Summary
After infections, thromboembolism is considered by many experts to be the most significant life-threatening complication of nephrotic syndrome. The purpose of this review is to summarize the epidemiology, clinical and molecular pathophysiology, and management of this complication. Children (2.8%) are less likely than adults (26.7%) with nephrotic syndrome to develop thromboembolism. However, infants and children aged >12 years are at much greater risk. Membranous histologic changes increase thromboembolic risk at all ages; in particular, adults with membranous nephropathy have the highest reported risk (37.0%) and children with membranous histology have a rate (25%) that approaches the overall adult rate. There are striking, but variable, pathologic alterations of molecular hemostasis associated with nephrotic syndrome. No clear molecular therapeutic targets have been identified, but most studies show that the major pathologic changes involve antithrombin, fibrinogen, and factors V and VIII. There is inadequate evidence to support routine prophylactic therapy. Therapy includes anticoagulation in all cases, with thrombolysis reserved for those with the most severe thromboembolic disease. Future hemostatic research in nephrotic syndrome should focus on identifying cohorts at highest risk for thrombosis through the use of clinical markers and biomarkers as well as searching for molecular targets to correct the prothrombotic pathophysiology of this disease.
Introduction
Thromboembolism is among the most serious complications of nephrotic syndrome (NS) (1–3). Thrombosis results from intravascular blood coagulation leading to thrombus formation that obstructs blood flow. Thrombosis may form in either arteries or veins. Embolism occurs when all or a portion of the thrombus breaks free and flows downstream in the circulation, blocking flow to vital organs. Collectively these phenomena are referred to as thromboembolism.
As early as 1840, renal vein thrombosis was the first thromboembolism recognized to be associated with NS (4,5). Since that time, it has been recognized that NS-associated thromboembolism may be seen in essentially any major blood vessel (4). Before the development of immunosuppressive regimens able to effectively induce remission of NS, many texts discussed thromboembolism complications at length (6,7). This was likely because thromboembolism was one of the major life-threatening complications of NS (3,8). Although modern anticoagulant and thrombolytic therapies may decrease the risk of thromboembolism-related mortality, thromboembolism remains a common complication of NS in adults and a less common complication in children (2,9).
Epidemiology
The epidemiology of NS-associated thromboembolism differs significantly between children and adults, between “primary” and “secondary” causes of NS, and also on the basis of the underlying renal histopathology. Here we discuss some of the clinical parameters that may be important candidate risk markers for impending thromboembolism in patients with NS.
Overall, thromboembolism is much more common in adults with NS, in whom the incidence of thromboembolism is approximately 25%, compared with children, in whom the overall incidence is about 3% (Table 1) (1–3,8,10–28). However, the incidence of thromboembolism within each of these groups varies with the type of NS, and may also be dependent on several other factors. For instance, in pediatric NS, thromboembolism seems to be more likely in those children with congenital NS (i.e., presenting in the first 3 months of life) (approximately 10%), and even more likely in those with “secondary” NS, such as may be seen in children with vasculitis (17.1%) (2,16,23). Perhaps those at highest risk, however, are children with membranous nephropathy or a histologically similar process (e.g., class V SLE nephritis), in whom the incidence of thromboembolism (25%) approaches that seen in adults (2).
Table 1.
Summary of reported incidence of nephrotic syndrome–associated thromboembolism
| Publication Year | Author | N | TE | %TE | Study Type | Notes |
|---|---|---|---|---|---|---|
| Childhood | ||||||
| 1973 | Egli et al. (15) | 3377 | 60 | 1.8 | Retrospective | Probably only symptomatic TE |
| 1984 | Mahan et al. (23) | 41 | 4 | 9.8 | Retrospective | Only congenital nephrotic syndrome |
| 1986 | Hoyer et al. (17) | 26 | 7 | 26.9* | Retrospective | V·Q evidence of PE |
| 1987 | Mehls et al. (24) | 204 | 9 | 4.4 | Retrospective | Primarily only symptomatic TE |
| 1991 | Tsau et al. (25) | 193 | 2 | 1 | Retrospective | Only symptomatic TE reported |
| 1997 | Schlegel (8) | 360 | 11 | 3.0 | Not stated | Primarily only symptomatic TE |
| 2000 | Citak et al.l (14) | 49 | 2 | 4.1 | Prospective | Only symptomatic TE reported |
| 2000 | Lilova et al. (19) | 447 | 9 | 2.0 | Retrospective | Only symptomatic TE reported |
| 2001 | Hamed et al. (16) | 30 | 4 | 13.0 | Retrospective | Only congenital nephrotic syndrome |
| 2009 | Kerlin et al. (2) | 326 | 30 | 9.2 | Retrospective | Only symptomatic TE reported; included secondary causes of NS |
| TOTAL | 5053 | 141 | 2.8 | |||
| Adult | ||||||
| 1975 | Bennet et al. (11) | 21 | 6 | 28.6 | Prospective | No histology data |
| 1980 | Llach et al. (22) | 151 | 33 | 21.9 | Prospective | |
| 1980 | Andrassy et al. (10) | 84 | 29 | 34.5 | Prospective | RVT, DVT, and PE studied |
| 1981 | Chugh et al. (13) | 44 | 11 | 25.0 | Retrospective | |
| 1981 | Kuhlmann et al. (18) | 17 | 4 | 23.5 | Prospective | RVT, DVT, and PE studied |
| 1983 | Wagoner et al.l (27) | 27 | 14 | 51.9 | Prospective | Membranous nephropathy only |
| 1988 | Velasquez et al. (26) | 26 | 11 | 42.3 | Prospective | |
| 2000 | Cherng et al. (12) | 89 | 29 | 32.6 | Prospective | V·Q evidence of PE |
| 2008 | Wysokinski et al. (28) | 218 | 44 | 20.2 | Retrospective | RVT and DVT |
| TOTAL | 677 | 181 | 26.7 |
TE, thromboembolism; PE, pulmonary embolism; RVT, renal vein thrombosis; DVT, deep vein thrombosis; V·Q, ventilation:perfusion mismatch scanning; NS, nephrotic syndrome.
