Summary
Background and objectives
Rapid discontinuation of prednisone after kidney transplantation potentially allows for minimization of steroid-related side effects. Although intermediate-term data with rapid discontinuation of prednisone have been promising, concern still exists regarding long-term outcomes. The 10-year experience is reported herein.
Design, setting, participants, & measurements
Between October 1, 1999 and December 31, 2010, 1241 adult primary kidney transplants (791 living donor and 450 deceased donor) were performed using a protocol in which prednisone is discontinued after postoperative day 5. The 10-year actuarial recipient and graft survival rates and prednisone-related side effects were studied.
Results
Ten-year actuarial patient survival was 71% for living donor transplants and 62% for deceased donor transplants; 10-year graft survival was 61% for living donor transplants and 51% for deceased donor transplants, and was comparable to 10-year Scientific Registry of Transplant Recipients national data. Ten-year death-censored graft survival was 79% for living donor transplants and 80% for deceased donor transplants. Ten-year acute rejection rates were 25% for deceased donor transplants and 31% for living donor transplants; 10-year chronic rejection (interstitial fibrosis/tubular atrophy) rates were 39% for deceased donor transplants and 47% for living donor transplants. For nondiabetic recipients of living donor or deceased donor allografts, the incidence of new-onset diabetes was significantly lower than in historical controls on prednisone (P<0.001). We also found significantly reduced rates of cataracts, avascular necrosis, and cytomegalovirus infection in some subgroups.
Conclusions
Prednisone-related side effects can be minimized in a protocol incorporating rapid discontinuation of prednisone for maintenance immunosuppression. Ten-year patient and graft outcomes remain acceptable.
Introduction
Prolonged use of high-dose prednisone is associated with well described side effects, including hypertension, post-transplant glucose intolerance, new-onset diabetes mellitus (NODM), hyperlipidemia, cataracts, loss of bone mineral density, increased rates of fractures and avascular necrosis, appearance changes, mood swings, and in children, growth retardation. Before 2000, most immunosuppressive protocols for kidney transplant recipients incorporated large doses of prednisone (up to 2 mg/kg at transplant with a slow taper to 0.1–0.15 mg/kg at 1 year). These protocols were associated with significant prednisone-related morbidity (1–3). When queried, transplant recipients stated that the immunosuppressive drug that they would most like to avoid was prednisone (4).
Recognition of the morbidity associated with prolonged high-dose prednisone after transplant led to numerous trials to minimize or eliminate prednisone from post-transplant immunosuppressive protocols. Initial attempts of late prednisone minimization in selected (clinically well and rejection-free) recipients taking cyclosporine and azathioprine were associated with increased acute rejection (AR) rates and decreased graft survival (5,6). With introduction of new and more powerful maintenance immunosuppressive agents, there were new trials of late prednisone minimization. Again, these trials were generally associated with significantly increased AR rates (7–9).
In contrast to these late withdrawal trials, recent protocols have incorporated rapid discontinuation of prednisone (RDP; prednisone rapidly tapered and discontinued within 1 week) after kidney transplant. Reports of these protocols, including registry reports and meta-analyses, have noted an increase in AR rates after RDP but no decrease in patient or graft survival rates compared with recipients treated with long-term maintenance prednisone (10–13). RDP has been associated with decreased rates of NODM and decreased cardiovascular risks (14,15).
A limitation of previously published reports is the relatively short follow-up time. The longest follow-up of a randomized trial of RDP has been 5 years (16), and most other single- or multicenter trials have had <5 years of follow-up. Herein, we report actuarial 10-year outcomes for 1241 adult primary kidney transplant recipients on a RDP protocol.
Materials and Methods
Between October 1, 1999 and December 31, 2010, a total of 1241 adult primary kidney transplants were done at our center (minimum follow-up of 3 months). Immunosuppression incorporated a rapid prednisone taper and discontinuation on postoperative day (POD) 6. Initially, our protocol (approved by the Human Subjects Committee) was limited to primary living donor (LD) transplant recipients; in October of 2000, we expanded our entry criteria to include all first and second LD and deceased donor (DD) recipients. Our only exclusion criteria were patients with more than or equal to three transplants and those patients already taking prednisone at the time of their transplant.
Our protocol has been described in detail (17). Briefly, all recipients received thymoglobulin (Genzyme Corp., Cambridge, MA) at a dose of 1.25–1.5 mg/kg per day for 5 days (the first dose was given in the operating room), a calcineurin inhibitor (either tacrolimus or cyclosporine), and a secondary agent (either mycophenolate mofetil or sirolimus). Prednisone was given for a total of six doses: 500 mg were given in the operating room followed by 1 mg/kg on POD 1, 0.5 mg/kg on POD 2 and 3, and 0.25 mg/kg on POD 4 and 5. For recipients with delayed graft function, defined as the need for dialysis within the first week after transplant, we extended the course of thymoglobulin (to a maximum of 10 doses) and delayed introduction of the calcineurin inhibitor; prednisone (5 mg/d) was given during thymoglobulin administration and discontinued when thymoglobulin was discontinued. For recipients not on our RDP protocol, prednisone was tapered to 5 mg/d by POD 6 and maintained at that dose.
All recipients were treated with prophylactic ganciclovir or valganciclovir for 3 months post-transplant. For patients treated for an AR episode, an additional 6 weeks of cytomegalovirus (CMV) prophylaxis was given. Pneumocystis prophylaxis was with trimethoprim-sulfamethoxazole; in patients with sulfa allergies, dapsone or aerosolized pentamidine was used. Fungal prophylaxis was with oral clotrimazole or nystatin for 3 months post-transplant.
