Abstract
Oral candidiasis is often accompanied by severe inflammation, resulting in a decline in the quality of life of immunosuppressed individuals and elderly people. To develop a new oral therapeutic option for candidiasis, a nonpathogenic commensal oral probiotic microorganism, Streptococcus salivarius K12, was evaluated for its ability to modulate Candida albicans growth in vitro, and its therapeutic activity in an experimental oral candidiasis model was tested. In vitro inhibition of mycelial growth of C. albicans was determined by plate assay and fluorescence microscopy. Addition of S. salivarius K12 to modified RPMI 1640 culture medium inhibited the adherence of C. albicans to the plastic petri dish in a dose-dependent manner. Preculture of S. salivarius K12 potentiated its inhibitory activity for adherence of C. albicans. Interestingly, S. salivarius K12 was not directly fungicidal but appeared to inhibit Candida adhesion to the substratum by preferentially binding to hyphae rather than yeast. To determine the potentially anti-infective attributes of S. salivarius K12 in oral candidiasis, the probiotic was administered to mice with orally induced candidiasis. Oral treatment with S. salivarius K12 significantly protected the mice from severe candidiasis. These findings suggest that S. salivarius K12 may inhibit the process of invasion of C. albicans into mucous surfaces or its adhesion to denture acrylic resins by mechanisms not associated with the antimicrobial activity of the bacteriocin. S. salivarius K12 may be useful as a probiotic as a protective tool for oral care, especially with regard to candidiasis.
INTRODUCTION
The overgrowth of Candida albicans, which is one of the members of the oral microbial flora in a healthy human, causes pathogenic symptoms such as oral candidiasis. Oral candidiasis accompanied with severe inflammation can significantly degrade the quality of life of immunosuppressed individuals and elderly people (9). It can cause a variety of mucosal infections in the gastrointestinal, respiratory, and genital tracts and is a major cause of oral and esophageal infections (9, 23, 29). Oral candidiasis is common in patients with advanced AIDS, hyposalivation, and diabetes mellitus, those on antibiotic therapy or immunosuppressive drugs, and those who have poor oral hygiene (9, 22, 23, 29).
The probiotic strain Streptococcus salivarius K12 was originally isolated from the saliva of a healthy child and produces several megaplasmid-encoded bacteriocin-like inhibitory substances (BLISs), such as the lantibiotics salivaricin A and salivaricin B (11, 13, 31). It has been used commercially as a probiotic for more than a decade and has numerous studies supporting its safety (3, 4, 5). S. salivarius strains have been reported to inhibit the biofilm formation of Streptococcus mutans (13, 19, 28), and Streptococcus salivarius K12 has been shown to have the ability to inhibit various potentially deleterious upper respiratory tract bacteria, such as Streptococcus pyogenes and Streptococcus pneumoniae (13, 31), and decrease oral malodor (2). These properties suggest that S. salivarius K12 might be widely applied as a management tool for oral health applications.
C. albicans is a polymorphic yeast and grows predominantly as yeast, pseudohyphae, or hyphae (23). Mycelial growth of C. albicans is often observed in mucosal infection and is considered to contribute to pathogenesis by biofilm formation (22). In this study, we aimed to elucidate the potential mechanisms of Streptococcus salivarius K12 suppression of the mycelial growth of the C. albicans first by in vitro analysis and then by testing an experimental oral candidiasis model with mice with furry white tongues.
MATERIALS AND METHODS
Candida albicans and Streptococcus salivarius.
The C. albicans strain TIMM1768 was isolated clinically from the blood of a candidiasis patient and maintained at Teikyo University Institute of Medical Mycology; this strain, which was shown to induce oral candidiasis in a murine model, has been used for animal experiments (12, 14). Cultures were stored at −80°C in Sabouraud dextrose broth (Becton Dickinson, MD) containing 0.5% yeast extract (Becton Dickinson, MD) and 10% glycerol (vol/vol, final concentration) until use. Strain TIMM1768 was cultured on a Sabouraud dextrose agar plate for 18 h at 37°C, and the cells were harvested with a microspatula and suspended in RPMI 1640 medium containing 2.5% fetal calf serum (RPMI 1640 medium). The cultured C. albicans cells were used for in vitro germ tube formation, the mycelial growth experiment, and also in vivo oral inoculation of Candida.
S. salivarius K12 is a commercially available probiotic that was originally isolated from the oral cavity of a child. It was supplied as a freeze-dried powder at 2 × 1011 CFU per gram of material tested and was used with CAB K12 agar, which consisted of Columbia blood agar base (Becton Dickinson, MD), 0.5% yeast extract (Becton Dickinson, MD), 0.25% glucose, and 0.1% calcium carbonate (pH 7.3 ± 0.2).
Measurement of antimicrobial activity of bacteriocins produced by S. salivarius K12.
