Abstract
Objective
To determine event free survival (EFS) of children with Wilms tumor (WT) and metastatic liver disease at diagnosis.
Summary and Background data
We reviewed patients with stage IV Wilms tumor treated on National Wilms Tumor Study 4 and -5 to ascertain if they have a worse prognosis than other Stage IV disease.
Methods
742 patients (pts) with stage IV disease were assessed for EFS (95% confidence interval [CI]) at 5 years after diagnosis. Cohorts included those who underwent resection of the liver lesions compared with those who received only chemotherapy and radiotherapy (XRT).
Results
Seven hundred and forty two patients with stage IV Wilms tumor were enrolled on NWTS-4 and 5, 111 of who had liver metastases. Of these, 96 had favorable histology disease and are the focus of this analysis. Twenty-two patients had a primary liver resection (wedge resection - 18 and lobectomy – 4). After chemotherapy and/or radiation, 13 patients underwent liver resection (wedge resection - 7, lobectomy - 5 and trisegmentectomy – 1). Seventy-one patients (67%) did not undergo surgery for their liver disease. In 14 patients the liver disease disappeared with chemotherapy only. Eighty-two patients received abdominal radiation. EFS for the patients with metastatic FH Wilms tumor was 75% (95% confidence interval [CI]: (71%, 78%), EFS by Stage IV category was: lung only 76% (95% CI: 72%, 80%)(513 patients); liver, not lung 76% (95% CI: 58%, 87%)(34 patients); liver and lung 70% (95% CI: 57%, 80%)(62 patients) and other sites 64% (95% CI: 42%, 79%)(25 patients). There were no significant differences among stage IV groups (p=0.60). EFS (95% CI) for the patients with primary resection of the liver metastases (22 patients) was 86% (63%, 95%) compared to 68% (56%, 78%) (p=0.09) for the 74 with no primary resection of liver metastases. There was no significant difference in EFS for patients with FH Wilms tumor treated with chemotherapy compared to that of patients treated with chemotherapy and radiation (p=0.63). The EFS (95% CI) for each of the subsets was; no abdominal radiation: 64% (34%, 83%); abdominal radiation, no boost: 77% (55%, 89%); abdominal radiation, boost: 72% (58%, 82%) (p=0.05).
Conclusion
Liver metastasis at diagnosis is not an adverse prognostic factor for stage IV metastatic FH WT.
Introduction
Metastatic disease (Stage IV) is recognized as a poor prognostic factor for children with Wilms tumor.1 Published data from the National Wilms Tumor Study Group (NWTSG) showed equivalent outcomes regardless of the site of metastatic disease although no study focused specifically on stage IV disease due to hepatic metastases.2–5 Recent reports, however, suggested that liver involvement at diagnosis in infants and children with Wilms tumor indicated a worse prognosis than lung or other sites of Stage IV disease.6–8 The International Society of Pediatric Oncology (SIOP) and the German Pediatric Oncology Group (GPOH) studied 29 patients with liver metastases at diagnosis, including some adult patients with Wilms tumor.7 The overall survival was less than 60%, but all patients who had complete resection of the hepatic lesions survived. Another report from Varan et al of 18 patients with liver metastases also noted a poorer outcome than for patients with pulmonary disease (50.2% vs. 16.6%).6 These authors suggested, based on the poor outcomes of patients with liver metastases, that more intensive chemotherapy and more aggressive surgical treatment was warranted. This would be a significant change in approach that potentially could increase morbidity and mortality for these patients.
To address the recommendations suggested by these investigators and their studies, we reviewed patients with stage IV Wilms tumor treated on NWTS-4 and -5. We hypothesized that children with Wilms tumor metastatic to the liver had outcomes similar to those with pulmonary metastases alone. Furthermore, we hypothesized that survival for children with Wilms tumor and hepatic metastases was equivalent with or without resection of the hepatic tumors.
