Summary
Background and objectives
ESRD is associated with substantial cognitive deficits but whether earlier kidney dysfunction predicts cognitive decline is less well defined.
Design, setting, participants, & measurements
More than 1700 women aged ≥70 years in the Nurses' Health Study had plasma creatinine and urinary albumin/creatinine ratios (ACRs) measured in 2000, within 12 months of their initial cognitive testing. These participants had repeated assessments of cognition administered by phone every 2 years, including tests for general cognition, verbal memory, verbal fluency, and working memory for up to 6 years of follow-up. Mixed-effects regression analysis was applied to calculate mean differences in the rate of cognitive decline between women with an estimated GFR <60 ml/min per 1.73 m2 or an ACR ≥5 mg/g versus referent levels.
Results
The median age was 74 years at initial cognitive testing, 99% of women were Caucasian, median plasma creatinine was 0.8 mg/dl, and 25% had an ACR ≥5 mg/g. The difference in cognitive decline with a baseline ACR ≥5 mg/g versus an ACR <5 mg/g was equivalent to the difference observed with 2–7 years of aging; that is, a higher ACR was associated with 2–7 times faster decline in all four cognitive domains assessed (all P values <0.05) than that attributed to each 1 year of aging alone. No associations were observed between an eGFR <60 ml/min per 1.73 m2 and cognitive decline.
Conclusions
A baseline urinary ACR ≥5 mg/g, a level not traditionally considered clinically significant, is independently associated with faster decline in cognitive function.
Introduction
CKD has reached epidemic proportions in the United States in part due to the increasing population of older adults (1). Cognitive deficits have been well documented in patients with ESRD (2,3), but how early kidney dysfunction (as reflected by a moderately decreased estimated GFR [eGFR] or albuminuria) may be related to subsequent cognitive decline is less well described.
Albuminuria is considered a marker of small vessel disease and has been independently associated with risk of hypertension and mortality (4,5). Because cognitive decline is frequently attributed to microvascular disease in the brain (6,7), and vascular disease is often a prominent feature of advancing renal insufficiency (8), progressive small vessel disease may be a key component linking albuminuria with progressive kidney dysfunction and cognitive decline. A number of investigators have reported cross-sectional associations between albuminuria and worse cognitive function (9,10); however, longitudinal data on change in cognitive function with albuminuria are sparse.
Similarly, limited data are available on the association between moderately decreased eGFR and cognitive function, with the majority of studies being cross-sectional in design and showing an increased prevalence of impaired cognitive function in participants with decreased kidney function (11,12). Therefore, we prospectively analyzed associations between albuminuria and an eGFR <60 ml/min per 1.73 m2 in >1700 women participating in the Nurses’ Health Study (NHS).
Materials and Methods
Study Population
The NHS began in 1976, when 121,700 female registered nurses (aged 30–55 years, living in 11 US states) completed a mailed questionnaire on lifestyle and health. Every 2 years, follow-up questionnaires are mailed to participants to update their information, and >90% follow-up of the total possible person-time has been maintained (13).
For the study of cognitive function, we selected participants aged ≥70 years who were free of diagnosed stroke. We contacted 21,085 eligible women for a baseline telephone cognitive assessment, and 19,415 women agreed to have the initial testing done (1995–2001) (14). Follow-up cognitive assessments were conducted every 2 years. Women who underwent at least two cognitive assessments were included in the study. Of these participants, 1764 women had plasma creatinine measured in 2000, within 12 months of their initial cognitive testing, and 1743 women had data on urinary albumin/creatinine ratios (ACRs).
Estimated GFR
An eGFR <60 ml/min per 1.73 m2 was one of the primary exposures of interest. Blood samples were collected in 2000. Each participant was sent a blood collection kit containing instructions and needed supplies. The participants made arrangements for the blood to be drawn locally and then sent the samples back by overnight courier. A total of 97% of samples arrived in our laboratory within 26 hours of being drawn. After centrifugation, the cryotubes were stored in the vapor phase of liquid nitrogen freezers at −130°C. Plasma collected from the study participants was thawed and sent to the laboratory at Children’s Hospital (Boston, MA). Plasma creatinine (PCr) was measured using a modified kinetic Jaffe reaction. The percent coefficient of variation (CV) for creatinine in masked quality control samples in the NHS was 5.2%. The GFR was estimated using the following Chronic Kidney Disease Epidemiology Collaboration equation (15):
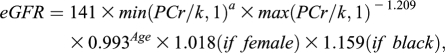 |
where k is 0.7 for females and 0.9 for males, a is −0.329 for females and −0.411 for males, min indicates the minimum of PCr/k or 1, and max indicates the maximum of PCr/K or 1.
