Summary
Background and objectives
With the advent of fetal screening ultrasonography, the detection of congenital anomalies of the kidney and urinary tract (CAKUT) in utero has permitted early management of these conditions. This study aims to describe the clinical course of a large cohort of patients with prenatally detected nephrouropathies.
Design, setting, participants, & measurements
In this retrospective cohort study, 822 patients were prenatally diagnosed with CAKUT and systematically followed up at a tertiary Renal Unit for a median time of 43 months. Variables included in the analysis were sex, laterality, fetal ultrasonography (isolated versus associated hydronephrosis), and presence/absence of nephrouropathies. The events of interest were urinary tract infection, surgical interventions, hypertension, CKD, and death. Survival analyses were performed to evaluate time until occurrence of the events of interest.
Results
Urinary tract infection occurred in 245 (29.8%) children, with higher risk in females (hazard ratio=1.30, 95% confidence interval=1.02–1.70, P=0.05); 22 patients (2.7%) had hypertension, and 49 (6%) patients developed CKD. The risk of CKD was greater in patients with associated hydronephrosis (hazard ratio=5.20, 95% confidence interval=2.90–9.30, P<0.001). Twelve patients (1.5%) died during follow-up. Death was significantly associated with being born during the first period of the study (hazard ratio=6.00, 95% confidence interval=1.60–22.50, P<0.001), associated hydronephrosis (hazard ratio=9.30, 95% confidence interval=2.90–29.30, P<0.001), and CKD (hazard ratio=170.00, 95% confidence interval=41.00–228.00, P<0.001).
Conclusions
In our series, the clinical course of prenatally detected CAKUT was heterogeneous, and those infants with associated hydronephrosis at baseline were identified as a high-risk subgroup.
Introduction
With the advent of fetal screening ultrasonography, the detection of congenital anomalies of the kidney and urinary tract (CAKUT) in utero has permitted early management of these conditions (1). CAKUT comprise a spectrum of malformations that occur at the level of the kidney (e.g., hypoplasia and dysplasia), collecting system (e.g., hydronephrosis and megaureter), bladder (e.g., ureterocele and vesicoureteral reflux), or urethra (e.g., posterior urethral valves) (2). Antenatal hydronephrosis (ANH) affects 1–5% of all pregnancies and is one of the most common birth defects (3–5).
There has been a continuous advance in the understanding of pathophysiology, genetic basis, and natural history of CAKUT (2,6–13). However, there are still many controversies regarding the clinical significance of prenatally detected CAKUT. In addition, the current literature about ANH lacks a systematic analysis of clinical outcomes, which might provide additional insight into unresolved issues and highlight areas where consensus does exist (13). The aim of this retrospective study was to describe the clinical course of a large cohort of patients with prenatally detected nephrouropathies.
Materials and Methods
Patients
In this retrospective cohort study, the records of 832 patients with prenatally detected CAKUT who were admitted at the Pediatric Nephrology Unit of Clinics Hospital from Federal University of Minas Gerais (Brazil) between 1989 and 2009 were reviewed. At the Fetal Medicine Unit, each fetus underwent a detailed anatomic study aimed at detecting CAKUT and other organ abnormalities. Specific ultrasound findings, such as the presence of renal, ureteral, and bladder involvement, and volume of amniotic fluid were recorded. Fetuses with aneuploidy or multiple malformations were excluded from the analysis as well 10 patients who were lost to follow-up soon after birth.
Study Protocol
During the last 20 years of systematic approach to and follow-up of infants with prenatally detected CAKUT at our unit, the postnatal investigation of these infants has changed. In the first decade of the study, infants were investigated according to a comprehensive systematic protocol described elsewhere (14–16). After 2000, we developed a more tailored clinical protocol (17–19). Briefly, a renal ultrasonography (US) was performed after the first week of postnatal life, and all infants with confirmed hydronephrosis underwent a voiding cystourethrogram (VCUG). After 2009, VCUG was performed only in a selected subgroup of patients. Antibiotic prophylaxis was started on the first postnatal day and maintained according to the definitive diagnosis. Clinical examination (including growth and BP measurements), US, and laboratory reviews (including urine culture and serum creatinine) were scheduled at 6-month intervals. When the VCUG was normal but postnatal US showed renal pelvis dilatation (RPD) ≥10 mm, renal scintigraphy was performed after the first month (11,18,19).
