Summary
Background and objectives
The optimal maintenance immunosuppressive regimen to improve long-term renal allograft function and graft survival is yet to be determined.
Design, setting, participants, & measurements
This observational study prospectively compared tacrolimus/sirolimus with tacrolimus/mycophenolate mofetil in renal transplant recipients using a prednisone-free regimen with over 8.5 years of follow-up. Patients received methylprednisonlone and anti-IL2 receptor antagonist (Basiliximab) induction and were blindly randomized to either the tacrolimus/mycophenolate mofetil (n=45) or tacrolimus/sirolimus (n=37) groups. Outcome measures included patient and renal allograft survival, incidence of acute rejection, and estimated GFR.
Results
The tacrolimus/mycophenolate mofetil group compared with the tacrolimus/sirolimus group had overall better renal allograft survival (91% versus 70%, P=0.02); 13 patients (35.1%) in the tacrolimus/sirolimus group and 8 patients (17.8%) in the tacrolimus/mycophenolate mofetil group experienced biopsy-proven acute cellular rejection (P=0.07). By 3 months post-transplant, estimated GFR was significantly lower in the tacrolimus/sirolimus group compared with the tacrolimus/mycophenolate mofetil group (47.7 versus 59.6 ml/min per 1.73 m2, P=0.0002), and this trend persisted throughout the follow-up period. Also, the slope of decline in the tacrolimus/sirolimus group was significantly steeper than in the tacrolimus/mycophenolate mofetil group.
Conclusions
This study shows that, in a prednisone-free immunosuppressive regimen, long-term renal graft survival and function are significantly worse in the tacrolimus/sirolimus group than the tacrolimus/mycophenolate mofetil group. The synergistic nephrotoxic effect and higher acute rejection rates in the tacrolimus/sirolimus compared with the tacrolimus/mycophenolate mofetil group adversely affect graft survival.
Introduction
There exists tremendous variation among transplant centers in the United States regarding immunosuppressive regimens in an effort to improve patient and graft survival while minimizing toxicity. Newer immunosuppressive agents have improved early acute rejection rates; however, the optimal maintenance immunosuppressive regimen to promote long-term renal allograft function and improve survival is yet to be determined (1).
Calcineurin inhibitors (CNIs) are an important cause of post-transplant renal function decline and graft loss (2–8). Consequently, CNI minimization and withdrawal strategies are being aggressively investigated. Agents such as the mammalian target of rapamycin inhibitor sirolimus (SRL) and purine biosynthesis inhibitor mycophenolate mofetil (MMF) are being used in conjunction with CNIs in an effort to reduce the dose of CNI and related toxicity. Tacrolimus (Tac) seems to be less toxic than cyclosporine (9–11). Scientific Registry of Transplant Recipients data reveal that the Tac/MMF combination is the most common maintenance immunosuppression for renal transplant patients in the United States (12).
Few studies have attempted to delineate which antiproliferative agent (MMF versus SRL, both combined with Tac) is superior. These studies are limited in scope by their retrospective nature, the inadequacy of registry data, and the lack of long-term follow-up (13–15). One multicenter randomized study had a follow-up of only 12 months post-transplant and used a prednisone-based maintenance regimen, limiting, in part, its implications regarding the impact on long-term graft function (16). Our group was the first, to our knowledge, to prospectively compare Tac/SRL with Tac/MMF in renal transplant recipients using a prednisone-free regimen (17). During a 3-year follow-up, we showed that renal graft survival and graft function were significantly lower in the Tac/SRL combination compared with Tac/MMF.
We present here an extended follow-up to our initial study, with over 8.5 years of follow-up.
Materials and Methods
Detailed methods have been outlined previously (17).
Study Design and Aim
This trial is a single-center, randomized trial aimed at evaluating the impact of two Tac-based prednisone-free maintenance immunosuppressive protocols (Tac/SRL versus Tac/MMF) on long-term graft function and survival.
Patient Recruitment
Patients were recruited between October of 2000 and September of 2001. All patients provided written informed consent. The Institutional Review Board of Northwestern University approved the protocol.
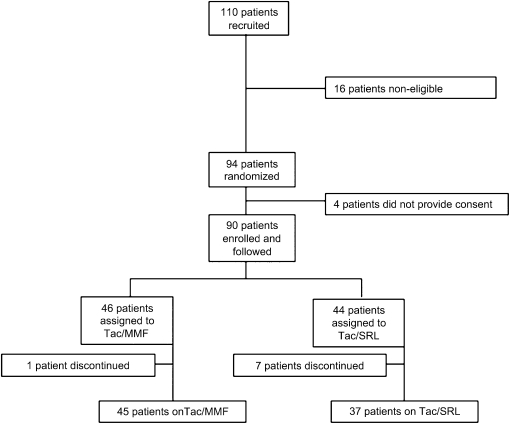
Figure 1 shows the Consolidated Standards of Reporting Trials flow diagram (18). In the final analysis, 45 patients were included in the Tac/MMF arm, and 37 patients were included in the Tac/SRL arm. No additional late discontinuations of either immunosuppressive regimen were encountered aside from the early changes described previously (one MMF and seven SRL) (17).