Methods
NWTS-4 and -5 were prospective studies with NWTS-4 being a randomized controlled study.2,9,10 The primary hypotheses for NWTS-4 and -5 have been described previously.2,9–11 Patients underwent nephrectomy before chemotherapy using previously described surgical guidelines unless the primary tumor was considered to be unresectable by the treating surgeon, in which case a biopsy was obtained followed by initial chemotherapy. A tumor stage was assigned using the NWTSG surgical-pathologic staging system. Patients received flank or whole abdominal radiation therapy (XRT) according the local extent of disease (local stage). The liver irradiation guidelines were similar in NWTS-4 and -5. Liver irradiation was indicated only for metastatic lesions that were not completely resected at diagnosis. The radiation treatment portal included the lesion/s with a margin of 2cm into adjacent uninvolved liver. If the entire organ was known to be involved or is suspected of being diffusely involved, then a dose of 19.8Gy was delivered to the entire liver. Lesser volumes could get 5.4 – 10.8Gy at the discretion of the radiation oncologist. It was recommended that a dose of 30.6Gy should not be given to > 75% of the entire liver volume. If patients underwent primary resection of > 25% of the liver and still required liver irradiation, it was recommended that actinomycin-D and radiation therapy be withheld until there was evidence that liver regeneration had been established with serial liver function tests, and this generally required an interval of at least 10 days from the date of surgery. There were no specific surgical guidelines for liver disease
NWTS-4 opened August 1986 and closed September 1994 with 2,345 patients enrolled; 2,006 had favorable histology (FH) tumors. NWTS-5 opened January 1996 and closed June 2002; 2,596 patients were enrolled with 2,397 having FH tumors. All patients registered on NWTS-4 and -5 with stage IV disease were identified and reviewed. A standardized data collection instrument (“COG Wilms Tumor Hepatic Metastasis Project”) was used to collect information from the patient charts available at the Data and Statistical Center of the NWTSG in Seattle, WA. This included the patient demographics, initial surgical procedure, kidney tumor characteristics, pathology, treatment regimen (chemotherapy and radiation therapy), extent of liver disease, the surgical therapy for the liver disease and any complications. Patients with Stage IV disease were divided into 4 groups: those with lung disease only, those with liver disease only, those with both liver and lung metastases and those with Stage IV disease due to other sites of metastasis (brain, spine, bone marrow). Patients with rhabdoid tumor of the kidney or clear cell sarcoma (and other renal tumor pathologies) were excluded as well as those with incomplete or indeterminate records. Analysis of outcome data was restricted to those patients for whom the review diagnosis was known and was either Stage 4 FH or anaplastic Wilms tumor. Patients that have direct invasion of the liver from the primary renal tumor into the liver were also excluded.
Statistical analysis
Event-free survival (EFS) and overall survival (OS) at 5 years after diagnosis were estimated by actuarial methods of Kaplan and Meier.12 Comparisons of EFS and OS between patient subgroups were made with the log-rank test.13 Comparisons of mean age at diagnosis by histology were made using the t test. Comparisons of sex and stage distribution by histology were made using the Fisher's exact and related tests14 Cohort comparisons included those who underwent resection of the liver lesions compared with those who received only chemotherapy, and those who received chemotherapy and radiotherapy.
Results
Seven hundred and forty-two patients with stage IV Wilms tumor were enrolled on NWTS-4 and -5 (Table 1). Of these, 111 had liver metastases at diagnosis. The patient demographics and tumor characteristics of the patients are shown in table 2. The extent of liver disease at presentation was right lobe only in 43 patients (40%), left lobe only in 10 patients (9%) and both lobes in 54 patients (50%). In four cases the extent of liver disease was unknown. Metastatic workup was done using a variety of imaging modalities. Computed tomography (CT) scans correctly identified liver involvement in 84/96 cases, ultrasound in 21/50 cases, and magnetic resonance imaging (MRI) in 9/35 cases. In 17 cases liver disease was diagnosed only at surgery. One hundred six of the 111 cases with Wilms tumor with hepatic involvement had complete data and were able to be analyzed. The final histologic subtypes of these patients were; favorable histology (FH) 96 (91%), focal anaplasia 1 (1%), and diffuse anaplasia 9 (8%). Due to the small number of anaplastic tumors and their potentially confounding influence on prognosis, the analysis was restricted to the 96 patients with FH Wilms tumor.
Table 1.