Recipients with >25% increase in serum creatinine level from baseline underwent percutaneous allograft biopsy. Mild to moderate AR episodes were treated with a rapid steroid taper; steroid-resistant rejection episodes and histologically severe rejection episodes were treated with antibody (OKT3 or thymoglobulin) therapy. After antirejection therapy, most recipients had 5 mg prednisone daily added to their maintenance immunosuppression; some insisted on returning to prednisone-free immunosuppression (18).
For the 1241 recipients, we studied actuarial patient, graft, and death-censored graft survival rates; biopsy-proven AR and chronic rejection (interstitial fibrosis and tubular atrophy) rates; yearly estimated GFR (eGFR); and actuarial rates of select steroid- and immunosuppression-related side effects. The Modification of Diet in Renal Disease study equation was used to calculate eGFR. Individual analyses were done for LD and DD recipients.
Actuarial patient survival, graft survival and AR rates, and rates of selected side effects were compared with historical control subjects at our institution and current data (patient and graft survival rates) reported from the Scientific Registry of Transplant Recipients (SRTR) (19). Our historical cohort consisted of 459 primary kidney transplant recipients from January 1, 1996 to December 31, 2000 (168 LD recipients [89 nondiabetic and 79 diabetic patients] and 291 DD recipients [186 nondiabetic and 105 diabetic patients]) treated with polyclonal antibody induction, a calcineurin inhibitor, mycophenolate mofetil, and prednisone (1 mg/kg per day tapered to 0.4 mg/kg per day by 1 month and 0.15 mg/kg per day by 1 year). Of the 1241 primary kidney transplants, 120 (109 LD and 11 DD) underwent a pancreas transplant after their kidney transplant. Outcome analyses were censored at the time of pancreas transplant. Similarly, for patients lost to follow-up, all analyses were censored at the last known function and/or survival date.
Post-transplant side effects examined included NODM (defined as postdischarge new need for insulin or oral hypoglycemic agents), cataracts, fractures, avascular necrosis, CMV disease (defined as treatment with valganciclovir or ganciclovir), post-transplant lymphoproliferative disease, and other malignancy. Side effects were analyzed individually for pretransplant diabetic and nondiabetic recipients and LD and DD transplant recipients. The side effects in recipients on our RDP maintenance protocol were compared with the historical cohort as defined above.
For demographic data, categorical variables were analyzed using the chi-squared test and Fisher exact test (for small sample size). Continuous variables were analyzed using the t test. We assumed variables to be significant for corresponding P<0.05 at a 95% confidence interval. We estimated actuarial patient, graft, and death-censored graft survival rates and acute and chronic rejection rates (intention to treat analysis) by using Kaplan–Meier life table analyses. To compare differences between groups, we used the log-rank test.
Results
Demographics
Of the 1241 adult primary kidney transplant recipients treated with RDP, 791 were LD recipients (456 related and 335 unrelated donors), and 450 were DD recipients. Median follow-up time (and interquartile range [IQR]) was 63 (IQR = 28–92) months for LD recipients and 36 (IQR = 19–75) months for DD recipients. Patient characteristics are shown in Table 1.
Table 1.
Summary of study demographic data for living donor and deceased donor kidney transplants using rapid discontinuation of prednisone for maintenance immunosuppression
| LD | DD | |
|---|---|---|
| Total transplants (n, % of total) | 791 (64) | 450 (36) |
| LRD | 456 (37) | |
| LURD | 335 (27) | |
| Median follow-up in months (IQR) | 63 (28–92) | 36 (19–75) |
| Recipient demographics | ||
| age | ||
| median age in years (IQR) | 48 (39–57) | 55 (46–63) |
| gender (n, % DD/LD) | ||
| male | 488 (62) | 266 (59) |
| race (n, % DD/LD) | ||
| Caucasian | 714 (90) | 337 (75) |
| African-American | 32 (4) | 38 (8) |
| primary renal disease (n, % DD/LD) | ||
| type 1 diabetes | 211 (27) | 97 (22) |
| type 2 diabetes | 90 (11) | 88 (20) |
| hypertensive nephropathy | 58 (7) | 44 (10) |
| IgA nephropathy | 62 (8) | 23 (5) |
| Peak PRA level (n, % DD/LD) | ||
| 0 | 473 (60) | 199 (44) |
| 1–10 | 196 (25) | 122 (27) |
| 11–50 | 75 (9) | 67 (15) |
| 51–100 | 45 (6) | 60 (13) |
| pretransplant type 1 diabetes (n, % DD/LD) | 214 (27) | 99 (22) |
| Donor demographics | ||
| age | ||
| median age in years (IQR) | 44 (35–50) | 41 (22–53) |
| sex (n, % DD/LD) | ||
| male | 312 (39) | 263 (58) |
| race (n, % DD/LD) | ||
| Caucasian | 723 (92) | 413 (92) |
| African American | 32 (4) | 19 (4) |
LD, living donor; DD, deceased donor; LRD, living related donor; LURD, living unrelated donor; IQR, interquartile range; PRA, panel reactive antibody.
Median age was 48 (IQR = 39–57) years for LD recipients and; 55 (IQR = 46–63) years for DD recipients. Most of our recipients were Caucasian (90% of LD and 75% of DD) and male (62% of LD and 59% of DD). Among recipients, the most common primary renal disease was type 1 diabetes (27% of LD and 22% of DD). Peak panel reactive antibody was >10% in 15% of LD recipients and 28% of DD recipients.