Outcomes
The events of interest were urinary tract infection (UTI), surgical interventions, hypertension, CKD, and death.
Covariates
The following variables were included in the analysis: sex, RPD laterality (unilateral versus bilateral), fetal US findings (isolated fetal hydronephrosis versus hydronephrosis associated with lower urinary tract abnormalities), period of diagnosis (1989–1999 versus 2000–2009), and presence/absence of urinary tract anomalies.
Definitions
Combined data obtained by VCUG, renal scintigraphy, and sequential US were considered for the diagnosis of urinary tract abnormalities. Data from postmortem examinations were also considered for the diagnosis when appropriate. The absence of any recognized uropathy was classified as idiopathic hydronephrosis. Isolated hydronephrosis was defined as the presence of RPD without any other alterations of the urinary tract. Associated hydronephrosis was defined as the presence of RPD combined with other alterations such as megaureter and oligohydramnios. Ureteropelvic junction obstruction (UPJO) was defined as the presence of moderate or severe hydronephrosis on US associated with an abnormal diethylenetriamine pentaacetic acid scan pattern. Patients with UPJO were conservatively managed if renal unit function was higher than 40% as ascertained by dimercaptosuccinic acid scan. Patients with severe dilation who exhibited renal uptake lower than 40% were surgically managed by pyeloplasty. CKD was defined as a GFR ≤89 ml/min per 1.73 m2 in two consecutive exams, and CKD stages were classified according to the National Kidney Foundation practice guidelines (20). GFR was estimated by the formula in the work by Schwartz et al. (21). UTI was defined as growth of at least 100,000 cfu/ml of a single bacterium in urine sample associated with fever (≥38.0°C) or urinary symptoms. BP measurements were performed as recommended by the Working Group of the National High Blood Pressure Education Program (17). Reference values and definitions of normal BP were based on The Fourth Report on High Blood Pressure in Children and Adolescents (22). For patients above 17 years of age, we considered sustained hypertension values to be consistently above 140/90 mmHg.
Statistical Analyses
The values are expressed as medians and interquartile ranges (IQs) or means and SDs when appropriate. The Mann–Whitney or Kruskal–Wallis test was used to compare nonparametric continuous variables. Odds ratio (ORs) and 95% confidence intervals (95% CIs) were used for group risk comparison. Survival analyses were performed by the Kaplan–Meier method to evaluate time until occurrence of outcomes: CKD, hypertension, death, and UTI. Hazard ratios (HRs) and respective 95% CIs were assessed by univariate Cox regression analysis. Differences between subgroups were assessed by the two-sided log-rank test.
Ethical Aspects
The study was approved by the Ethics Committee of Federal University of Minas Gerais.
Results
Baseline Findings
A total of 822 patients were included in the analysis. The main baseline clinical characteristics are summarized in Table 1. There were higher proportions of males and patients with isolated unilateral hydronephrosis. After the postnatal clinical and imaging investigation, the most common CAKUT identified were UPJO, multicystic dysplastic kidney, and primary vesicoureteral reflux (VUR). Children diagnosed between 2000 and 2009 had a five times greater chance of exhibiting idiopathic hydronephrosis compared with those children diagnosed between 1989 and 1999 (OR=5.20, 95% CI=3.60–7.50, P<0.001).
Table 1.
Baseline clinical characteristics of 822 infants with prenatally detected nephrouropathies
| N (%) | |
|---|---|
| Sex | |
| male | 557 (67.8) |
| female | 265 (32.2) |
| Laterality—fetal ultrasonography | |
| unilateral | 491 (59.7) |
| bilateral | 331 (40.3) |
| Period | |
| 1989–1999 | 273 (33.2) |
| 2000–2009 | 549 (66.8) |
| Fetal ultrasonography findings | |
| unilateral hydronephrosis | 355 (43.2) |
| bilateral hydronephrosis | 263 (32.0) |
| cystic kidneys | 97 (11.8) |
| hydronephrosis + megaureter | 49 (6.0) |
| hydronephrosis + megaureter + urinary tract alterations | 45 (5.5) |
| others | 13 (1.5) |
| Postnatal diagnosis | |
| idiopathic hydronephrosis | 308 (37.5) |
| ureteropelvic junction obstruction | 157 (19.1) |
| multicystic dysplastic kidney | 89 (10.8) |
| vesicoureteral reflux | 67 (8.2) |
| primary megaureter | 60 (7.3) |
| posterior urethral valves | 46 (5.6) |
| ureterocele | 28 (3.4) |
| hypodysplastic kidney | 20 (2.4) |
| prune belly syndrome | 10 (1.2) |
| others | 38 (4.6) |
Clinical Course
Median follow-up time was 43 months (IQ=18.6–94.7 months) for those patients who survived the neonatal period; 310 (37.7%) patients were followed up for more than 5 years, and 126 (15%) patients were followed up for more than 10 years. Median time of antibacterial prophylaxis was 29 months (IQ=18–38 months) for patients with uropathy. Regarding imaging examinations, 811 (98.7%) patients underwent at least one postnatal US, 641 (77.9%) patients underwent VCUG, 470 (57.2%) patients underwent a dimercaptosuccinic acid scan, and 399 (48.5%) patients underwent a diethylenetriamine pentaacetic acid scan.