Site of distant metastasis in children with Stage IV disease from NWTS-4 and 5
| Stage 4 Disease | Histology | ||
|---|---|---|---|
| FH | Focal Anaplasia | Diffuse Anaplasia | |
| Lung only | 513 | 25 | 71 |
| Liver no lung(1) | 34 | 0 | 1 |
| Liver and Lung(2) | 62 | 1 | 8 |
| Other(3) | 25 | 1 | 1 |
No lung metastasis, but could have metastatic disease elsewhere
Could have metastatic disease elsewhere
Metastatic disease other than lung and liver
Table 2.
Patient characteristics of the 111 patients with Wilms tumor and hepatic metastasis
| Patient Characteristic | Number | percent |
|---|---|---|
| Age at diagnosis | ||
| 0–23 months | 4 | (4%) |
| 24–59 months | 45 | (41%) |
| 60–119 | 51 | (46%) |
| 120+months | 11 | (10%) |
| Sex | ||
| Male | 48 | (43%) |
| Female | 63 | (57%) |
| Final Histologic subtype | ||
| Favorable histology | 96 | (86.6%) |
| Focal anaplasia | 1 | (0.9%) |
| Diffuse anaplasia | 9 | (8%) |
| Unknown | 5 | (4.5% |
| Primary tumor site | ||
| Left kidney | 67 | (60.4%) |
| Right kidney | 40 | (36%) |
| Single kidney | 1 | (0.9%) |
| Horseshoe kidney | 2 | (1.8%) |
| Unknown | 1 | (0.9%) |
The initial surgical management of the 96 FH patients with complete data is shown in table 3. Twenty-two patients had a primary liver resection (wedge resection - 18 and lobectomy - 4. After initial surgery, the adjuvant therapy was determined based on the pathology, stage and the protocol. The management of the liver metastases is detailed in Table 4. After chemotherapy and/or radiation, 13 patients underwent liver resection (wedge resection - 7, lobectomy - 5 and trisegmentectomy – 1). Seventy-one patients (67%) did not undergo resection of their liver metastasis(es). Liver disease disappeared with chemotherapy only in 14 patients. Abdominal radiation was used in 82 patients with or without a liver boost.(Table 5).
Table 3.
Initial surgical treatment for all 96 children with a diagnosis of Stage IV FH Wilms tumor and liver metastasis.
| Surgical treatment | Number |
|---|---|
| Complete nephrectomy | 21 |
| Complete nephrectomy and primary liver resection | 22 |
| Complete nephrectomy and liver biopsy | 16 |
| Biopsy of kidney only | 26 |
| Biopsy of liver and kidney | 11 |
Table 4.
Final treatment for the 96 patients with Stage IV FH Wilms tumor and liver metastasis.
| Chemotherapy only | Chemotherapy and XRT | |
|---|---|---|
| Complete nephrectomy with 1° liver resection | 8 | 13 |
| Complete nephrectomy with 2° liver resection | 2 | 11 |
| Complete nephrectomy | 4 | 58 |
Table 5.
Radiation therapy for the 96 patients with FHWT, Stage IV and liver metastasis.
| Standard XRT | Standard plus Liver XRT | |
|---|---|---|
| Complete nephrectomy with 1° liver resection | 12 | 1 |
| Complete nephrectomy with 2° liver resection | 9 | 2 |
| Complete nephrectomy | 31 | 27 |
Legend
FH = favorable histology
XRT = radiation therapy
The estimated 5-year EFS (95% confidence interval [CI]) for the 634 patients with metastatic FH Wilms tumor was 75%: (71%, 78%). The 5-year EFS (95% CI) by Stage IV category was : lung only 76% (72%, 80%)(513 patients); liver, not lung 76% (58%, 87%)(34 patients); liver and lung 70% (57%, 80%)(62 patients) and other sites 64% (42%, 79%)(25 patients) (Fig.1). There were no significant differences among stage IV categories (p=0.60).
1.
Estimated 5-year event-free survival (EFS) of patients with Stage IV disease by site of metastasis. The x axis is time and the y axis is proportion.