The eGFR was also calculated using previously published equations, using serum cystatin C (cys C) (16). Cys C levels were measured using an immunoturbidimetric assay on the Hitachi 917 analyzer from Roche Diagnostics (Indianapolis, IN), with reagents and calibrators from Genzyme (Stillwater, MN). The CV for cys C was 3.8%. GFR was calculated using only cys C and then also with both cys C and plasma creatinine using the following equations (16):
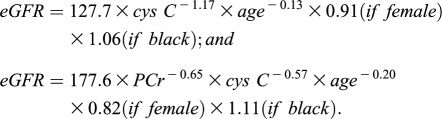 |
Albumin/Creatinine Ratio
Spot urine samples were also collected in 2000 at the same time as the blood samples. Urine albumin was measured by an immunoassay using the Hitachi 911 analyzer and Roche Diagnostics reagents. Urine albumin concentrations as low as 1 mg/L were reported reliably; values <1 mg/L were considered to be 0. In split blinded quality control samples, the CV for urinary albumin was 8.0%. Urine creatinine was measured using a modified Jaffe method (CV 2.0%). For each participant, urinary albumin concentration was divided by urinary creatinine concentration to obtain the ACR in milligrams per gram.
Because only 104 (6.3%) participants in our study population met the criterion for microalbuminuria using a sex-specific cutpoint of an ACR ≥25 mg/g, we examined associations of an ACR ≥10 mg/g (recently identified as significantly associated with mortality in a meta-analysis of over 100,000 individuals) (17) and also an ACR ≥5 mg/g (because ACR ≥4.3 mg/g has been associated with incident hypertension in NHS women) (18).
Cognitive Function Assessments
The telephone battery included the following six tests (with previously reported population distributions in parentheses): (1) the Telephone Interview for Cognitive Status (TICS), which is a telephone adaptation of the Mini-Mental State Exam (MMSE) (mean 33.8; SD 2.7; range, 8–41) (19); (2) East Boston Memory Test Immediate Recall, in which participants are read a brief paragraph and asked to repeat 12 elements immediately (mean 9.4; SD 1.7; range, 0–12) (20); (3) East Boston Memory Test Delayed Recall (EBMT), in which participants are asked to repeat 12 elements from a brief paragraph 15 minutes later (mean 9.0; SD 2.0; range, 0–12) (20); (4) verbal fluency, in which participants are asked to name as many animals as they can in 1 minute (mean 16.9; SD 4.6; range, 0–38) (21); (5) TICS 10-word list delayed recall, which is a test of verbal memory in which participants are asked to recall the TICS 10-word list 15 minutes after the initial recall (mean 2.3; SD 2; range, 0–10) (19); and (6) digit span backward, in which participants are asked to repeat in reverse order 12 increasingly long series of numbers (mean 6.7; SD 2.4; range, 0–12) (19).
The cognitive tests were repeated biennially. Participation rates were identical across all cognitive tests. For our primary outcome, we calculated a global composite score by averaging the z scores from the six tests among women who completed all of them. Because composite scores integrate information from multiple tests, they are stable measures of cognitive function (22). We also calculated verbal memory composite scores, in which we averaged the z scores of four tests of verbal memory (the immediate and delayed recalls of the TICS 10-word list, and the immediate and delayed recalls of the EBMT). Verbal fluency was also one of the primary outcome measures because it partially assesses executive function and is thus of particular interest for variables that affect vascular health.
Validity and Reliability of Telephone Assessments
We found a correlation of 0.81 comparing the global scores of the telephone versus extensive in-person assessments in a validation of our battery among 61 educated older women. We also found high reliability of test performance among 35 women who were given the TICS twice, 31 days apart (test–retest correlation = 0.7). Finally, among 88 older female health professionals, poor cognitive performance as determined by our telephone assessment was strongly associated with dementia diagnosis after 3 years; poor performance in the TICS and in verbal memory were both associated with significant 8- and 12-fold increases, respectively, of dementia (14).