Surgical Interventions
Two hundred thirty (28%) patients were submitted to surgical procedures. The most common surgical interventions were as follows: 105 patients with UPJO were submitted to pyeloplasty, 38 patients with posterior urethral valves (PUVs) underwent neonatal diversions, 28 infants underwent heminephrectomy and other procedures for ureterocele, and 20 patients with primary megaureter and 17 patients with VUR were submitted to ureteral reimplantation.
UTIs
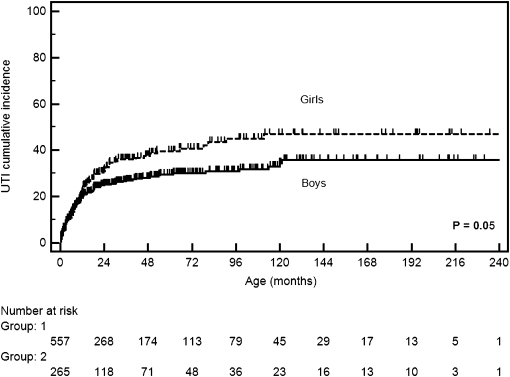
During follow-up, UTIs occurred in 245 (29.8%) children. A total of 135 (16.4%) children presented one UTI episode, 54 (6.6%) children had two episodes, and only 56 (6.8%) children had three or more episodes. By survival analysis, the cumulative incidence of UTIs for the whole series was estimated as 14% at 6 months, 22% at 12 months, and 29% at 24 months. Females had a higher risk of UTI during follow-up (HR=1.30, 95% CI=1.02–1.70, P=0.05). It was estimated that, by 3 years of age, 36% of girls would present with UTIs, whereas for boys, this rate was about 30%. Of note, up to 12 months, the estimated risk of UTI was quite similar for boys (21%) and girls (24%) (Figure 1). As expected, there was a difference in the occurrence of UTIs between patients who had urinary tract anomalies and children with idiopathic hydronephrosis (HR=5.20, 95% CI=2.90– 9.30, P<0.001). By 3 years, it was estimated that 37% of children with urinary tract anomalies would present with UTIs, whereas for idiopathic hydronephrosis, this rate was about 15%. UTIs occurred more frequently in prune belly syndrome (90% of cases), ureterocele (64.3%), PUVs (58.7%), primary megaureter (50%), and VUR (43.3%).
Figure 1.
Kaplan–Meier survival curves showing the cumulative incidence of urinary tract infection (UTI) according to sex.
Hypertension
Twenty-two (2.7%) patients presented BP persistently above the 95th percentile according to age, sex, and height. However, the prevalence of sustained hypertension increased with age. The prevalence of hypertension was 0.8% for 516 patients under 5 years, 2.2% for 182 patients between 5 and 10 years, 8.2% for 90 patients between 10 and 17 years, and 19.4% for 31 patients above 17 years. There was no difference in the occurrence of hypertension between sexes (HR=0.93, 95% CI=0.38–2.20, P=0.54). However, hypertension was strongly associated with CKD. Of 49 patients with CKD, 9 (18.4%) patients developed hypertension, whereas only 13 (1.7%) of 773 patients with normal renal function presented high BP (HR=3.90, 95% CI=1.50–10.00, P=0.006). The rate of hypertension was greater for autosomal recessive polycystic kidney disease (16.7%), PUVs (13%), ureterocele (7%), and VUR (6%).