To determine if the bacteriocins or other secretory molecules from S. salivarius K12 inhibited C. albicans TIMM1768, a deferred antagonism assay was employed. This was conducted essentially as described by Tagg and Bannister (25), in duplicate, using the nine bacterial indicator strains described to be positive controls for S. salivarius K12 bacteriocin production and also applying the C. albicans TIMM1768 strain. In brief, S. salivarius K12 was preliminarily cultured on a CAB agar (with 5% blood, 0.1% CaCO3) plate to form a 1-cm-wide streak. After incubation of the plate at 37°C under 5% CO2 for 18 to 24 h, the culture of S. salivarius K12 was removed from the plate using a clean microscope slide and sterilized with chloroform vapors for 30 min. The plates were aired for 30 min in an extraction hood. Indicator bacterial strains as well as C. albicans were then inoculated horizontally across the original but now sterile S. salivarius K12 streak. Plates were then reincubated for 18 h. The inhibitory effect of microbial growth was evaluated as follows: −, no inhibition of the test organism; +, inhibition of the test organism only over the primary inoculation; ++, inhibition of the test organism just beyond the primary inoculation; +++, inhibition of the test organism much beyond the primary inoculation.
In vitro assay of germ tube formation and mycelial growth of Candida albicans.
The ability of C. albicans cells to undergo germ tube formation or mycelial growth with S. salivarius K12 was assessed as described below.
(i) Germ tube formation analysis.
One hundred microliters of C. albicans cells was aliquoted into 96-well microtiter plates (1 × 104 CFU per well for morphological analysis, 5 × 105 CFU per well for crystal violet [CV] staining), 100-μl serial dilutions of freeze-dried S. salivarius K12 powder were then added to the plates, which made final concentrations of 30 mg/ml (3.0 × 109 CFU/ml) to 0.12 mg/ml (1.2 × 107 CFU/ml), and the plates were incubated at 37°C in 5% CO2 in air for 3 h. Germ tube formation was assessed microscopically: cells were fixed with 70% ethanol and stained with CV as described by Abe et al. (1) and Kamagata-Kiyoura et al. (14).
(ii) Mycelial growth analysis.
Mycelial growth analysis was carried out as described for the germ tube formation assay, except that the inoculum per well was 500 cells in 100 μl and the culture period was lengthened to 16 h. Mycelial growth of C. albicans cells was determined as described by Abe et al. (1). Culture medium for in vitro assays was composed of diluted RPMI 1640 (1:3; Sigma Chemical Co., St. Louis, MO) containing 0.8% fetal calf serum, 20 mM HEPES buffer, pH 7.2, 2 mM urea, and 10 mg/ml d-glucose with or without antibiotics (60 μg/ml of benzylpenicillin potassium [Wako, Japan] and kanamycin sulfate [Wako, Japan]), according to the nutritional requirements of S. salivarius (6). The planktonic cells were centrifuged, stained with 50% lactophenol blue solution (containing 1 mg/ml of methyl blue [C.I. 42780], 204 mg/ml of phenol, 247 mg/ml of lactic acid, and 502 mg/ml of glycerol; Merck, Germany) in saline, and observed by microscopy.
Yeast viability assay using fluorescence microscopy.
The effect of S. salivarius K12 on C. albicans viability was detected by use of a two-color fluorescent probe (FUN1; F-7030; Molecular Probes, Eugene, OR), a live/dead yeast viability kit, and fungal surface labeling with a reagent of a third color (calcofluor white M2R; Molecular Probes, Eugene, OR). C. albicans and S. salivarius K12 were cultured as described above. In brief, C. albicans and S. salivarius K12 were combined in adequate culture medium and cultured for 1 to 3 h in a CO2 incubator. After centrifugation at 3,000 rpm for 3 min and one-time washing with GH solution (2% glucose in 10 mM HEPES buffer, pH 7.2), the GH solution was replaced with GH solution containing 20 μM FUN1 with 5 μM calcofluor white M2R. After incubation for 30 min at room temperature, cells were observed with a fluorescence microscope (BH50; Olympus, Japan) equipped with a WU (wide range of UV excitation), (WU), WBV (wide range of blue-violet excitation), WG (wide range of green excitation), and NB (narrow range of UV excitation) filter assortment. Staining with FUN1 was observed using NB and calcofluor white WU. All images were taken as digital data with a DC200 camera (Leica, Germany), and the digital data were inserted into the IM50 program and recorded.
Murine oral candidiasis model.
All animal experiments were performed in accordance with the guidelines for the care and use of animals approved by Teikyo University. The derivation of the murine oral candidiasis model has been described previously (15, 27). Six-week-old female ICR mice (Charles River Japan, Inc., Yokohama, Kanagawa, Japan) were used for all animal experiments. The mice were randomized, kept in cages housing 3 to 4 individuals, and given food and water ad libitum. During the experimental period, the photoperiods were adjusted to 12 h of light and 12 h of darkness daily, and the environmental temperature was maintained at 21°C. To induce an immunosuppressed condition, 100 mg of prednisolone (Mitaka Pharmaceutical Co., Japan) per kg of body weight was injected subcutaneously to mice 20 to 24 h before oral inoculation. Prior to prednisolone administration, 15 mg/ml of tetracycline hydrochloride (Takeda Shering Purau Animal Health Co., Japan) was administered in drinking water for 24 h. On the day of infection, animals were anesthetized by intramuscular injection with 14.4 mg/kg of chlorpromazine chloride in the femur, after which they were orally inoculated with 2.0 × 108 CFU/ml of C. albicans TIMM1768 in modified RPMI 1640 medium. Oral inoculation was performed by means of rubbing and rolling a cotton swab (baby cotton buds; Johnson & Johnson Co., Tokyo, Japan) inside all parts of the mouth. The number of Candida cells inoculated in the oral cavity was calculated to be 1 × 106 CFU/mouse on the basis of the difference in viable cell number adhering to the cotton swabs before and just after oral inoculation, as described by Takakura et al. (27).
