Summary
Background and objectives
Guidelines promote early fistula creation to avoid central venous catheter use. This practice may lead to fistula creations in patients who never receive dialysis. The objective of this study was to estimate the risk of fistula nonuse with long-term follow-up.
Design, setting, participants, & measurements
Administrative health data identified 1929 predialysis adults who had their first fistula creation between April of 2002 and March of 2006. Patients were followed for a minimum of 2 years or until they began dialysis, received a kidney transplant, or died.
Results
The median follow-up times in patients who started dialysis, died without receiving dialysis, and remained in predialysis were 6.1, 11.5, and 38.7 months, respectively. Eighty-one percent of patients initiated dialysis; 9% of patients died without receiving dialysis, and 10% of patients remained predialysis. Forty percent of patients had their first fistula creation 3–12 months before initiating dialysis (the recommended window). Thirty percent were created within 90 days of starting dialysis; 30% were created more than 1 year before starting dialysis, and 10% were created more than 2 years before starting dialysis. Older patients, females, and patients with less comorbidity were not as likely to initiate dialysis after incident fistula creation.
Conclusions
Most patients who underwent fistula creation before starting dialysis eventually received dialysis with extended follow-up, but the risk was significantly modified by age, sex, and comorbidity. Many patients had fistula creations earlier or later than recommended.
Introduction
Clinical practice guidelines recommend that patients with GFRs from 15 to 30 ml/min per 1.73 m2 be referred for fistula creation (1,2). However, likelihood of initiating dialysis is highly variable in this population (3). Strictly applying such guidelines could, therefore, increase the creation of fistulae that are never used or created too early in the course of CKD. Indeed, the likelihood of starting dialysis ranges from 57–70% depending on the duration of follow-up (4,5). In one of these studies, fistulae were also created when the GFR was between 11 and 14 ml/min per 1.73 m2, which is much later in the course of CKD than recommended (4). This evidence suggests that the risk of fistula nonuse may be substantial, and therefore, the objective of this study was to estimate this risk in a large, generalizable CKD cohort that could be followed for an extended period of time. The latter caveat is important to determine whether relatively short follow-up is overestimating the risk of fistula nonuse. Secondary objectives were to estimate the risk of fistula creation too early in the course of CKD and determine whether age, sex, diabetes, and other comorbidities significantly modified the likelihood of initiating dialysis.
Materials and Methods
The study was a population-based retrospective cohort study using provincial healthcare databases housed at the Institute for Clinical Evaluative Sciences (ICES) in Ontario, Canada (www.ices.on.ca). Ontario has a population of approximately 13 million people with access to a single-payer public health insurance program that covers all hospital and physician services. We identified all adult Ontarians with an incident fistula creation between April 1, 2002 and March 31, 2006 who were not yet receiving dialysis. This accrual period permitted a minimum potential follow-up of at least 2 years from the date of fistula creation. Fistula creations were identified from the Ontario Health Insurance Plan (OHIP) Physician Claims Database (using the fee code R827), the Canadian Institute for Health Information (CIHI) Hospital Discharge Abstract Database (using the Canadian Classification of Health Interventions procedure code 1KY76LA), and the National Ambulatory Care Reporting System (using the Canadian Classification of Health Interventions code 1KY76LA). The OHIP Database and the CIHI Discharge Abstract Database were used to identify dialysis records (Supplemental Material). Using these data sources, the accuracy of detecting fistula placement was found to be 96% (6), and the accuracy of detecting dialysis initiation (within 28 days) was 88% (7). We excluded patients who had any evidence of dialysis therapy, fistula creation, or arteriovenous graft creation in the 5 years preceding the incident fistula creation date.
Baseline Assessment
At the time of fistula creation, each patient’s age and sex were obtained from the OHIP Registered Persons Database, which has an accuracy of 99% for identifying demographic information (7). The presence of diabetes was determined from the Ontario Diabetes Database, which has a sensitivity of 90% and a specificity of 97% (8). Comorbidity was determined from hospital discharge abstracts captured over the 5 years preceding creation of each patient’s first fistula using an adaptation of the Deyo-Charlson Comorbidity Index (9,10). The receipt of nephrology care before fistula creation was determined over the 5 years preceding the fistula creation using first OHIP billing record by a nephrologist according to main specialty category of the ICES physician database.