In adults, estimating the overall frequency of thromboembolism from the literature is somewhat difficult because the early studies focused only on investigations of renal vein thrombosis (RVT), whereas more recent studies have focused either on the epidemiology of non-RVT thrombi, the combination of RVT and non-RVT thrombi, or the correlation between thromboembolism and various NS histopathologies. Regardless, adults with membranous nephropathy seem to be at greatest risk for development of thromboembolism. Indeed, in this subset of adults, the incidence of RVT may be as high as 37%, whereas the cumulative incidence is only about 24% in the remaining common histologies (membranoproliferative glomerulonephritis, minimal change NS, and FSGS) (Table 2) (9,13,20,22,26,27). The physiologic reasons for the apparent membranous nephropathy thromboembolism predilection are unknown (29).
Table 2.
Membranous nephropathy is associated with the highest incidence of RVT by histologic diagnosis in adult nephrotic syndrome
| Study | MN | MPGN | MCD | FSGS | Other |
|---|---|---|---|---|---|
| Llach et al. (22) | 230.0 (20/69) | 22.2 (6/27) | 20.0 (2/10) | 25.0 (1/4) | 9.8 (11/41) |
| Chugh et al. (13) | 42.9 (3/7) | 20.0 (1/5) | 26.3 (5/19) | 0 (0/5) | 25 (2/8) |
| Wagoner et al. (27) | 51.9 (14/27) | NS | NS | NS | NS |
| Velasquez et al. (26) | 60.0 (3/5) | 40.0 (4/10) | NS | 28.6 (2/7) | 50 (2/4) |
| TOTAL | 37.0 (40/108) | 26.2 (11/42) | 24.1 (7/29) | 18.8 (3/16) | 28.3 (15/53) |
| TOTAL for combined MPGN, MCD, and FSGS | — | ----------24.1 (21/87)---------- | — | ||
Data are shown as percentages with thromboembolism (number with thromboembolism/number of patients in category). MN, membranous nephropathy; MPGN, membranoproliferative GN; MCD, minimal change disease; NS, not studied.
Age is an important modifier of both thromboembolism risk and thromboembolism presentation in NS. For adult patients, it is now well recognized that RVT is more likely to present as an acute phenomenon, with the classic symptoms including flank pain and macroscopic hematuria in young adults (mean age 20 years) (9). Older adults (mean age 38 years) are more likely to develop occult chronic RVT (9,22). In these older patients, the latter presentation also seems to be the more common form of RVT and these patients are also more likely to develop extrarenal thromboembolisms (22). In contrast, for children, congenital NS presenting in infancy is associated with an estimated 10% incidence of thromboembolism (23). After the first year, thromboembolism risk seems to correlate well with increasing age, with the univariate odds ratio (OR) increasing by 1.16 (95% confidence interval [95% CI], 1.08, 1.25) for each additional year of age advancement (2). As a result, adolescents seem to be at highest risk, with an OR of 8.59 (95% CI, 3.31, 22.28) for those aged >12 years at diagnosis compared with children aged <12 years (2). These data are consistent with the known age-related thromboembolism epidemiology seen in both children and adults, which show that thromboembolism risk increases with advancing age (30–33).
In both adults and children, thromboembolism is most prevalent early in the disease course. In our recent study of children with NS and thromboembolism, the median time from NS diagnosis to first thromboembolism was 70.5 days (2). This is similar to previous reports demonstrating that 61% of thromboembolism events in children occur within 3 months after NS diagnosis (34). In adults, the majority of venous thromboembolism events occur within the first 6 months after NS diagnosis (35). Moreover, the absolute risk of arterial thromboembolism in adults with NS correlates well with the known risk factors for atherosclerotic disease, but remains roughly eight times that of the general population, suggesting that NS has a strong influence on the risk for arterial thromboembolism (35). In addition, the risk for both arterial and venous thromboembolism in adults with NS has a secondary peak about 20 years after NS diagnosis that is likely related to aging more than to NS (32,35,36).
Thromboembolism prevalence estimates in both adult and childhood NS are subject to methodological limitations including retrospective study designs, small sample sizes, objectivity of thromboembolism definitions, NS histopathology distribution, and imaging methods used to detect thromboembolism (9). For a comprehensive discussion of current methods for diagnosis of thromboembolism, the reader is referred to Manco-Johnson for childhood thromboembolism (37) and Hirsch and Lee for adult thromboembolism (38).