Oral administration of Streptococcus salivarius K12.
Fifty microliters of S. salivarius K12 solution, fluconazole (2 mg/ml), or distilled water was administered into the oral cavity of the Candida-inoculated mice at five time points: 24 h and 3 h before and 3, 24, and 27 h after C. albicans inoculation. The total numbers of mice in each group during two different trials were as follows: water control, n = 15; S. salivarius K12 at 7.5 mg/ml, n = 7; S. salivarius K12 at 15 mg/ml, n = 12; 30 mg/ml, n = 15; and fluconazole at 2 mg/ml, n = 6. Administration was undertaken using a rounded-top needle to spread the treatment over all parts of the mouth. An active control of 50 μl of fluconazole solution (2 mg/ml) was similarly administered.
Scoring severity of oral infection.
The procedure of scoring the severity of oral infection was performed as described previously (27). Forty-eight hours after inoculation, mice were sacrificed by cervical dislocation and the severity of the lesion of the tongue was evaluated by scoring the fur coating on each tongue and the squamous disorder as follows: 0, normal; 1, fur on less than 20% of the tongue; 2, fur on more than 21% but less than 90% of the tongue; 3, fur on more than 91% of the tongue and on the squamous layer; 4, thick fur on more than 91% of the tongue and on the squamous layer (12, 27).
Evaluation of number of viable Candida cells on murine tongues.
At 48 h after inoculation, the cheek, tongue, and soft palate of each mouse was swabbed uniformly using a cotton swab, and the swab was used for microbiological evaluation. After swabbing, the cotton end was cut off and placed in 3 ml of sterile saline. Candida cells were resuspended by mixing on a vortex mixer and diluted with a series of 20-fold and 100-fold dilutions of sterile saline. Fifty microliters of each dilution was incubated on a Candida GS agar plate (selection medium for Candida; Eiken Chemical Co. Ltd., Japan) for 20 h at 37°C. The Candida cells were counted, and then the total numbers per swab were calculated and reported as numbers of CFU.
Histology.
For histological study, the tongues were resected at the base of the tongue, fixed with 4% paraformaldehyde (pH 7.4) at 4°C, dehydrated by ethanol series, and embedded in paraffin in accordance with common procedure. Specimens were sectioned to an 8-μm thickness along the longitudinal center line. Sections on the slide were deparaffinized by xylene, rehydrated by ethanol series, and stained with periodic acid-Schiff (PAS).
Statistical analysis.
The score data were compared by the nonparametric Mann-Whitney U test. Statistical analysis of the log10 number of CFU of C. albicans isolated from each mouse part was compared using a Student's t test. P values of <0.05 were considered statistically significant. All mean values given in the text include the standard deviation of the mean.
RESULTS
Inhibition of Candida albicans attachment to plastic substratum by S. salivarius BLIS K12.
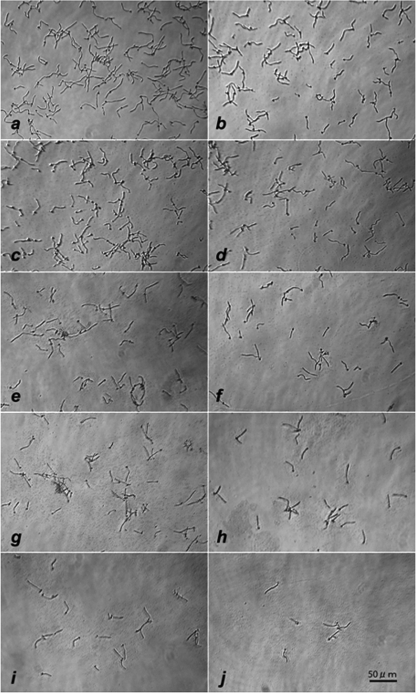
Mycelial growth of C. albicans is considered to contribute to the pathogenesis of mucosal candidiasis. The first step to make mycelia is germ tube formation, followed by an increase of the adherent capacity by hydrophobicity. We investigated the in vitro effects of S. salivarius K12 on the germ tube formation of C. albicans. Figure 1a shows that C. albicans cells cultured in control culture medium formed germ tube-like hyphae within 3 h. In the experimental group, where C. albicans was cultured in the presence of S. salivarius K12 (Fig. 1b to j), the morphological shape and size of the Candida cells appeared to be almost the same as those for the control group; however, the adherence of the mycelial form to the plastic substratum was weaker and the mycelial numbers on the plastic bottom were dose-dependently reduced in the presence of more than 0.94 mg/ml of freeze-dried S. salivarius K12 starting material, equating to approximately 1 × 108 CFU/ml.
Fig 1.
