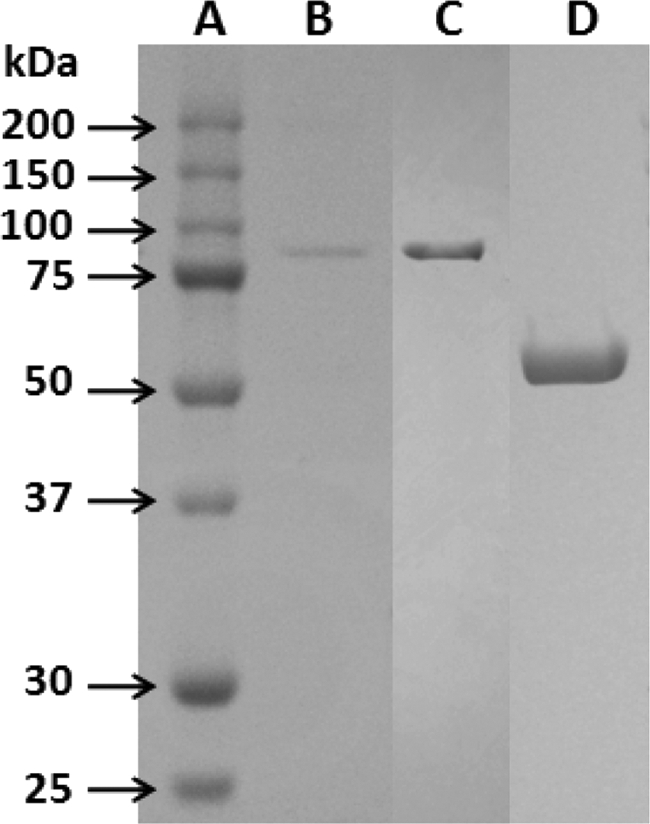
SDS-PAGE of purified IN33, rIN33, and rFF33. Lane A, molecular mass marker; lane B, purified IN33 of Microbulbifer sp. strain JAM-3301 (0.25 μg); lane C, purified rIN33 (1.2 μg); lane D, purified rFF (8.6 μg).
The inulinase gene was amplified by PCR using degenerate primers. Primer A, 5′-CCNGGNARNARRTCNARGTT-3′, was designed from the lysylendopeptidase-digested fragment. Primer B, 5′-TGGATGAAYGANCCNMAYGG-3′, was designed from a conserved amino acid sequence, WMNDPHG, among other endo-inulinases belonging to the GH32 family. The amplified 3.8-kb DNA fragment encoded a part of inulinase and another protein. To obtain an entire gene cluster, inverse PCR was carried out with the appropriate primers and self-circularized SalI- or BsrGI-digested strain JAM-3301 genomic DNA as the template. Consequently, a large DNA fragment of approximately 4.9 kb was sequenced. Three open reading frames (ORFs) were found in the fragment: ORF1 is composed of 1,458 bp encoding a protein of 485 amino acids, ORF2 is 954 bp encoding a protein of 317 amino acids, and ORF3 is 2,214 bp encoding a protein of 737 amino acids. The deduced amino acid sequences of ORF1, -2, and -3 show the highest sequence identity to GH family 32 proteins of Spirosoma linguale (48%; GenBank accession no. ADB39193.1), the 6-phosphofructokinase of Glaciecola sp. (45%; GenBank accession no. ZP_03560346.1), and the endo-inulinase of Arthrobacter sp. (43%; GenBank accession no. CAB63119.1), respectively. The inulinase (IN33) gene (ORF3) was amplified by PCR using the primers 5′-AATGGATCCCTTGGAAGGGGTCTGGCTTTTG-3′ and 5′-AATTCTAGACCGTGGAATTGTCAACGCATGG-3′ (underlining indicates additional BamHI and XbaI sites). The β-fructofuranosidase (FF33) gene (ORF 1) was amplified using the primers 5′-AATGGATCCTGTCCAACGGTGGACATTGTCG-3′ and 5′-AATTCTAGACCAAACACAGAAACGCGCAGA-3′. Each fragment was ligated into pUC18 vectors that were digested by the corresponding restriction enzymes. The resulting plasmids (pUCIN and pUCFF) were introduced into Escherichia coli DH5α cells. Each transformant was propagated aerobically in Luria-Bertani broth containing 100 μg/ml ampicillin at 30°C for 20 h. After disruption of cells by a sonicator, ammonium sulfate was added to the centrifugal cell extracts of recombinant IN33 (rIN33) and FF33 (rFF33) at 75% saturation. The precipitates were dialyzed against 10 mM Tris-HCl buffer plus 1 mM CaCl2. rIN33 was purified by the procedures used for the inulinase purification described above. rFF33 was purified by procedures similar to those used for rIN33 purification, except that the final step used a hydroxyl apatite column (Bio-Rad, Hercules, CA) equilibrated with 10 mM phosphate buffer (pH 7.0). rFF33 was purified by a gradient elution of 10 to 80 mM phosphate buffer. All purified enzymes were