Median age was 44 (IQR = 35–50) years for LD recipients and 41 (IQR = 22–53) years for DD recipients. The majority of LDs were female (61%), whereas the majority of DDs were male (58%). The vast majority of donors were Caucasian for both LD (92%) and DD (92%) recipients.
Patient, Graft, and Rejection-Free Graft Survival
Patient Survival.
For LD recipients on RDP, actuarial patient survival was 98% at 1 year, 90% at 5 years, and 71% at 10 years (Figure 1A). For DD recipients on RDP, actuarial patient survival was 95% at 1 year, 86% at 5 years, and 62% at 10 years (Figure 1B).
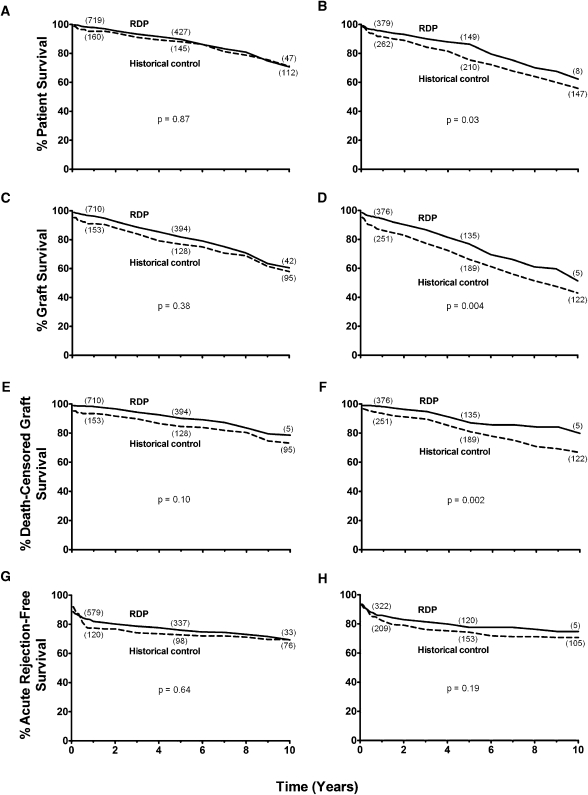
Figure 1.
Summary of survival outcomes following rapid discontinuation of prednisone (RDP). Patient (A and B), graft (C and D), death-censored graft survival (E and F), and acute cellular rejection-free survival (G and H) in recipients after transplant of living (A, C, E, and G) and deceased (B, D, F, and H) donor kidneys and RDP (solid line). Recipients treated with RDP are compared with historical controls treated with maintenance prednisone (dashed line). The number of recipients remaining at 1-, 5-, and 10-year follow-up is depicted in parentheses near each line for each cohort. Statistical analysis reveals significant differences between the RDP cohort and historical controls for patient (P=0.03), graft (P=0.004), and death-censored graft (P=0.002) survival for deceased donor kidney transplant recipients.
For LD historical controls (on prednisone), actuarial patient survival was 95% at 1 year, 88% at 5 years, and 71% at 10 years (P=0.87 versus RDP) (Figure 1A). For DD historical controls, actuarial patient survival was 91% at 1 year, 75% at 5 years, and 66% at 10 years (P=0.03 versus RDP) (Figure 1B). In addition, patient survival for RDP-treated recipients is comparable with contemporary 1-, 5-, and 10-year US national outcome for first transplants as reported to the SRTR (19) (Table 2).
Table 2.
Summary of survival data for living donor and deceased donor kidney transplants using rapid discontinuation of prednisone for maintenance immunosuppression along with Scientific Registry of Transplant Recipients national survival data
| 1-/5-/10-year rates (%) | ||
|---|---|---|
| LD | DD | |
| RDP patient survival | 98/90/71 | 95/86/62 |
| SRTR patient survivala | 99/92/77 | 97/86/65 |
| RDP graft survival | 96/82/61 | 94/77/51 |
| SRTR graft survivala | 96/81/60 | 92/72/45 |
| RDP death-censored graft survival | 98/90/79 | 98/87/80 |
LD, living donor; DD, deceased donor; RDP, rapid discontinuation of prednisone; SRTR, Scientific Registry of Transplant Recipients.
2009 Organ Procurement and Transplant Network/SRTR annual report adjusted 10-year data (http://www.ustransplant.org/annual_Reports/current/default.htm).
Graft Survival.
For LD recipients on RDP, actuarial graft survival was 96% at 1 year, 82% at 5 years, and 61% at 10 years (Figure 1C). For DD recipients on RDP, actuarial graft survival was 94% at 1 year, 77% at 5 years, and 51% at 10 years (Figure 1D).
For LD historical controls, actuarial graft survival was 91% at 1 year, 77% at 5 years, and 58% at 10 years (P=0.38 versus RDP) (Figure 1C). For DD historical controls, actuarial graft survival was 86% at 1 year, 66% at 5 years, and 43% at 10 years (P=0.004 versus RDP) (Figure 1D). In addition, graft survival for RDP-treated recipients is comparable with contemporary 1-, 5-, and 10-year US national outcome for first transplants as reported to the SRTR (19) (Table 2).
Death-Censored Graft Survival.
For LD recipients on RDP, actuarial death-censored graft survival was 98% at 1 year, 90% at 5 years, and 79% at 10 years (Figure 1E and Table 2). For DD recipients on RDP, actuarial death-censored graft survival was 98% at 1 year, 87% at 5 years, and 80% at 10 years (Figure 1F and Table 2).