CKD
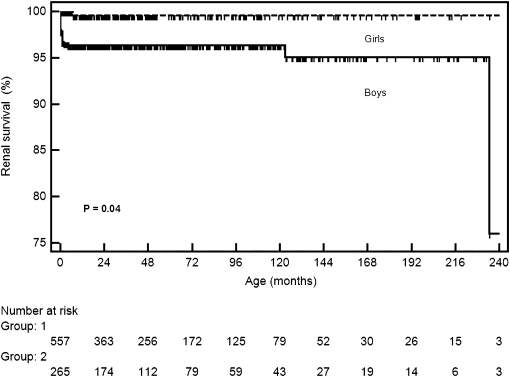
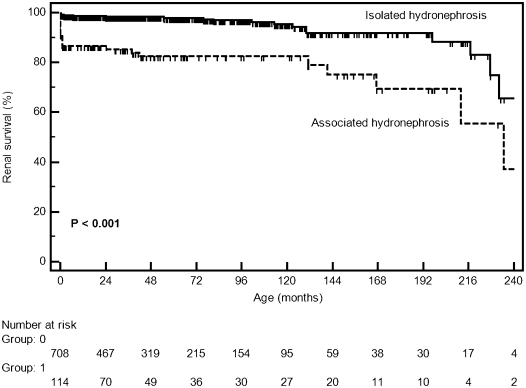
Of 822 patients, 49 (6%) patients developed CKD with a median GFR of 27.0 ml/min per 1.73 m2 (IQ=11.4–73.2). The clinical characteristics of these patients are summarized in Table 2. At the end of follow-up, 14 patients had CKD stage 2, 9 patients had CKD stage 3, 2 patients had CKD stage 4, and 23 (2.8%) patients reached CKD stage 5, with a median GFR of 10.8 ml/min per 1.73 m2 (IQ=7.6–17.7). Of 23 patients, 11 patients died within the first months of life, 10 patients were on peritoneal dialysis, and 3 patients underwent renal transplantation. It was estimated by survival analysis that the probability of CKD for patients with prenatally detected nephrouropathies was 3% at 2 years, 4% at 5 years, and 6% at 10 years. There was no difference in the incidence of CKD between the two periods of the study (HR=1.40, 95% CI=0.70–2.80, P=0.32). There was a significant difference between sexes regarding the occurrence of CKD. The probability of renal survival at 10 years of age was about 95% for boys and 99.5% for girls (HR=1.50, 95% CI=1.02–2.10, P=0.04) (Figure 2). CKD was also related to hydronephrosis associated with other alterations in fetal US. The probability of CKD at 2 years of age was 15% for patients with fetal hydronephrosis associated with other abnormalities and only about 2% for children with isolated hydronephrosis (HR=5.20, 95% CI=2.90–9.30, P<0.001) (Figure 3). The laboratory evaluation at the end of follow-up revealed a median serum creatinine of 0.4 mg/dl (IQ=0.34–0.54) for patients who did not present CKD. The mean estimated GFR was 127.7 ml/min per 1.73 m2 (IQ=106.0–152.7).
Table 2.
Baseline features and clinical course of 49 children with CKD and 23 children with CKD stage 5
| CKD (%; n=49) | CKD Stage 5 (%; n=23) | |
|---|---|---|
| Sex | ||
| male | 39 (79.6) | 22 (95.5) |
| female | 10 (20.4) | 1 (4.5) |
| Period | ||
| 1987–1999 | 32 (65.3) | 13 (56.5) |
| 2000–2009 | 17 (34.7) | 10 (43.5) |
| Fetal ultrasonography findings | ||
| isolated hydronephrosis | 24 (49%) | 8 (34.8) |
| associated hydronephrosis | 25 (51%) | 15 (65.2) |
| Postnatal diagnosis | ||
| posterior urethral valves | 21 (42.9) | 14 (61%) |
| ureteropelvic junction obstruction | 9 (18.4) | — |
| multicystic dysplastic kidney | 5 (10.2) | — |
| prune belly | 4 (8.2) | 4 (17.4) |
| primary megaureter | 3 (6.1) | — |
| hypodysplastic kidneys | 3 (6.1) | 3 (13.0) |
| others | 4 (8.2) | 2 (8.7) |
| Hypertension | ||
| present | 10 (20.4) | 2 (8.7) |
| absent | 39 (79.6) | 21 (91.3) |
| Death | ||
| yes | 10 (20.4) | 9 (39.1) |
| no | 39 (79.6) | 14 (60.9) |
Figure 2.
