Summary
Background and objectives
Primary hyperoxaluria (PH) as a cause of ESRD in children is believed to have poor outcomes. Data on management and outcomes of these children remain scarce.
Design, setting, participants, & measurements
This study included patients aged <19 years who started renal replacement therapy (RRT) between 1979 and 2009 from 31 countries providing data to a large European registry.
Results
Of 9247 incident patients receiving RRT, 100 patients had PH. PH children were significantly younger than non-PH children at the start of RRT. The median age at RRT of PH children decreased from 9.8 years in 1979–1989 to 1.5 years in 2000–2009. Survival was 86%, 79%, and 76% among PH patients at 1, 3, and 5 years after the start of RRT, compared with 97%, 94%, and 92% in non-PH patients, resulting in a three-fold increased risk of death over non-PH patients. PH and non-PH patient survival improved over time. Sixty-eight PH children received a first kidney (n=13) or liver-kidney transplantation (n=55). Although the comparison was hampered by the lower number of kidney transplantations primarily derived from the earlier era of RRT, kidney graft survival in PH patients was 82%, 79%, and 76% at 1, 3, and 5 years for liver-kidney transplantation and 46%, 28%, and 14% at 1, 3, and 5 years for kidney transplantation alone, compared with 95%, 90%, and 85% in non-PH patients.
Conclusions
The outcomes of PH children with ESRD are still poorer than in non-PH children but have substantially improved over time.
Introduction
Primary hyperoxaluria (PH) type 1, the most common form of PH, is a rare autosomal recessive disorder caused by the functional defect of the liver-specific peroxisomal enzyme alanine:glyoxylate aminotransferase, leading to oxalate overproduction (1). Oxalate is eliminated in the urine, where it forms complexes and crystals with calcium. Because calcium oxalate is insoluble in urine, PH1 usually presents with symptoms referable to the urinary tract such as tubular injury, stone formation, and nephrocalcinosis (2). Along with progressive decline of GFR due to renal parenchymal involvement, continued overproduction of oxalate by the liver and reduced oxalate excretion by the kidneys lead to systemic involvement, termed oxalosis (3). Oxalosis affects many organs, including bone, retina, vessels, myocardium, nerves, and joints, damage to which results in poor quality of life and death.
Patients with PH1 may benefit from conservative treatment measures, including aggressive hydration, calcium oxalate crystallization inhibitors, and pyridoxine (4,5), but usually progress to ESRD over time at a median age of 24–35 years (6–8). Children who are symptomatic during infancy have a more severe course and most of them reach ESRD before the age of 3 years (9).
In theory, dialysis is ineffective for patients who have reached ESRD because it cannot overcome the continuous production of oxalate by the liver (10). However, in clinical practice, dialysis is required as a temporary therapy for a number of patients. The ultimate management is centered on organ transplantation. Isolated kidney transplantation can reduce plasma oxalate levels and has been reported in the United States and in Europe (with fewer living donors) (11–13); however, disease recurrence often leads to poor graft survival. Liver transplantation completely corrects the enzyme defect. In Europe, with the poor results of isolated kidney transplantation reported 2 decades ago (13), combined or sequential liver-kidney transplantation has predominated thereafter (14). Early reports from the European Dialysis and Transplant Association (EDTA) Registry suggested a patient survival of 79% at 3 years after the start of renal replacement therapy (RRT) and a 3-year kidney graft survival ranging from 25% to 50% in recipients aged <15 years (13,15). Liver-kidney transplantation allograft and patient survival have improved over time in the United States and may currently approach that of transplant patients with kidney transplantation alone (16,17). However, because most registries’ reports combine adult and children, data focusing on management and outcomes of ESRD in children with PH are limited to small case series (18–21). The objective of this study was to describe the incidence, characteristics, treatment, and outcomes in a large cohort of children starting RRT for primary oxalosis.
Materials and Methods
Study Population
This study was based on pediatric patients starting RRT recorded in the European Society for Pediatric Nephrology/European Renal Association-European Dialysis and Transplant Association (ESPN/ERA-EDTA) Registry (22,23). Patients whose primary renal disease was reported to be “primary oxalosis” (ERA-EDTA primary renal disease code 53) will be termed as PH patients in this study. All others will be termed as non-PH patients. Patients aged <19 years at the start of RRT who started RRT from 1979 were included in the analyses. Only patients from countries and periods in which follow-up data were complete were included in the analyses. The periods of inclusions differed by country (Table 1). Patients with infantile oxalosis were defined as PH patients with ESRD before 2 years of age.
Table 1.