For LD historical controls, actuarial death-censored graft survival was 93% at 1 year, 85% at 5 years, and 73% at 10 years (P=0.10 versus RDP) (Figure 1E). For DD historical controls, actuarial death-censored graft survival was 93% at 1 year, 81% at 5 years, and 67% at 10 years (P=0.002 versus RDP) (Figure 1F).
AR-Free Graft Survival.
For LD recipients on RDP, actuarial AR-free survival was 82% at 1 year, 76% at 5 years, and 69% at 10 years (Figure 1G). For DD recipients on RDP, actuarial AR-free survival was 86% at 1 year, 78% at 5 years, and 75% at 10 years (Figure 1H).
For LD historical controls, actuarial AR-free survival was 77% at 1 year, 73% at 5 years, and 69% at 10 years (P=0.64 versus RDP) (Figure 1G). For DD historical controls, actuarial AR-free survival was 82% at 1 year, 74% at 5 years, and 71% at 10 years (P=0.19 versus RDP) (Figure 1H).
Graft Survival by AR Status
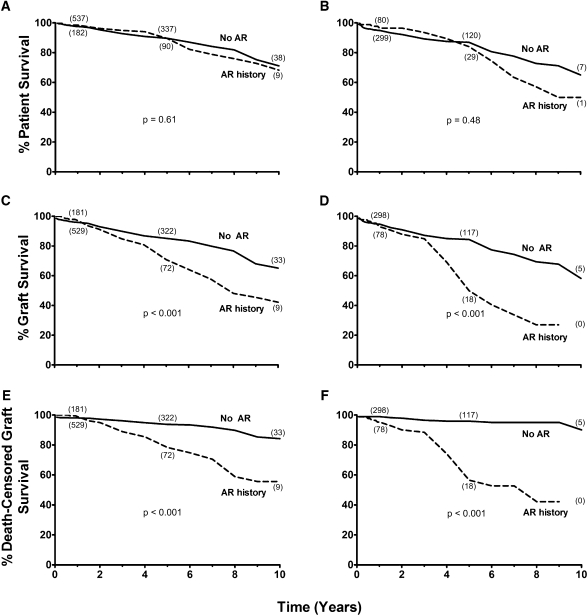
For LD recipients without AR, actuarial patient survival was 98% at 1 year, 90% at 5 years, and 71% at 10 years; for LD recipients with AR, survival was 98% at 1 year, 90% at 5 years, and 68% at 10 years (P=0.61) (Figure 2A). For DD recipients without AR, actuarial patient survival was 95% at 1 year, 87% at 5 years, and 65% at 10 years; for DD recipients with AR, survival was 96% at 1 year, 84% at 5 years, and 50% at 10 years (P=0.48) (Figure 2B).
Figure 2.
Summary of survival outcomes following rapid discontinuation of prednisone (RDP) as stratified by history of acute rejection. Patient (A and B), graft (C and D), and death-censored graft survival (E and F) in RDP recipients after transplant of living (A, C, and E) and deceased (B, D, and F) donor kidneys stratified by history of overall acute rejection (AR). The number of recipients remaining at 1-, 5-, and 10-year follow-up is depicted in parentheses near each line for each cohort. Statistical analysis reveals significant differences between RDP recipients with AR history and without AR history for living donor graft (P<0.001) and death-censored graft (P<0.001) survival, and deceased donor graft (P<0.001) and death-censored graft (P<0.001) survival.
For LD recipients without AR, actuarial graft survival was 96% at 1 year, 85% at 5 years, and 65% at 10 years; for LD recipients with AR, survival was 97% at 1 year, 71% at 5 years, and 42% at 10 years (P<0.001) (Figure 2C). For DD recipients without AR, actuarial graft survival was 95% at 1 year, 84% at 5 years, and 58% at 10 years; for DD recipients with AR, survival was 93% at 1 year, 50% at 5 years, and 27% at 9 years (P<0.001) (Figure 2D).
For LD recipients without AR, actuarial death-censored graft survival was 98% at 1 year, 94% at 5 years, and 84% at 10 years; for LD recipients with AR, survival was 99% at 1 year, 78% at 5 years, and 56% at 10 years (P<0.001) (Figure 2E). For DD recipients without AR, actuarial death-censored graft survival was 99% at 1 year, 96% at 5 years, and 90% at 10 years; for DD recipients with AR, survival was 95% at 1 year, 56% at 5 years, and 42% at 9 years (P<0.001) (Figure 2F).
Other Outcome Measures
A total of 165 cases of graft failure occurred in LD recipients. Of these failures, the most common causes were death with function (50%), chronic rejection (23%), biopsy-proven recurrent disease (4%), and biopsy proven AR (2%). A total of 97 graft failures occurred in DD recipients. Of these failures, the most common causes were death with function (61%), chronic rejection (12%), and AR (6%).
For LD recipients, actuarial chronic rejection (interstitial fibrosis and tubular atrophy) rates were 13% at 1 year, 29% at 5 years, and 47% at 10 years. For DD recipients, actuarial chronic rejection (interstitial fibrosis and tubular atrophy) rates were 19% at 1 year, 33% at 5 years, and 39% at 10 years. Delayed graft function rates were 2.5% and 17% for LD and DD recipients, respectively.