Outcome Assessment
The primary outcome was the initiation of any form of dialysis therapy during follow-up. Other outcomes included death, which was detected by the OHIP Registered Persons Database, and pre-emptive transplantation, which was detected in the CIHI Discharge Abstract Database (using the Canadian Classification of Health Interventions codes 1PC85LAXXJ and 1PC85LAXXK). Patients were followed from incident fistula creation until their first dialysis treatment, kidney transplantation, death, or end of the study period (March 31, 2008). Catheter use at the time of dialysis was detected by the occurrence of any catheter code within 7, 14, and 30 days of the initiation of dialysis (codes in Supplemental Material).
Statistical Analyses
Patients were classified into three groups according to whether they experienced an outcome event (dialysis or death) or not (predialysis). Patients receiving preemptive transplants were excluded from the analysis. Baseline differences between dialysis recipients and nonrecipients were compared using a t or chi-squared test as appropriate. Using the method in the work by Fine and Gray (11), we developed a semiparametric Cox proportional hazards model that estimated the risk of starting dialysis compared with remaining in predialysis; death was treated as a competing event. This model adjusted for age, sex, diabetes, and the Deyo-Charlson Comorbidity Index. Baseline comparisons were performed using SAS version 9.1 for UNIX (SAS Institute, Cary, NC), and the competing risk model was developed using R version 2.9.1 (R Foundation for Statistical Computing). All analyses assumed a two-sided, type I error probability set at <0.05. The research protocol was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre in Toronto, Ontario, Canada.
Results
Patient Characteristics
Incident fistulae creations occurred in 1929 patients who met the study inclusion criteria. Their baseline characteristics are shown in Table 1. Two-thirds of patients were male. The average age was 66 years, and 60% of patients were 65 years or older. Fifty-four percent of patients had diabetes. The mean Deyo-Charlson Comorbidity Index was 3.4; 16 (0.8%) of 1929 patients received a pre-emptive transplant and were excluded from additional analysis. The duration of nephrology care was 0–5, 6–11, 11–23, and 24 or more months for 11.5%, 10.3%, 17%, and 56.3%, respectively. Five percent of patients had no nephrologist care detected in the administrative datasets.
Table 1.
Baseline characteristics of patients according to their first event after incident fistula creation
| Dialysis (n=1552) (80.5%) | Death (n=164) (8.5%) | Predialysis (n=197) (10.2%) | |
|---|---|---|---|
| Time to event: median (IQR) days | 186 (72–423) | 345 (158–757) | 1179 (889–1466) |
| Sex: n (%) male | 1039 (66.9) | 111 (67.7) | 113 (57.4)a |
| Age: mean (SD) years | 64.9 (14.1) | 74.3 (10.7)b | 69.0 (12.8)b |
| n (%) years | |||
| 18–54 | 353 (22.8) | 9 (5.4) | 31 (15.7) |
| 55–64 | 313 (20.2) | 19 (11.6) | 29 (14.7) |
| 65–74 | 428 (27.6) | 38 (23.2) | 52 (26.4) |
| 75–84 | 398 (25.6) | 77 (47.0) | 73 (37.1) |
| ≥85 | 60 (3.9) | 21 (12.8) | 12 (6.1) |
| Diabetes: n (%) | 848 (54.6) | 96 (58.5) | 93 (47.2) |
| Deyo-Charlson Comorbidity Index: mean (SD) | 3.4 (1.9) | 4.1 (2.1)b | 3.0 (1.6)b |
| n (%) | |||
| 0–2 | 673 (43.4) | 42 (25.6) | 97 (49.2) |
| 3–4 | 489 (31.5) | 57 (34.8) | 75 (38.1) |
| ≥5 | 390 (25.1) | 65 (39.6) | 25 (12.7) |
Excludes 16 (0.8%) patients whose first event was a preemptive kidney transplant. IQR, interquartile range.