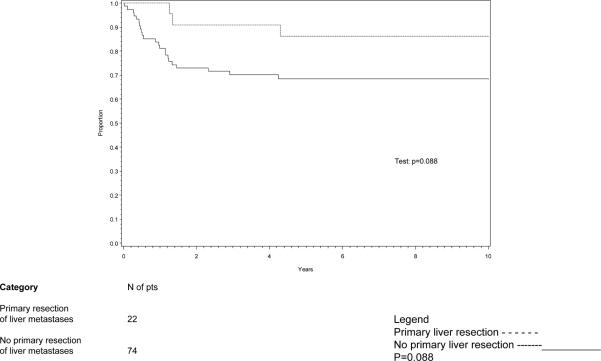
EFS for children with FH Wilms tumor and hepatic metastasis with or without primary resection of the hepatic tumors is shown in Figure 2. Event-free survival (EFS, 95% CI) for the patients with liver metastases and a primary resection of the liver metastases (22 patients) was 86% (63%, 95%) compared to 68% (56%, 78%) (p=0.09) for the 74 patients who did not undergo primary resection of the liver metastases (Figure 2).
2.
Event-free survival (EFS) for FH patients with liver metastases, by whether primary resection of metastases.
The EFS for patients with FH Wilms tumor treated with chemotherapy only, chemotherapy with standard abdominal radiation therapy and chemotherapy with radiation boost is shown in Figure 3. There were no significant differences among groups (p=0.63). The estimated 5-year EFS (95% CI) for each of the subsets was: no abdominal radiation: 64% (34%, 83%); abdominal radiation, no boost: 77% (55%, 89%); abdominal radiation, boost: 72% (58%, 82%).
3.
Estimated event-free survival (EFS) correlated with radiation treatment.
Discussion
Risk stratification is increasingly sophisticated using tools such as molecular tests, genetic analysis and large clinical trials.1,11,15 Current prognostic factors used by the Children's Oncology Group (COG) renal tumors committee include; pathology (favorable vs. unfavorable), stage (I–IV), age less then 2 years and tumor weight less then 550 grams for children with stage I or II, and loss of heterozygosity at loci on 1p and 16q.11,16,17 Subtypes of stage IV disease such as stage IV due to liver metastasis and stage IV due to pulmonary metastasis have not been identified previously as independent prognostic factors. What has been reported is that there is no difference in survival according to metastatic site (liver +/− lung vs. lung only) if present prior to treatment. By contrast, patients who developed liver metastases during or after treatment had an especially poor chance for survival as compared with those who developed lung deposits at those times.18
We formulated three hypotheses to address liver metastasis in a child with a Wilms tumor. The first was that children with Wilms tumor metastatic to the liver had outcomes similar to those with pulmonary metastasis. In this analysis of 129 patients from NWTS-4 and -5, we did not find that liver metastasis was an independent adverse prognostic factor for children with stage IV FH Wilms tumor. Our results, which are consistent with previous NWTS reports, would not support a more aggressive initial surgical approach for a child with Wilms tumor and liver metastasis. Varan et al. reported poor outcomes of patients with liver metastases.6 This single institutional report included 542 patients treated over thirty years, 57 of whom had stage IV disease, including 18 with liver metastases. The overall survival rate was 50.2% for those with lung metastases compared to 16.6% for those with liver metastases. The overall survival was only 56.2% for those treated after 1990 compared to 75% EFS for patients with stage IV disease in the present study. This difference suggests that other factors may have contributed to the poorer outcomes of the patients reported by Varan et al., such as timing and dosing of chemotherapy and radiation. These authors acknowledged the limitations of their single institution report of a rare disease compared to that of a large randomized controlled trial.
The second hypothesis was that survival for children with Wilms tumor and hepatic metastasis was equivalent with or without primary resection of the hepatic tumors. We assessed the therapy and outcome of patients with regard to treatment of their hepatic metastases. Although EFS for the patients who had primary resection of their liver metastases was higher than for those not resected, this was not statistically significant. If the cohort of patients with a primary resection of their liver metastases had been larger, we might have been able to demonstrate that overall outcome was improved. However, these patients may be highly selected (e.g. ease with which surgery could result in complete resection of liver disease). The extent of the hepatic metastatic disease and other factors which lead the surgeon to proceed with primary resection may be additional predictors of a favorable outcome. Thus, the improved outcome may be the result of having limited hepatic disease and being a more appropriate surgical candidate. Furthermore we did not conduct central radiology review and therefore cannot provide an estimate of how many could have undergone primary resection (as is the current practice). Only a prospective study in which patients were randomized between immediate versus delayed resection of liver metastases could answer this question.