Figure 1.
Consolidated Standards of Reporting Trials flow diagram.
Inclusion Criteria
Consecutive kidney transplant recipients between 30 and 70 years transplanted at Northwestern University between October of 2000 and September of 2001 were included.
Exclusion Criteria
The study excluded pediatric recipients, multiorgan transplants, ABO blood group–incompatible, positive crossmatch, donation after cardiac death donors, and expanded criteria donors. We also excluded patients with a pretransplant fasting serum cholesterol >350 mg/dl, patients with a body mass index>35 kg/m2, patients who were pregnant/lactating, patients who were HIV-positive, and patients with a known sensitivity to Tac, SRL, or MMF.
Immunosuppression
All patients were induced with methylprednisolone and the anti-IL2 receptor antagonist Basiliximab with no additional steroids (rapid steroid elimination). Tac (Prograf) and MMF (Cellcept) or SRL (Rapamune) were started on postoperative day 1.
Drug Monitoring
Target 12-h trough levels for Tac were 8–10 ng/ml during the first 3 months, 7–9 ng/ml from 4 to 6 months, and 6–8 ng/ml thereafter. The target 24-h trough levels for SRL were 7–10 ng/ml during the entire study period.
Outcomes
The primary end points of the study were patient and graft survival. The secondary end points were (1) incidence and severity (by histologic grade) of acute renal allograft rejection, (2) graft function determined by the Modification of Diet in Renal Disease equation, and (3) incidence of hyperlipidemia, infections, hematologic abnormalities, and malignancy.
Data Collection
Recipient demographics included but were not limited to age, race, sex, comorbidities, and need for dialysis pretransplant. Donor characteristics such as donor type (living versus deceased), age, and race were also collected. Post-transplant data included delayed graft function (DGF), episodes of acute rejection, graft loss, patient survival, serial GFRs, new-onset hypertension, diabetes mellitus, incidence of cardiovascular events, anemia, infections, malignancy, and hyperlipidemia.
Rejection Monitoring and Treatment
All rejection episodes were biopsy-proven and graded using the Banff 97 classification. Acute cellular rejections were treated, based on severity, with methylprednisolone followed by a 7-day course of tapered prednisone or antilymphocyte antibody therapy (Thymoglobulin) for up to 14 days. No modification of immunosuppression was made on diagnosis of biopsy-proven chronic allograft nephropathy. Patients who developed acute cellular rejection requiring therapy were maintained on prednisone 5 mg one time per day.
Renal Allograft Function Measurement
GFR was calculated at different time points (1, 3, 6, and every 6 months up to 8.5 years) using the abbreviated Modification of Diet in Renal Disease equation (19).
Statistical Analyses
Data were summarized using descriptive statistics (means and standard deviation for continuous variables; frequency and count for categorical variables). Baseline patient characteristics and postoperative outcomes were compared between the Tac/MMF and Tac/SRL groups. Chi-squared or Fisher exact tests were used to compare categorical variables, whereas the two-sample t or nonparametric Mann–Whitney U tests were used for continuous variables. Patient survival, graft survival, and occurrence of acute rejection were determined using the Kaplan–Meier survival estimates, whereas the cumulative curves were compared between the Tac/MMF and Tac/SRL groups using the log-rank test.
Variables that were found significantly different between the two treatment groups at baseline were included in the risk adjustment model using Cox proportional hazards regression. Finally, a mixed effects model was used to compare estimated GFR (eGFR) slopes over time between the Tac/MMF and Tac/SRL groups. Our power analysis indicated that, using Cox regression of the log hazard ratio with an anticipated patient morality rate and graft failure rate of 5% and 20%, respectively, a sample of 82 patients will achieve 81% power at a two-tailed 0.05-significance level to detect a minimum hazard ratio of 4.33 for any given covariate. Thus, our study is powered to detect any hazard ratios greater than 4.33 or smaller than 0.23 (1/4.33) between the Tac/MMF and Tac/SRL groups. All analyses were conducted using SAS 9.2 (SAS Inc., Cary, NC). All P values were two-sided, and P<0.05 was considered statistically significant.
Results
Patient Characteristics
Eighty-two kidney transplant recipients (Tac/SRL, n=37; Tac/MMF, n=45) were included. Donor and recipient baseline demographics are depicted in Table 1. There were no differences between the two groups, comparing recipient age, race, sex, degree of HLA mismatch, pre-existing diabetes, and donor type (deceased versus living). However, the mean donor age in the Tac/SRL group was significantly higher (39.7±13.3 versus 33.3±11.5 years, P=0.03). Similarly, the rate of DGF in the Tac/SRL group was higher (10.8% versus 0%, P=0.02).
Table 1.