P<0.01 compared with patients who started dialysis.
P<0.001 compared with patients who started dialysis.
The median times to dialysis, death, and end of follow-up were 6.1 (interquartile range=2.4–13.9), 11.6 (interquartile range=5.2–24.9), and 38.7 months (interquartile range=29.2–48.2), respectively. Table 1 presents the patients’ baseline characteristics by outcome group. Patients who started dialysis were significantly younger (mean age=64.9 years) than both those patients who died (mean age=74.3 years) and those patients who remained in the predialysis group (mean age=69.0 years; both P<0.001). Patients who started dialysis were more likely to be male than patients who remained in the predialysis group (66.9% versus 57.4%, respectively; P<0.01). Patients who started dialysis had fewer comorbidities than those patients who died without receiving dialysis (mean Deyo-Charlson Comorbidity Indices was 3.4 versus 4.1, respectively; P<0.001) but more comorbidities than those patients who remained in the predialysis group (mean Deyo-Charlson Comorbidity indices was 3.4 versus 3.0, respectively; P<0.001).
Risks of Dialysis and Death
Eighty-one percent of study patients started dialysis; 9% died, and 10% were alive and had not received dialysis by the end of follow-up. When outcomes were analyzed at 1 year of follow-up (the duration studied in the work by O’Hare et al. [4]), 57% of patients had started dialysis, 5% died without receiving dialysis, and 38% remained predialysis. In the patients who started dialysis, catheter use was detected within 7 days of the dialysis start date in 29.9% of patients. If the window is expanded to 14 or 30 days, the percentages increased to 33.5% and 36.7% of patients, respectively, suggesting that a significant proportion of the fistulae could not independently provide vascular access for hemodialysis.
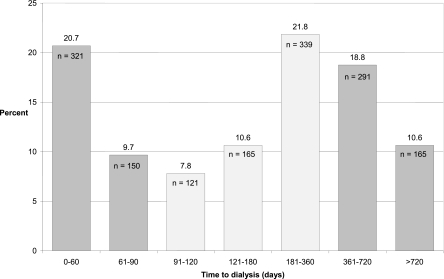
Figure 1 shows the distribution of time from fistula creation to dialysis initiation for the 1552 subjects who began dialysis. Forty percent of patients received dialysis within 4 months of incident fistula creation. One-third of patients began dialysis within 4–12 months of fistula creation, 30% began more than 1 year after fistula creation, and 10% began over 2 years after fistula creation.
Figure 1.
Time from incident fistula creation to initiation of dialysis. The light gray bars are considered optimal timing of fistula creation according to vascular access guidelines.
In a proportional hazards regression model that treated death as a competing event, increasing age was associated with a decreased likelihood of starting dialysis (Table 2). For example, adjusting for sex and comorbidity, patients aged 65–74, 75–84, and 85 years or greater had relative risks of dialysis of 0.79 (95% confidence interval [CI]=0.69–0.91, P=0.0008), 0.67 (95% CI=0.58–0.78, P<0.0001), and 0.55 (95% CI=0.41–0.75, P=0.0001), respectively, compared with patients aged 54 years or less. Women had a relative risk of 0.90 (95% CI=0.81–0.99, P=0.04) compared with men, and patients with a Deyo-Charlson Comorbidity Index of five or greater had a relative risk of 1.22 (95% CI=1.07–1.39, P=0.003) of starting dialysis compared with patients with a Deyo-Charlson Comorbidity Index of two or less. After adjusting for the other variables, the presence of diabetes was not associated with an altered risk of starting dialysis.
Table 2.