Number of patients with RRT due to PH during periods of complete follow-up, by country
| Country | Number of Children Starting RRT | |
|---|---|---|
| Total | PH | |
| Austria (1979–2008) | 348 | 1 |
| Belgium (1994–2008) | 216 | 6 |
| Croatia (1979–2008) | 131 | 2 |
| Denmark (1990–2008) | 227 | 8 |
| France (2004–2009) | 582 | 11 |
| Greece (1980–2008) | 497 | 2 |
| Italy (1990–2009) | 847 | 12 |
| Lithuania (2006–2009) | 21 | 1 |
| Netherlands (1979–2008) | 1046 | 11 |
| Norway (1980–2008) | 283 | 6 |
| Poland (2006–2008) | 174 | 1 |
| Russia (2006–2008) | 205 | 2 |
| Serbia (2006–2009) | 24 | 1 |
| Spain (1982–2009) | 1330 | 16 |
| Sweden (1991–2008) | 320 | 1 |
| Switzerland (1979–2009) | 351 | 10 |
| United Kingdom (1990–2008) | 1636 | 9 |
| Other countriesa | 809 | 0 |
| Total | 9247 | 100 (1.1%) |
RRT, renal replacement therapy; PH, primary hyperoxaluria.
Including Belarus (2008), Czech Republic (2006–2008), Estonia (2006–2008), Finland (1979–2008), Former Yugoslav Republic of Macedonia (2006–2008), Hungary (2006–2008), Iceland (1979–2009), Latvia (2006–2008), Montenegro (2006–2008), Portugal (2006–2008), Romania (2005–2008), Slovenia (2006–2008), Slovakia (2006–2008), and Turkey (2002).
Data Collection
Within the ESPN/ERA-EDTA Registry, data are collected annually from national pediatric registries and via the ERA-EDTA Registry. Information includes the following: date of birth, sex, primary renal disease, date of start of RRT, treatment modality, change in RRT modality, death, transfer out of the registry, and, for some countries, clinical parameters such as height, BP, albumin, hemoglobin, and parathyroid hormone blood levels. Because height and BP depend on age and sex, SD scores were used. We defined arterial hypertension as either systolic or diastolic BP ≥95th percentile for age, height, and sex or the use of antihypertensive medication (24). SD scores for height were calculated using the Centers for Disease Control and Prevention growth charts (25). Additional data on transplantation procedures and causes of death were obtained for PH patients via the use of specific questionnaires.
Statistical Analyses
Incidence of RRT for PH patients <19 years of age and point prevalence on December 31, 2008, were studied and expressed per 100 million age-related population. Data on the general population were derived from Eurostat (26).
The characteristics of PH patients were compared with the overall incident RRT population of children <19 years of age. We determined 5-year patient and first kidney graft survival by performing Kaplan–Meier and Cox regression analyses, thereby censoring follow-up time when a patient reached the age of 20 years, the end of the study, or 5-year follow-up, whichever came first. Analyses were adjusted for age, sex, and decade of start of RRT using the adjusted hazard ratio (aHR).
To determine whether there were differences in general clinical characteristics, we matched three non-PH patients to one PH patient by age at the start of RRT (1-year interval), period of start of RRT (5-year interval), and country (or neighboring country if no eligible patient was available). Missing data for height SD score (40%), BP measurements (47%), hemoglobin (50%), albumin (59%), and log-transformed parathyroid hormone levels (55%) were imputed using a multiple imputation approach as recommended by the STrenghtening the Reporting of OBservational Studies in Epidemiology (STROBE) guidelines (27). Linear and logistic mixed models, which are methods for repeated measurements, were used to compare clinical parameters of the PH patients with those of the matched population (28). Statistical analyses were performed using SAS 9.2 software.
Results
Incidence and Prevalence
Of the 9247 children starting RRT between 1979 and 2009, 100 PH patients (1.1% of all patients) were reported (Table 1). Overall incidence of RRT for PH was 7.1 per 100 million age-related population in 1990–2008, with a 10-times higher incidence in infants (30.1 pmarp) than in the group aged 15–18 years (3.1 pmarp). The incidence was lower in Eastern and Central Europe compared with Western European countries. Point prevalence on December 31, 2008, of RRT for PH in children aged <19 years was 38.7 per 100 million age-related population.