Patient and donor characteristics by treatment group: Tac/SRL versus Tac/MMF
| Characteristics | Tac/SRL (n=37) | Tac/MMF (n=45) | P Valuea | ||
|---|---|---|---|---|---|
| n | Percent | n | Percent | ||
| Age in years (mean±SD) | 45.7±13.4 | 42.3±11.9 | 0.24 | ||
| Race | 0.87 | ||||
| white | 25 | 67.6 | 30 | 66.7 | |
| African American | 10 | 27.0 | 11 | 24.4 | |
| Hispanic | 1 | 2.7 | 1 | 2.2 | |
| Asian | 1 | 2.7 | 3 | 6.7 | |
| Sex | 0.80 | ||||
| male | 22 | 59.5 | 28 | 62.2 | |
| female | 15 | 40.5 | 17 | 37.8 | |
| Diabetes | 0.21 | ||||
| yes | 7 | 18.9 | 4 | 8.9 | |
| no | 30 | 81.1 | 41 | 91.1 | |
| Degree of mismatch in number of Ags (mean±SD) | 3.1±2.0 | 3.6±1.8 | 0.25 | ||
| PRA (percent) >25% | 3 | 8 | 0 | 0 | 0.99 |
| Donor type | 0.77 | ||||
| deceased | 10 | 27.0 | 15 | 33.3 | |
| living | 20 | 54.1 | 21 | 46.7 | |
| living (unrelated) | 7 | 18.9 | 9 | 20.0 | |
| Donor age in years (mean±SD) | 39.7±13.3 | 33.3±11.5 | 0.03 | ||
| DGF | 4 | 10.8 | 0 | 0.0 | 0.02 |
Tac, tacrolimus; SRL, sirolimus; MMF, mycophenolate mofetil; Ags, antigens; PRA, Panel reactive antibody; DGF, delayed graft function.
t test for continuous variables; chi-squared or Fisher exact test for categorical variables.
Patient and Graft Survival
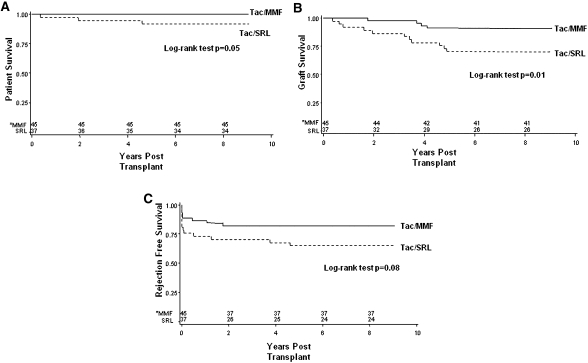
Median follow-up in the Tac/SRL and Tac/MMF groups was 8.5 years (interquartile range=8.1–9.1) and 8.6 years (interquartile range=4.3–9.1), respectively (Table 2). Patient survival at 8.5 years post-transplant tended to be worse among Tac/SRL- compared with Tac/MMF-treated recipients, but it failed to achieve statistical significance (P=0.05) (Figure 2A). Similarly, there were fewer deaths in the Tac/MMF group compared with the Tac/SRL group (0/45 versus 3/37, P=0.09, respectively) (Table 2). Causes of death included leukemia (n=1), lung adenocarcinoma (n=1; patient expired less than 1 year post-transplantation), and myocardial infarction (n=1; patient with known coronary artery disease pretransplant).
Table 2.
Comparison of outcomes between the two groups: Tac/SRL versus Tac/MMF
| Patient Outcome | Tac/SRL (n=37) | Tac/MMF (n=45) | P Valuea | ||
|---|---|---|---|---|---|
| n | Percent | n | Percent | ||
| Length of follow-up (years) | |||||
| mean ± SD | 8.5±0.3 | 8.5±0.7 | 0.99 | ||
| median (range) | 8.5 (8.1–9.1) | 8.6 (4.3–9.1) | 0.99 | ||
| Death | 3 | 8.1 | 0 | 0.0 | 0.09 |
| Kidney loss | 11 | 29.7 | 4 | 8.9 | 0.02 |
| Rejection | 13 | 35.1 | 8 | 17.8 | 0.07 |
| Infection | 9 | 24.3 | 11 | 24.4 | 0.99 |
| Post-Tx CAD | 1 | 2.7 | 2 | 4.4 | 0.99 |
| CAN | 8 | 21.6 | 3 | 6.7 | 0.05 |
| Malignancy | 2 | 5.4 | 0 | 0.0 | 0.19 |
| Hypertension | 36 | 97.3 | 39 | 86.7 | 0.12 |
| Hyperlipidemia | 19 | 51.4 | 24 | 53.3 | 0.86 |
| Anemia (Hgb<11) | 18 | 48.6 | 15 | 33.3 | 0.16 |
| NODAT | 9 | 24.3 | 6 | 13.3 | 0.25 |
Tac, tacrolimus; SRL, sirolimus; MMF, mycophenolate mofetil; CAD, coronary artery disease; CAN, chronic allograft nephropathy; Hgb, hemoglobin; NODAT, new-onset diabetes after transplantation.
t test or Mann–Whitney U test for continuous variables; chi-squared or Fisher exact test for categorical variables.
Figure 2.