Pathophysiology
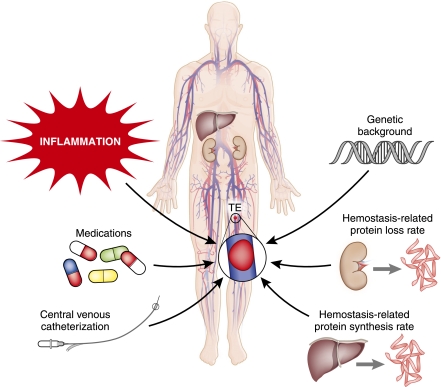
The pathophysiology of thrombogenesis in NS is not completely understood, but seems to be multifactorial (Figure 1). The first consideration is patients’ underlying genetic background, which may be unrelated to their renal disease but influences their likelihood of developing thromboembolism (39). This genetic predisposition includes the known mutations and single nucleotide polymorphisms associated with thrombophilia (e.g., congenital antithrombin deficiency or factor V Leiden [F5 R506Q]), as well as genetic predispositions that may be as yet unknown. These factors combine with environmental and acquired thromboembolism risk factors (e.g., inflammation, medications, central venous catheters; Figure 1) to determine the likelihood that an individual will ever exceed the thrombotic threshold (39).
Figure 1.
Thromboembolism in nephrotic syndrome is due to multifaceted pathophysiology.
The area of pathophysiology that has garnered the most attention in NS has been the study of disease-related hemostatic derangements. Because the primary glomerular defect of NS results in the leakage of high molecular mass proteins, at least the size of albumin (approximately 66 kD), many important hemostatic proteins of similar sizes are also pathologically excreted in the urine (1,40). Prominent among these are the loss of important coagulation regulatory proteins, including antithrombin and protein S (8,41). Counterbalancing these urinary losses is the synthetic rate at which the liver (primarily) produces hemostatic proteins (Figure 1) (8). As a result, during active nephrotic-range proteinuria, there is a net shift in the hemostatic balance toward a prothrombotic milieu (Figure 2) (42–51). These alterations are thought to be the primary mechanism that produces the increased risk of thromboembolism in patients with NS. Unfortunately, the evidence to support this conclusion is somewhat lacking because the majority of studies examining the hemostatic derangement of NS have not utilized thromboembolism as an outcome. Rather, most studies have compared the hemostatic state of patients with active NS to either patients with NS in remission or to control samples from healthy individuals. As a result, there are very few studies directly examining the link between these derangements and the clinical outcome of interest, thromboembolism. This is likely because conducting these studies for such an infrequent event would require very large sample sizes in order to adequately power the study, while accounting for the remaining confounding variables.
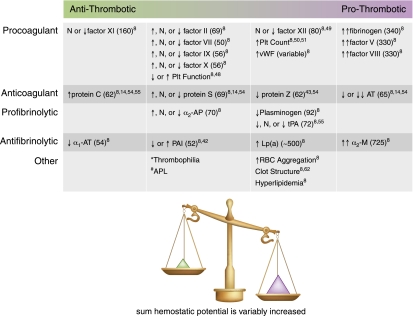
Figure 2.
Nephrotic syndrome is a prothrombotic state of variable magnitude. Marked decrease (↓↓) through normal (N) to marked increase (↑↑). Numbers in parentheses are molecular masses of proteins given in kilodaltons (45,47). *Thrombophilic genetic polymorphisms (Factor V Leiden, Prothrombin G20210A, MTHFR C677T) may increase thromboembolism risk, but have not been shown to occur with higher prevalence in NS with VTE; other thrombophilic polymorphisms have not yet been studied in NS (8,44,55). #APL has been inadequately studied in NS, but some thromboembolism patients are positive for APL (2,46). Plt, platelet; vWF, von Willebrand factor; AT, antithrombin; α2-AP, α2-antiplasmin; tPA, tissue-type plasminogen activator; α1-AT, α1-antitrypsin; PAI, plasminogen activator inhibitor; Lp(a), lipoprotein(a); α2-M, α2-macroglobulin; APL, antiphospholipid antibodies; RBC, red blood cell.
The important changes in plasma hemostatic protein content with active NS are summarized in Figure 2. The most striking feature is that with few exceptions, there is a considerable amount of variability in the changes from study to study, likely related to differing study designs. However, some consistencies do appear. Plasma concentrations of the procoagulant proteins with higher molecular weights are generally markedly elevated, including fibrinogen and factors V and VIII. These changes are predicted to amplify the coagulation cascade resulting in a prothrombotic state (8,52,53). Interestingly, however, plasma concentrations of factor XI, which is considerably larger than albumin (approximately 160 kD), have been reported to be decreased (8).
The anticoagulant proteins are similarly shifted toward a prothrombotic state. Perhaps the best known of these derangements is a marked urinary loss of antithrombin (65 kD) (8,14,54). Although slightly smaller than albumin, plasma concentrations of protein C (62 kD) are preserved and/or upregulated to the point that the levels are generally increased (8,14,54,55). The protein S data must be interpreted cautiously due to the complex metabolism of this molecule (8,56). Protein S is an important co-factor required for efficient activity of protein C; it is generally bound to its carrier protein, C4b-binding protein (C4BP), a component of the complement system. However, only the free, nonbound form of protein S is biologically active (approximately 30%–40% of the total circulating protein S) (56). Free protein S has a molecular mass of 69 kD, whereas that of C4BP is approximately 570 kD. Free protein S is thus lost in the urine in the nephrotic state, whereas C4BP is preserved, binding up any remaining protein S. The expected consequence of this is that although the total protein S plasma content may remain normal or even increase, the free, biologically active form of the protein should be deficient. However, studies of free protein S have not shown consistently deficient levels, with some studies even reporting increased free protein S plasma concentrations during the nephrotic state (8,14,54,57).