Population Characteristics
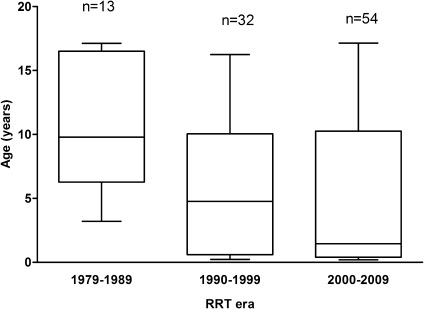
PH children were significantly younger than non-PH children at the start of RRT (Table 2). Patients with infantile oxalosis accounted for almost 40% of the PH population. Median age of PH children at start of RRT decreased significantly (P=0.01) from 9.8 (interquartile range [IQR], 7.2–16.3) in 1979–1989 to 4.6 (IQR, 0.6–10.0) in 1990–1999 and 1.5 years (IQR, 0.4–9.9) in 2000–2009 (Figure 1). Sex, decade of start of RRT, and initial treatment modality were similar in both PH and non-PH patients (Table 2).
Table 2.
Characteristics of PH and non-PH patients starting renal replacement therapy in 1979–2009
| Characteristic | PH Patients | Non-PH Patients | P Value |
|---|---|---|---|
| Number of patients | 100 | 9147 | |
| Male sex, n (%) | 61 (61.0) | 5263 (57.5) | 0.48 |
| Age (yr), median (IQR) | 5.3 (0.5–10.2) | 11.4 (5.8–15.1) | <0.001 |
| Age category (yr), n (%) | <0.001 | ||
| 0–1.9 | 38 (38.0) | 1132 (12.4) | |
| 2–6.9 | 15 (15.0) | 1526 (16.7) | |
| 7–11.9 | 25 (25.0) | 2203 (24.1) | |
| ≥12 | 21 (21.0) | 4261 (46.6) | |
| missing | 1 (1.0) | 25 (0.2) | |
| Decade of entry, n (%) | 0.75 | ||
| 1979–1989 | 13 (13.0) | 1369 (15.0) | |
| 1990–1999 | 32 (32.0) | 3018 (33.0) | |
| 2000–2009 | 54 (54.0) | 4736 (51.8) | |
| missing | 1 (1.0) | 24 (0.2) | |
| Initial treatment, n (%) | 0.07 | ||
| hemodialysis | 55 (55.0) | 3691 (40.3) | |
| peritoneal dialysis | 31 (31.0) | 3567 (39.0) | |
| transplantation | 6 (6.0) | 1401 (15.3) | |
| missing/unknown | 9 (9.0) | 588 (6.4) |
PH, primary hyperoxaluria; IQR, interquartile range.
Figure 1.
Age at start of RRT in patients with primary hyperoxaluria according to RRT era. The horizontal line in the middle of the box represents the median, the bottom and top of the box represent the lower and upper quartiles, respectively, and the ends of the whiskers represent the minimum and the maximum. RRT, renal replacement therapy.
Patient Survival
Within 5 years after commencing RRT, 18 of 100 PH patients (18%) and 491 of 9147 non-PH patients (5%) died. Four additional deaths were recorded in PH patients >5 years after RRT. Among the 22 deaths in PH patients, 12 occurred while on dialysis, 8 after transplantation, and 2 after graft failure and return to dialysis. Median age at death was 4.2 years (range, 1.4–10.9). The causes of death in PH patients are summarized in Table 3.
Table 3.
Causes of death in 22 patients with primary hyperoxaluria on renal replacement therapy
| Cause of Death | n (%) |
|---|---|
| Acute complications of dialysis (fluid overload, hyperkalemia) | 4 (18) |
| Cardiovascular disease | 3 (14) |
| ESRD treatment refused by patient or withdrawn for medical reasons | 3 (14) |
| Complications of liver transplantation | 3 (14) |
| Infections (pneumonia, bacterial sepsis) | 2 (9) |
| Malignancy | 1 (4) |
| Cachexia | 1 (4) |
| Other/unknown | 5 (23) |
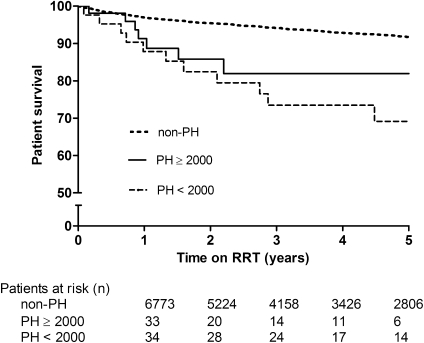
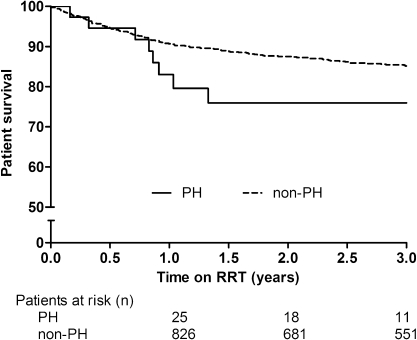
Overall PH patient survival was 86%, 79%, and 76% at 1, 3, and 5 years after the start of RRT, respectively, compared with 97%, 94%, and 92% in non-PH patients (Figure 2). PH children had a nearly three-fold (aHR. 2.70; 95% CI, 1.73–4.23) higher risk of death within 5 years compared with non-PH patients (P<0.01). In children aged <2 years, 1- and 3-year survival on RRT was 81% and 74% in PH patients versus 90% and 85% in non-PH patients, respectively (aHR, 2.05; 95% CI, 1.08–3.91; P=0.02) (Figure 3).