Covariates
Demographic and clinical characteristics were obtained through the information collected on biennial questionnaires obtained near the baseline cognitive assessment. We included the following potential confounders for associations between kidney dysfunction and cognitive decline: age, high BP, body mass index, diabetes, cigarette smoking, cardiovascular disease, alcohol intake, physical activity, high cholesterol, participant education level, spouse’s education level, and postmenopausal hormonal use. Systolic BP was reported in nine categories (<105, 105–114, 115–124, 125–134, 135–144, 145–154, 155–164, 165–174, and >175 mmHg), and diastolic BP was reported in seven categories (<65, 65–74, 75–84, 85–89, 90–94, 95–104, and >105 mmHg). A participant’s BP was defined as the middle systolic and middle diastolic value of the reported category. Self-reported hypertension has been previously validated through direct review of medical records (23).
Statistical Analyses
Demographic and clinical characteristics were compared using the Wilcoxon test for continuous variables and the chi-squared test for categorical variables. We applied mixed-effects modeling to compare the rate of change in cognitive decline between groups with higher versus lower eGFR and higher versus lower urinary ACR. We evaluated the mean performance at each cognitive assessment by eGFR or ACR category using repeated analysis of means with random intercepts and random slopes, which accounts for intra-individual correlations of cognitive function over time. All models were fitted by maximum likelihood, incorporating the longitudinal correlation within study participants using unstructured covariance structures; for statistical testing, we used Wald tests (24). This longitudinal analysis approach takes into account the individual baseline cognitive scores in the assessment of change of scores over time. These analyses were performed using the Proc Mixed command in SAS software (release 9.1; SAS Institute Inc, Cary, NC).
This study was approved by the Institutional Review Board of the Brigham and Women’s Hospital.
Results
Our prospective analyses included 1764 older women from the NHS, 1743 of whom had ACR data. Of these, 23% had an eGFR <60 ml/min per 1.73 m2, and 25% had an ACR ≥5 mg/g. Because our main findings were in those with higher albuminuria, we also present demographic and clinical characteristics stratified by an ACR ≥5 mg/g (Table 1). Compared with women with an ACR <5 mg/g, there was a higher prevalence of diabetes, hypertension, and cardiovascular disease in women with an ACR ≥5 mg/g. Women with an ACR ≥5 mg/g were also less physically active.
Table 1.
Demographic and clinical characteristics of participants in the Nurses’ Health Study
| All (N=1764) | ACR <5 mg/g (n=1303) | ACR ≥5 mg/g (n=440) | P Valuea | |
|---|---|---|---|---|
| Age (yr) | 74.0 (72.3, 75.8) | 73.9 (72.1, 75.6) | 74.5 (72.8, 76.3) | <0.001 |
| Caucasian | 98.8 | 98.8 | 98.6 | 0.82 |
| Hypertension | 58.1 | 55.3 | 65.2 | <0.001 |
| Systolic BP (mmHg) | 130 (120, 140) | 130 (120, 140) | 140 (130, 150) | <0.001 |
| Diastolic BP (mmHg) | 80 (70, 80) | 80 (70, 80) | 80 (70, 80) | 0.06 |
| Diabetes | 12.4 | 7.9 | 25.9 | <0.001 |
| History of cardiovascular disease | 7.3 | 5.7 | 12.1 | <0.001 |
| Current smoker | 4.6 | 4.1 | 6.4 | 0.05 |
| Alcohol intake (g/d) | 0.9 (0.0, 5.8) | 0.9 (0, 6.0) | 0 (0, 4.0) | 0.06 |
| Activity level (METs/wk) | 11.5 (4.2, 23.7) | 12.8 (4.9, 25.5) | 7.0 (2.4, 20.2) | <0.001 |
| BMI (kg/m2) | 24.8 (22.3, 28.3) | 24.7 (22.2, 27.9) | 24.9 (22.1, 29.2) | 0.19 |
| Depression | 6.4 | 6.5 | 6.1 | 0.82 |
| Hypertension medication use | 49.2 | 47.8 | 55.9 | 0.003 |
| Depression medication use | 7.9 | 7.8 | 8.4 | 0.65 |
| Analgesic medication use | 76.3 | 76.8 | 74.8 | 0.38 |
| Insulin use | 2.5 | 1.3 | 5.9 | <0.001 |
| Postmenopausal hormone use | 0.03 | |||
| current | 48.0 | 47.8 | 39.6 | |
| past | 27.0 | 25.1 | 28.4 | |
| never | 24.7 | 22.6 | 26.6 | |
| Participant highest education level | 0.11 | |||
| RN | 73.2 | 73.8 | 71.8 | |
| BA | 19.7 | 19.7 | 20.2 | |
| MA or DR | 7.1 | 6.6 | 8.0 | |
| Spouse’s highest education level | 0.22 | |||
| less than high school | 1.6 | 1.4 | 2.5 | |
| some high school | 5.0 | 4.7 | 5.5 | |
| high school graduate | 37.0 | 36.2 | 39.1 | |
| college graduate | 25.4 | 26.6 | 22.5 | |
| grad school | 20.1 | 20.5 | 18.4 | |
| missing | 10.9 | 10.7 | 12.1 | |
| PCr in 2000 (mg/dl) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.8 (0.7, 1.0) | 0.02 |
| eGFR in 2000 by CKD-EPI (ml/min per 1.73 m2) | 72 (61, 83) | 72 (61, 84) | 70 (57, 82) | 0.01 |
| eGFR in 2000 by cystatin C (ml/min per 1.73 m2) | 57 (49, 65) | 57.8 (50, 66) | 54 (43, 62) | <0.001 |
| eGFR in 2000 by cystatin C + PCr (ml/min per 1.73 m2) | 66 (58, 75) | 67 (59, 76) | 63 (51, 73) | <0.001 |
Results are expressed as median (interquartile range) or percentage. ACR, albumin/creatinine ratio; BMI, body mass index; PCr, plasma creatinine; eGFR, estimated GFR; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation.