Cumulative patient survival, renal allograft survival, and rejection-free survival in tacrolimus/mycophenolate mofetil (Tac/MMF) and Tac/sirolimus (Tac/SRL) groups. Kaplan–Meier patient survival curves. Log-rank test (A, P=0.05; B, P=0.01; C, P=0.08). *Numbers at risk in each group.
Graft survival was significantly worse in the Tac/SRL group compared with the Tac/MMF group (P=0.01) (Figure 2B). Similarly, there were significantly greater graft losses in the Tac/SRL group compared with the Tac/MMF group (11/37 versus 4/45, P=0.02, respectively) (Table 2). Causes of graft loss in the Tac/SRL group included chronic allograft nephropathy (n=8) and death with a functioning graft (n=3). In the Tac/MMF group, the causes of graft loss were acute rejection in the setting of nonadherence (n=1) and chronic allograft nephropathy (n=3). After excluding death with a functioning graft, the difference in graft survival between the Tac/SRL and Tac/MMF groups was 24% versus 9%, respectively, but it failed to achieve statistical significance (P=0.11).
Acute Rejection Rate
Thirteen patients (35.1%) in the Tac/SRL group experienced acute cellular rejection compared with eight patients (17.8%) in the Tac/MMF group (Table 2) (P=0.07), which corresponded with a similar trend in rejection-free survival for the Tac/MMF group (P=0.08) (Figure 2C).
Renal Allograft Function
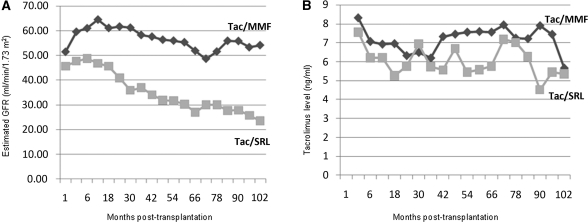
eGFR was consistently lower in the Tac/SRL group compared with the Tac/MMF group throughout the entire long-term follow-up period (Figure 3A, Table 3). At 1 month post-transplantation, eGFR was similar in the two groups (45.7 ml/min per 1.73 m2 in the SRL group compared with 51.4 ml/min per 1.73 m2 in the Tac/MMF group, P=0.14). From 3 months on, graft function was significantly worse in the Tac/SRL group (Table 3). At 3, 5, and 8.5 years of follow-up, eGFR in the Tac/SRL and Tac/MMF groups were 36.9 versus 58.3, 30.3 versus 55.3, and 23.5 versus 54.1 ml/min per 1.73 m2, respectively (Table 3). Moreover, the slope of decline in the SRL group was significantly steeper than in the MMF group (significant group×time interaction effect, P=0.02; data not shown).
Figure 3.
Estimated GFR and Tac trough levels according to treatment groups; Tac/MMF versus Tac/SRL. (A) The GFR slope was significantly steeper in the Tac/SRL group (P=0.02). (B) There was no difference in Tac level over time between Tac/MMF and Tac/SRL groups (P>0.05).
Table 3.
Estimated GFR (ml/min per 1.73 m2) at different time points during the course of follow-up in the Tac/MMF and Tac/SRL groups
| Estimated GFR: Months Post-Transplant | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 | 60 | 66 | 72 | 78 | 84 | 90 | 96 | 102 | |
| Estimated GFR | ||||||||||||||||||
| MMF | 51.4 | 59.6 | 61.0 | 64.5 | 61.1 | 61.7 | 61.2 | 58.3 | 57.6 | 56.3 | 55.3 | 51.8 | 48.6 | 51.5 | 55.9 | 55.8 | 53.3 | 54.1 |
| SRL | 45.7 | 47.7 | 48.7 | 46.8 | 45.6 | 40.9 | 35.9 | 36.9 | 34.1 | 31.8 | 30.3 | 27.0 | 30.1 | 30.0 | 27.7 | 27.9 | 25.7 | 23.5 |
| P value (t test) | 0.14 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.01 | 0.007 | <0.001 | 0.001 | <0.001 | <0.001 |
Adverse Events
There was no significant difference between the two groups with regard to anemia (hemoglobin<11 g/dl), infections, malignancy, new-onset diabetes after transplantation, hypertension, heart disease, or hyperlipidemia (Table 2); 9 of 37 patients (24.3%) in the Tac/SRL group developed >1 g/day proteinuria, and only 3 of 37 patients in the Tac/SRL group developed >3.5 g/day (one with active crescents and recurrent IgA nephropathy and another with chronic allograft nephropathy). In the Tac/MMF group, five patients (11.1%) developed >1 g/day proteinuria, and three patients developed >3.5 g/day (one patient with focal segmental glomerulosclerosis, one patient with recurrent focal segmental glomerulosclerosis, and one patient with recurrent membranoproliferative glomerulonephritis).
Drug Monitoring
Dosing and trough blood levels of Tac during the entire follow-up post-transplantation were similar in the two groups (Figure 3B). SRL levels were between 6 and 10 ng/ml throughout follow-up.