Mean eGFR (±SD) for LD recipients was 52 (±15) ml/min per 1.73 m2 at 1 year, 50 (±17) ml/min per 1.73 m2 at 5 years, and 45 (±16) ml/min per 1.73 m2 at 10 years. Mean eGFR (±SD) for DD recipients was 55 (±20) ml/min per 1.73 m2 at 1 year, 51 (±21) ml/min per 1.73 m2 at 5 years, and 55 (±21) ml/min per 1.73 m2 at 10 years.
Of the RDP recipients with functioning grafts and without having a pancreas transplant, 83% were prednisone-free at their latest follow-up. The most common reason for these recipients to resume prednisone has been treatment of an AR episode.
Side Effects
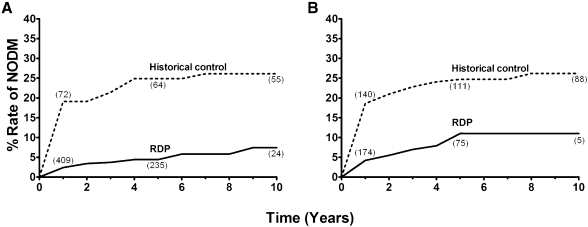
Rates of posttransplant side effects are shown in Table 3. For nondiabetic LD recipients on RDP, NODM developed in 2% at 1 year, 4% at 5 years, and 7% at 10 years (P<0.001 versus historical controls) (Figure 3A); for DD recipients, NODM developed in 4% at 1 year, 11% at 5 years, and 11% at 10 years (P<0.001 versus historical controls) (Figure 3B).
Figure 3.
New-onset diabetes mellitus (NODM) following rapid discontinuation of prednisone (RDP). Rates of NODM for living (A) and deceased donors (B) comparing RDP cohort with historical control (on steroid maintenance). The number of recipients at 1-, 5-, and 10-year follow-up is depicted in parentheses near each line for each cohort. Statistical analysis reveals significant differences in rates of NODM between RDP recipients and historical controls for living (P<0.001) and deceased (P<0.001) donor kidney recipients.
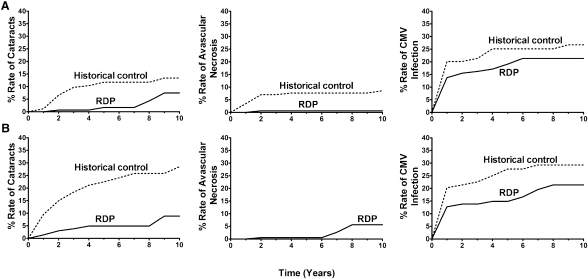
Diabetic and nondiabetic RDP-treated recipients (LD and DD) had significantly lower rates of cataracts (P<0.001) (Figures 4 and 5). Avascular necrosis was significantly decreased in RDP-treated LD nondiabetic (P=0.02) (Figure 4A) and DD nondiabetic (P<0.001) (Figure 5A) recipients. CMV infection rates were significantly decreased in RDP-treated LD nondiabetic (P<0.001) (Figure 4A) and DD diabetic (P=0.02) (Figure 5B) recipients. There was a trend to lower rates of fractures in RDP-treated DD nondiabetic recipients (P=0.06) (Figure 5A) and CMV infection in LD diabetic recipients (P=0.07) (Figure 4B). There were no significant differences between RDP recipients and historical controls in other studied side effects (Figures 4 and 5).
Figure 4.
Complications following rapid discontinuation of prednisone (RDP) in living donor kidney transplantation as stratified by history of diabetes mellitus. Rates of cataracts, avascular necrosis, and cytomegalovirus (CMV) infection in living donor transplants without recipient pretransplant diabetes mellitus (A) or with diabetes mellitus (B). Statistical analysis reveals significant differences in the rates of cataracts (P<0.001), avascular necrosis (P=0.02), and CMV infection (P<0.001) between the RDP cohort and historical controls among nondiabetics and in the rates of cataracts (P<0.001) between the RDP cohort and historical controls among those with diabetes.
Figure 5.
Complications following rapid discontinuation of prednisone (RDP) in deceased donor kidney transplantation as stratified by history of diabetes mellitus. Rates of cataracts, avascular necrosis, and CMV infection in deceased donor transplants without recipient pretransplant diabetes mellitus (A) or with diabetes mellitus (B). Statistical analysis reveals significant differences in the rates of cataracts (P<0.001) and avascular necrosis (P<0.001) between the RDP cohort and historical controls among nondiabetics and in the rates of cataracts (P<0.001) and CMV infection (P=0.02) between the RDP cohort and historical controls among those with diabetes.
Discussion
To the best of our knowledge, our study is the first to report 10-year outcomes for kidney transplant recipients treated with RDP. We found that RDP-treated patient survival rates were similar to the rates of historical controls at our institution and contemporary SRTR national averages (19). RDP-treated LD recipients had graft survival rates similar to the rates for historical controls and contemporary SRTR national averages (19); RDP-treated DD recipients had better graft survival rates than historical controls but similar rates as contemporary SRTR averages. Meta-analyses of shorter-term studies have shown no impact of RDP on patient and graft survival (12,14), and a registry report, although potentially limited by selection bias, found that patient and graft survival is improved in those patients on RDP (20). In the randomized trial with the longest follow-up (5 years), Woodle et al. (16) found no differences of patient and graft survival in those patients treated with RDP versus maintenance prednisone.