Comparison between ACR <5 and ≥5 mg/g.
We also examined the demographic and clinical characteristics of women with at least two cognitive assessments, who had urinary ACR and plasma creatinine data available (n=1746) compared with those who did not have these data (n=13,444), and we concluded that our study cohort seems to be reasonably representative of the overall group that underwent cognitive function testing. For example, for those with ACR and plasma creatinine data compared with those without these data, the median age was 74 versus 74.8 years, body mass index was 24.8 kg/m2 versus 25.1 kg/m2, 58% versus 61% had hypertension, 12.6% versus 11.9% had diabetes, and 6.4% had depression in each group. Antihypertensive, antidepressant, and analgesic medication use was not meaningfully different between the groups.
Among 1764 women who completed the baseline test and at least one follow-up test, 1761 women (99.8%) had three cognitive tests (4 years of follow-up) and 1376 (78%) had four cognitive tests (6 years of follow-up). Because the majority of the women had up to three cognitive tests, we chose to examine cognitive decline across three assessments of cognition for our analyses. We first examined the rate of decline in cognitive function associated with aging alone. The mean annual decline in global composite, verbal memory composite, verbal fluency, and TICS scores associated with each year of aging were −0.006 (95% CI, −0.009 to −0.003), −0.007 (95% CI, −0.010 to −0.003), −0.026 (95% CI, −0.039 to −0.012), and −0.040 (95% CI, −0.064 to −0.017) standard units, respectively.
No significant difference was seen in cognitive decline in participants with an eGFR <60 ml/min per 1.73 m2 compared with eGFR ≥60 ml/min per 1.73 m2 in either age-adjusted or multivariable models (Table 2). Significantly worse decline in cognitive function was seen in participants with an ACR ≥5 mg/g compared with those with an ACR <5 mg/g in age-adjusted models. Multivariable adjustment did not influence the results meaningfully (Table 3), and no individual covariate changed the associations observed when added separately. Additional adjustment for BP and insulin did not change the results (mean annual decline of −0.03 [95% CI, −0.05 to −0.01] in global cognitive score). The associations with an ACR ≥10 mg/g were almost identical to those with an ACR ≥5 mg/g, although the verbal memory composite score was no longer significant (Table 3).
Table 2.