Cox Proportional Hazard Model
After risk adjustment, the Tac/MMF group remained protective against graft failure compared with the Tac/SRL group (hazard ratio=0.20, 95% confidence interval [CI]=0.05–0.77, P=0.02) (Table 4). There were no deaths in the Tac/MMF group compared with three deaths in the Tac/SRL group (0/45 versus 3/37, respectively, P=0.09). There was a tendency to a lower hazard of acute rejection (HR=0.37, 95% CI=0.13–1.01, P=0.05) for the Tac/MMF group after adjusting for donor age and DGF (significant baseline differences were observed for these two variables between the groups).
Table 4.
Cox proportional hazard model for patient mortality, graft failure, and acute rejection adjusting for donor age and delayed graft function
| Patient Mortality | Graft Failure | Acute Rejection | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Treatment (MMF versus SRL) | 0.00 | — | 0.08 | 0.20 | 0.05–0.77 | 0.02 | 0.37 | 0.13–1.01 | 0.05 |
| Donor age | 1.71 | 0.62–4.69 | 0.29 | 0.95 | 0.90–1.01 | 0.08 | 0.99 | 0.95–1.03 | 0.54 |
| DGF (no versus yes) | 0.12 | 0.001–10.10 | 0.35 | 0.88 | 0.11–7.29 | 0.99 | 1.28 | 0.16–10.20 | 0.81 |
HR, hazard ratio; CI, confidential interval; MMF, mycophenolate mofetil; SRL, sirolimus; DGF, delayed graft function.
Discussion
The impact of MMF versus SRL combined with Tac on renal allograft function over the longer term remains poorly characterized. Tac/MMF and Tac/SRL are commonly used immunosuppressive maintenance regimens. Herein, we describe the long-term follow-up of kidney transplant recipients showing that, in steroid-free maintenance immunosuppressive regimens, Tac/SRL was associated with poorer graft survival and poorer renal allograft function compared with Tac/MMF. This finding might be explained on the basis of a trend to a higher acute rejection rate in the Tac/SRL group compared with the Tac/MMF group. The lack of statistical significance observed in our study may be because of insufficient power and a limited sample size. These data are consistent with our previous early observations after limited follow-up (17). Extended follow-up of cohorts is important, because it is often difficult to extrapolate long-term outcomes based on trends from early data (1). Moreover, improvements in short-term outcomes do not seem to translate into improved long-term survival.
Similarly, in a retrospective analysis of adult renal transplant patients from the Scientific Registry of Transplant Recipients between 2000 and 2004, Meier-Kriesche et al. (13) showed that Tac/SRL was associated with significantly worse graft survival compared with Tac/MMF. However, registry data, often lacking in granularity, might not adequately capture changes in immunosuppression, complicating interpretation of studies employing this data source. In another retrospective study of patients treated with Tac or cyclosporine combined with SRL, withdrawal of SRL and substitution with MMF led to improvement of graft function, suggesting a possible synergistic toxicity between SRL and CNIs (20). However, this work was a small, uncontrolled observational study with only 17 patients. The work by Gralla and Wiseman (15) analyzed 518 primary renal recipients transplanted between 2000 and 2006. All patients were maintained on prednisone; 211 patients received Tac/MMF, and 307 patients received Tac/SRL. In addition to the immunosuppressive regimen, outcomes were compared for two eras (2000–2002 and 2003–2006). The Tac/SRL 2000–2002 group had significantly lower 3-year patient and graft survival compared with the Tac/SRL 2003–2006 group and Tac/MMF groups irrespective of the era. Despite similar acute rejection rates, 1-year calculated GFR was significantly lower in the Tac/SRL group compared with the Tac/MMF group (57.6 versus 63.1 mL/min per 1.73 m2, P<0.01) during the 2003–2006 period, which further supports the theory of synergistic nephrotoxicity for the Tac/SRL combination.
The work by Mendez et al. (16) compared Tac/SRL (n=185) and Tac/MMF (n=176) combined with prednisone in renal transplant patients in a multicenter randomized study. Despite only a short term of follow-up, at 12 months post-transplantation, renal function was significantly lower in the SRL group compared with the MMF group. These data and our own data support the notion that the nephrotoxic effect of Tac can be augmented by concurrent use of SRL. Decline in renal allograft function is rapid and persistent when Tac and SRL are used together. In our experience, the reduction in GFR became apparent as early as 3 months post-transplant and persisted throughout the entire follow-up period. The earliest difference noted was the absence of a normal physiologic compensatory hyperfiltration among the Tac/SRL patients indicated by the early flattening of the Tac/SRL eGFR curve (Figure 3A). This finding was closely coupled with a steeper decline in eGFR thereafter. Short-term allograft function has been shown to correlate with long-term graft survival (21–23). CKD has been associated with higher risk of cardiovascular disease, stroke, and subsequent mortality (24–26). However, in our study, we did not observe a greater number of cardiovascular events or cardiac-related mortality in the Tac/SRL group. This finding may be because of the relatively small sample size in our study.
