Abstract
One and two dimensional NMR techniques have been used together with molecular modelling to obtain the solution structure for the photoproduct d(TpA)*. The NMR data confirm that the cyclobutane linkage is formed between the bonds thymine C6-C5 and adenine C5-C6. The 2D NOE data are used as constraints in a distance geometry calculation. The structures obtained show a trans-syn cyclobutane linkage and the glycosidic angles are SYN and ANTI for thymidine and deoxyadenosine, respectively. The coupling constant data are used to check the backbone torsion angles of the obtained structures. Typical torsion angles are a gamma+ and beta t for the deoxyadenosine residue. A free molecular dynamics simulation of a trans-syn d(TpA) photoproduct confirmed all these structural characteristics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bose S. N., Davies R. J., Sethi S. K., McCloskey J. A. Formation of an adenine-thymine photoadduct in the deoxydinucleoside monophosphate d(TpA) and in DNA. Science. 1983 May 13;220(4598):723–725. doi: 10.1126/science.6836308. [DOI] [PubMed] [Google Scholar]
- Bose S. N., Kumar S., Davies R. J., Sethi S. K., McCloskey J. A. The photochemistry of d(T-A) in aqueous solution and in ice. Nucleic Acids Res. 1984 Oct 25;12(20):7929–7947. doi: 10.1093/nar/12.20.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J., Voituriez L., Hruska F. E., Grand A. Crystal structure of the cis-syn photodimer of thymidylyl (3'-5') thymidine cyanoethyl ester. Biopolymers. 1985 May;24(5):897–903. doi: 10.1002/bip.360240512. [DOI] [PubMed] [Google Scholar]
- Cheng D. M., Sarma R. H. Intimate details of the conformational characteristics of deoxyribodinucleoside monophosphates in aqueous solution. J Am Chem Soc. 1977 Oct 26;99(22):7333–7348. doi: 10.1021/ja00464a038. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B. On the conformational dependence of the proton chemical shifts in nucleosides and nucleotides. I. Proton shifts in the ribose ring of pyrimidine nucleosides as a function of the torsion angle about the glycosyl bond. J Theor Biol. 1977 Mar 7;65(1):171–188. doi: 10.1016/0022-5193(77)90082-0. [DOI] [PubMed] [Google Scholar]
- Kan L., Voituriez L., Cadet J. Nuclear magnetic resonance studies of cis-syn, trans-syn, and 6-4 photodimers of thymidylyl(3'-5')thymidine monophosphate and cis-syn photodimers of thymidylyl(3'-5')thymidine cyanoethyl phosphotriester. Biochemistry. 1988 Jul 26;27(15):5796–5803. doi: 10.1021/bi00415a060. [DOI] [PubMed] [Google Scholar]
- Kaptein R., Zuiderweg E. R., Scheek R. M., Boelens R., van Gunsteren W. F. A protein structure from nuclear magnetic resonance data. lac repressor headpiece. J Mol Biol. 1985 Mar 5;182(1):179–182. doi: 10.1016/0022-2836(85)90036-1. [DOI] [PubMed] [Google Scholar]