Association of age, sex, and comorbidity on the likelihood of initiating dialysis after incident fistula creation compared with remaining in predialysis, adjusting for the competing risk of death
| Covariate | Hazard Ratios | 95% CI | P Value |
|---|---|---|---|
| Age: 18–54 (reference) | |||
| 55–64 | 0.90 | 0.78–1.05 | 0.17 |
| 65–74 | 0.79 | 0.69–0.91 | <0.001 |
| 75–84 | 0.67 | 0.58–0.78 | <0.001 |
| 85+ | 0.56 | 0.41–0.75 | <0.001 |
| Sex: male (reference) | |||
| Female | 0.90 | 0.81–1.00 | 0.04 |
| Deyo-Charlson Comorbidity Index: 0–2 (reference) | |||
| 3–4 | 1.04 | 0.92–1.16 | 0.55 |
| ≥5 | 1.22 | 1.07–1.39 | 0.003 |
CI, confidence interval.
Table 3 illustrates how the absolute risk of initiating dialysis varied by age, sex, and comorbidity over a fixed follow-up period of 2 years. The risk of dialysis in men who were aged 85 years or older was 64.7%. This risk increased to 87.7% for men aged 54 years or younger. The risk for women 85 years or older was 64.0%, and this risk increased to 84.6% for those women aged 54 years or younger. The effect of comorbidity across age strata was variable.
Table 3.
Likelihood of initiating dialysis following fistula creation according to age, sex, and comorbidity category at 2 years of follow-up
| Age (yr) | Male | Female | ||||
|---|---|---|---|---|---|---|
| Lower Comorbidity (n, %)a | Higher Comorbidity (n, %)b | Total (n, %) | Lower Comorbidity (n, %)a | Higher Comorbidity (n, %)b | Total (n, %) | |
| 18–54 | 204/234, 87.2 | 39/43, 90.7 | 243/277, 87.7 | 98/113, 86.7 | 12/17, 70.6 | 110/130, 84.6 |
| 55–64 | 148/174, 85.1 | 54/63, 85.7 | 202/237, 85.2 | 88/99, 88.9 | 23/26, 88.5 | 111/125, 88.8 |
| 65–74 | 208/246, 84.6 | 83/104, 79.8 | 291/350, 83.1 | 94/120, 78.3 | 43/49, 87.8 | 137/169, 81.1 |
| 75–84 | 174/239, 72.8 | 85/104, 81.7 | 259/343, 75.5 | 104/155, 67.1 | 35/50, 70.0 | 139/205, 67.8 |
| ≥85 | 31/48, 64.6 | 13/20, 65.0 | 44/68, 64.7 | NR | NR | 16/25, 64.0 |
Table cells with counts <6 are suppressed in accordance with Ontario privacy laws. NR, not reportable.
Deyo-Charlson Comorbidity Index = 0–4.
Deyo-Charlson Comorbidity Index ≥5.
Discussion
This study found that 81% of CKD patients who underwent incident fistula creation eventually initiated dialysis when followed for an extended period of time. Although this finding is reassuring, fistulae were often created earlier or later than the 4- to 12-month recommended lead time (1,2,12). Catheter use was evident in nearly one-third of patients at the start of dialysis, indicating that many fistulae were likely not usable to initiate dialysis. Older patients, women, and patients with less comorbidity were less likely to start dialysis after fistula creation. When follow-up was fixed at 2 years, the percentage of patients starting dialysis after fistula creation varied from a low of 64% to a high of 91% depending on an individual’s risk profile.
The finding that most patients with predialysis fistula creation eventually started dialysis suggests that clinicians are selecting appropriate patients but that fistula creations are occurring later in the course of CKD than most guidelines recommend. Studies from Canada and Australia have found that incident fistulae were created between a GFR of 7 and 14 ml/min per 1.73 m2—well below the recommended window of 15–30 ml/min per 1.73 m2 (5,13). This practice may occur because there is tension between the competing risks of starting dialysis without a fistula in place and creating fistulae in patients who never require dialysis. Clinicians may wait until the probability of starting dialysis is very high and the GFR is quite low. Carefully selecting predialysis patients for fistula creation minimizes the risk of fistula nonuse but may contribute to the high catheter use among incident hemodialysis recipients—estimated to be as high as 70% in Canada (14). Selection bias was also evidenced by the low risk of death in our cohort of patients with advanced CKD (9%).