The fibrinolytic system is not as profoundly altered by NS. Both plasminogen (92 kD) and its primary activator, tissue-type plasminogen activator (tPA; 72 kD), are mildly decreased in concentration (8,55). Meanwhile, both α2-macroglobulin (725 kD) and lipoprotein (a) (approximately 500 kD), which are important inhibitors of fibrinolysis, are increased in concentration (8). The net result of these changes during NS is diminished fibrinolytic activity to combat the prothrombotic activity of an inadequately regulated prohemostatic coagulation cascade. For further reading on hemostatic molecular biology in NS, see the excellent reviews by Schlegel (for children) (8) and also Singhal and Brimble (for adults) (9).
The platelet count is almost universally elevated in NS (1,3). However, the prothrombotic consequences of such an increase are debatable. For instance, a reactive thrombocytosis, such as is seen in NS, is rarely a cause for alarm in children (58). Similarly, thrombocytosis is generally pathologic in adults only in the setting of a hematopathologic state such as with essential thrombocythemia or other myeloproliferative syndromes (59). However, there is also evidence that platelets may be constitutively activated in NS (60,61). The latter may be particularly problematic in adults with atherosclerotic disease, in whom the combination of thrombocytosis, hyperaggregability, and hyperlipidemia secondary to NS may increase their risk for arterial thrombotic disease (35).
Lastly, there is evolving literature surrounding several other hemostatic issues and perihemostatic mechanisms that may be physiologically important in the setting of NS. There are confocal scanning laser microscopy data demonstrating that the fibrin clot structure formed by nephrotic plasma has an altered molecular structure that is less porous than typical thrombi (62). The potential implication of this “closed” structure is that the clot is less permeable and therefore more resistant to fibrinolysis. In addition, the study of prothrombotic microparticles has become an emerging field of hemostatic science (63). These are small particles, measuring <1 μm in diameter, that may derive from a variety of cells, including platelets, leukocytes, or endothelial cells. In addition to providing a prothrombotic phospholipid surface, they may also express important procoagulant moieties on their surface, such as phosphatidylserine, p-selectin, or functional tissue factor. Indeed, elevated levels of microparticles have recently been identified in children with idiopathic NS (64). In addition, antiphospholipid antibody syndrome is an autoimmune disorder that activates hemostasis, leading to recurrent thromboembolism (65,66). There are multiple case reports of co-morbid antiphospholipid antibody syndrome with NS; however, some of these may have included transient antiphospholipid antibody detection that is not always pathologic. Therefore, the significance of these findings remains unclear. Lastly, there is a significant amount of literature on red blood cell hyperaggregability in NS (8,67,68). It is suspected that this phenomenon may be related to hypoalbuminemia and/or intravascular volume depletion, because reconstituting the samples with albumin abolishes the hyperaggregation. Conversely, there are ample data demonstrating that dehydrated red cells, such as may occur in sickle cell disease, express additional cell adhesion molecules that may slow blood flow and enhance hemostasis (69,70). It would therefore not be unreasonable to hypothesize that red cell dehydration, secondary to volume contraction and sodium retention, may contribute to NS thrombogenicity (1).
There is an apparent predilection, at least in adults, for RVT, with RVT being nearly twice as common as other types of thromboembolism (3,29). Although the pathophysiologic reasons for this predilection are unclear, they may be related to glomerular injury stimulating thrombin production within the efferent vasculature and/or decreased renal vein perfusion pressure (29).
Prevention and Management
Debate continues regarding the appropriateness of prophylactic anticoagulation to prevent NS-associated thromboembolism (71). This is due in large part to the lack of any large, prospective randomized trials to determine the efficacy and safety of such an approach. This is of substantial concern given that the majority of patients do not develop thromboembolism, because a significant number of patients would receive prophylaxis unnecessarily. Therefore any potential adverse effects (i.e., anticoagulant-related bleeding) need to be carefully balanced against the expected benefit of thromboembolism prevention. The possibility that statin therapy may decrease NS-associated thromboembolism risk was recently described in a retrospective cohort study (72). Statin therapy would have a much more tolerable adverse bleeding profile if it were proven to be thromboembolism protective in prospective studies.
An alternative approach would be to identify only those patients at highest risk for thromboembolism and target them for prophylactic therapy. This would potentially greatly diminish the number needed to treat to prevent a large proportion of the thromboembolism events and avoid the therapeutic risks to those who are unlikely to develop thromboembolism. For instance, using Markov decision analysis, it has been estimated that adults with membranous nephropathy may benefit from a prophylactic strategy (73). However, this type of study is subject to significant limitations because unknown adverse events or therapeutic failures cannot be incorporated into the analysis. Therefore, even after defining parameters that identify a high-risk group of patients, randomized studies will be needed. Recent studies have begun to elucidate additional clinical parameters that may be useful to better identify such high-risk patients (2,35). For adults, these parameters include proteinuria/serum albumin ratio for venous thromboembolism and traditional atherosclerotic disease risks (sex, age, hypertension, diabetes, smoking) and estimated GFR for arterial thromboembolism (35). In children, adolescent age (>12 years) and proteinuria severity correlate with thromboembolism risk (2). Although proteinuria was an important risk factor in both studies, there are several limitations that apply, including retrospective study design and potential treatment-related influences on proteinuria. Early studies in adults linked serum hypoalbuminemia with thromboembolism risk (74); however, this association did not reach statistical significance in recent studies (35). Additional research incorporating laboratory coagulation and/or inflammatory biomarkers correlated with the outcome of thromboembolism will likely be very helpful to provide insight into which objectively measured laboratory parameters may indicate an increased risk of thromboembolism.