concentrated by ultrafiltration (Amicon Ultra-15; Millipore). A typical summary of the purification of each enzyme is shown in Table 1. Inulinase activity was measured in 100 mM MOPS buffer (pH 6.5), 0.2% inulin, and an enzyme solution in a total volume of 0.5 ml. After incubation at 30°C for 1 h, the reducing sugars released were quantified (3). One unit of inulinase activity was defined as the amount of the enzyme that released reducing sugar equivalent to 1 μmol of d-fructose under the assay conditions. β-Fructofuranosidase activity was measured in 100 mM acetate buffer (pH 5.8), 40 mM sucrose, and an enzyme solution in a total volume of 0.5 ml. After 30 min incubation at 30°C, the reducing sugars produced were measured (3). One unit of β-fructofuranosidase activity was defined as the amount of the enzyme that released reducing sugar equivalent to 1 μmol of mixture of glucose and fructose under the assay condition. Protein was quantified using a protein assay kit (Bio-Rad) with bovine serum albumin as the standard.
Table 1.
Purification of rIN33 and rFF33
| Step | Total protein (mg) |
Total activity (U) |
Sp act (U/mg) |
Yield (%) |
Fold purification |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| rIN33 | rFF33 | rIN33 | rFF33 | rIN33 | rFF33 | rIN33 | rFF33 | rIN33 | rFF33 | |
| Crude extract | 1,120 | 620.0 | 54.8 | 12,454.2 | 0.05 | 20.1 | 100 | 100 | 1 | 1 |
| DEAE Toyopearl | 51.8 | 67.2 | 37.0 | 7,736.7 | 0.71 | 115.1 | 67.5 | 62.1 | 14.2 | 5.73 |
| Phenyl Toyopearl | 13.0 | 10.5 | 0.81 | 19.2 | 16.2 | |||||
| Butyl Toyopearl | 2.6 | 2.2 | 3.7 | 1,387.5 | 1.42 | 630.7 | 6.8 | 11.1 | 28.4 | 31.4 |
| DEAE Toyopearl | 0.073 | 2.2 | 30.1 | 4.0 | 602 | |||||
| Hydroxyl apatite | 1.7 | 1,165.5 | 685.6 | 9.4 | 34.1 | |||||
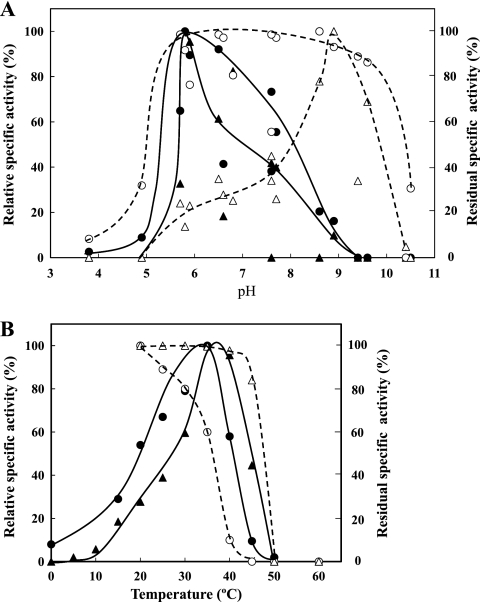
The molecular masses of the purified rIN33 and rFF33 were around 80 kDa and 50 kDa, respectively (Fig. 1). N-terminal amino acid sequences of rIN33 and rFF33 were AEEXAY (X is an unidentified amino acid residue) and MDLEVETGVVE, respectively. Both sequences were found in each deduced amino acid sequence. Thus, rIN33 was identical to the purified inulinase of strain JAM-3301. The optimal pH values of rIN33 and rFF33 were around pH 6 in 100 mM acetate buffer. rIN33 was stable from pH 6 to 9, whereas rFF33 was very stable in a narrow pH region (pH 8 to 9) (Fig. 2A). The optimal temperature for both enzymes was 35°C. rIN33 gradually lost its activity above 30°C, whereas rFF33 was stable up to 45°C (Fig. 2B). rIN33 hydrolyzed only inulin among fructose-containing oligosaccharides, whereas rFF33 degraded sucrose, nystose, raffinose, 1-kestose, and inulin with relative hydrolysis rates of 100%, 77%, 22%, 21%, and 0.9%. rIN33 (1.28 μg) and rFF33 (1.30 μg) individually hydrolyzed inulin to 0.17 ± 0.005 μmol and 0.13 ± 0.004 μmol of