The risk of starting dialysis in this study is remarkably similar to risk noted in the work by O’Hare et al. (4), which was a retrospective analysis of US veterans. The mean GFR was 17.7 ml/min per 1.73 m2 at study entry and 1-year follow-up; 57.5% of subjects who underwent arteriovenous access creation (fistula or graft) began dialysis, 4.2% of patients died, and 38.3% of patients remained in predialysis (A.M. O’Hare, unpublished data) compared with 56.8%, 4.7%, and 38.1%, respectively, in this study (4). Weber et al. (5) performed a retrospective analysis of predialysis patients at a single center in Vancouver, Canada. When followed for 2 years, 70% of patients who underwent fistula creation (GFR ranged from 11 to 14 ml/min per 1.73 m2 at fistula creation) eventually started dialysis compared with 64–91% (depending on their risk profile) in this study. We confirmed the finding in the work by O’Hare et al. (4) that older patients were less likely to start dialysis. In contrast, the work by O’Hare et al. (4) found that age was not associated with the likelihood of receiving a permanent access before starting dialysis, but the work by Weber et al. (5) found that younger patients were more likely to have a predialysis fistula creation. Our results, therefore, have many similarities to previous studies; however, our results are more generalizable, because the work by O’Hare et al. (4) studied predominantly males and the work by Weber et al. (5) was a single center study.
An important limitation of our work is that we did not have information about patients’ renal function, proteinuria, or CKD progression. The work by O’Hare et al. (4) modeled the percentage of patients in whom fistula creation would be unnecessary and found it increased when fistulae were created at higher GFR (4). The risk factors identified in this study (age, sex, and comorbidity) may be less significant if we adjusted for GFR. Another limitation is that we could not measure fistula use directly. The work by Weber et al. (5) found that 61% of patients who undergo a predialysis fistula creation used their fistula to initiate dialysis (5). We found that approximately two-thirds of patients likely used their fistulae to start dialysis, because they had no evidence of catheter use; however, we could not measure fistula use directly. Because the risk of primary failure for fistulae ranges from 29% to 61%, it is not unexpected that a portion of fistulas created in the predialysis period will not be used, even if the patient starts dialysis (15–17). Finally, we did not assess the impact of early fistulae creation on patient outcomes, such as death, hospitalization, or costs—the main purported benefits of fistulae. However, a previous Ontario study found that earlier fistula creation was associated with lower rates of hospitalization caused by sepsis and death (18).
The main implications of this study are that vascular access guidelines, which recommend that CKD patients be referred for fistula creation at a fixed GFR cut point, may be too simplistic. Promoting early fistula creation in an effort to reduce catheter use without considering risk factors for starting dialysis may increase the risk of unnecessary fistula surgery or surgeries performed too early in the course of CKD. The risk of unnecessary or early surgery should be, therefore, measured by programs that promote early fistula creation. Of note, the 2006 National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines no longer use a fixed GFR cutoff but rather, recommend that fistulae be created at least 6 months before the expected start of dialysis. This expected time to dialysis guideline may reduce fistula nonuse but be difficult to implement. For example, some prediction models of ESRD require knowledge of age, sex, comorbidity, GFR, albuminuria, serum calcium, serum phosphate, serum bicarbonate, and serum albumin (19). Lack of proteinuria, in particular, seems to identify a group of patients who, despite having advanced CKD, are at low risk for progression (20). In addition, the clinician must anticipate the lead time required for vascular surgery and the additional time for procedures to mature fistulae if required. Backing up fistula referrals to allow for this lead time inevitably introduces more prediction error and therefore, fistula nonuse.