Once thromboembolism has developed, clinical management is similar to that utilized in the non-nephrotic patient (1,3,75,76). Anticoagulation should be promptly initiated. The current mainstay is to begin with heparinization. This may be accomplished through the use of standard intravenous heparin or, alternatively, subcutaneous low molecular weight heparins or synthetic pentasaccharides may be employed. Warfarin can begin simultaneously for long-term management. The total recommended course of anticoagulation for a first venous thromboembolism is at least 3–6 months and until the provocating illness (NS) has resolved or is in remission (75,76). Warfarin is hepatically cleared so that additional renal adjustments should not be needed. However, many of the heparin compounds are cleared renally; thus, dose adjustment may be necessary if the patient has evidence of renal dysfunction. There are several additional anticoagulant compounds in the drug development pipeline. An example is dabigatran, an orally administered direct thrombin inhibitor, recently approved for prevention of stroke in patients with atrial fibrillation (77). Unfortunately, there are inadequate data at this time to determine the safety and efficacy of these new agents in the nephrotic population.
Thrombolysis has not been formally studied in NS-associated thromboembolism. Most evidence for its use has been derived from case reports and series (78–85), which are generally of limited value because negative outcomes may be subject to publication bias (86). Conversely, several reports have documented rapid symptomatic improvement of RVT with anticoagulation alone (78–82). Therefore, most experts recommend reserving thrombolytic therapy for severe bilateral RVT (9). This treatment should also be considered for patients with massive pulmonary embolism (87). Utilization of thrombolytic agents requires careful consideration of the bleeding risks before initiation of therapy. Although a thorough discussion of thrombolytic therapy is beyond the scope of this review, interested readers are encouraged to keep abreast of developing recommendations for both anticoagulation management and thrombolysis through the periodically updated American College of Chest Physicians guidelines on antithrombotic and thrombolytic therapy (88).
In summary, thromboembolic disease is a significant complication of NS in both adults and children that usually presents early in the NS disease course. Advanced age, membranous nephropathy, and severe proteinuria are all now recognized to be correlated with an increased risk of thromboembolism development. Abundant data illustrate that massive urinary protein loss triggers significant hemostatic derangement, but no clinically relevant biomarker has yet emerged from this knowledge. Treatment of thromboembolism in NS is similar to that in other affected individuals, and includes standard anticoagulation, with thrombolysis reserved for severe cases. We are only recently beginning to understand the complex pathophysiology of thromboembolism in NS at the cellular and molecular levels. Future studies should focus on identifying clinically useful biomarkers of thromboembolism risk, determining the clinical efficacy and safety of prevention strategies, and on appropriate therapy of thrombotic events in patients with NS.
Disclosures
None.
Acknowledgments
This project was supported by Grant 239409 from the Nationwide Children's Hospital Research Institute (to B.A.K.) and Award UL1RR025755 from the National Center for Research Resources.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Eddy AA, Symons JM: Nephrotic syndrome in childhood. Lancet 362: 629–639, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Kerlin BA, Blatt NB, Fuh B, Zhao S, Lehman A, Blanchong C, Mahan JD, Smoyer WE: Epidemiology and risk factors for thromboembolic complications of childhood nephrotic syndrome: A Midwest Pediatric Nephrology Consortium (MWPNC) study. J Pediatr 155: 105–110, 110.e1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orth SR, Ritz E: The nephrotic syndrome. N Engl J Med 338: 1202–1211, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Zumberg M, Kitchens CS: Consultative Hemostasis and Thrombosis, Philadelphia, Saunders Elsevier, 2007 [Google Scholar]
- 5.Rayer P: Traite des maladies des reins et des alteret lens de la secretions urinaire. Baillieve 2: 550–559, 1840 [Google Scholar]
- 6.Welch TR: Nephrosis and clots. J Pediatr 155: A2, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Cameron JS: Five hundred years of the nephrotic syndrome: 1484-1984. Ulster Med J 54[Suppl]: S5–S19, 1985 [PMC free article] [PubMed] [Google Scholar]
- 8.Schlegel N: Thromboembolic risks and complications in nephrotic children. Semin Thromb Hemost 23: 271–280, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Singhal R, Brimble KS: Thromboembolic complications in the nephrotic syndrome: Pathophysiology and clinical management. Thromb Res 118: 397–407, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Andrassy K, Ritz E, Bommer J: Hypercoagulability in the nephrotic syndrome. Klin Wochenschr 58: 1029–1036, 1980 [DOI] [PubMed] [Google Scholar]
- 11.Bennett WM: Letter: Renal vein thrombosis in nephrotic syndrome. Ann Intern Med 83: 577–578, 1975 [DOI] [PubMed] [Google Scholar]