In another multicenter trial, 318 patients were randomized to Tac/SRL, and 316 patients were randomized to Tac/MMF. Renal function at 6 months of follow-up was comparable; however, the study was limited by short follow-up (27). The Edmonton protocol for islet cell transplantation consists of Tac/SRL maintenance and has been associated with nephrotoxicity (28). The mechanism by which the Tac/SRL combination is potentially more nephrotoxic is unclear. The mechanism implicated includes a complex cascade of events leading to perturbation of glomerular hemodynamics in the setting of Tac/SRL combination therapy (29).
In both animal studies and in humans, SRL has been shown to prolong DGF by impairing recovery from ischemia reperfusion injury (30,31). Although there were not a large number of deceased donor kidney transplants in our analysis, we did observe a higher incidence of DGF in the Tac/SRL group compared with the Tac/MMF group (10.8% versus 0%, P=0.02).
Combination of immunosuppressive agents depends on their individual pharmacokinetics and interactions between them (32). SRL increases the bioavailability of cyclosporine, thereby potentially increasing its nephrotoxic effect (33). The impact of SRL on Tac in renal transplant recipients is less consistent (32,34–37). In contrast, MMF has not been shown to impact Tac levels (38). In our study, the trough Tac levels combined with either SRL or MMF were comparable.
SRL has been shown to cause renal tubular collapse, vacuolization in the proximal tubules, and nephrocalcinosis in animal models (39). The production of the cytokine TGF-β in the rat kidney increased with SRL administration (40), which may lead to an increase in fibrosis (41). The work by Han et al. (42) showed, in a mouse model, that SRL accelerates the CNI-induced oxidative process by downregulating the renal antioxidant Klotho expression in the kidney.
The work by Andoh et al. (43) showed that SRL combined with cyclosporine enhances the nephrotoxic effects of the CNI after 4 weeks in a rat model (43). However, patients on SRL have a lower incidence of interstitial fibrosis and tubular atrophy compared with patients on cyclosporine (44). In a prospective randomized study using steroid-free maintenance, at 5 years post-transplantation, chronic CNI toxicity was observed more frequently in CNI combinations with SRL (45).
Studies using low-dose CNI with mammalian target of rapamycin inhibitors have yielded favorable results. A randomized study compared 56 patients on everolimus (C0 trough levels=8–12 ng/ml) and low-dose cyclosporine (C2 levels=250–300 ng/ml) with 50 patients with mycophenolate sodium and standard exposure cyclosporine (C2 levels=500–700 ng/ml). Both groups were also maintained on steroids. The everolimus group was associated with lower DGF, better 1-year graft survival, and significantly higher GFR (81.6 versus 62.6 ml/min per 1.73m2, P<0.001) (46).
Another recent trial randomized 833 patients to everolimus at 1.5 or 3 mg/day (target C0=3–8 and 6–12 ng/ml, respectively) with reduced exposure cyclosporine or mycophenolic acid with standard exposure cyclosporine (47). All patients received basiliximab ± corticosteroids. At 12 months, eGFR was similar in the everolimus groups versus the mycophenolic acid group. Additional studies with longer follow-up are needed to delineate the optimal immunosuppression regimen and optimal dose.
The randomized nature of the study and the long duration of follow-up contribute to the strengths of our study. However, our study is limited to a single center and a relatively small sample size. Although we presume that the decline in GFR in the Tac/SRL group is likely caused by additive nephrotoxicity, we do not have histologic or mechanistic evidence.
In conclusion, our study shows that, in a prednisone-free immunosuppressive regimen for renal transplant recipients induced with an IL2 receptor antagonist, MMF combined with Tac seems to confer superior allograft function and improved long-term graft survival compared with the Tac/SRL combination. The nephrotoxicity associated with Tac is augmented by combination with SRL in a steroid-free immunosuppressive regimen. Given the significant difference in graft function and survival between the two maintenance immunosuppressive regimens evaluated in this study, full-dose Tac and SRL should be reserved for patients unable to tolerate MMF.
Disclosures
None.