Inhibitory effect of BLIS K12 on germ tube formation of C. albicans cultured with different doses of S. salivarius K12 for 3 h at 37°C in 5% CO2 in air. Starting concentrations of Streptococcus salivarius K12 freeze-dried material were control (a), 0.12 mg/ml (b), 0.23 mg/ml (c), 0.47 mg/ml (d), 0.94 mg/ml (e), 1.88 mg/ml (f), 3.75 mg/ml (g), 7.5 mg/ml (h), 15 mg/ml (i), and 30 mg/ml (j) (1.2 × 107 to 3 × 109 CFU/ml).
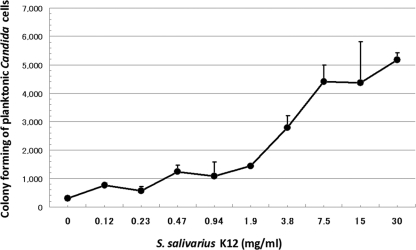
Figure 2 shows the results of the number of viable C. albicans cells growing in planktonic form, which were found to increase, according to the concentration of S. salivarius K12, to more than 1.9 to 3.8 mg/ml (approximately 2.0 × 108 to 4.0 × 108 CFU/ml). These results indicate that S. salivarius K12 increased the number of planktonic Candida cells in culture medium. The planktonic cells, including unattached mycelia, were centrifuged and stained with lactophenol blue. Figure 3 shows that mycelial cells of Candida attached to and were surrounded by S. salivarius K12.
Fig 2.
Number of planktonic cells after 3 h culture of C. albicans with various concentrations of S. salivarius K12. C. albicans was cultured with different doses of S. salivarius K12 for 3 h at 37°C in 5% CO2 in air. After the cultured plate was shaken, the supernatant was collected, diluted, and seeded on a GS agar plate for determining the number of planktonic cells. The experiments were performed in duplicate.
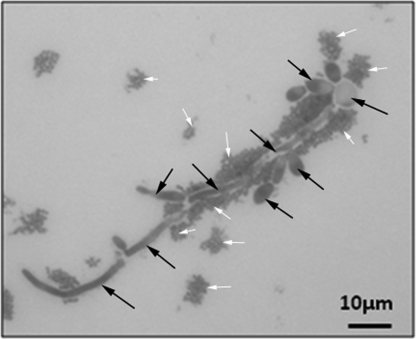
Fig 3.
Microscopic observation of interaction between S. salivarius K12 and Candida planktonic cells after 3 h culture and staining with lactophenol blue. Black arrows, Candida cells; white arrows, S. salivarius.
Effective inhibition of C. albicans attachment to substratum by viable S. salivarius K12.
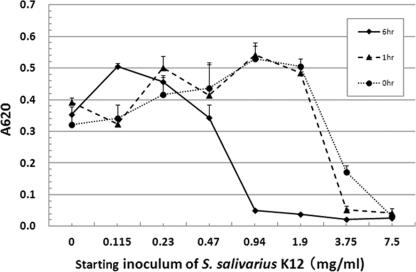
Although S. salivarius K12 was shown to bind to mycelial growth of Candida at 3 h of culture and inhibit Candida adherence to plastic plates, it is not clear whether these effects continue for longer periods of culture with Candida. Mycelial growth of C. albicans for 16 h of culture was quantified using the crystal violet staining method (1), and the results are shown in Fig. 4. When S. salivarius K12 existed at 3.75 mg/ml (3.75 × 108 CFU/ml), there were no Candida hyphae attached to the plastic plate.
Fig 4.
Inhibitory effect of S. salivarius K12 on hyphal growth of C. albicans. S. salivarius K12 was preliminarily cultured for 1 h or 6 h and then cultured with C. albicans for 3 h and stained with crystal violet. The optical absorbance at 620 nm (A620) was then detected. The experiments were performed in triplicate.
S. salivarius K12 was obtained as a lyophilized ingredient. To determine the effect of the probiotic in an active culture, different inoculum or dose sizes were tested to see if hyphal growth and the subsequent adherence ability were inhibited. When S. salivarius K12 was preliminarily grown for 6 h, it appeared to enhance the inhibition of Candida adherence; with concentrations as low as 0.94 mg/ml (approximately 1 × 108 CFU/ml), S. salivarius K12 completely inhibited adherence of Candida (Fig. 4). Shorter incubation periods with higher inoculum doses also appeared to greatly affect hyphal growth and adhesion. In contrast, when Candida was preliminarily grown for 3 h, the inhibitory effect of S. salivarius K12 at concentrations as low as 15 mg/ml (1.5 × 109 CFU/ml; data not shown) on Candida adherence after an additional 3 h culture appeared to decrease.
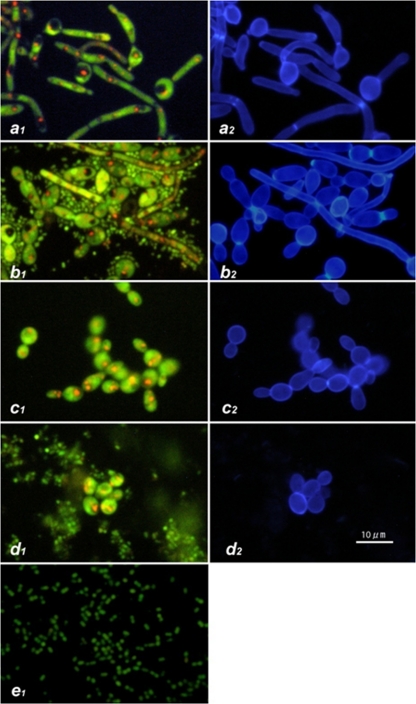
Preferential binding of S. salivarius to hyphae of C. albicans.