Similarly, previous studies have also reported no difference in renal function for those patients on RDP versus maintenance steroids (21–23). RDP-treated recipients do, however, have an increased rate of AR but not an increase in AR episodes requiring antibody treatment (12,14,16). Rejection episodes, in recipients treated with prednisone (24,25) or after RDP (Figure 2) (16), are a risk factor for graft loss. Given the increased early AR rates but no difference in graft survival (12,14,16), it may be that mild rejections on an RDP protocol do not always have the same negative consequences as rejection episodes for patients taking prednisone. Alternatively, longer follow-up with larger numbers may be necessary.
We observed a significant reduction in NODM and other prednisone-related complications for those patients treated with RDP (Figures 3–5 and Table 3). Others have similarly shown decreased rates of NODM and individual prednisone-related side effects after RDP (14–16). Decreased cardiovascular risk factors were reported in the works by Woodle et al. (16) (triglycerides, NODM requiring insulin, and weight gain) and Knight and Morris (14) (hypertension, NODM, and hypercholesterolemia). It is interesting that many known steroid-related side effects, including appearance and mood, bone mineral density, or quality of life changes, have not been studied in RDP protocols. This lack of study may be because of cost or difficulties with quantification, or it may be because most investigators felt that it would be intuitively obvious that these side effects would be minimized in the absence of prednisone.
Table 3.
Summary of complications data for living donor and deceased donor kidney transplants using rapid discontinuation of prednisone for maintenance immunosuppression with and without pretransplant diabetes
| 1-/5-/10-year rates (%) | ||
|---|---|---|
| Recipients with Diabetes | Recipients without Diabetes | |
| Complication rate after LD transplant | ||
| NODM | — | 2/4/7a |
| cataracts | 4/11/17a | 0.7/2/4a |
| fracture | 4/16/33 | 1/5/10 |
| AVN | 0/0/1 | 0/0.4/3b |
| CMV infection | 7/16/22 | 8/11/12a |
| PTLD | 0.3/0.3/0.3 | 0/0.6/2 |
| non-PTLD malignancy | 0/3/11 | 1/6/13 |
| Complication rate after DD transplant | ||
| NODM | — | 4/11/11a |
| cataracts | 1/5/9a | 0/2/7a |
| fracture | 6/15/23 | 3/7/9 |
| AVN | 0/0.5/6 | 0/0.6/0.6a |
| CMV infection | 13/15/21b | 14/19/21 |
| PTLD | 1/3/6 | 0.5/1/1 |
| non-PTLD malignancy | 3/10/13 | 1/2/8 |
NODM, new-onset diabetes mellitus; AVN, avascular necrosis; CMV, cytomegalovirus; PTLD, post-transplant lymphoproliferative disease.
P<0.001 (vs. historical control).
P<0.05 (vs. historical control).
An important consideration in RDP protocols is the choice of induction and maintenance immunosuppression. All of our recipients were treated with polyclonal antibody. In the study by Woodle et al. (16), centers were allowed to choose which induction agent to use (IL-2R inhibitor or polyclonal antibody). Lower AR rates were observed when polyclonal antibody was used. Similarly, data from a prospective randomized study by Vitko et al. (26) suggest that both induction and dual agent maintenance therapy may be important for successful RDP.
RDP has been used with a number of different maintenance protocols (27–30). A recent meta-analysis by Pascual et al. (12) suggested that RDP with cyclosporine, but not tacrolimus-based protocols, was associated with increased AR rates, whereas RDP with tacrolimus, but not cyclosporine-based protocols, was associated with increased NODM.
There are a number of limitations to our data. First, it is a single center report and not a randomized trial. Although we have compared patient and graft survival at our institution with SRTR results, there are limitations to this comparison. Our demographics are different than the demographics of the SRTR, and the SRTR report also includes recipients on RDP. However, our SRTR-reported, center-specific adjusted results are similar to national averages (19). Second, most recipients in our study were Caucasian, with only a small proportion of African Americans. Although successful short-term outcome has been reported for African Americans on RDP (31–34), additional studies with larger numbers and longer follow-up need to be done. Third, we have not done protocol biopsies. Although mean eGFR levels remain stable in our RDP recipients, it is possible that progressive fibrosis caused by the absence of prednisone maintenance therapy may be missed in some grafts. Finally, we have compared prednisone-related side effects with a historical cohort taking relatively high maintenance doses of prednisone. One major impact of prednisone-free immunosuppression trials is that most centers, even when not using RDP, now use much lower doses of prednisone than they did 10 years ago. Ideally, there should be a prospective randomized trial of RDP versus a rapid prednisone taper to 5 mg/day maintenance therapy.
The role of RDP in kidney transplant immunosuppressive protocols requires additional definition. Most large studies (ours is one of the exceptions) have limited RDP to low-risk recipients. However, RDP has been successfully used in higher risk subgroups such as African Americans, children, patients with potentially recurrent disease, and high immunologic risk recipients (31–41). The accumulation of available data to date suggests that RDP protocols are associated with an increase in mild rejection episodes (not requiring antibody treatment) and a decrease in steroid-related side effects, including cardiovascular risk factors. In addition, a recipient may elect an RDP protocol with an increased risk of rejection for the benefit of minimizing appearance changes or loss in bone mineral density.
It is interesting that, given similar data, recent reviews have had a diversity of opinion on the role of RDP (10, 42–44). These opinions have ranged from recommendations for caution in using RDP to recommendations for widespread use in low-risk populations while continuing to study higher-risk populations. Our data provide additional support for the use of RDP protocols. RDP use in an unselected population of primary transplant recipients has resulted in significant reduction of prednisone-related side effects while maintaining acceptable patient and graft survival.