Acknowledgment
This work was presented at the American Transplant Congress, San Diego, California, May 1–5, 2010.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Meier-Kriesche HU, Schold JD, Kaplan B: Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 4: 1289–1295, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Bertani T, Ferrazzi P, Schieppati A, Ruggenenti P, Gamba A, Parenzan L, Mecca G, Perico N, Imberti O, Remuzzi A, Remuzzi G: Nature and extent of glomerular injury induced by cyclosporine in heart transplant patients. Kidney Int 40: 243–250, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Davies DR, Bittmann I, Pardo J: Histopathology of calcineurin inhibitor-induced nephrotoxicity. Transplantation 69[Suppl]: SS11–SS13, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Bennett WM: Insights into chronic cyclosporine nephrotoxicity. Int J Clin Pharmacol Ther 34: 515–519, 1996 [PubMed] [Google Scholar]
- 5.Pascual M, Swinford RD, Ingelfinger JR, Williams WW, Cosimi AB, Tolkoff-Rubin N: Chronic rejection and chronic cyclosporin toxicity in renal allografts. Immunol Today 19: 514–519, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Remuzzi G, Perico N: Cyclosporine-induced renal dysfunction in experimental animals and humans. Kidney Int Suppl 52: S70–S74, 1995 [PubMed] [Google Scholar]
- 7.Solez K, Vincenti F, Filo RS: Histopathologic findings from 2-year protocol biopsies from a U.S. multicenter kidney transplant trial comparing tarolimus versus cyclosporine: A report of the FK506 Kidney Transplant Study Group. Transplantation 66: 1736–1740, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Kaplan B, Schold JD, Meier-Kriesche HU: Long-term graft survival with neoral and tacrolimus: A paired kidney analysis. J Am Soc Nephrol 14: 2980–2984, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM: Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 349: 931–940, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Paramesh AS, Roayaie S, Doan Y, Schwartz ME, Emre S, Fishbein T, Florman S, Gondolesi GE, Krieger N, Ames S, Bromberg JS, Akalin E: Post-liver transplant acute renal failure: Factors predicting development of end-stage renal disease. Clin Transplant 18: 94–99, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Meier-Kriesche HU, Li S, Gruessner RWG, Fung JJ, Bustami RT, Barr ML, Leichtman AB: Immunosuppression: Evolution in practice and trends, 1994–2004. Am J Transplant 6: 1111–1131, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Meier-Kriesche HU, Schold JD, Srinivas TR, Howard RJ, Fujita S, Kaplan B: Sirolimus in combination with tacrolimus is associated with worse renal allograft survival compared to mycophenolate mofetil combined with tacrolimus. Am J Transplant 5: 2273–2280, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Srinivas TR, Schold JD, Guerra G, Eagan A, Bucci CM, Meier-Kriesche HU: Mycophenolate mofetil/sirolimus compared to other common immunosuppressive regimens in kidney transplantation. Am J Transplant 7: 586–594, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Gralla J, Wiseman AC: Tacrolimus/sirolimus versus tacrolimus/mycophenolate in kidney transplantation: Improved 3-year graft and patient survival in recent era. Transplantation 87: 1712–1719, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Mendez R, Gonwa T, Yang HC, Weinstein S, Jensik S, Steinberg S; Prograf Study Group: A prospective, randomized trial of tacrolimus in combination with sirolimus or mycophenolate mofetil in kidney transplantation: Results at 1 year. Transplantation 80: 303–309, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Gallon L, Perico N, Dimitrov BD, Winoto J, Remuzzi G, Leventhal J, Gaspari F, Kaufman D: Long-term renal allograft function on a tacrolimus-based, pred-free maintenance immunosuppression comparing sirolimus vs. MMF. Am J Transplant 6: 1617–1623, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D; for the CONSORT Group: CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Int J Surg 9: 672–677, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Gaspari F, Ferrari S, Stucchi N, Centemeri E, Carrara F, Pellegrino M, Gherardi G, Gotti E, Segoloni G, Salvadori M, Rigotti P, Valente U, Donati D, Sandrini S, Sparacino V, Remuzzi G, Perico N; MY.S.S. Study Investigators: Performance of different prediction equations for estimating renal function in kidney transplantation. Am J Transplant 4: 1826–1835, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kaplan B, Schold J, Srinivas T, Womer K, Foley DP, Patton P, Howard R, Meier-Kriesche HU: Effect of sirolimus withdrawal in patients with deteriorating renal function. Am J Transplant 4: 1709–1712, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Giral M, Taddei C, Nguyen JM, Dantal J, Hourmant M, Cantarovich D, Blancho G, Ancelet D, Soulillou JP: Single-center analysis of 468 first cadaveric kidney allografts with a uniform ATG-CsA sequential therapy. Clin Transpl 1996: 257–264, 1996 [PubMed] [Google Scholar]
- 22.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP: Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int 62: 311–318, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Cecka JM: The UNOS Scientific Renal Transplant Registry. Clin Transpl 1998: 1–16, 1998 [PubMed] [Google Scholar]
- 24.McCullough PA, Li S, Jurkovitz CT, Stevens LA, Wang C, Collins AJ, Chen SC, Norris KC, McFarlane SI, Johnson B, Shlipak MG, Obialo CI, Brown WW, Vassalotti JA, Whaley-Connell AT; KEEP Investigators Kidney Early Evaluation Program Investigators: CKD and cardiovascular disease in screened high-risk volunteer and general populations: The Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis 51[Suppl 2]: S38–S45, 2008 [DOI] [PubMed] [Google Scholar]