Earlier experiments indicated that S. salivarius K12 inhibited C. albicans mycelial adhesion to plastic plates and that possible interactions between S. salivarius K12 and C. albicans were occurring; these findings were further investigated using staining techniques. C. albicans was cultured on a poly-l-lysine-coated glass slide with or without S. salivarius K12 for 3 h and then stained by FUN1 to determine its viability by detecting metabolic activity. Slides were also stained by calcofluor white to identify the cell wall of Candida, which is composed of β-glucans. FUN1 staining showed that the hyphae were surrounded by numerous small green particles (Fig. 5). These particles were not stained with calcofluor white (and thus not composed of β-glucans) and were bacterial bodies of S. salivarius K12. Concurrently with staining of C. albicans with calcofluor white, the green and red fluorescence of FUN1 was also applied. In this system, green fluorescence accumulates throughout the cytoplasm and red particles transfer and concentrate in the vacuoles in the cytoplasm, indicating metabolic activity. The red pigments appeared to be concentrated in vacuoles, which indicated that the mycelial form of C. albicans was alive, although it was surrounded by S. salivarius K12. These results suggest that C. albicans is not killed by S. salivarius K12 but that C. albicans and S. salivarius K12 form some sort of interaction with each other (Fig. 5). This interaction appears to occur preferentially for the mycelial form of C. albicans rather than the yeast form.
Fig 5.
Vital staining of C. albicans by FUN1 (1) and calcofluor white M2R (2). C. albicans was cultured with or without S. salivarius K12 at 30°C or 37°C for 3 h. (a) Hyphal form of control culture at 37°C; (b) hyphal form of culture at 37°C with S. salivarius K12; (c) yeast form of control culture at 30°C; (d) yeast form of culture with S. salivarius K12 at 30°C; (e) S. salivarius K12.
No susceptibility of Streptococcus salivarius K12 bacteriocins to Candida albicans TIMM1768.
To examine the possibility that Streptococcus salivarius K12 bacteriocins interfere with the growth of C. albicans, the susceptibility of C. albicans to S. salivarius K12 was tested by the deferred antagonism test (11, 25, 26). S. salivarius K12 inhibited all of the bacteria which were used as indicators of bacteriocin inhibitory activity but not the C. albicans strain, when tested in duplicate, as shown in Table 1. To further confirm a lack of bacteriocin inhibitory activity on the Candida strain, a simultaneous antagonism test was also performed in the liquid RPMI 1640 medium used in the other experiments described above, and again, no activity of live or heat-killed supernatants against C. albicans was shown (data not shown).
Table 1.
Detection of inhibitory properties of S. salivarius K12 against test organisms using deferred antagonism testing
| Test organism | K12 deferred antagonisma |
|---|---|
| Candida albicans TIMM1768 | − |
| Micrococcus luteus T-18 | +++ |
| Streptococcus pyogenes FF22, M type 52, T pattern 3/13 | +++ |
| Streptococcus anginosus T-29 | +++ |
| Streptococcus uberis ATCC 27958 | +++ |
| Streptococcus pyogenes 71-679, M type 4, T pattern 4 | +++ |
| Lactococcus lactis T-21 | +++ |
| Streptococcus pyogenes 71-698, M type 28 | +++ |
| Streptococcus pyogenes W-1, T pattern 6 | +++ |
| Streptococcus agalactiae T-148 | +++ |
−, no inhibition of the test organism; +++, inhibition of the test organism much beyond the primary inoculation.
Effect of treatment with oral S. salivarius K12 on oral candidiasis model.
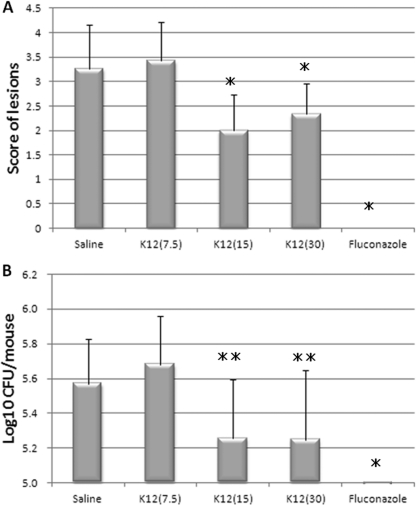
The effects of S. salivarius K12 on murine oral candidiasis were examined. S. salivarius K12 was orally administrated to the mice at 24 and 3 h before and 3, 24, and 27 h after Candida inoculation. It appeared that S. salivarius K12 application caused a dose-dependent improvement in symptom score and fungal burden (Fig. 6). Although the oral administration of 7.5 × 108 CFU/ml of S. salivarius K12 (score = 3.4 ± 0.79, n = 7) resulted in no significant difference in symptom score from that for the saline-treated control group (score = 3.3 ± 0.88, n = 15), oral administration of 1.5 × 109 CFU/ml and 3 × 109 CFU/ml of S. salivarius K12 (scores = 2.0 ± 0.74 [n = 12] and 2.3 ± 0.62 [n = 15], respectively) resulted in an obviously significant difference from the saline-treated control group (P < 0.01) (Fig. 6).