Figure 2.
Unadjusted 5-year survival on RRT for PH patients according to period of RRT versus non-PH patients (log-rank P<0.001). RRT, renal replacement therapy; PH, primary hyperoxaluria.
Figure 3.
Unadjusted 3-year survival on RRT in children aged <2 years for PH patients versus non-PH patients (log-rank P=0.03). RRT, renal replacement therapy; PH, primary hyperoxaluria.
Among PH patients, those with infantile oxalosis had a higher risk of death (aHR, 3.44; 95% CI, 1.15–10.28; P=0.02) compared with PH patients aged >2 years at RRT. In addition, survival among PH patients improved over time. Patient survival at 1, 3, and 5 years was 88%, 83%, and 83% in patients who started RRT in 2000–2009 versus 83%, 75%, and 71% in those starting RRT before 2000. Mortality was significantly higher in 1979–1989 (aHR, 4.5; 95% CI, 1.2–16.2; P=0.02) and in 1990–1999, although not statistically significant (aHR, 1.8; 95% CI, 0.7–5.1; P=0.20) compared with those starting RRT from 2000 onward. Survival in non-PH patients did not improve to the same extent during the same period.
The survival on dialysis was significantly lower at 1 (91%) and 2 years (83%) after the start of dialysis in PH patients, compared with 97% and 95% in non-PH patients (aHR, 1.99; 95% CI, 1.06–3.75; P=0.03). This was also true for post-transplantation patient survival, in which survival was 89% and 87% at 1 and 3 years in PH versus 98% and 96%, respectively, among non-PH patients (aHR, 3.00; 95% CI, 1.48–6.07; P<0.01). No effect of dialysis duration on post-transplantation patient survival was found.
Transplantation Outcome
Among PH patients, 68 (68%) received a first kidney transplantation, including 55 liver-kidney transplantations (53 combined and 2 sequential) and 13 kidney transplantations, at a median age of 7.4 years (IQR, 3.1–13.1). The proportions of kidney transplantation were 7 to 8 (87%) from 1979 to 1989, 5 to 22 (23%) from 1990 to 1999, and 1 to 38 (3%) from 2000 onward. The median time on dialysis before first kidney transplantation was 13.9 months (IQR, 7.2–22.6), whereas six patients received a pre-emptive kidney graft. Although not significantly different, the time spent on dialysis before transplantation for PH increased over time from 8.4 months in 1979–1989 to 11.6 months in 1990–1999 and 16.7 months in 2000–2009, whereas it remained unchanged at 12 months in the non-PH patients.
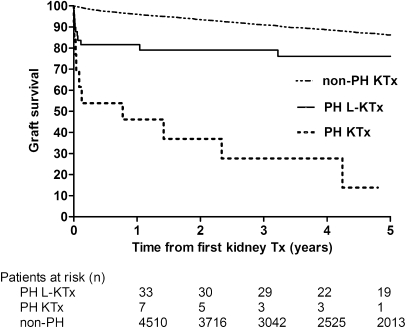
Kidney graft survival in PH patients was poor at 3 months post-transplantation (54% for kidney transplantation and 82% for liver-kidney transplantation) but stabilized thereafter for liver-kidney transplantation (82%, 79%, and 76% at 1, 3, and 5 years, respectively), whereas it declined steeply for kidney transplantation alone (46%, 28%, and 14% at 1, 3, and 5 years, respectively), compared with a graft survival of 95%, 90% and 85% at 1, 3, and 5 years post-transplantation in non-PH patients (Figure 4). Liver-kidney transplantation was associated with a lower risk of graft failure than kidney transplantation (aHR, 0.14; 95% CI, 0.05–0.41; P<0.001). Among the 55 liver-kidney transplantations, kidney graft survival did not change over time.
Figure 4.
Unadjusted 5-year kidney graft survival for PH patients versus non-PH patients (log-rank P<0.001). PH, primary hyperoxaluria; KTx, kidney transplant; L-KTx, liver-kidney transplant; Tx, transplant.