Mean differences in annual rate of cognitive decline for an eGFR <60 ml/min per 1.73 m2 compared with an eGFR ≥60 ml/min per 1.73 m2
| Age-Adjusted Only (95% CI) | Multivariable Adjusteda (95% CI) | |
|---|---|---|
| eGFR <60 ml/min per 1.73 m2 (CKD-EPI) (n=408) | ||
| global cognitive score | −0.003 (−0.02, 0.02) | −0.002 (−0.02, 0.02) |
| verbal memory composite score | −0.003 (−0.03, 0.02) | −0.002 (−0.02, 0.02) |
| verbal fluency score | −0.006 (−0.14, 0.13) | 0.002 (−0.14, 0.15) |
| TICS score | −0.06 (−0.14, 0.02) | −0.05 (−0.14, 0.03) |
| eGFR < 60 ml/min per 1.73 m2 (cystatin C)b (n=665) | ||
| global cognitive score | 0.004 (−0.02, 0.02) | 0.0005 (−0.02, 0.02) |
| verbal memory composite score | 0.004 (−0.02, 0.03) | −0.001 (−0.03, 0.02) |
| verbal fluency score | 0.03 (−0.12, 0.17) | 0.01 (−0.004, 0.004) |
| TICS score | −0.03 (−0.12, 0.06) | −0.02 (−0.11, 0.07) |
| eGFR < 60 ml/min per 1.73 m2 (PCr + cystatin C)b (n=329) | ||
| global cognitive score | −0.01 (−0.03, 0.008) | −0.02 (−0.04, 0.01) |
| verbal memory composite score | −0.02 (−0.04, 0.01) | −0.02 (−0.05, 0.01) |
| verbal fluency score | 0.02 (−0.14, 0.18) | −0.01 (−0.18, 0.16) |
| TICS score | −0.03 (−0.10, 0.04) | −0.04 (−0.14, 0.05) |
95% CI, 95% confidence interval; eGFR, estimated GFR; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration equation; TICS, telephone interview for cognitive status; PCr, plasma creatinine.
Adjusted for age, hypertension, body mass index, diabetes, cigarette smoking, cardiovascular disease, alcohol intake, physical activity (METs/wk), high cholesterol, participant education level, spouse’s education level, and postmenopausal hormone use.
Cystatin C was measured in 1110 participants.
Table 3.
Mean differences in annual rate of cognitive decline for an ACR ≥5 mg/g and ≥10 mg/g compared with lower levels
| Age-Adjusted Only (95% CI) | Multivariable Adjusteda (95% CI) | |
|---|---|---|
| ACR ≥5 mg/g (n=440) | ||
| global cognitive score | −0.03 (−0.05, −0.01) | −0.03 (−0.05, −0.01) |
| verbal memory composite score | −0.03 (−0.05, −0.004) | −0.02 (−0.05, −0.001) |
| verbal fluency score | −0.18 (−0.31, −0.04) | −0.19 (−0.34, −0.05) |
| TICS score | −0.09 (−0.16, −0.009) | −0.09 (−0.17, −0.004) |
| ACR ≥10 mg/g (n=228) | ||
| global cognitive score | −0.03 (−0.05, −0.009) | −0.04 (−0.06, −0.01) |
| verbal memory composite score | −0.02 (−0.05, 0.005) | −0.02 (−0.05, 0.01) |
| verbal fluency score | −0.16 (−0.33, 0.02) | −0.19 (−0.38, −0.01) |
| TICS score | −0.13 (−0.23, −0.03) | −0.13 (−0.24, −0.02) |
95% CI, 95% confidence interval; ACR, urinary albumin/creatinine ratio; TICS, telephone interview for cognitive status.
Adjusted for age, hypertension, body mass index, diabetes, cigarette smoking, cardiovascular disease, alcohol intake, physical activity (METS/wk), high cholesterol, participant education level, spouse’s education level, and postmenopausal hormone use.
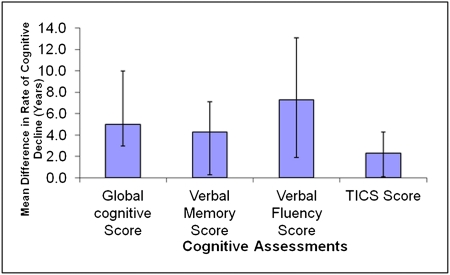
For ease of interpretation, we used the rate of cognitive decline associated with 1 year of aging in this cohort (data shown above) as a metric for understanding the apparent effects of ACR level on cognitive decline. The difference in the rate of cognitive decline with an ACR ≥5 mg/g versus an ACR ≤5 mg/g was equivalent to that seen with 2–7 years of aging; that is, a higher ACR accelerated cognitive decline such that the decline was 2–7 times faster than that associated with 1 year of aging alone (Figure 1).
Figure 1.
Mean difference in the rate of cognitive function decline for women with an ACR ≥5 mg/g versus women with an ACR <5 mg/g (with 95% confidence interval) standardized to rate seen with per-year aging alone. ACR, albumin/creatinine ratio; TICS, Telephone Interview for Cognitive Status.