- 25.McCullough PA, Steigerwalt S, Tolia K, Chen SC, Li S, Norris KC, Whaley-Connell A; KEEP Investigators: Cardiovascular disease in chronic kidney disease: Data from the Kidney Early Evaluation Program (KEEP). Curr Diab Rep 11: 47–55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP: Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int 76: 652–658, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Gurp E, Bustamante J, Franco A, Rostaing L, Becker T, Rondeau E, Czajkowski Z, Rydzewski A, Alarcon A, Bachleda P, Samlik J, Burmeister D, Pallardo L, Moal MC, Rutkowski B, Wlodarczyk Z: Comparable renal function at 6 months with tacrolimus combined with fixed-dose sirolimus or MMF: Results of a randomized multicenter trial in renal transplantation. J Transplant 2010: 731426, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmann R, Spinas GA, Moritz W, Weber M: Has time come for new goals in human islet transplantation? Am J Transplant 8: 1096–1100, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Saurina A, Campistol JM, Piera C, Diekmann F, Campos B, Campos N, de las Cuevas X, Oppenheimer F: Conversion from calcineurin inhibitors to sirolimus in chronic allograft dysfunction: Changes in glomerular haemodynamics and proteinuria. Nephrol Dial Transplant 21: 488–493, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Fuller TF, Freise CE, Serkova N, Niemann CU, Olson JL, Feng S: Sirolimus delays recovery of rat kidney transplants after ischemia-reperfusion injury. Transplantation 76: 1594–1599, 2003 [DOI] [PubMed] [Google Scholar]
- 31.McTaggart RA, Gottlieb D, Brooks J, Bacchetti P, Roberts JP, Tomlanovich S, Feng S: Sirolimus prolongs recovery from delayed graft function after cadaveric renal transplantation. Am J Transplant 3: 416–423, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Cattaneo D, Merlini S, Pellegrino M, Carrara F, Zenoni S, Murgia S, Baldelli S, Gaspari F, Remuzzi G, Perico N: Therapeutic drug monitoring of sirolimus: Effect of concomitant immunosuppressive therapy and optimization of drug dosing. Am J Transplant 4: 1345–1351, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Napoli KL, Taylor PJ: From beach to bedside: History of the development of sirolimus. Ther Drug Monit 23: 559–586, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Undre NA: Pharmacokinetics of tacrolimus-based combination therapies. Nephrol Dial Transplant 18[Suppl 1]: i12–i15, 2003 [DOI] [PubMed] [Google Scholar]
- 35.McAlister VC, Mahalati K, Peltekian KM, Fraser A, MacDonald AS: A clinical pharmacokinetic study of tacrolimus and sirolimus combination immunosuppression comparing simultaneous to separated administration. Ther Drug Monit 24: 346–350, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Kuypers DR, Claes K, Evenepoel P, Maes B, Vanrenterghem Y: Long-term pharmacokinetic study of the novel combination of tacrolimus and sirolimus in de novo renal allograft recipients. Ther Drug Monit 25: 447–451, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Kuypers DR: Influence of interactions between immunosuppressive drugs on therapeutic drug monitoring. Ann Transplant 13: 11–18, 2008 [PubMed] [Google Scholar]
- 38.Undre NA, van Hooff J, Christiaans M, Vanrenterghem Y, Donck J, Heeman U, Kohnle M, Zanker B, Land W, Morales JM, Andrés A, Schäfer A, Stevenson P: Pharmacokinetics of FK 506 and mycophenolic acid after the administration of a FK 506-based regimen in combination with mycophenolate mofetil in kidney transplantation. Transplant Proc 30: 1299–1302, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Andoh TF, Burdmann EA, Fransechini N, Houghton DC, Bennett WM: Comparison of acute rapamycin nephrotoxicity with cyclosporine and FK506. Kidney Int 50: 1110–1117, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Shihab FS, Bennett WM, Yi H, Choi SO, Andoh TF: Sirolimus increases transforming growth factor-beta1 expression and potentiates chronic cyclosporine nephrotoxicity. Kidney Int 65: 1262–1271, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Border WA, Noble NA: Transforming growth factor beta in tissue fibrosis. N Engl J Med 331: 1286–1292, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Han DH, Piao SG, Song JH, Ghee JY, Hwang HS, Choi BS, Kim J, Yang CW: Effect of sirolimus on calcineurin inhibitor-induced nephrotoxicity using renal expression of KLOTHO, an antiaging gene. Transplantation 90: 135–141, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Andoh TF, Lindsley J, Franceschini N, Bennett WM: Synergistic effects of cyclosporine and rapamycin in a chronic nephrotoxicity model. Transplantation 62: 311–316, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Flechner SM, Kurian SM, Solez K, Cook DJ, Burke JT, Rollin H, Hammond JA, Whisenant T, Lanigan CM, Head SR, Salomon DR: De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. Am J Transplant 4: 1776–1785, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Anil Kumar MS, Irfan Saeed M, Ranganna K, Malat G, Sustento-Reodica N, Kumar AM, Meyers WC: Comparison of four different immunosuppression protocols without long-term steroid therapy in kidney recipients monitored by surveillance biopsy: Five-year outcomes. Transpl Immunol 20: 32–42, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Bertoni E, Larti A, Rosso G, Zanazzi M, Di Maria L, Salvadori M: Good outcomes with cyclosporine very low exposure with Everolimus high exposure in renal transplant patients. J Nephrol 24: 613–618, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Tedesco Silva H, Jr, Cibrik D, Johnston T, Lackova E, Mange K, Panis C, Walker R, Wang Z, Zibari G, Kim YS: Everolimus plus reduced-exposure CsA versus mycophenolic acid plus standard-exposure CsA in renal-transplant recipients. Am J Transplant 10: 1401–1413, 2010 [DOI] [PubMed] [Google Scholar]