Fig 6.
Effect of S. salivarius K12 on the symptom score (A) and fungal burden (B) in the murine model of oral candidiasis. Groups of immunosuppressed mice (control, n = 15; S. salivarius K12 at 7.5 mg/ml, n = 7; S. salivarius K12 at 15 mg/ml, n = 12; S. salivarius K12 at 30 mg/ml, n = 15; fluconazole at 2 mg/ml, n = 6) were inoculated with C. albicans TIMM1768, and S. salivarius K12 was administered as described in Materials and Methods. Symptom scores (A) and fungal burden (B) were assessed after 48 h, as described in Materials and Methods. ⁎ and ⁎⁎, significant differences (P < 0.01 and P < 0.05, respectively) with no S. salivarius K12 (control), as determined using Student's t test.
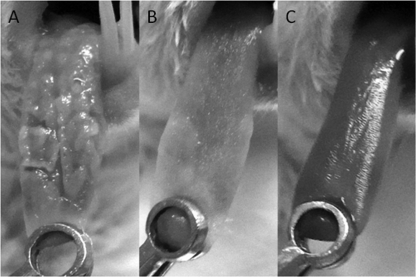
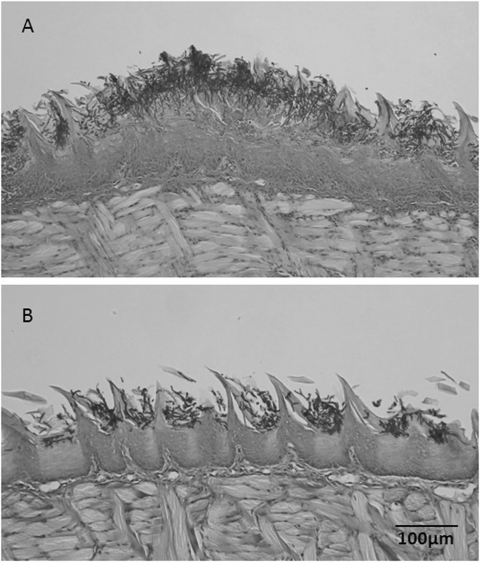
The tongues of mice administered S. salivarius K12 showed fewer lesions than the tongues of the control mice (Fig. 7A and B). Even though there was no total eradication, as observed in the control using fluconazole, in the mice that were administered S. salivarius K12 at 30 mg/ml (3 × 109 CFU/ml), there was a significant decrease in fungal burden compared to that for the untreated control. The reduced pathogenicity of C. albicans cells when mice were given S. salivarius K12 was illustrated by the histopathology of tongue sections (Fig. 8A and B). Fewer PAS-staining mycelial elements were found to invade the oral epithelium of tongues treated with S. salivarius K12 than the tongues in the control group.
Fig 7.
Typical images of tongues from mice inoculated with C. albicans TIMM1768. (A) control; (B) S. salivarius K12 (30 mg/ml; 3 × 109 CFU/ml); (C) fluconazole (2 mg/ml).
Fig 8.
Histology of longitudinal formalin-fixed paraffin-embedded (FFPE) sections of mouse tongues inoculated with C. albicans TIMM1768. (A) Representative control mouse; (B) representative mouse given S. salivarius K12 (30 mg/ml; 3 × 109 CFU/ml). Sections were stained with periodic acid-Schiff.
DISCUSSION
The in vitro culture experiments that were conducted showed that S. salivarius K12 bound directly to Candida cells and inhibited the adherence of Candida cells to the plastic petri dish. We also report that S. salivarius K12 had a protective effect against Candida invasion, indicated by the results obtained with the in vivo experimental model of oral candidiasis.
The results of in vitro culture experiments showed that S. salivarius K12 bound Candida cells at both the stage of germ tube formation and the stage of mycelial expansion. When S. salivarius K12 was preliminarily cultured aerobically in a low-ionic-strength medium with properties like those of saliva, the absorption at 620 nm increased, binding to Candida cells was enhanced, and the adherence of Candida cells to the petri dish was further inhibited.
The planktonic cells from mixed culture of S. salivarius K12 and Candida cells proportionally increased according to the concentration of S. salivarius K12 in the medium. The planktonic cells were composed of the mycelial form of Candida cells and appeared to be surrounded by S. salivarius, which may decrease its adhesive ability and pathogenic potential. To analyze the cross-kingdom interaction of Candida cells and S. salivarius in detail, vital staining with FUN1 for viability check and calcofluor white for yeast body analysis was undertaken, and it was confirmed that Candida cells were surrounded by S. salivarius. Interestingly, almost all of these were the mycelial form, and the staining indicated that they were viable. These findings, i.e., an increase in the number of planktonic Candida cells able to form colonies with the presence of S. salivarius K12 in the medium, were in agreement with the results observed in Fig. 2. This interaction was kinetically observed from 30 min to 6 h, and the results showed that more than 30 min was needed for adhesion and 1 h was enough for interaction (data not shown).