Overall, PH children had a two-fold (aHR, 2.18; 95% CI, 1.44–3.30) higher risk of graft failure than non-PH patients (P<0.001). Dialysis modality and duration were not significantly associated with graft survival.
Clinical Parameters
General clinical parameters were compared between 99 PH patients on RRT and their matched control group (n=297) (Table 4). PH patients on dialysis had significant lower hemoglobin and albumin levels compared with the matched population. There were no differences between PH and the other patients regarding the other parameters.
Table 4.
Clinical parameters in PH patients versus matched pediatric RRT population
| Dialysis | Transplantation | |||
|---|---|---|---|---|
| PH Patients | Non-PH Patients | PH Patients | Non-PH Patients | |
| Height, mean SD score | −1.5 (−2.9 to −0.1) | −1.4 (−2.1 to −0.7) | −1.3 (−2.0 to −0.6) | −1.4 (−1.7 to −1.1) |
| Laboratory values | ||||
| hemoglobin (g/dl), mean | 9.4 (8.8–10.0)a | 10.4 (10.1–10.7) | 11.7 (11.0–12.4) | 12.0 (11.7–12.3) |
| parathyroid hormone (pg/ml), median | 438 (184–711) | 417 (214–621) | 122 (59–303) | 174 (124–224) |
| albumin (g/L), mean | 32.1 (29.4–34.9)a | 36.0 (34.9–37.2) | 39.4 (37.0–41.9) | 42.4 (40.2–44.5) |
| BP | ||||
| % hypertensive | 59 | 58 | 39 | 50 |
| systolic BP, mean SD score | 1.4 (0.7–2.2) | 1.3 (1.1–1.5) | 0.8 (0.3–1.3) | 1.0 (0.8–1.2) |
| diastolic BP, mean SD score | 1.4 (1.0–1.8) | 1.2 (1.0–1.4) | 0.7 (0.3–1.0) | 0.8 (0.6–0.9) |
Data in parentheses are 95% confidence intervals. PH, primary hyperoxaluria; RRT, renal replacement therapy;
Significantly lower in PH patients than in non-PH patients (P<0.05).
Discussion
PH accounts for 1% of pediatric ESRD with an incidence of 7 per 100 million age-related population, which indicates 6 to 7 additional children with oxalosis in Europe per year requiring RRT. During the past decades, there has been a dramatic change in the age distribution of PH patients. Even though patient and graft survival improved among PH patients, these are still much lower than among non-PH patients.
The age distribution of oxalosis on RRT has changed dramatically; no infantile cases were reported in 1979–1989, whereas they represent half of the new cases in 2000–2009. There is likely to be an underestimation of the incidence of PH in children aged 15–18 years because some adolescents with ESRD are treated in adult dialysis units and therefore might not have been recorded in the ESPN/ERA-EDTA Registry. The higher proportion of infantile cases in the more recent period may reflect the increasing acceptance of infants into RRT programs over the past 25 years (29), as well as increased accuracy in diagnosis. Furthermore, older children are more frequently diagnosed before ESRD than infants and therefore might benefit from aggressive conservative treatment that may preserve renal function until adulthood (5,30). Conversely, infantile oxalosis usually progresses rapidly to ESRD. A previous study of infantile PH1 including patients from both developing and developed countries showed that ESRD was present in 50% of children at the time of diagnosis and >80% had ESRD by the age of 3 years (9).
We found that outcomes of RRT for PH remain poor in children. PH children had an almost three-fold increased risk for death over non-PH patients. The survival of PH children was lower on both dialysis and transplantation. A major determinant of survival among PH children was age with a considerably higher risk for death in infants. Fortunately, patient survival of PH children on RRT has significantly improved in the last decade. Five-year survival increased by 12% in PH patients who started RRT in 2000 or later compared with those who started RRT before 2000. Survival now approaches that of infants and young children with RRT from other causes (31), suggesting a better management of oxalosis.
The choice of organ transplantation strategy is still challenging in children with oxalosis (32). Because the alanine:glyoxylate aminotransferase enzyme is specific to hepatocytes, complete removal and replacement of the native liver is required to correct the metabolic defect causing PH1. Thus, pre-emptive isolated liver transplantation before the occurrence of advanced CKD might be considered in carefully selected patients (18,33), but must be weighed against the additional mortality associated with hepatectomy and liver transplantation. Indeed, some patients in this study, in particular infants, died of liver transplant complications early after combined liver-kidney transplantation or liver retransplantation, whereas older PH patients were more likely to die on dialysis.