In sensitivity analyses, we used the data from all four waves of cognitive function testing; results were not meaningfully changed for either eGFR or albuminuria, although participation was lower in the fourth wave and thus there were fewer data available. In alternate analyses of 1068 women after excluding women with diabetes, we did not observe meaningful changes in any of our results (i.e., an ACR ≥5 mg/g was associated with a multivariable-adjusted decline of −0.02 [95% CI, −0.04 to −0.004] in the global cognitive score). We also analyzed women without hypertension (n=873) and found consistent results (annual global cognitive score decline −0.03 [95% CI, −0.06 to −0.01]). Lastly, including an eGFR <60 ml/min per 1.73 m2 (using plasma creatinine alone or plasma creatinine plus cystatin C estimations) in models with an ACR ≥5 mg/g or ≥10 mg/g did not affect the associations with ACR.
Discussion
This study reports that albuminuria, even at levels not traditionally considered clinically significant, is independently associated with worse cognitive decline. Interestingly, the effect of an ACR ≥5 mg/g was not influenced by adjustment for multiple covariates, suggesting that it may be a useful measure for risk stratification in isolation. The independent association of an ACR ≥5 mg/g observed in this study require confirmation in further studies; however, the public health effect of our findings could be substantial because estimates from the 1999–2004 National Health and Nutrition Examination Survey data suggest that up to 19% of individuals aged ≥60 years in the United States have microalbuminuria (25). The prevalence of an ACR ≥5 mg/g in population studies has not been routinely reported, but will certainly be higher than for microalbuminuria (ACR 30–300 mg/g). The associations between an ACR ≥10 mg/g and cognitive decline were similar to those observed with an ACR >5 mg/g, except the result for verbal memory composite score was not significant presumably because of reduced statistical power due to the smaller number of participants with an ACR ≥10 mg/g.
There is currently limited literature on the relation between albuminuria, eGFR, and cognitive function. In the recently published Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial and the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease of 28,384 participants with diabetes or vascular disease, Brazilay et al. (26) found that microalbuminuria was modestly associated with a rapid decline in MMSE scores over 5 years of follow-up (odds ratio [OR], 1.22; 95% CI, 1.07–1.38). Jassal et al. (27) also reported that an ACR of ≥30 mg/g was independently associated with more rapid decline in MMSE and category fluency scores in men, but not in women, in a prospective analysis of 759 participants from southern California. Consistent with these prospective studies, Weiner et al. (28) performed a cross-sectional study of 335 participants from New England and reported that individuals were more likely to be in the lowest tertile of executive functioning (OR, 1.18; 95% CI, 1.06–1.32) per every doubling of ACR. Similarly, an analysis of the Cardiovascular Health Study reported that each doubling of albuminuria was significantly associated with increased odds of incident dementia (OR, 1.22; 95% CI, 1.15 –1.29) (29).
Using a large sample of generally healthy participants and a prospective design, our study adds to the growing evidence that albuminuria is an important early predictor of cognitive decline. Higher levels of albuminuria are likely an early marker of diffuse vascular endothelial cell dysfunction (30), as supported by the observation that microalbuminuria is a strong independent predictor of cardiovascular death in patients with and without diabetes (31). Moreover, because microalbuminuria is linearly associated with high-grade white matter hyperintensity changes on brain magnetic resonance imaging (28), it is likely that albuminuria reflects diffuse vascular endothelial dysfunction, which, in turn, may result in cognitive impairment.
Our study did not show a significant association between moderate CKD, defined as an eGFR <60 ml/min per 1.73 m2, and cognitive decline. In contrast to albuminuria, which may primarily reflect glomerular (vascular) dysfunction, moderately reduced eGFR may also result from nonvascular intrarenal processes including tubular and interstitial fibrosis, which may explain the lack of association between moderate CKD and cognitive decline. Consistent with our study, Weiner et al. (28) did not find an eGFR of <60 ml/min per 1.73 m2 to be associated with poor cognitive performance in older community-dwelling adults (mean age 73.4 years). Similarly, Slinin et al. (32) did not find an association between an eGFR <60 ml/min per 1.73 m2 and cognitive decline over 4 years of follow-up in 3722 men aged ≥65 years and participating in the Osteoporotic Fractures in Men Study. In contrast, a prospective analysis of 2172 participants reported that a creatinine clearance (CrCl) <60 ml/min per 1.73 m2 was associated with a rapid annual decline in TICS score compared with participants with a CrCl ≥90 ml/min per 1.73 m2 (P<0.001) (33). This study population was, however, quite different from our study because it included 59% Hispanics and 20% blacks. The largest cross-sectional study to date by Kurella et al. (34) included 23,405 adults enrolled in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort and reported significantly increased odds of cognitive impairment (OR, 1.11; 95% CI, 1.04–1.19) with each 10 ml/min per 1.73 m2 lower eGFR <60 ml/min per 1.73 m2. The REGARDS cohort had a relatively high prevalence of stroke (18.6%), coronary artery disease (24.9%), diabetes (33%), and obesity (41.7%). The cohort was cross-sectional in design with participants who were demographically and clinically different from those in the NHS; thus, it may not be directly comparable to our results.