In conclusion, our findings confirm that most patients who underwent predialysis fistula creation eventually initiated dialysis. This practice reduced the risk of nonuse but also may explain the high use of central venous catheters in Canada. A more nuanced approach to selecting patients and timing fistula creation may reduce the risk of unnecessary surgery while also reducing incident catheter use. The risk of unnecessary fistula creation and fistula creations too early in the course of CKD should be monitored if fistulae are aggressively promoted in the CKD population.
Disclosures
R.W. was supported by an unrestricted educational grant from Amgen.
Supplementary Material
Acknowledgments
We thank Peter Austin, Angie Wong, and Ping Li for advice and assistance with data analysis and Anne O’Hare for providing additional data tables from their study (4).
M.J.O. is supported by a Sunnybrook Health Sciences Centre Department of Medicine Research Award. R.R.Q. was supported by a Canadian Institutes of Health Research (CIHR) Institute for Health Services and Policy Research Fellowship. A.X.G. and J.K. are supported by CIHR Clinician Scientist Awards. R.W. was supported by the CIHR Randomized Controlled Trials Mentoring Program and an unrestricted educational grant from Amgen. The study was supported by the Institute for Clinical Evaluative Sciences (ICES), a nonprofit research institute funded by the Ontario Ministry of Health and Long-Term Care (MOHLTC).
The opinions, results, and conclusions are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08920811/-/DCSupplemental.
References
- 1.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Culleton BF; Canadian Society of Nephrology Committee for Clinical Practice Guidelines: Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol 17[Suppl 1]: S1–S27, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG on vascular access. Nephrol Dial Transplant 22[Suppl 2]: ii88–ii117, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M; Alberta Kidney Disease Network: Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 4.O’Hare AM, Bertenthal D, Walter LC, Garg AX, Covinsky K, Kaufman JS, Rodriguez RA, Allon M: When to refer patients with chronic kidney disease for vascular access surgery: Should age be a consideration? Kidney Int 71: 555–561, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Weber CL, Djurdjev O, Levin A, Kiaii M: Outcomes of vascular access creation prior to dialysis: Building the case for early referral. ASAIO J 55: 355–360, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Quinn RR: Validating Administrative Data Algorithms for Identifying Dialysis-Related Variables, Institute for Clinical Evaluative Sciences, 2009 [Google Scholar]
- 7.Quinn RR, Laupacis A, Austin PC, Hux JE, Garg AX, Hemmelgarn BR, Oliver MJ: Using administrative datasets to study outcomes in dialysis patients: A validation study. Med Care 48: 745–750, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Hux JE, Ivis F, Flintoft V, Bica A: Diabetes in Ontario: Determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 25: 512–516, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45: 613–619, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA: New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 57: 1288–1294, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Fine JP, Gray RJ: A proportional hazards model for the substitution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 12.Vascular Access Work Group: Clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S248–S273, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Vargas PA, Craig JC, Gallagher MP, Walker RG, Snelling PL, Pedagogos E, Gray NA, Divi MD, Gillies AH, Suranyi MG, Thein H, McDonald SP, Russell C, Polkinghorne KR: Barriers to timely arteriovenous fistula creation: A study of providers and patients. Am J Kidney Dis 57: 873–882, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Mendelssohn DC, Ethier J, Elder SJ, Saran R, Port FK, Pisoni RL: Haemodialysis vascular access problems in Canada: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS II). Nephrol Dial Transplant 21: 721–728, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Moll FL, Blankestijn PJ; CIMINO Study Group: Hemodialysis arteriovenous fistula patency revisited: Results of a prospective, multicenter initiative. Clin J Am Soc Nephrol 3: 714–719, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI; Dialysis Access Consortium Study Group: Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliver MJ, Rothwell DM, Fung K, Hux JE, Lok CE: Late creation of vascular access for hemodialysis and increased risk of sepsis. J Am Soc Nephrol 15: 1936–1942, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS: A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Tonelli M, Muntner P, Lloyd A, Manns BJ, James MT, Klarenbach S, Quinn RR, Wiebe N, Hemmelgarn BR; Alberta Kidney Disease Network: Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: A cohort study. Ann Intern Med 154: 12–21, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.