Combined liver-kidney transplantation is currently the most widely used procedure. Since the first report of combined liver-kidney transplantation in 1985 (34), early studies from Europe suggested better outcomes with liver-kidney transplantation (13,14), whereas those from the United States suggested equally or even better outcomes with kidney transplantation alone (11,12). Our data show a poor kidney graft survival in kidney transplantation primarily derived from the earliest era of RRT; yet, even in liver-kidney transplant recipients, early kidney graft survival was poorer than in non-PH patients. Most of the kidney graft failures occurred in the first 3 months after transplantation and included deaths related to liver transplant complications in patients with a functioning kidney graft. One may speculate that mobilization of the oxalate pool from tissue stores with return of renal function leads to a persistently high oxalate load presented to the new kidney, thereby exposing the graft to the consequences of high urinary oxalate excretion. It has been shown that urine oxalate levels can remain markedly elevated as long as several years after successful kidney-liver transplantation (17). Moreover, PH patients are frequently in a critical condition due to systemic oxalosis at the time of kidney-liver transplantation. This stresses the importance of the specific perioperative intensive care of PH patients as well as postoperative continuation of high fluid intake and crystallization inhibitors to prevent disease recurrence in the new kidney (2,3,35). Unfortunately, causes of graft failure are not available in the registry; thus, whether early graft losses were related to hyperoxaluria or to other causes is unknown. However, in children who survived the early period after transplantation, successful liver-kidney transplantation was associated with similar long-term kidney survival as in non-PH patients, as has been noted previously (19–21).
We did not observe any difference between PH and non-PH patients regarding clinical parameters except in hemoglobin and serum albumin levels among dialysis patients. Anemia may be attributed to bone marrow involvement of systemic oxalosis.
Outcomes of PH patients on RRT have improved over time, although many more infants were taken into therapy (16,17). Earlier diagnosis, greater availability of genetic testing (36), appropriate supportive therapy (5), adjusted dialysis techniques such as frequent hemodiafiltration or combined hemodialysis and peritoneal dialysis (10,11), consideration of timing of liver-kidney transplantation (17), and specific perioperative management (35) may have contributed to better management of oxalosis. Although we found no association of dialysis duration on patient or kidney graft survival, unlike in other studies (7,14), the increasing time spent on dialysis by PH children may jeopardize some of the progress seen in this study. This time may reflect the younger age of the population as well as the longer waiting time for combined liver-kidney transplantation.
The limitations of this study include the large amount of missing data and the disease classification (PH1 and non-PH1 might be reported under the same code of primary oxalosis). However, given the available information on the diagnosis of PH1 for almost two-thirds of patients, the age distribution, and the need for liver-kidney transplantation, we believe that our assumption that all cases were PH1 is most likely correct.
The occurrence of ESRD in children with PH who require RRT is challenging, and in the past has been characterized by poor outcomes. Although outcomes of RRT remain poorer in PH children than in other children with ESRD, there has been substantial improvement over time.
Disclosures
None.
Acknowledgments
We thank the patients, their parents, and the staff of all of the dialysis and transplant units who have contributed their national registries and contact persons. We also thank R. Coppo, M.A. Lewis, and D. Haffner for being members of the ESPN/ERA-EDTA Registry Committee. In addition, we thank the following individuals for contributing data to the ESPN/ERA-EDTA Registry: D. Shitza, R. Kramar, R. Oberbauer, S. Baiko, A. Sukalo, K. van Hoeck, F. Collart, J.M. des Grottes, R. Lombaerts, D. Pokrajac, D. Roussinov, Z. Puretić, D. Batinić, T. Seeman, K. Vondrak, J. Heaf, U. Toots, P. Finne, C. Grönhagen-Riska, C. Holmberg, C. Couchoud, P. Niaudet, L. le Mignot, E. Sahpazova, G. Gersdorf, C. Scholz, G. Ioannidis, G. Reusz, S. Túri, L. Szabó, T. Szabó, B. Gianoglio, G. Leozappa, F. Mencarelli, B. Minale, F. Puteo, A. Jankauskiene, S. Pavićević, T. Leivestad, D. Brackman, A. Zurowska, I. Zagozdzon, C. Mota, M. Almeida, C. Afonso, G. Mircescu, L. Garneata, E. Podgoreanu, M. Gafencu, E.A. Molchanova, N.A. Tomilina, B.T. Bikbov, A. Peco-Antic, M. Kostic, B. Spasojevic-Dimitrijeva, D. Paripovic, L. Podracka, G. Kolvek, J. Buturovic-Ponikvar, G. Novljan, N. Battelino, A. Alonso Melgar and the Spanish Pediatric Registry, S. Schön, J.K.G. Prütz, A. Seeberger, L. Backmän, M. Herthelius, C.E. Kuenhi, E. Maurer, A. Hoitsma, A. Hemke, W.F. Tromp, N. Schoenmaker, A. Kramer, M.W. van de Luijtgaarden, R. Topaloglu, D. Ivanov, D. Ansell, and C. Inward.