In summary, we have reported 10-year patient and graft survival rates for primary transplants on RDP that are similar to national averages. Over 80% of recipients remain prednisone-free with stable GFR. Most importantly, RDP significantly reduces the rate of steroid-related side effects.
Disclosures
A.J.M. is a consultant for Bristol-Myers Squibb.
Acknowledgments
The authors thank Ms. Stephanie Daily for assistance with the preparation of the manuscript.
This work was supported by National Institutes of Health Grant DK-13083.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Are Maintenance Corticosteroids No Longer Necessary after Kidney Transplantation?” on pages 383–384.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1.Citterio F: Steroid side effects and their impact on transplantation outcome. Transplantation 72[Suppl]: S75–S80, 2001 [PubMed] [Google Scholar]
- 2.Morris PJ, Chan L, French ME, Ting A: Low dose oral prednisolone in renal transplantation. Lancet 1: 525–527, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Vanrenterghem YF, Claes K, Montagnino G, Fieuws S, Maes B, Villa M, Ponticelli C: Risk factors for cardiovascular events after successful renal transplantation. Transplantation 85: 209–216, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Prasad GV, Nash MM, McFarlane PA, Zaltzman JS: Renal transplant recipient attitudes toward steroid use and steroid withdrawal. Clin Transplant 17: 135–139, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Hricik DE, O’Toole MA, Schulak JA, Herson J: Steroid-free immunosuppression in cyclosporine-treated renal transplant recipients: A meta-analysis. J Am Soc Nephrol 4: 1300–1305, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Kasiske BL, Chakkera HA, Louis TA, Ma JZ: A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol 11: 1910–1917, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Ahsan N, Hricik D, Matas A, Rose S, Tomlanovich S, Wilkinson A, Ewell M, McIntosh M, Stablein D, Hodge E, Steroid Withdrawal Study Group : Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil—a prospective randomized study. Transplantation 68: 1865–1874, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Vanrenterghem Y, Lebranchu Y, Hené R, Oppenheimer F, Ekberg H: Double-blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation 70: 1352–1359, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Pascual J, Quereda C, Zamora J, Hernández D, Spanish Group for Evidence-Based Medicine in Renal Transplantation : Steroid withdrawal in renal transplant patients on triple therapy with a calcineurin inhibitor and mycophenolate mofetil: A meta-analysis of randomized, controlled trials. Transplantation 78: 1548–1556, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Luan FL, Steffick DE, Ojo AO: Steroid-free maintenance immunosuppression in kidney transplantation: Is it time to consider it as a standard therapy? Kidney Int 76: 825–830, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matas AJ: Minimization of steroids in kidney transplantation. Transpl Int 22: 38–48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascual J, Zamora J, Galeano C, Royuela A, Quereda C: Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev 1: CD005632, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Schiff J, Cole EH: Renal transplantation with early steroid withdrawal. Pediatr Nephrol 24: 243–251, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Knight SR, Morris PJ: Steroid avoidance or withdrawal after renal transplantation increases the risk of acute rejection but decreases cardiovascular risk. A meta-analysis. Transplantation 89: 1–14, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Luan FL, Steffick DE, Ojo AO: New-onset diabetes mellitus in kidney transplant recipients discharged on steroid-free immunosuppression. Transplantation 91: 334–341, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P, Astellas Corticosteroid Withdrawal Study Group : A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 248: 564–577, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Matas AJ, Kandaswamy R, Humar A, Payne WD, Dunn DL, Najarian JS, Gruessner RW, Gillingham KJ, McHugh LE, Sutherland DE: Long-term immunosuppression, without maintenance prednisone, after kidney transplantation. Ann Surg 240: 510–516, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humar A, Gillingham K, Kandaswamy R, Payne W, Matas A: Steroid avoidance regimens: A comparison of outcomes with maintenance steroids versus continued steroid avoidance in recipients having an acute rejection episode. Am J Transplant 7: 1948–1953, 2007 [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services HRSA : 2009 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1999–2008, Rockville, MD, U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, 2009
- 20.Luan FL, Steffick DE, Gadegbeku C, Norman SP, Wolfe R, Ojo AO: Graft and patient survival in kidney transplant recipients selected for de novo steroid-free maintenance immunosuppression. Am J Transplant 9: 160–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boots JM, Christiaans MH, Van Duijnhoven EM, Van Suylen RJ, Van Hooff JP: Early steroid withdrawal in renal transplantation with tacrolimus dual therapy: A pilot study. Transplantation 74: 1703–1709, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Kumar MS, Heifets M, Moritz MJ, Saeed MI, Khan SM, Fyfe B, Sustento-Riodeca N, Daniel JN, Kumar A: Safety and efficacy of steroid withdrawal two days after kidney transplantation: Analysis of results at three years. Transplantation 81: 832–839, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Montagnino G, Sandrini S, Iorio B, Schena FP, Carmellini M, Rigotti P, Cossu M, Altieri P, Salvadori M, Stefoni S, Corbetta G, Ponticelli C: A randomized exploratory trial of steroid avoidance in renal transplant patients treated with everolimus and low-dose cyclosporine. Nephrol Dial Transplant 23: 