A number of limitations in this study deserve mention. First, the study results may not be generalizable to men or different ethnic groups, because the study population was composed almost entirely of older Caucasian women. Second, we measured albuminuria using only a single spot urine sample; however, it is likely that this would bias our results toward the null because repeat testing generally reveals substantial intraperson variation in albuminuria on subsequent assessments (35,36). In clinical practice, however, risk stratification is often based on a single assessment and previous studies have shown that even if one of three random urine tests shows the presence of albuminuria, it may increase the risk for overall morbidity (37). Third, we do not have detailed data on glycemic control in these study participants. Finally, telephone-based cognitive testing cannot assess all cognitive domains (e.g., visuospatial memory), and ours has a focus on verbal memory; however, verbal memory is the best predictor of Alzheimer’s disease, which is the primary cause of dementia in the United States, and thus is particularly clinically relevant.
Our study has unique strengths. Many prior studies examining the association between albuminuria with cognitive function were cross-sectional, making it difficult to establish temporal relations. Previous studies have not reported results for levels of albuminuria below the traditional microalbuminuria cut-off (ACR 30 mg/g). In addition, we used a large battery of tests to assess cognitive function, allowing us to study the association of albuminuria with cognitive domains, including one that partially assesses executive function.
In summary, a urinary ACR ≥5 mg/g, a level not traditionally considered clinically significant, was independently associated with more rapid decline in cognitive functioning over 4–6 years of follow-up in older community-dwelling women. A ACR ≥5 mg/g may be important as a prognostic marker for subsequent cognitive decline.
Disclosures
None.
Acknowledgments
We thank Gideon Aweh for statistical programming support for this project.
This work was supported by the National Institutes of Health Grants R01 DK078202 (to J.L.), R01 AG015424 (to F.G.), and R01 CA087969 (parent NHS grant).
A portion of this work was given as an oral presentation at the annual meeting of the American Society of Nephrology, Denver, Colorado, November 18–22, 2010.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Albuminuria and Cognitive Impairment,” on pages 376–378.
References
- 1.Stevens LA, Li S, Wang C, Huang C, Becker BN, Bomback AS, Brown WW, Burrows NR, Jurkovitz CT, McFarlane SI, Norris KC, Shlipak M, Whaley-Connell AT, Chen S-C, Bakris GL, McCullough PA: Prevalence of CKD and comorbid illness in elderly patients in the United States: Results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 55[Suppl 2]: S23–S33, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP: Cognitive impairment and 7-year mortality in dialysis patients. Am J Kidney Dis 56: 693–703, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Agganis BT, Weiner DE, Giang LM, Scott T, Tighiouart H, Griffith JL, Sarnak MJ: Depression and cognitive function in maintenance hemodialysis patients. Am J Kidney Dis 56: 704–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McClellan WM, Langston RD, Presley R: Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J Am Soc Nephrol 15: 1912–1919, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S; HOPE Study Investigators: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Knopman DS: Invited commentary: Albuminuria and microvascular disease of the brain—a shared pathophysiology. Am J Epidemiol 171: 287–289, author reply 290–291, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalaria RN: Vascular factors in Alzheimer’s disease. Int Psychogeriatr 15[Suppl 1]: 47–52, 2003 [DOI] [PubMed] [Google Scholar]
- 8.McCullough PA, Steigerwalt S, Tolia K, Chen S-C, Li S, Norris KC, Whaley-Connell A: Cardiovascular Disease in Chronic Kidney Disease: Data from the Kidney Early Evaluation Program (KEEP). Curr Diab Rep 11: 47–55, 2011 [DOI] [PMC free article] [PubMed]