Kaplan–Meier curves showing the probability of renal survival according to sex.
Figure 3.
Kaplan–Meier curves showing the probability of renal survival according to the fetal US findings (isolated versus associated hydronephrosis).
Death
Of 822 patients, 12 (1.5%) patients died during follow-up. The median age at death was 1 month (IQ=3.0 days to 1.6 months ranging from 1.0 day to 5.7 months). There was a tendency to a higher occurrence of death in male than female patients with CKD (HR=2.30, 95% CI=0.89–6.40, P=0.07). Death was significantly associated with the occurrence of CKD (HR=170.00, 95% CI=41.00–228.00, P<0.001) (Figure 4), being born during the first period of the study (HR=6.00, 95% CI=1.60–22.50, P=0.002), and hydronephrosis associated with other abnormalities (HR=9.30, 95% CI=2.90–29.30, P<0.001).
Figure 4.
Kaplan–Meier curves showing the probability of patient survival according to the presence of CKD.
Discussion
In this retrospective cohort study, we report the clinical outcome of a nonselected group of pediatric patients with prenatally detected nephrouropathies. The main finding of our cohort is that the clinical course of CAKUT is heterogeneous. Undoubtedly, there is a subgroup of high-risk patients for renal impairment and other adverse events among children with prenatally detected CAKUT. As expected, the probability of CKD was higher for patients with hydronephrosis associated with lower urinary tract abnormalities and dysplastic kidneys (23). However, the clinical course is relatively benign, particularly for children with isolated fetal hydronephrosis. Furthermore, our series highlighted the importance of long-term follow-up, because about 30% of these children needed surgical intervention or had recurrent UTIs during subsequent visits.
At baseline, the clinical data for our series were similar to the data reported in observational studies by showing a higher proportion of males and mild to moderate ANH (24–26). There has been an agreement among several studies that the degree of ANH is associated with postnatal outcome (8,13). For instance, Lee et al. (4), in a meta-analysis study, have shown that the risk of any postnatal pathology was 11.9% for mild, 45.1% for moderate, and 88.3% for severe dilatation. Interestingly, after 1999, we established a prospective protocol in which all fetuses with pelvic dilatation equal to or greater than 5 mm were enrolled to be followed up at our unit (6,19,27). By adopting this criterion, the chance of identifying an infant without any congenital anomaly of the urinary tract increased five times compared with the first period of our study. Of note, recently, Hothi et al. (28) performed a systematic review of cohort studies of fetuses with RPD<15 mm. The work by Hothi et al. (28) showed that the estimated risks of obstruction and VUR were quite low, particularly in the mild group. Therefore, there are still many controversies regarding the clinical significance of mild isolated ANH. Consequently, there has been an abundant and somewhat controversial literature about the best work-up and follow-up for these patients (29,30). A recent consensus statement from the Society for Fetal Urology recommends at least 1 year of follow-up for mild dilatation (31).
The rationale for antibiotic prophylaxis in children with a history of prenatally detected uropathies includes prevention of UTIs, because infants with hydronephrosis are at increased risk (31,32). UTI rates seem to be as high as 40% in children with severe hydronephrosis (33). In a previous study, we estimated a cumulative incidence of UTIs as 39% and 11% at 36 months of age for severe and mild RPD, respectively (27). However, despite early antibiotic prophylaxis and careful follow-up, UTIs occurred at a significant rate in our series (29.8%). UTI was significantly associated with female sex and urinary tract anomaly. Furthermore, most episodes of UTI occurred during the first 3 years of life (Figure 1). Thus, a careful clinical follow-up must be considered besides antibiotic prophylaxis for young children with urinary tract anomaly, especially girls. It is important to note, nevertheless, that the accurate incidence of UTIs is a difficult question to ascertain in a retrospective study (34).
To our knowledge, our study is the first to estimate renal survival in a large series of prenatally detected nephrouropathies. The probability of CKD was estimated at 6% at 10 years of age. The impairment of renal function occurred mainly in patients with ANH associated with alterations in the lower urinary tract or those patients with hypodysplastic kidneys. As expected, there was a higher incidence of CKD in boys. This finding is related to the fact that the main CAKUT involving early development of renal impairment, such as PUVs, occur almost exclusively in boys (35). Nevertheless, it should be pointed out that this cohort is a relative short-time cohort, and some disorders, such as reflux nephropathy, may take years to impair renal function (36–38).