The SIOP/GPOH group reported the outcomes of 29 patients treated on SIOP93-01/GPOH and SIOP2001/GPOH that enrolled 1,365 patients between April 1994 and September 2004. The five-year OS was 62.6% for those with hepatic metastases while the OS for all stage IV patients was 76.3%. The difference was not analyzed statistically. The SIOP histology and classification of these cases included four low risk, 18 intermediate risk, four high risk (two with diffuse anaplasia). Three tumors could not be classified because death occurred before therapy was started. Within the group of 29 children with hepatic involvement at presentation, 14 did not undergo a liver resection. In 4/29 (13%) patients, the liver metastasis responded to chemotherapy (three of whom are still alive). This response to chemotherapy was similar to our data in which 14.5%(14/96) of the cases responded to treatment of the liver metastasis with chemotherapy only. Fifteen patients had liver surgery (primary liver resection – 11; complete resection - 6 and incomplete resection – 5). All six who had a complete resection survived. Four patients underwent liver resection after adjuvant therapy, three of whom had a complete resection and all three survived. The improved survival for their patients with complete resection may reflect the same selection bias as for the NWTSG group. Alternatively, it may suggest that those tumors that responded to chemotherapy and were amenable to resection had a more favorable biology.
In both our study and the SIOP study, adult patients comprised part of the study population. In our study less then 10% were over 12 years old and 3% were over 16. In some studies older age itself has been an adverse prognostic factor19–21 although other studies showed that adults treated by stage-appropriate combined modality therapy had similar outcomes to children.22,23
A subanalysis of the liver lesions comparing those who received only chemotherapy to those who received chemotherapy and radiotherapy (with and without boost) was also performed. There was no statistical difference in survival based on the use of abdominal radiation, but the cohort of patients for analysis was small (N=14). There was no difference in outcomes for those children who received abdominal boost radiation compared to those who did not. However the doses of boost radiation were not uniform, making any assessment of efficacy difficult.
The use of radiation therapy is a concern for both short term hepatic toxicity and long term development of complications. Hepatotoxicity is a problem for children being treated for Wilms tumor.24–26 Both chemotherapy (dactinomycin) and abdominal irradiation have been shown to be important factors. Previous reports have shown that the risk of developing hepatic toxicity is significantly increased in patients receiving abdominal irradiation involving the liver, with a relative risk (RR) of 2.6.24 Long term complications from abdominal radiation are musculoskeletal problems (muscular hypoplasia, limb length inequality, kyphosis, and iliac wing hypoplasia) and second malignancy.27 Two studies of long term cancer survivors have shown that radiation is the principal contributing factor for second malignancy in patients treated for Wilms tumors.28,29 We could not detect based on the sample size available, whether routine use of a radiation boost to the liver impacted outcome. The absence of a statistically significant effect does not mean that a significant effect is not present – only that we cannot detect it.
The third hypothesis was that resection of hepatic metastasis from Wilms tumor was associated with a significant risk of surgical complications. The data for surgical complications could not be accurately analyzed in this retrospective review. Surgery was not one of the primary questions addressed in the protocols thus the data were not collected prospectively.
The SIOP/GPOH report recommended an aggressive initial surgical approach to patients with liver disease.7 It is essential to note the SIOP patients receive preoperative chemotherapy before nephrectomy and or resection of metastatic lesions. This is in contrast to the patients in this report treated with immediate nephrectomy with or without resection of metastatic lesions before chemotherapy with or without radiation therapy is given.