- 9.Kuo H-K, Lin L-Y, Yu Y-H: Microalbuminuria is a negative correlate for cognitive function in older adults with peripheral arterial disease: Results from the U.S. National Health and Nutrition Examination Survey 1999-2002. J Intern Med 262: 562–570, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Vupputuri S, Shoham DA, Hogan SL, Kshirsagar AV: Microalbuminuria, peripheral artery disease, and cognitive function. Kidney Int 73: 341–346, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Kurella M, Chertow GM, Luan J, Yaffe K: Cognitive impairment in chronic kidney disease. J Am Geriatr Soc 52: 1863–1869, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, Smith GE, Hochhalter AK, Collins AJ, Kane RL: Cognitive impairment in hemodialysis patients is common. Neurology 67: 216–223, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Curhan GC: Kidney function decline and physical function in women. Nephrol Dial Transplant 23: 2827–2833, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang JH, Grodstein F: Postmenopausal hormone therapy, timing of initiation, APOE and cognitive decline [published online ahead of print November 29, 2010]. Neurobiol Aging doi: doi:10.1016/j.neurobiolaging.2010.10.007 [DOI] [PMC free article] [PubMed]
- 15.Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forman JP, Fisher NDL, Schopick EL, Curhan GC: Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol 19: 1983–1988, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo EH, Lee DY, Kim SG, Kim KW, Kim H, Kim BJ, Kim M-D, Kim SY, Kim YH, Kim J-L, Kim JW, Moon SW, Park JH, Ryu S-H, Yoon JC, Lee NJ, Lee CU, Jhoo JH, Choo LH, Woo JI: Validity of the telephone interview for cognitive status (TICS) and modified TICS (TICSm) for mild cognitive impairment (MCI) and dementia screening. Arch Gerontol Geriatr 52: e26–e30, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH: Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci 57: 167–178, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A: The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 44: 609–614, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J: Natural history of mild cognitive impairment in older persons. Neurology 59: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Hu FB, Willett WC, Colditz GA, Ascherio A, Speizer FE, Rosner B, Hennekens CH, Stampfer MJ: Prospective study of snoring and risk of hypertension in women. Am J Epidemiol 150: 806–816, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Fitzmaurice GM, Laird NM, Ware JH: Modelling the mean: Analyzing response profiles. In: Applied Longitudinal Analysis, Hoboken, NJ, John Wiley & Sons Inc, 2004, pp 103–139 [Google Scholar]
- 25.Castro AF, Coresh J: CKD surveillance using laboratory data from the population-based National Health and Nutrition Examination Survey (NHANES). Am J Kidney Dis 53[Suppl 3]: S46–S55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barzilay JI, Gao P, O’Donnell M, Mann JFE, Anderson C, Fagard R, Probstfield J, Dagenais GR, Teo K, Yusuf S; ONTARGET and TRANSCEND Investigators: Albuminuria and decline in cognitive function: The ONTARGET/TRANSCEND studies. Arch Intern Med 171: 142–150, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Jassal SK, Kritz-Silverstein D, Barrett-Connor E: A prospective study of albuminuria and cognitive function in older adults: The Rancho Bernardo study. Am J Epidemiol 171: 277–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiner DE, Bartolomei K, Scott T, Price LL, Griffith JL, Rosenberg I, Levey AS, Folstein MF, Sarnak MJ: Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis 53: 438–447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barzilay JI, Fitzpatrick AL, Luchsinger J, Yasar S, Bernick C, Jenny NS, Kuller LH: Albuminuria and dementia in the elderly: A community study. Am J Kidney Dis 52: 216–226, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A: Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 32: 219–226, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S: Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 286: 421–426, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Slinin Y, Paudel ML, Ishani A, Taylor BC, Yaffe K, Murray AM, Fink HA, Orwoll ES, Cummings SR, Barrett-Connor E, Jassal S, Ensrud KE; Osteoporotic Fractures in Men Study Group: Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc 56: 2082–2088, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khatri M, Nickolas T, Moon YP, Paik MC, Rundek T, Elkind MSV, Sacco RL, Wright CB: CKD associates with cognitive decline. J Am Soc Nephrol 20: 2427–2432, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, Allman RM, Warnock DG, McClellan W: Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 52: 227–234, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 36.de Jong PE, Hillege HL, Pinto-Sietsma SJ, de Zeeuw D: Screening for microalbuminuria in the general population: A tool to detect subjects at risk for progressive renal failure in an early phase? Nephrol Dial Transplant 18: 10–13, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Romundstad S, Holmen J, Kvenild K, Hallan H, Ellekjaer H: Microalbuminuria and all-cause mortality in 2,089 apparently healthy individuals: A 4.4-year follow-up study. The Nord-Trøndelag Health Study (HUNT), Norway. Am J Kidney Dis 42: 466–473, 2003 [DOI] [PubMed] [Google Scholar]