- 12.Cherng SC, Huang WS, Wang YF, Yang SP, Lin YF: The role of lung scintigraphy in the diagnosis of nephrotic syndrome with pulmonary embolism. Clin Nucl Med 25: 167–172, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Chugh KS, Malik N, Uberoi HS, Gupta VK, Aggarwal ML, Singhal PC, Suri S, Jain SK: Renal vein thrombosis in nephrotic syndrome—a prospective study and review. Postgrad Med J 57: 566–570, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Citak A, Emre S, Sâirin A, Bilge I, Nayir A: Hemostatic problems and thromboembolic complications in nephrotic children. Pediatr Nephrol 14: 138–142, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Egli F, Elmiger P, Stalder G: Thromboembolism in the nephrotic syndrome [abstract]. Pediatr Res 8: 903, 1973 [Google Scholar]
- 16.Hamed RM, Shomaf M: Congenital nephrotic syndrome: A clinico-pathologic study of thirty children. J Nephrol 14: 104–109, 2001 [PubMed] [Google Scholar]
- 17.Hoyer PF, Gonda S, Barthels M, Krohn HP, Brodehl J: Thromboembolic complications in children with nephrotic syndrome. Risk and incidence. Acta Paediatr Scand 75: 804–810, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Kuhlmann U, Steurer J, Rhyner K, von Felten A, Briner J, Siegenthaler W: Platelet aggregation and beta-thromboglobulin levels in nephrotic patients with and without thrombosis. Clin Nephrol 15: 229–235, 1981 [PubMed] [Google Scholar]
- 19.Lilova MI, Velkovski IG, Topalov IB: Thromboembolic complications in children with nephrotic syndrome in Bulgaria (1974-1996). Pediatr Nephrol 15: 74–78, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Llach F, Arieff AI, Massry SG: Renal vein thrombosis and nephrotic syndrome. A prospective study of 36 adult patients. Ann Intern Med 83: 8–14, 1975 [DOI] [PubMed] [Google Scholar]
- 21.Llach F, Koffler A, Finck E, Massry SG: On the incidence of renal vein thrombosis in the nephrotic syndrome. Arch Intern Med 137: 333–336, 1977 [PubMed] [Google Scholar]
- 22.Llach F, Papper S, Massry SG: The clinical spectrum of renal vein thrombosis: Acute and chronic. Am J Med 69: 819–827, 1980 [DOI] [PubMed] [Google Scholar]
- 23.Mahan JD, Mauer SM, Sibley RK, Vernier RL: Congenital nephrotic syndrome: Evolution of medical management and results of renal transplantation. J Pediatr 105: 549–557, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Mehls O, Andrassy K, Koderisch J, Herzog U, Ritz E: Hemostasis and thromboembolism in children with nephrotic syndrome: Differences from adults. J Pediatr 110: 862–867, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Tsau YK, Chen CH, Tsai WS, Sheu JN: Complications of nephrotic syndrome in children. J Formos Med Assoc 90: 555–559, 1991 [PubMed] [Google Scholar]
- 26.Velasquez Forero F, Garcia Prugue N, Ruiz Morales N: Idiopathic nephrotic syndrome of the adult with asymptomatic thrombosis of the renal vein. Am J Nephrol 8: 457–462, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Wagoner RD, Stanson AW, Holley KE, Winter CS: Renal vein thrombosis in idiopathic membranous glomerulopathy and nephrotic syndrome: Incidence and significance. Kidney Int 23: 368–374, 1983 [DOI] [PubMed] [Google Scholar]
- 28.Wysokinski WE, Gosk-Bierska I, Greene EL, Grill D, Wiste H, McBane RD, 2nd: Clinical characteristics and long-term follow-up of patients with renal vein thrombosis. Am J Kidney Dis 51: 224–232, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Nickolas TL, Radhakrishnan J, Appel GB: Hyperlipidemia and thrombotic complications in patients with membranous nephropathy. Semin Nephrol 23: 406–411, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Andrew M, Monagle PT, Brooker L: Thromboembolic Complications during Infancy and Childhood, Hamilton, Ontario, B.C. Decker, 2000 [Google Scholar]
- 31.Raffini L, Huang YS, Witmer C, Feudtner C: Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics 124: 1001–1008, 2009 [DOI] [PubMed] [Google Scholar]
- 32.White RH: The epidemiology of venous thromboembolism. Circulation 107[Suppl 1]: I4–I8, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Setty BA, O'Brien SH, Kerlin BA: Pediatric venous thromboembolism in the United States: A tertiary care complication of chronic diseases [published online ahead of print October 28, 2011]. Pediatr Blood Cancer doi:10.1002/pbc.23388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrew M, Brooker LA: Hemostatic complications in renal disorders of the young. Pediatr Nephrol 10: 88–99, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Mahmoodi BK, ten Kate MK, Waanders F, Veeger NJ, Brouwer JL, Vogt L, Navis G, van der Meer J: High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: Results from a large retrospective cohort study. Circulation 117: 224–230, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Kannel WB: Hazards, risks, and threats of heart disease from the early stages to symptomatic coronary heart disease and cardiac failure. Cardiovasc Drugs Ther 11[Suppl 1]: 199–212, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Manco-Johnson MJ: How I treat venous thrombosis in children. Blood 107: 21–29, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsh J, Lee AY: How we diagnose and treat deep vein thrombosis. Blood 99: 3102–3110, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Lijfering WM, Rosendaal FR, Cannegieter SC: Risk factors for venous thrombosis - current understanding from an epidemiological point of view. Br J Haematol 149: 824–833, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Valentini RP, Smoyer WE: Nephrotic syndrome. In: Clinical Pediatric Nephrology, 2nd Ed., edited by Kher KK, Schnaper HW, and Makker SP, Abingdon, Oxon, Informa Healthcare, 2006, pp 155–194 [Google Scholar]