- Kemmink J., Boelens R., Kaptein R. Two-dimensional 1H NMR study of two cyclobutane type photodimers of thymidylyl-(3'----5')-thymidine. Eur Biophys J. 1987;14(5):293–299. doi: 10.1007/BF00254894. [DOI] [PubMed] [Google Scholar]
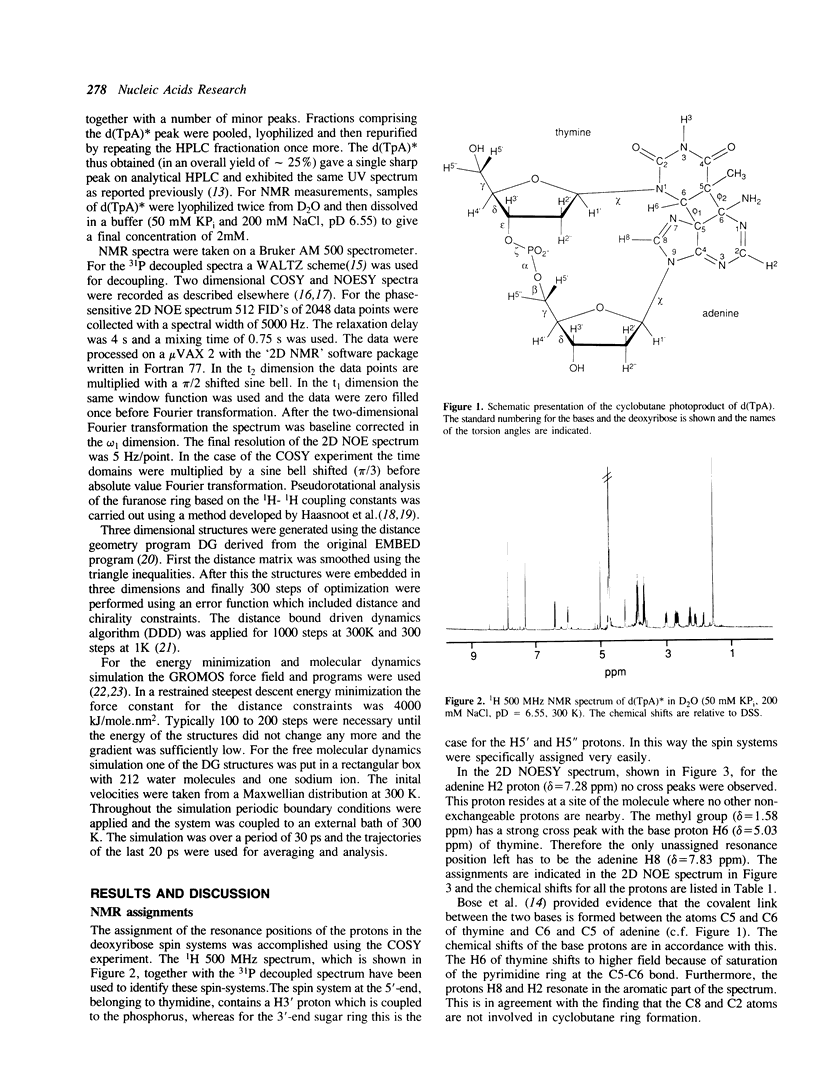
- Kemmink J., Boelens R., Koning T. M., Kaptein R., van der Marel G. A., van Boom J. H. Conformational changes in the oligonucleotide duplex d(GCGTTGCG) x d(CGCAACGC) induced by formation of a cis-syn thymine dimer. A two-dimensional NMR study. Eur J Biochem. 1987 Jan 2;162(1):37–43. doi: 10.1111/j.1432-1033.1987.tb10538.x. [DOI] [PubMed] [Google Scholar]
- Kumar S., Sharma N. D., Davies R. J., Phillipson D. W., McCloskey J. A. The isolation and characterisation of a new type of dimeric adenine photoproduct in UV-irradiated deoxyadenylates. Nucleic Acids Res. 1987 Feb 11;15(3):1199–1216. doi: 10.1093/nar/15.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankhorst P. P., Haasnoot C. A., Erkelens C., Altona C. Carbon-13 NMR in conformational analysis of nucleic acid fragments. 2. A reparametrization of the Karplus equation for vicinal NMR coupling constants in CCOP and HCOP fragments. J Biomol Struct Dyn. 1984 Jun;1(6):1387–1405. doi: 10.1080/07391102.1984.10507527. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Yang N. C. Photochemistry of cytosine derivatives. 1. Photochemistry of thymidylyl-(3' leads to 5')-deoxycytidine. Biochemistry. 1978 Nov 14;17(23):4865–4876. doi: 10.1021/bi00616a003. [DOI] [PubMed] [Google Scholar]
- Pörschke D. A specific photoreaction in polydeoxyadenylic acid. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2683–2686. doi: 10.1073/pnas.70.9.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycyna R. E., Alderfer J. L. UV irradiation of nucleic acids: formation, purification and solution conformational analysis of the '6-4 lesion' of dTpdT. Nucleic Acids Res. 1985 Aug 26;13(16):5949–5963. doi: 10.1093/nar/13.16.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycyna R. E., Wallace J. C., Sharma M., Alderfer J. L. Ultraviolet irradiation of nucleic acids: formation, purification, and solution conformational analyses of oligothymidylates containing cis-syn photodimers. Biochemistry. 1988 May 3;27(9):3152–3163. doi: 10.1021/bi00409a006. [DOI] [PubMed] [Google Scholar]
- Taylor J. S., Garrett D. S., Cohrs M. P. Solution-state structure of the Dewar pyrimidinone photoproduct of thymidylyl-(3'----5')-thymidine. Biochemistry. 1988 Sep 20;27(19):7206–7215. doi: 10.1021/bi00419a007. [DOI] [PubMed] [Google Scholar]
- Taylor J. S., Garrett D. S., Wang M. J. Models for the solution state structure of the (6-4) photoproduct of thymidylyl-(3'----5')-thymidine derived via a distance- and angle-constrained conformation search procedure. Biopolymers. 1988 Oct;27(10):1571–1593. doi: 10.1002/bip.360271004. [DOI] [PubMed] [Google Scholar]