fructose at 30°C for 3 h. When the enzymes were mixed, they produced 0.63 ± 0.021 μmol of fructose. The patterns of inulin hydrolysis by rIN33 and rFF33 were analyzed by thin-layer chromatography on a silica gel 60 (Merck, Darmstadt, Germany) using a solvent system of 1-buthanol–ethanol–water (2:1:1, vol/vol). Spots were visualized by heating after spraying with anisaldehyde reagent (Fig. 3). rIN33 produced fructosyl oligomers, and rFF33 formed a small amount of fructose. After hydrolysis of inulin by rIN33, rFF33 was added to the reaction mixture and incubated for another 3 h at 30°C, and inulin was finally degraded to fructose. To clarify whether IN33 and FF33 are inducible, strain JAM-3301 was propagated aerobically in marine broth 2216 with or without 0.2% inulin at 30°C for 48 h. Enzyme activities in cell extracts or culture broths were measured at 30°C for 1 h. As expected, IN33 and FF33 were clearly produced by adding inulin (Table 2). It is concluded that the gene cluster works as an inulin operon in the presence of inulin.
Fig 2.
Effects of pH and temperature on rIN33and rFF33. (A) Effects of pH on activity and stability. Enzyme activities were measured in various buffers. The highest specific activity of rIN33 (●) (30.0 U/mg) and that of rFF33 (▲) (685.2 U/mg) were taken as 100%. The residual activity of rIN33 after incubation at 20°C for 30 min in various buffers and that of rFF33 after incubation at 15°C for 4 h in various buffers were measured under standard assay conditions. The highest residual specific activity of rIN33 (○) (15.4 U/mg) and that of rFF33 (▵) (657.6 U/mg) were taken as 100%. The buffers used were acetate (pH 3.9 to 5.8), phosphate (pH 5.9 to 7.7), MOPS (pH 5.7 to 7.6), Tris-HCl (pH 6.6 to 8.6), glycine-NaOH (pH 8.0 to 10.5), and carbonate (pH 9.4 to 11.0). (B) Effects of temperature on activity and stability. The purified rIN33 and rFF33 activities were measured at the indicated temperatures under the standard assay conditions. The highest specific activity of rIN33 (●) (38.2 U/mg) and that of rFF33 (▲) (1,135.8 U/mg) were taken as 100%. The residual activities after incubation at the indicated temperatures for 15 min were measured under standard assay conditions. The highest residual specific activity of rIN33 (○) (12.9 U/mg) and that of rFF33 (▵) (661.3 U/mg) were taken as 100%.
Fig 3.
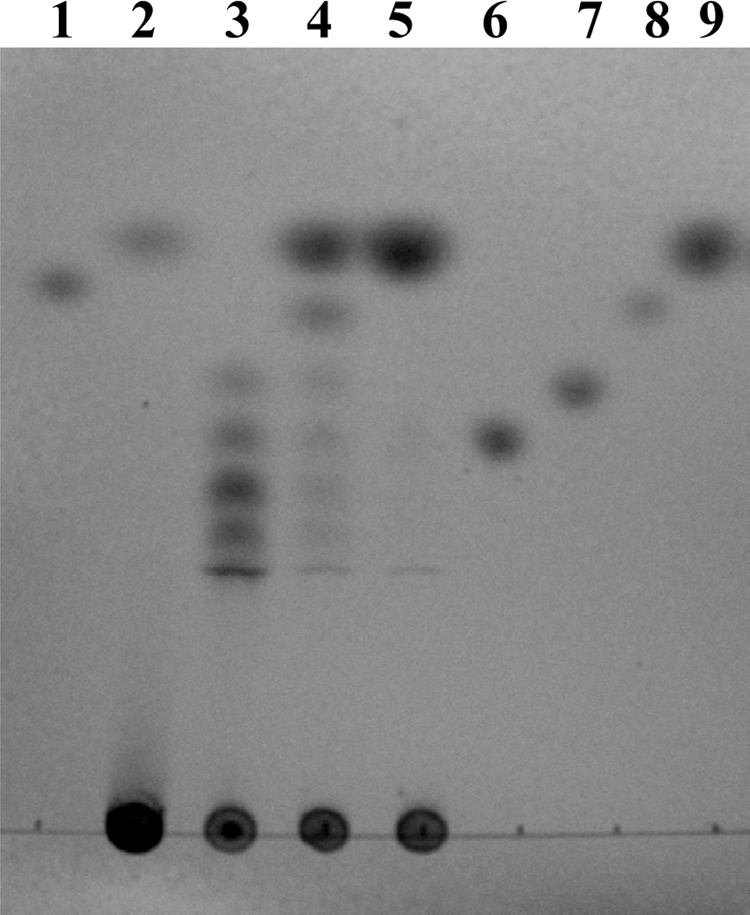
Degradation of inulin by rIN33 and rFF33. Lane 2, degradation product of rFF33 (1.3 μg) after 3 h incubation; lane 3, product of rIN33 (0.7 μg) after 3 h incubation, lane 4, product of rIN33 followed by rFF33 (0.11 μg) after another 3 h incubation; lane 5, product of rIN33 followed by rFF33 (2.1 μg) after another 3 h incubation. Authentic samples used were sucrose (lanes 1 and 8), nystose (lane 6), 1-kestose (lane 7), and fructose (lane 9).