- 41.Hanevold CD, Lazarchick J, Constantin MA, Hiott KL, Orak JK: Acquired free protein S deficiency in children with steroid resistant nephrosis. Ann Clin Lab Sci 26: 279–282, 1996 [PubMed] [Google Scholar]
- 42.al-Mugeiren MM, Gader AM, al-Rasheed SA, Bahakim HM, al-Momen AK, al-Salloum A: Coagulopathy of childhood nephrotic syndrome–a reappraisal of the role of natural anticoagulants and fibrinolysis. Haemostasis 26: 304–310, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Corral J, González-Conejero R, Hernández-Espinosa D, Vicente V: Protein Z/Z-dependent protease inhibitor (PZ/ZPI) anticoagulant system and thrombosis. Br J Haematol 137: 99–108, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Fabri D, Belangero VM, Annichino-Bizzacchi JM, Arruda VR: Inherited risk factors for thrombophilia in children with nephrotic syndrome. Eur J Pediatr 157: 939–942, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Nathan DG, Oski FA: Nathan and Oski's Hematology of Infancy and Childhood, Philadelphia, Saunders, 2003 [Google Scholar]
- 46.Sá H, Freitas L, Mota A, Cunha F, Marques A: Primary antiphospholipid syndrome presented by total infarction of right kidney with nephrotic syndrome. Clin Nephrol 52: 56–60, 1999 [PubMed] [Google Scholar]
- 47.Stamatoyannopoulos G: The Molecular Basis of Blood Diseases, Philadelphia, W.B. Saunders, 2001 [Google Scholar]
- 48.Svetlov SI, Moskaleva ES, Pinelis VG, Daikhin Y, Serebruany VL: Decreased intraplatelet Ca2+ release and ATP secretion in pediatric nephrotic syndrome. Pediatr Nephrol 13: 205–208, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Varga EA, Kerlin BA, Wurster MW: Social and ethical controversies in thrombophilia testing and update on genetic risk factors for venous thromboembolism. Semin Thromb Hemost 34: 549–561, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Wasilewska A, Zoch-Zwierz WM, Tomaszewska B, Zelazowska B: Relationship of serum interleukin-7 concentration and the coagulation state in children with nephrotic syndrome. Pediatr Int 47: 424–429, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Wasilewska AM, Zoch-Zwierz WM, Tomaszewska B, Biernacka A: Platelet-derived growth factor and platelet profiles in childhood nephrotic syndrome. Pediatr Nephrol 20: 36–41, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Kuipers S, Cannegieter SC, Doggen CJ, Rosendaal FR: Effect of elevated levels of coagulation factors on the risk of venous thrombosis in long-distance travelers. Blood 113: 2064–2069, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Tracy RP, Aleksic N, Folsom AR: Coagulation factors, inflammation markers, and venous thromboembolism: The longitudinal investigation of thromboembolism etiology (LITE). Am J Med 113: 636–642, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Ozkaya O, Bek K, Fişgin T, Aliyazicioğlu Y, Sultansuyu S, Açikgöz Y, Albayrak D, Baysal K: Low protein Z levels in children with nephrotic syndrome. Pediatr Nephrol 21: 1122–1126, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Ozkayin N, Mir S, Kavakli K: Hypercoagulability risk factors in children with minimal change disease and the protective role of protein-C activity. Int Urol Nephrol 36: 599–603, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Dahlback B, Stenflo J: The protein C anticoagulant system. In: The Molecular Basis of Blood Diseases, Philadelphia, W.B. Saunders, 2001, pp 614–656 [Google Scholar]
- 57.Gouault-Heilmann M, Gadelha-Parente T, Levent M, Intrator L, Rostoker G, Lagrue G: Total and free protein S in nephrotic syndrome. Thromb Res 49: 37–42, 1988 [DOI] [PubMed] [Google Scholar]
- 58.Dame C, Sutor AH: Primary and secondary thrombocytosis in childhood. Br J Haematol 129: 165–177, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Finazzi G, Xu M, Barbui T, Hoffman R: Hematology Basic Principles and Practice, Philadelphia, Churchill Livingstone/Elsevier, 2009 [Google Scholar]
- 60.Remuzzi G, Marchesi D, Mecca G, de Gaetano G, Silver M: Platelet hypersensitivity in the nephrotic syndrome. Proc Eur Dial Transplant Assoc 16: 487–494, 1979 [PubMed] [Google Scholar]
- 61.Walter E, Deppermann D, Andrassy K, Koderisch J: Platelet hyperaggregability as a consequence of the nephrotic syndrome. Thromb Res 23: 473–479, 1981 [DOI] [PubMed] [Google Scholar]
- 62.Colle JP, Mishal Z, Lesty C, Mirshahi M, Peyne J, Baumelou A, Bensman A, Soria J, Soria C: Abnormal fibrin clot architecture in nephrotic patients is related to hypofibrinolysis: Influence of plasma biochemical modifications: A possible mechanism for the high thrombotic tendency? Thromb Haemost 82: 1482–1489, 1999 [PubMed] [Google Scholar]