The results of tests of the in vivo effects of S. salivarius K12 against murine experimental oral candidiasis indicated that S. salivarius K12 had the ability to protect against severe fungal infection in a dose-dependent manner in the model used. The symptom scoring of mouse tongues and histological studies of their fungal burdens indicated the appearance of an infection less severe than that in the control group. However, those in the fluconazole group showed no symptoms of infection after treatment. Further studies may ascertain whether the S. salivarius K12 treatment over a longer period of time will reduce the infection.
The protective or therapeutic efficacy against oral candidiasis was evaluated, as multiple possible probiotic mechanisms were thought to be involved. These mechanisms include not only the fungicidal effect or the inhibitory effect on germ tube formation but also blocking of the attachment of mycelium to the host epithelial cells. Additionally, the reduction of C. albicans attachment to artificial dentition or acrylic resin was previously presumed to be an important mechanism for infection prevention (9). Saliva, which commonly contains S. salivarius, also has defensive effects and may play a key role in the process. Previous studies showed that adhesion of C. albicans germ tubes to polystyrene is decreased by saliva, whereas C. albicans yeast cell adhesion to the same material is enhanced (8, 14). One may postulate that the possible action of S. salivarius K12 in vivo might involve the latter type of effect, whereby the rolled up Candida mycelial form prevents adhesion to mucosal surfaces of the oral cavity, resulting in the fungus then traveling harmlessly through to the esophagus and beyond. This was an interesting result, in that previous studies of the antimicrobial activity of S. salivarius K12 against bacteria indicated that activity resulted from its bacteriocin production, since the percolation liquid in the culture, including bacteriocins from S. salivarius K12, did not appear to affect C. albicans when tested here, as shown in Table 1.
This is the first report that the direct interaction between bacteria and Candida induces a protective effect against oral candidiasis in an animal model and in vitro assay systems. Previous studies of the cross-kingdom interaction of bacteria and fungi have focused upon direct antimicrobial interactions or interactions through chemical mechanisms, such as quorum-sensing molecules or terpenoids (10, 17, 18). Despite the abundance of bacterium-fungus interactions in nature and the clinical environment, very little is known about the molecular mechanisms underlying these interactions and their importance to human health (7, 16, 19, 21, 24). Human microbial infections are often found to be polymicrobial in composition and may include bacteria and fungi. These complex microbial consortiums are also usually structured into biofilms, which have increased resistance against antimicrobials, enhanced colonization, and enhanced interspecies antagonism (7, 16). There are examples in the literature where polymicrobial combinations of opportunistic pathogens are thought to be much more deleterious than monoculture alone, such as S. mutans and Candida, which have been reported to produce a mixed biofilm and to make candidiasis more severe (20).
While there are various reports on the antibacterial activities of S. salivarius K12 and other strains with bacteriocin action in the literature (28, 30, 31), Candida albicans was not directly inhibited by bacteriocin action, and it appears that yeast cell-to-bacterial cell contact may be required. In this study, S. salivarius K12 directly interacted with Candida, as demonstrated by in vitro assays, and also showed a protective effect in a murine model of Candida infection. S. salivarius K12 appeared to inhibit the colonization of Candida by both direct and indirect mechanisms. It is not known if these properties are unique to this particular strain of S. salivarius or to the species in general; however, this strain has a history of safe use, and a human clinical study is warranted. The data obtained in this study suggest that the use of S. salivarius K12 as an oral probiotic for the prevention or treatment of oral candidiasis may have merit and warrants further clinical investigations. The mechanisms of the therapeutic effect of S. salivarius K12 against oral candidiasis will be studied in detail in future experiments.
ACKNOWLEDGMENTS
J. P. Burton was a member of the finders of S. salivarius K12 and established the company BLIS Technologies Ltd. at the Centre for Innovation of Otago University. Y. Mastushita is a member of the branch of BLIS Technologies Ltd. in Japan.