Table 2.
Induction of IN33 and FF33 in strain JAM-3301
| Substrate | Sp acta in: |
Fold induction |
||||
|---|---|---|---|---|---|---|
| Marine broth |
Marine broth plus 0.2% inulin |
|||||
| Broth | Cell extract | Broth | Cell extract | Broth | Cell extract | |
| Inulin | 0.0031 | 0.0096 | 0.034 | 0.137 | 11.0 | 14.3 |
| Sucrose | 0.0051 | 0.135 | 0.042 | 0.745 | 8.2 | 5.5 |
| Raffinose | 0.0042 | 0.009 | 0.014 | 0.275 | 3.3 | 30.6 |
| 1-Kestose | 0.0034 | 0.005 | 0.0047 | 0.170 | 1.4 | 34.0 |
Protein concentrations in broths and cell extracts were 0.67 and 1.35 mg/ml in marine broth and 0.94 and 1.53 mg/ml in marine broth plus inulin.
Nucleotide sequence accession numbers.
The nucleotide sequences of the 16S rRNA gene of strain JAM-3301 was submitted to the GenBank/EMBL/DDBJ databases under the accession number AB669003. The nucleotide sequences of inulinase gene cluster, inulinase (IN33) gene, and β-fructofuranosidase (FF33) gene were submitted to the GenBank/EMBL/DDBJ databases under the accession numbers AB669413, AB669414, and AB669415, respectively.
Footnotes
Published ahead of print 27 January 2012
REFERENCES
- 1. Baba A, Miyazaki M, Nagahama T, Nogi Y. 2011. Microbulbifer chitinilyticus sp. nov. and Microbulbifer okinawensis sp. nov., chitin-degrading bacteria isolated from mangrove forests. Int. J. Syst. Evol. Microbiol. 61:2215–2220 [DOI] [PubMed] [Google Scholar]
- 2. Gong F, et al. 2008. Purification and characterization of extracellular inulinase from a marine yeast Pichia guilliermondii and inulin hydrolysis by the purified inulinase. Biotechnol. Bioprocess Eng. 13:533–539 [DOI] [PubMed] [Google Scholar]
- 3. Ito S, Kobayashi T, Hatada Y, Horikoshi K. 2005. Enzymes in modern detergents. Methods Biotechnol. 17:151–163 [Google Scholar]
- 4. Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–175 In Stackebrandt E, Goodfellow M. (ed), Nucleic acid techniques in bacterial systematics. Wiley & Sons, New York, NY [Google Scholar]
- 5. Neagu C, Bahrim G. 2011. Inulinases—a versatile tool for biotechnology. Innovat. Rom. Food Biotechnol. 9:1–11 [Google Scholar]
- 6. Sheng J, Chi Z, Gong F, Li J. 2008. Purification and characterization of extracellular inulinase from a marine yeast Cryptococcus aureus G7a and inulin hydrolysis by the purified inulinase. Appl. Biochem. Biotechnol. 144:111–121 [DOI] [PubMed] [Google Scholar]
- 7. Vandamme EJ, Derycke DG. 1983. Microbial inulinases: fermentation process, properties, and applications. Adv. Appl. Microbiol. 29:139–176 [DOI] [PubMed] [Google Scholar]