707–714, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Matas AJ, Gillingham KJ, Payne WD, Najarian JS: The impact of an acute rejection episode on long-term renal allograft survival (t1/2). Transplantation 57: 857–859, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Humar A, Hassoun A, Kandaswamy R, Payne WD, Sutherland DE, Matas AJ: Immunologic factors: The major risk for decreased long-term renal allograft survival. Transplantation 68: 1842–1846, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Vítko S, Klinger M, Salmela K, Wlodarczyk Z, Tydèn G, Senatorski G, Ostrowski M, Fauchald P, Kokot F, Stefoni S, Perner F, Claesson K, Castagneto M, Heemann U, Carmellini M, Squifflet JP, Weber M, Segoloni G, Bäckman L, Sperschneider H, Krämer BK: Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: Results of the Atlas study. Transplantation 80: 1734–1741, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kandaswamy R, Melancon JK, Dunn T, Tan M, Casingal V, Humar A, Payne WD, Gruessner RW, Dunn DL, Najarian JS, Sutherland DE, Gillingham KJ, Matas AJ: A prospective randomized trial of steroid-free maintenance regimens in kidney transplant recipients—an interim analysis. Am J Transplant 5: 1529–1536, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Anil Kumar MS, Heifets M, Fyfe B, Saaed MI, Moritz MJ, Parikh MH, Kumar A: Comparison of steroid avoidance in tacrolimus/mycophenolate mofetil and tacrolimus/sirolimus combination in kidney transplantation monitored by surveillance biopsy. Transplantation 80: 807–814, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Gallon L, Perico N, Dimitrov BD, Winoto J, Remuzzi G, Leventhal J, Gaspari F, Kaufman D: Long-term renal allograft function on a tacrolimus-based, pred-free maintenance immunosuppression comparing sirolimus vs. MMF. Am J Transplant 6: 1617–1623, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Gallon LG, Winoto J, Chhabra D, Parker MA, Leventhal JR, Kaufman DB: Long-term renal transplant function in recipient of simultaneous kidney and pancreas transplant maintained with two prednisone-free maintenance immunosuppressive combinations: Tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. Transplantation 83: 1324–1329, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Anil Kumar MS, Moritz MJ, Saaed MI, Heifets M, Sustento-Reodica N, Fyfe B, Kumar A: Avoidance of chronic steroid therapy in African American kidney transplant recipients monitored by surveillance biopsy: 1-year results. Am J Transplant 5: 1976–1985, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Boardman RE, Alloway RR, Alexander JW, Buell JF, Cardi M, First MR, Hanaway MJ, Munda R, Rogers CC, Roy-Chaudhury P, Susskind B, Trofe J, Woodle ES: African-American renal transplant recipients benefit from early corticosteroid withdrawal under modern immunosuppression. Am J Transplant 5: 356–365, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Haririan A, Sillix DH, Morawski K, El-Amm JM, Garnick J, Doshi MD, West MS, Gruber SA: Short-term experience with early steroid withdrawal in African-American renal transplant recipients. Am J Transplant 6: 2396–2402, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Zeng X, El-Amm JM, Doshi MD, Singh A, Morawski K, Cincotta E, Losanoff JE, West MS, Gruber SA: Intermediate-term outcomes with early steroid withdrawal in African-American renal transplant recipients undergoing surveillance biopsy. Surgery 142: 538–544, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Alloway RR, Hanaway MJ, Trofe J, Boardman R, Rogers CC, Hanaway MJ, Buell JF, Munda R, Alexander JW, Thomas MJ, Roy-Chaudhury P, Cardi M, Woodle ES: A prospective, pilot study of early corticosteroid cessation in high-immunologic-risk patients: The Cincinnati experience. Transplant Proc 37: 802–803, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Khwaja K, Asolati M, Harmon JV, Melancon JK, Dunn TB, Gillingham KJ, Kandaswamy R, Humar A, Gruessner RW, Payne WD, Najarian JS, Dunn DL, Sutherland DE, Matas AJ: Rapid discontinuation of prednisone in higher-risk kidney transplant recipients. Transplantation 78: 1397–1399, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Chavers BMCC, Gillingham KJ, Matas AJ: Pediatric kidney transplantation using a novel protocol of rapid (6-day) discontinuation of prednisone: 2-year results. Transplantation 88: 237–241, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birkeland SA, Larsen KE, Rohr N: Pediatric renal transplantation without steroids. Pediatr Nephrol 12: 87–92, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Oberholzer J, John E, Lumpaopong A, Testa G, Sankary HN, Briars L, Kraft KA, Knight PS, Verghese P, Benedetti E: Early discontinuation of steroids is safe and effective in pediatric kidney transplant recipients. Pediatr Transplant 9: 456–463, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Ibrahim H, Rogers T, Casingal V, Sturdevant M, Tan M, Humar A, Gillingham K, Matas A: Graft loss from recurrent glomerulonephritis is not increased with a rapid steroid discontinuation protocol. Transplantation 81: 214–219, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Boardman R, Trofe J, Alloway R, Rogers C, Roy-Chaudhury P, Cardi M, Safdar S, Groene B, Buell J, Hanaway M, Thomas M, Alexander W, Munda R, Woodle ES: Early steroid withdrawal does not increase risk for recurrent focal segmental glomerulosclerosis. Transplant Proc 37: 817–818, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Desai NM, Schnitzler M, Jendrisak MD, Brennan DC: Maintenance steroid therapy for kidney recipients—not ready for relegation. Am J Transplant 9: 1263–1264, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Knight SR.Morris PJ: Steroid avoidance or withdrawal in renal transplantation. Transplantation 91: e25–e27, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Augustine JJ, Hricik DE: Steroid withdrawal: Moving on to the next questions. Am J Transplant 9: 3–4, 2009 [DOI] [PubMed] [Google Scholar]