Hypertension was observed in 2.7% of our patients. Probably, the low prevalence of sustained hypertension in our series may have been caused by the young age of our patients. It is well known that the risk of hypertension increases with age and length of follow-up (39). Of note, 6 (19.4%) of 31 patients above 17 years of age at the end of follow-up had sustained hypertension. As expected, the risk of hypertension was greater for patients with polycystic kidneys, urinary tract obstruction, VUR, and CKD. Urinary tract obstruction and reflux nephropathy have been regarded as the most common disorders leading to hypertension in childhood (40). In this regard, the work by Parkhouse et al. (41) showed that 7 (8.7%) of 80 patients with PUVs in an average follow-up of 5 years were hypertensive. By survival analysis of a primary VUR cohort, the work by Simoes e Silva et al. (42) estimated that 50% of patients with unilateral and bilateral renal damage would have sustained hypertension at about 30 and 22 years of age, respectively.
In our series, 12 (1.5%) children died during follow-up. Death was significantly associated with being born during the first period of the study (1987–1999), presence of associated hydronephrosis, and occurrence of CKD. Of note, all deaths occurred within the first 6 months of life. Taken together, these data show that most deaths occurred during the first years of our cohort when renal replacement therapy had not been fully implemented at our institution. Of note, in 1990, a Predialysis Interdisciplinary Management Program was created in our unit with the aim to provide full clinical assistance to children with CKD (43). We believe that this program has contributed to improving the prognosis for these patients (44,45).
Our results must be considered in light of potential limitations associated with the retrospective design. The possible main weakness is the inevitable heterogeneity in the management of patients through the decades because of the wide span of time of patient enrollment in our cohort. In addition, we had difficulty in verifying with certainty the occurrence of UTIs and its features in our series. Another limitation was the small number of deaths, which did not allow the use of multivariable analysis to identify possibly predictive factors. Moreover, we were not able to analyze variables, such as renal scarring at baseline and ethnicity, that can possibly contribute to the prediction of renal outcome. We are also aware of the limitations of the estimated GFR, especially for infants and higher GFR values (46). Nevertheless, some features of the study may increase the strength of our findings, such as the size of our sample, management by the same medical team, and relatively long follow-up.
After about 20 years of systematic approach to prenatally detected nephrouropathies, our understanding of clinical course and natural history of CAKUT has clearly improved. The main practical conclusion is that patients with CAKUT must be followed until adulthood with strict control of BP and renal function, especially for the high-risk subgroup of infants with associated hydronephrosis at baseline. Prediction of the risk of CKD and hypertension in individual cases is difficult, and therefore, regular follow-up remains the only way of recognizing these subjects. Additional prospective studies are necessary to establish risk stratification in children with CAKUT and propose a tailored approach.
Disclosures
None.
Acknowledgments
T.J.L., G.M.P., N.N.B., and L.C.F. were recipients of Brazilian National Research Council (CNPq) fellowships. A.C.S.e.S. and E.A.O. received a research grant from the CNPq. This study was partially supported by CNPq Grant 401949/2010-9, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) Grants PPM-00152-09 and PPM-00345-11, and a National Institute of Science and Technology Medicine Molecular Grant (FAPEMIG CBB-APQ-00075-09/CNPq 573646/2008-2).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Carr MC, Kim SS: Prenatal management of urogenital disorders. Urol Clin North Am 37: 149–158, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Song R, Yosypiv IV: Genetics of congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 26: 353–364, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Blyth B, Snyder HM, Duckett JW: Antenatal diagnosis and subsequent management of hydronephrosis. J Urol 149: 693–698, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Lee RS, Cendron M, Kinnamon DD, Nguyen HT: Antenatal hydronephrosis as a predictor of postnatal outcome: A meta-analysis. Pediatrics 118: 586–593, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Livera LN, Brookfield DS, Egginton JA, Hawnaur JM: Antenatal ultrasonography to