Footnotes
Published ahead of print 20 January 2012
REFERENCES
- 1. Abe S, Satoh T, Tokuda Y, Tansho S, Yamaguchi H. 1994. A rapid colorimetric assay for determination of leukocyte-mediated inhibition of mycelial growth of Candida albicans. Microbiol. Immunol. 38:385–388 [DOI] [PubMed] [Google Scholar]
- 2. Burton JP, Chilcott CN, Tagg JR. 2005. The rationale and potential for the reduction of oral malodour using Streptococcus salivarius probiotics. Oral Dis. 11(Suppl 1):29–31 [DOI] [PubMed] [Google Scholar]
- 3. Burton JP, et al. 2011. Evaluation of safety and human tolerance of the oral probiotic Streptococcus salivarius K12: a randomized, placebo-controlled, double-blind study. Food Chem. Toxicol. 49:2356–2364 [DOI] [PubMed] [Google Scholar]
- 4. Burton JP, Chilcott C, Wescombe PA, Tagg JR. 2010. Extended safety data for the oral cavity probiotic Streptococcus salivarius K12. Probiot. Antimicrob. Prot. 2:135–144 [DOI] [PubMed] [Google Scholar]
- 5. Burton JP, Wescombe PA, Moore CJ, Chilcott CN, Tagg JR. 2006. Safety assessment of the oral cavity probiotic Streptococcus salivarius K12. Appl. Environ. Microbiol. 72:3050–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carlsson J. 1971. Nutritional requirements of Streptococcus salivarius. J. Gen. Microbiol. 67:69–76 [DOI] [PubMed] [Google Scholar]
- 7. De Sordi L, Mühlschlegel FA. 2009. Quorum sensing and fungal-bacterial interactions in Candida albicans: a communicative network regulating microbial coexistence and virulence. FEMS Yeast Res. 9:990–999 [DOI] [PubMed] [Google Scholar]
- 8. Elguezabal N, Maza JL, Dorronsoro S, Ponton J. 2008. Whole saliva has a dual role on the adherence of Candida albicans to polymethylmetacrylate. Open Dent. J. 2:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellepola AN, Samaranayake LP. 2000. Oral candidal infections and antimycotics. Crit. Rev. Oral Biol. Med. 11:172–198 [DOI] [PubMed] [Google Scholar]
- 10. Hogan DA, Vik A, Kolter R. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 54:1212–1223 [DOI] [PubMed] [Google Scholar]
- 11. Hyink O, et al. 2007. Salivaricin A2 and the novel lantibiotic salivaricin B are encoded at adjacent loci on a 190-kilobase transmissible megaplasmid in the oral probiotic strain Streptococcus salivarius K12. Appl. Environ. Microbiol. 73:1107–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishijima SA, et al. 2011. N-Acetylglucosamine increases symptoms and fungal burden in a murine model of oral candidiasis. Med. Mycol [Epub ahead of print.] doi:10.3109/13693786.2011.598194 [DOI] [PubMed] [Google Scholar]
- 13. James SM, Tagg JR. 1991. The prevention of dental caries by BLIS-mediated inhibition of mutans streptococci. N. Z. Dent. J. 87:80–83 [PubMed] [Google Scholar]
- 14. Kamagata-Kiyoura Y, Abe S, Yamaguchi H, Nitta T. 2003. Detachment activity of human saliva in vitro for Candida albicans cells attached to a plastic plate. J. Infect. Chemother. 9:215–220 [DOI] [PubMed] [Google Scholar]
- 15. Kamagata-Kiyoura Y, Abe S. 2005. Recent studies on oral candidiasis using a murine model. J. Oral Biosci. 47:60–64 [Google Scholar]
- 16. Kumamoto CA, Vinces MD. 2005. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell. Microbiol. 7:1546–1554 [DOI] [PubMed] [Google Scholar]
- 17. Morales DK, Hogan DA. 2010. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 6:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nickerson KW, Atkin AL, Hornby JM. 2006. Quorum sensing in dimorphic fungi: farnesol and beyond. Appl. Environ. Microbiol. 72:3805–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ogawa A, et al. 2011. Inhibition of Streptococcus mutans biofilm formation by Streptococcus salivarius FruA. Appl. Environ. Microbiol. 77:1572–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peleg AY, Hogan DA, Mylonakis E. 2010. Medically important bacterial-fungal interactions. Nat. Rev. Microbiol. 8:340–349 [DOI] [PubMed] [Google Scholar]
- 21. Pereira-Cenci T, et al. 2008. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch. Oral Biol. 53:755–764 [DOI] [PubMed] [Google Scholar]
- 22. Sardi JC, et al. 2010. Candida spp. in periodontal disease: a brief review. J. Oral Sci. 52:177–185 [DOI] [PubMed] [Google Scholar]
- 23. Shapiro RS, Robbins N, Cowen LE. 2011. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 75:213–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shirtliff ME, Peters BM, Jabra-Rizk MA. 2009. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol. Lett. 299:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tagg JR, Bannister LV. 1979. “Fingerprinting” beta-haemolytic streptococci by their production of and sensitivity to bacteriocine-like inhibitors. J. Med. Microbiol. 12:397–411 [DOI] [PubMed] [Google Scholar]
- 26. Tagg JR, Dajani AS, Wannamaker LW. 1976. Bacteriocins of gram-positive bacteria. Bacteriol. Rev. 40:722–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takakura N, et al. 2003. A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiol. Immunol. 47:321–326 [DOI] [PubMed] [Google Scholar]
- 28. Tamura S, et al. 2009. Inhibiting effects of Streptococcus salivarius on competence-stimulating peptide-dependent biofilm formation by Streptococcus mutans. Oral Microbiol. Immunol. 24:152–161 [DOI] [PubMed] [Google Scholar]
- 29. Thompson GR, III, et al. 2010. Oropharyngeal candidiasis in the era of antiretroviral therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109:488–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walls T, Power DA, Tagg JR. 2003. Bacteriocin-like inhibitory substance (BLIS) production by the normal flora of the nasopharynx: potential to protect against otitis media? J. Med. Microbiol. 52:829–833 [DOI] [PubMed] [Google Scholar]
- 31. Wescomebe PA, Heng NCK, Burton JP, Chilcott CN, Tagg R. 2009. Streptococcus bacteriocins and the case for Streptococcus salivarius as model oral probiotics. Future Microbiol. 4:819–835 [DOI] [PubMed] [Google Scholar]