There are limitations in both the report from SIOP and the present study. We excluded 10 patients with anaplastic histology because of the small numbers, however if included the overall outcomes would not have changed. Furthermore this is a retrospective study and assessing early versus delayed liver resection was not part of the study objectives, therefore to definitively answer this question a future study with this as an objective would need to be conducted. The SIOP study did not report OS based on site of metastasis. The 5-year survival for all SIOP stage IV patients was similar to that of similar patients in the NWTSG studies. None of the data reported suggested that an initial aggressive surgical resection of the liver lesions in these patients was warranted. However, when considering liver resection as part of the initial therapy, complete resection was beneficial as all patients who had a complete resection survived compared to only one who had an incomplete resection. Nine of the 15 (66%) who had liver resection survived compared to 50% (7/14) of those who did not undergo liver resection. We do not know if this difference was statistically significant, why the 14 patients did not undergo a liver resection, nor what role adjuvant therapy had in converting an unresectable metastasis to a resectable one. Those that did not resolve with chemotherapy may have had more unfavorable histology.
In the SIOP study, tumor burden and response to therapy were not presented. We could not reliably comment in the present study on the extent of response of liver metastases to treatment based on the subsequent radiology reports.
The current study is the largest series in which the treatment and outcomes of patients with Wilms tumor metastatic to the liver were analyzed. We found no evidence that the presence of liver metastases was an adverse prognostic factor for patients with stage IV metastatic FH Wilms tumor treated on NWTS-4 and -5. There was no evidence to support an aggressive primary resection of the hepatic tumors. The impact of boost radiation to liver metastases on survival was not resolved. In both the present and the SIOP analyses, those patients with residual liver disease after treatment with chemotherapy and/or radiation that could be completely resected did well, suggesting there is a role for complete surgical resection of residual metastases after adjuvant therapy.
References
- 1.Dome JS, Perlman EJ, Ritchey ML, et al. Renal Tumors. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Lippincott Williams and Wilkins; Philadelphia: 2006. pp. 905–932. [Google Scholar]
- 2.Green DM, Breslow N, Evans I, et al. Treatment of children with stage IV favorable histology Wilms tumor: a report from the National Wilms Tumor Study Group. Med Pediatr Oncol. 1996;26:147–152. doi: 10.1002/(SICI)1096-911X(199603)26:3<147::AID-MPO1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Green DM, Thomas PRM, Schocat S. The treatment of Wilms tumor. Results of the National Wilms Tumor Studies. Hematol Oncol Clin North Am. 1995;9:1267–1274. [PubMed] [Google Scholar]
- 4.Malogolowkin M, Cotton CA, Green DM, et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine, actinomycin D, and doxorubicin. A report from the National Wilms Tumor Study Group. Pediatr Blood Cancer. 2008;50:236–241. doi: 10.1002/pbc.21267. [DOI] [PubMed] [Google Scholar]
- 5.Thomas PRM, Schocat S, Norkool P. Prognostic implications of hepatic adhesion, invasion, and metastases at diagnosis of Wilms' tumor. The National Wilms' Tumor Study Group. Cancer. 1991;68:2486–2488. doi: 10.1002/1097-0142(19911201)68:11<2486::aid-cncr2820681128>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Varan A, Büyükpamukçu, Caglar M. Prognostic significance of metastatic site at diagnosis in Wilms' tumor: results from a single center. J Pediatr Hematol Oncol. 2005;27:188–191. doi: 10.1097/01.mph.0000158531.12996.38. [DOI] [PubMed] [Google Scholar]
- 7.Szavay P, Luithle T, Graf N. Primary hepatic metastases in nephroblastoma--a report of the SIOP/GPOH Study. J Pediatr Surg. 2006;41:168–172. doi: 10.1016/j.jpedsurg.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs J, Szavay P, Luithle T, et al. Surgical implications for liver metastasis in nephroblastoma- data from the SIOP/GPOH study. Surg Oncol. 2008;17:33–40. doi: 10.1016/j.suronc.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Green DM. National Wilms Tumor Study Group 5 (NWTS-5) protocol. 1994. [Google Scholar]