detect fetal renal abnormalities: A prospective screening programme. BMJ 298: 1421–1423, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coelho GM, Bouzada MC, Pereira AK, Figueiredo BF, Leite MR, Oliveira DS, Oliveira EA: Outcome of isolated antenatal hydronephrosis: A prospective cohort study. Pediatr Nephrol 22: 1727–1734, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Grazioli S, Parvex P, Merlini L, Combescure C, Girardin E: Antenatal and postnatal ultrasound in the evaluation of the risk of vesicoureteral reflux. Pediatr Nephrol 25: 1687–1692, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Mallik M, Watson AR: Antenatally detected urinary tract abnormalities: More detection but less action. Pediatr Nephrol 23: 897–904, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Nakayama M, Nozu K, Goto Y, Kamei K, Ito S, Sato H, Emi M, Nakanishi K, Tsuchiya S, Iijima K: HNF1B alterations associated with congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 25: 1073–1079, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Penido Silva JM, Oliveira EA, Diniz JS, Bouzada MC, Vergara RM, Souza BC: Clinical course of prenatally detected primary vesicoureteral reflux. Pediatr Nephrol 21: 86–91, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Rabelo EA, Oliveira EA, Diniz JS, Silva JM, Filgueiras MT, Pezzuti IL, Tatsuo ES: Natural history of multicystic kidney conservatively managed: A prospective study. Pediatr Nephrol 19: 1102–1107, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Rumballe B, Georgas K, Wilkinson L, Little M: Molecular anatomy of the kidney: What have we learned from gene expression and functional genomics? Pediatr Nephrol 25: 1005–1016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sidhu G, Beyene J, Rosenblum ND: Outcome of isolated antenatal hydronephrosis: A systematic review and meta-analysis. Pediatr Nephrol 21: 218–224, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Oliveira EA, Diniz JS, Cabral AC, Leite HV, Colosimo EA, Oliveira RB, Vilasboas AS: Prognostic factors in fetal hydronephrosis: A multivariate analysis. Pediatr Nephrol 13: 859–864, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Oliveira EA, Diniz JS, Cabral AC, Pereira AK, Leite HV, Colosimo EA, Vilasboas AS: Predictive factors of fetal urethral obstruction: A multivariate analysis. Fetal Diagn Ther 15: 180–186, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Oliveira EA, Diniz JS, Vilasboas AS, Rabêlo EA, Silva JM, Filgueiras MT: Multicystic dysplastic kidney detected by fetal sonography: Conservative management and follow-up. Pediatr Surg Int 17: 54–57, 2001 [DOI] [PubMed] [Google Scholar]
- 17.National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents : Update on the 1987 Task Force Report on high blood pressure in children and adolescents: A working group report from the National High Blood Pressure Education Program. Pediatrics 98: 649–658, 1996 [PubMed] [Google Scholar]
- 18.Bouzada MC, Oliveira EA, Pereira AK, Leite HV, Rodrigues AM, Fagundes LA, Gonçalves RP, Parreiras R: Diagnostic accuracy of postnatal renal pelvic diameter as a predictor of uropathy: A prospective study. Pediatr Radiol 34: 798–804, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Bouzada MC, Oliveira EA, Pereira AK, Leite HV, Rodrigues AM, Fagundes LA, Gonçalves RP, Parreiras RL: Diagnostic accuracy of fetal renal pelvis anteroposterior diameter as a predictor of uropathy: A prospective study. Ultrasound Obstet Gynecol 24: 745–749, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G, National Kidney Foundation : National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz GJ, Brion LP, Spitzer A: The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 34: 571–590, 1987 [DOI] [PubMed] [Google Scholar]
- 22.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[2 Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 23.Staples AO, Greenbaum LA, Smith JM, Gipson DS, Filler G, Warady BA, Martz K, Wong CS: Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol 5: 2172–2179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damen-Elias HA, De Jong TP, Stigter RH, Visser GH, Stoutenbeek PH: Congenital renal tract anomalies: Outcome and follow-up of 402 cases detected antenatally between 1986 and 2001. Ultrasound Obstet Gynecol 25: 134–143, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Duncan KA: Antenatal renal pelvic dilatation; the long-term outlook. Clin Radiol 62: 134–139, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Wollenberg A, Neuhaus TJ, Willi UV, Wisser J: Outcome of fetal renal pelvic dilatation diagnosed during the third trimester. Ultrasound Obstet Gynecol 25: 483–488, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Coelho GM, Bouzada MC, Lemos