The ESPN/ERA-EDTA Registry is funded by ESPN, ERA-EDTA, and the NephroQUEST project. The NephroQUEST project has received funding from the European Union in the framework of the Public Health Program (Project 2006114). J.H. was supported by an ERA-EDTA QUEST initiative research fund and received travel assistance from Astellas Pharma, Fresenius Medical Care, Pfizer, and Sandoz. Amgen has provided an unrestricted educational grant to assist the ESPN in the financial support of the registry.
Preliminary results from this study were presented at the 48th Congress of the ERA-EDTA, Prague, June 23–26, 2011.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Danpure CJ, Jennings PR: Peroxisomal alanine:glyoxylate aminotransferase deficiency in primary hyperoxaluria type I. FEBS Lett 201: 20–24, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Hoppe B, Beck BB, Milliner DS: The primary hyperoxalurias. Kidney Int 75: 1264–1271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cochat P, Liutkus A, Fargue S, Basmaison O, Ranchin B, Rolland MO: Primary hyperoxaluria type 1: Still challenging! Pediatr Nephrol 21: 1075–1081, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Leumann E, Hoppe B, Neuhaus T, Blau N: Efficacy of oral citrate administration in primary hyperoxaluria. Nephrol Dial Transplant 10[Suppl 8]: 14–16, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Fargue S, Harambat J, Gagnadoux MF, Tsimaratos M, Janssen F, Llanas B, Berthélémé JP, Boudailliez B, Champion G, Guyot C, Macher MA, Nivet H, Ranchin B, Salomon R, Taque S, Rolland MO, Cochat P: Effect of conservative treatment on the renal outcome of children with primary hyperoxaluria type 1. Kidney Int 76: 767–773, 2009 [DOI] [PubMed] [Google Scholar]
- 6.van Woerden CS, Groothoff JW, Wanders RJ, Davin JC, Wijburg FA: Primary hyperoxaluria type 1 in The Netherlands: Prevalence and outcome. Nephrol Dial Transplant 18: 273–279, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Harambat J, Fargue S, Acquaviva C, Gagnadoux MF, Janssen F, Liutkus A, Mourani C, Macher MA, Abramowicz D, Legendre C, Durrbach A, Tsimaratos M, Nivet H, Girardin E, Schott AM, Rolland MO, Cochat P: Genotype-phenotype correlation in primary hyperoxaluria type 1: The p.Gly170Arg AGXT mutation is associated with a better outcome. Kidney Int 77: 443–449, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Lieske JC, Monico CG, Holmes WS, Bergstralh EJ, Slezak JM, Rohlinger AL, Olson JB, Milliner DS: International registry for primary hyperoxaluria. Am J Nephrol 25: 290–296, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Cochat P, Koch Nogueira PC, Mahmoud MA, Jamieson NV, Scheinman JI, Rolland MO: Primary hyperoxaluria in infants: Medical, ethical, and economic issues. J Pediatr 135: 746–750, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Illies F, Bonzel KE, Wingen AM, Latta K, Hoyer PF: Clearance and removal of oxalate in children on intensified dialysis for primary hyperoxaluria type 1. Kidney Int 70: 1642–1648, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Scheinman JI, Najarian JS, Mauer SM: Successful strategies for renal transplantation in primary oxalosis. Kidney Int 25: 804–811, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Saborio P, Scheinman JI: Transplantation for primary hyperoxaluria in the United States. Kidney Int 56: 1094–1100, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Broyer M, Brunner FP, Brynger H, Dykes SR, Ehrich JH, Fassbinder W, Geerlings W, Rizzoni G, Selwood NH, Tufveson G, Wing AJ: Kidney transplantation in primary oxalosis: Data from the EDTA Registry. Nephrol Dial Transplant 5: 332–336, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Jamieson NV, European PHI Transplantation Study Group : A 20-year experience of combined liver/kidney transplantation for primary hyperoxaluria (PH1): The European PH1 transplant registry experience 1984-2004. Am J Nephrol 25: 282–289, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Mehls O, Rigden S, Ehrich JH, Berthoux F, Jones EH, Valderrábano F, European Dialysis and Transplant Association-European Renal Association : Report on management of renal failure in Europe, XXV, 1994. The child-adult interface. The EDTA-ERA Registry. Nephrol Dial Transplant 11[Suppl 1]: 22–36, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Cibrik DM, Kaplan B, Arndorfer JA, Meier-Kriesche HU: Renal allograft survival in patients with oxalosis. Transplantation 74: 707–710, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Bergstralh EJ, Monico CG, Lieske JC, Herges RM, Langman