- 10.Dome JS, Cotton CA, Perlman EJ. Treatment of anaplastic histology Wilms' tumor: results from the fifth National Wilms' Tumor Study. J Clin Oncol. 2006;24:2352–2358. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 11.Grundy PE, Breslow N, Li S, et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2005;23:7312–7321. doi: 10.1200/JCO.2005.01.2799. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;33:457–481. [Google Scholar]
- 13.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc (A) 1972;135:185–206. [Google Scholar]
- 14.Fischer RA. On the interpretation of chi-squared from contingency tables, and the calculation of P. J Royal Stat Soc. 1922;855:87–94. [Google Scholar]
- 15.Grosfeld JL. Risk-based managment: current concepts of treating malignant solid tumors of childhood. Journal of the American College of Surgeons. 1999;189:407–435. doi: 10.1016/s1072-7515(99)00167-2. [DOI] [PubMed] [Google Scholar]
- 16.Dome JS, Grundy PE, Perlman EJ, Ehrlich PF, et al. Protocols for the Renal tumors Study. Childrens Oncology Group; 2007. www.childrensoncologygroup.org. [Google Scholar]
- 17.Ehrlich PF. Wilms tumor: progress to date and future considerations. Expert Review of Anticancer Therapy. 2001;1:555–564. doi: 10.1586/14737140.1.4.555. [DOI] [PubMed] [Google Scholar]
- 18.Breslow N, Churchill G, Nessmith B, et al. Clinicopathologic features and prognosis for Wilms' tumor patients with metastases at diagnosis. Cancer. 1986;58:2501–2511. doi: 10.1002/1097-0142(19861201)58:11<2501::aid-cncr2820581125>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Pritchard-Jones K, Kelsey A, Vujanic G, et al. Older age is an adverse prognostic factor in stage I, favorable histology Wilms' tumor treated with vincristine monochemotherapy: a study by the United Kingdom Children's Cancer Study Group, Wilm's Tumor Working Group. J Clin Oncol. 2003;21:3269–3275. doi: 10.1200/JCO.2003.01.062. [DOI] [PubMed] [Google Scholar]
- 20.Reinhard H, Furtwängler R, Siemer S. Wilms' tumor in adults. Urologe A. 2007;46:748–753. doi: 10.1007/s00120-007-1363-0. [DOI] [PubMed] [Google Scholar]
- 21.Terenziani M SFCP Adult Wilms' tumor: A mono-institutional experience and a review of the literature. Cancer. 2004;101:289–293. doi: 10.1002/cncr.20387. [DOI] [PubMed] [Google Scholar]
- 22.Reinhard H, Aliani S, Ruebe C. Wilms' tumor in adults: results of the Society of Pediatric Oncology (SIOP) 93-01/Society for Pediatric Oncology and Hematology (GPOH) Study. J Clin Oncol. 2004;22:4500–4506. doi: 10.1200/JCO.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 23.Kalapurakal JA, Nan B, Norkool P, et al. Treatment outcomes in adults with favorable histologic type Wilms tumor-an update from the National Wilms Tumor Study Group. Int J Radiat Oncol Biol Phys. 2004;60:1379–1384. doi: 10.1016/j.ijrobp.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig R, Weirich A, Abel U. Hepatotoxicity in patients treated according to the nephroblastoma trial and study SIOP-9/GPOH. Med Pediatr Oncol. 1999;3333:462–9. 462–469. doi: 10.1002/(sici)1096-911x(199911)33:5<462::aid-mpo5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Bisogno G, de Kraker J, Weirich A. Veno-occlusive disease of the liver in children treated for Wilms tumor. Med Pediatr Oncol. 1997;29:245–251. doi: 10.1002/(sici)1096-911x(199710)29:4<245::aid-mpo2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 26.Czauderna P, Katski K, Kowalczyk J. Venoocclusive liver disease (VOD) as a complication of Wilms' tumour management in the series of consecutive 206 patients. Eur J Pediatr Surg. 2000;10:300–303. doi: 10.1055/s-2008-1072380. [DOI] [PubMed] [Google Scholar]
- 27.Paulino AC, Wen BC, Brown CK, et al. Late effects in children treated with radiation therapy for Wilms tumor. Int J Rad Oncol. 2000;46:1239–1246. doi: 10.1016/s0360-3016(99)00534-9. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AJ, Winter DL, Pritchard-Jones K, et al. Second primary neoplasms in survivors of Wilms' tumour--a population-based cohort study from the British Childhood Cancer Survivor Study. Int J Cancer. 2008;122:2085–2093. doi: 10.1002/ijc.23333. [DOI] [PubMed] [Google Scholar]
- 29.Bassal M, Mertens AC, Taylor L. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:476–83. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]