GS, Pereira AK, Lima BP, Oliveira EA: Risk factors for urinary tract infection in children with prenatal renal pelvic dilatation. J Urol 179: 284–289, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Hothi DK, Wade AS, Gilbert R, Winyard PJ: Mild fetal renal pelvis dilatation: Much ado about nothing? Clin J Am Soc Nephrol 4: 168–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ismaili K, Avni FE, Piepsz A, Wissing KM, Cochat P, Aubert D, Hall M: Current management of infants with fetal renal pelvis dilation: A survey by French-speaking pediatric nephrologists and urologists. Pediatr Nephrol 19: 966–971, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Toiviainen-Salo S, Garel L, Grignon A, Dubois J, Rypens F, Boisvert J, Perreault G, Decarie JC, Filiatrault D, Lapierre C, Miron MC, Bechard N: Fetal hydronephrosis: Is there hope for consensus? Pediatr Radiol 34: 519–529, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Nguyen HT, Herndon CD, Cooper C, Gatti J, Kirsch A, Kokorowski P, Lee R, Perez-Brayfield M, Metcalfe P, Yerkes E, Cendron M, Campbell JB: The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol 6: 212–231, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Walsh TJ, Hsieh S, Grady R, Mueller BA: Antenatal hydronephrosis and the risk of pyelonephritis hospitalization during the first year of life. Urology 69: 970–974, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Lee JH, Choi HS, Kim JK, Won HS, Kim KS, Moon DH, Cho KS, Park YS: Nonrefluxing neonatal hydronephrosis and the risk of urinary tract infection. J Urol 179: 1524–1528, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Silva JM, Santos Diniz JS, Marino VS, Lima EM, Cardoso LS, Vasconcelos MA, Oliveira EA: Clinical course of 735 children and adolescents with primary vesicoureteral reflux. Pediatr Nephrol 21: 981–988, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, Innocenti ML, Somenzi D, Trivelli A, Caridi G, Izzi C, Scolari F, Mattioli G, Allegri L, Ghiggeri GM: Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int 76: 528–533, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Silva JM, Diniz JS, Silva AC, Azevedo MV, Pimenta MR, Oliveira EA: Predictive factors of chronic kidney disease in severe vesicoureteral reflux. Pediatr Nephrol 21: 1285–1292, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Smellie JM, Jodal U, Lax H, Möbius TT, Hirche H, Olbing H, Writing Committee, International Reflux Study in Children (European Branch) : Outcome at 10 years of severe vesicoureteric reflux managed medically: Report of the International Reflux Study in Children. J Pediatr 139: 656–663, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Smellie JM, Prescod NP, Shaw PJ, Risdon RA, Bryant TN: Childhood reflux and urinary infection: A follow-up of 10-41 years in 226 adults. Pediatr Nephrol 12: 727–736, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Gomes RS, Quirino IG, Pereira RM, Vitor BM, Leite AF, Oliveira EA, Simões e Silva AC: Primary versus secondary hypertension in children followed up at an outpatient tertiary unit. Pediatr Nephrol 26: 441–447, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Farnham SB, Adams MC, Brock JW, 3rd, Pope JC, 4th: Pediatric urological causes of hypertension. J Urol 173: 697–704, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Parkhouse HF, Barratt TM, Dillon MJ, Duffy PG, Fay J, Ransley PG, Woodhouse CR, Williams DI: Long-term outcome of boys with posterior urethral valves. Br J Urol 62: 59–62, 1988 [DOI] [PubMed] [Google Scholar]
- 42.Simoes e Silva AC, Silva JM, Diniz JS, Pinheiro SV, Lima EM, Vasconcelos MA, Pimenta MR, Oliveira EA: Risk of hypertension in primary vesicoureteral reflux. Pediatr Nephrol 22: 459–462, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Soares CM, Oliveira EA, Diniz JS, Lima EM, Vasconcelos MM, Oliveira GR: Predictive factors of progression of chronic renal insufficiency: A multivariate analysis. Pediatr Nephrol 18: 371–377, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Soares CM, Diniz JS, Lima EM, Oliveira GR, Canhestro MR, Colosimo EA, Simoes e Silva AC, Oliveira EA: Predictive factors of progression to chronic kidney disease stage 5 in a predialysis interdisciplinary programme. Nephrol Dial Transplant 24: 848–855, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Soares CM, Diniz JS, Lima EM, Silva JM, Oliveira GR, Canhestro MR, Colosimo EA, Simoes e Silva AC, Oliveira EA: Clinical outcome of children with chronic kidney disease in a pre-dialysis interdisciplinary program. Pediatr Nephrol 23: 2039–2046, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Schwartz GJ, Furth SL: Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol 22: 1839–1848, 2007 [DOI] [PubMed] [Google Scholar]