- 63.George FD: Microparticles in vascular diseases. Thromb Res 122[Suppl 1]: S55–S59, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Tkaczyk M, Baj Z: Surface markers of platelet function in idiopathic nephrotic syndrome in children. Pediatr Nephrol 17: 673–677, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA: International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4: 295–306, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, Brey R, Derksen R, Harris EN, Hughes GR, Triplett DA, Khamashta MA: International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: Report of an international workshop. Arthritis Rheum 42: 1309–1311, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Böhler T, Linderkamp O, Leo A, Wingen AM, Schärer K: Increased aggregation with normal surface charge and deformability of red blood cells in children with nephrotic syndrome. Clin Nephrol 38: 119–124, 1992 [PubMed] [Google Scholar]
- 68.Ozanne P, Francis RB, Meiselman HJ: Red blood cell aggregation in nephrotic syndrome. Kidney Int 23: 519–525, 1983 [DOI] [PubMed] [Google Scholar]
- 69.Hillery CA, Panepinto JA: Pathophysiology of stroke in sickle cell disease. Microcirculation 11: 195–208, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Wandersee NJ, Punzalan RC, Rettig MP, Kennedy MD, Pajewski NM, Sabina RL, Paul Scott J, Low PS, Hillery CA: Erythrocyte adhesion is modified by alterations in cellular tonicity and volume. Br J Haematol 131: 366–377, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Glassock RJ: Prophylactic anticoagulation in nephrotic syndrome: A clinical conundrum. J Am Soc Nephrol 18: 2221–2225, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Resh M, Mahmoodi BK, Navis GJ, Veeger NJ, Lijfering WM: Statin use in patients with nephrotic syndrome is associated with a lower risk of venous thromboembolism. Thromb Res 127: 395–399, 2011 [DOI] [PubMed] [Google Scholar]
- 73.Sarasin FP, Schifferli JA: Prophylactic oral anticoagulation in nephrotic patients with idiopathic membranous nephropathy. Kidney Int 45: 578–585, 1994 [DOI] [PubMed] [Google Scholar]
- 74.Robert A, Olmer M, Sampol J, Gugliotta JE, Casanova P: Clinical correlation between hypercoagulability and thrombo-embolic phenomena. Kidney Int 31: 830–835, 1987 [DOI] [PubMed] [Google Scholar]
- 75.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ; American College of Chest Physicians: Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133[Suppl]: 454S–545S, 2008 [DOI] [PubMed] [Google Scholar]
- 76.Monagle P, Chalmers E, Chan A, DeVeber G, Kirkham F, Massicotte P, Michelson AD; American College of Chest Physicians: Antithrombotic therapy in neonates and children: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133[Suppl]: 887S–968S, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Dabigatran etexilate (Pradaxa)—a new oral anticoagulant. Med Lett Drugs Ther 52: 89–90, 2010 [PubMed] [Google Scholar]
- 78.Laville M, Aguilera D, Maillet PJ, Labeeuw M, Madonna O, Zech P: The prognosis of renal vein thrombosis: A re-evaluation of 27 cases. Nephrol Dial Transplant 3: 247–256, 1988 [PubMed] [Google Scholar]
- 79.Markowitz GS, Brignol F, Burns ER, Koenigsberg M, Folkert VW: Renal vein thrombosis treated with thrombolytic therapy: Case report and brief review. Am J Kidney Dis 25: 801–806, 1995 [DOI] [PubMed] [Google Scholar]
- 80.Crowley JP, Matarese RA, Quevedo SF, Garella S: Fibrinolytic therapy for bilateral renal vein thrombosis. Arch Intern Med 144: 159–160, 1984 [PubMed] [Google Scholar]
- 81.Lam KK, Lui CC: Successful treatment of acute inferior vena cava and unilateral renal vein thrombosis by local infusion of recombinant tissue plasminogen activator. Am J Kidney Dis 32: 1075–1079, 1998 [DOI] [PubMed] [Google Scholar]
- 82.Jaar BG, Kim HS, Samaniego MD, Lund GB, Atta MG: Percutaneous mechanical thrombectomy: A new approach in the treatment of acute renal-vein thrombosis. Nephrol Dial Transplant 17: 1122–1125, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Ruud E, Holmstrøm H, Aagenaes I, Hafsahl G, Handeland M, Kyte A, Brosstad F: Successful thrombolysis by prolonged low-dose alteplase in catheter-directed infusion. Acta Paediatr 92: 973–976, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Thompson CS, Cohen MJ, Wesley JM: Endovascular treatment of obliterative hepatocavopathy with inferior vena cava occlusion and renal vein thrombosis. J Vasc Surg 44: 206–209, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Janda SP: Bilateral renal vein thrombosis and pulmonary embolism secondary to membranous glomerulonephritis treated with percutaneous catheter thrombectomy and localized thrombolytic therapy. Indian J Nephrol 20: 152–155, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parekh-Bhurke S, Kwok CS, Pang C, Hooper L, Loke YK, Ryder JJ, Sutton AJ, Hing CB, Harvey I, Song F: Uptake of methods to deal with publication bias in systematic reviews has increased over time, but there is still much scope for improvement. J Clin Epidemiol 64: 349–357, 2011 [DOI] [PubMed] [Google Scholar]
- 87.van Es J, Douma RA, Gerdes VE, Kamphuisen PW, Büller HR: Acute pulmonary embolism. Part 2: Treatment. Nat Rev Cardiol 7: 613–622, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ; American College of Chest Physicians: Executive summary: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133[Suppl]: 71S–109S, 2008 [DOI] [PubMed] [Google Scholar]