CB, Hoppe B, Milliner DS, IPHR Investigators : Transplantation outcomes in primary hyperoxaluria. Am J Transplant 10: 2493–2501, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nissel R, Latta K, Gagnadoux MF, Kelly D, Hulton S, Kemper MJ, Ruder H, Söderdahl G, Otte JB, Cochat P, Roquet O, Jamieson NV, Haffner D: Body growth after combined liver-kidney transplantation in children with primary hyperoxaluria type 1. Transplantation 82: 48–54, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Brinkert F, Ganschow R, Helmke K, Harps E, Fischer L, Nashan B, Hoppe B, Kulke S, Müller-Wiefel DE, Kemper MJ: Transplantation procedures in children with primary hyperoxaluria type 1: Outcome and longitudinal growth. Transplantation 87: 1415–1421, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Gagnadoux MF, Lacaille F, Niaudet P, Revillon Y, Jouvet P, Jan D, Guest G, Charbit M, Broyer M: Long term results of liver-kidney transplantation in children with primary hyperoxaluria. Pediatr Nephrol 16: 946–950, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Millan MT, Berquist WE, So SK, Sarwal MM, Wayman KI, Cox KL, Filler G, Salvatierra O, Jr, Esquivel CO: One hundred percent patient and kidney allograft survival with simultaneous liver and kidney transplantation in infants with primary hyperoxaluria: A single-center experience. Transplantation 76: 1458–1463, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Tizard EJ, Verrina E, van Stralen KJ, Jager KJ: Progress with the European Society for Paediatric Nephrology (ESPN)/ERA-EDTA Registry for children with established renal failure (ERF). Nephrol Dial Transplant 24: 2615–2617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Stralen KJ, Tizard EJ, Jager KJ, Schaefer F, Vondrak K, Groothoff JW, Podracká L, Holmberg C, Jankauskiené A, Lewis MA, van Damme-Lombaerts R, Mota C, Niaudet P, Novljan G, Peco-Antic A, Sahpazova E, Toots U, Verrina E: Determinants of eGFR at start of renal replacement therapy in paediatric patients. Nephrol Dial Transplant 25: 3325–3332, 2010 [DOI] [PubMed] [Google Scholar]
- 24.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 25.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL: Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics 109: 45–60, 2002 [DOI] [PubMed] [Google Scholar]
- 26.European Commission: Eurostat. Statistics database. Available at: http://epp.eurostat.ec.europa.eu/portal/page/portal/eurostat/home Accessed March 28, 2011
- 27.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR: Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 338: b2393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Twisk JWR: Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide, Cambridge, UK, Cambridge University Press, 2003, p 318 [Google Scholar]
- 29.van der Heijden BJ, van Dijk PC, Verrier-Jones K, Jager KJ, Briggs JD: Renal replacement therapy in children: Data from 12 registries in Europe. Pediatr Nephrol 19: 213–221, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Milliner DS, Eickholt JT, Bergstralh EJ, Wilson DM, Smith LH: Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N Engl J Med 331: 1553–1558, 1994 [DOI] [PubMed] [Google Scholar]
- 31.US Renal Data System : USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 32.Scheinman JI: Liver transplantation in oxalosis prior to advanced chronic kidney disease. Pediatr Nephrol 25: 2217–2222, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Perera MT, Sharif K, Lloyd C, Foster K, Hulton SA, Mirza DF, McKiernan PJ: Pre-emptive liver transplantation for primary hyperoxaluria (PH-I) arrests long-term renal function deterioration. Nephrol Dial Transplant 26: 354–359, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Watts RW, Calne RY, Williams R, Mansell MA, Veall N, Purkiss P, Rolles K: Primary hyperoxaluria (type I): Attempted treatment by combined hepatic and renal transplantation. Q J Med 57: 697–703, 1985 [PubMed] [Google Scholar]
- 35.Harps E, Brinkert F, Ganschow R, Briem-Richter A, van Husen M, Schmidtke S, Herden U, Nashan B, Fischer L, Kemper MJ: Immediate postoperative intensive care treatment of pediatric combined liver-kidney transplantation: Outcome and prognostic factors. Transplantation 91: 1127–1131, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Williams EL, Acquaviva C, Amoroso A, Chevalier F, Coulter-Mackie M, Monico CG, Giachino D, Owen T, Robbiano A, Salido E, Waterham H, Rumsby G: Primary hyperoxaluria type 1: Update and additional mutation analysis of the AGXT gene. Hum Mutat 30: 910–917, 2009 [DOI] [PubMed] [Google Scholar]