Abstract
Aging and early-stage Alzheimer disease (AD) have been shown to be associated with increased RT intraindividual variability (IIV, as reflected by the coefficient of variation) and an exaggeration of the slow tail of the RT distribution in attentional control tasks, based on ex-Gaussian analyses. The current study examined associations between white matter volume, IIV, and ex-Gaussian RT distribution parameters in cognitively normal aging and early-stage AD. Three RT attention tasks (Stroop, Simon, and a consonant-vowel odd-even switching task) in conjunction with MRI-based measures of cerebral and regional white matter volume were obtained in 133 cognitively normal and 33 early-stage AD individuals. Larger volumes were associated with less IIV and less slowing in the tail of the RT distribution, and larger cerebral and inferior parietal white matter volumes were associated with faster modal reaction time. Collectively, these results support a role of white matter integrity in IIV and distributional skewing, and are consistent with the hypothesis that IIV and RT distributional skewing are sensitive to breakdowns in executive control processes in normal and pathological aging.
Keywords: mu, sigma, tau, coefficient of variation, default network, attentional control
1. Introduction
An important challenge in the cognitive neuroscience of aging involves understanding the associations between cognitive and structural changes in the aging brain (Raz, 2004; Raz & Rodrigue, 2006) and distinguishing these changes in the context of normal and pathological aging (Johnson et al., 2009). Recent work suggests that examination of white matter integrity and potentially more sensitive metrics of cognitive performance beyond mean reaction time may facilitate understanding of brain-behavior associations in aging (Gunning-Dixon & Raz, 2000; Johnson et al., 2010; MacDonald, Li, & Bäckman, 2009; MacDonald, Nyberg, & Bäckman, 2006). The present paper focuses on the association between regional white matter volume and both cognitive intraindividual variability (IIV) and reaction time distribution parameters in cognitively normal and pathological aging.
White matter integrity has been shown to be compromised in both healthy aging and early-stage Alzheimer disease (AD; Gunning-Dixon et al., 2009; Johnson et al., 2010). First, consider the evidence regarding healthy aging. This work has revealed more anterior declines in white matter in healthy aging that may accelerate with time (e.g., Bartzokis et al., 2003; Gunning-Dixon et al., 2009; Head et al., 2004; Salat et al., 2009). Furthermore, altered regulation of functional activity in frontal regions may be tied to white matter integrity in aging (see Grady, 2008, for a review). Additionally, the importance of white matter integrity is revealed in its association with reduced functional connectivity of brain regions in aging. For example, Andrews-Hanna et al. (2007) reported a positive association between reduced functional connectivity and reduced white matter integrity, even after controlling for the influence of age. Associations between white matter integrity and cognitive performance have also been reported in the aging literature (Gunning-Dixon et al., 2009; Raz et al., 2008; Raz, Rodrigue, Kennedy, & Acker, 2007).
Turning to pathological aging, there may be a differential vulnerability in more posterior regions in AD (Head et al., 2004; Kavcic et al., 2008; Salat et al., 2009). Recently, Bartzokis (2009) has suggested that AD may begin with compromised myelin. This is supported by evidence demonstrating strong links between hippocampal atrophy, an early region of decline in AD, and atrophy of critical white matter regions (Villain et al., 2010; but see Bai et al., 2009). Furthermore, frontal, temporal, corpus callosal, and parietal white matter integrity have been associated with memory and executive function in AD (Amar et al., 1996; Anstey et al., 2007; Huang & Auchus, 2007; Kavcic et al., 2008).
Although Rabbitt (1966) early on noted the importance of RT variability in the study of aging, there has been a burgeoning interest in this topic in the recent literature (see Hultsch, Strauss, Hunter, & MacDonald, 2008, for a review of variability in aging). Typically, examinations of cognitive functioning tend to rely on mean reaction times (RT), a measure of central tendency that assumes stability within individuals or groups. Treating the mean as the dependent measure relegates RT variability to random noise, ignoring potentially systematic variations that may lead to a better understanding of cognitive and neurobiological processes. For purposes of the present paper, we focus on inconsistency, a type of IIV indicating fluctuations in RT task performance over very short intervals (i.e., variability on a trial-to-trial level). One needs to be concerned that the variability is above and beyond the increase in overall mean performance, since there is a strong relation between mean performance and SDs. Thus, researchers have turned to the coefficient of variation (CoV; SD/mean), which is emerging as a standard measure of inconsistency, due to its low bias from mean reaction time, relatively easy calculation, and high association with other measures of inconsistency (Hultsch et al., 2000).
There is accumulating evidence indicating that aging is associated with increased IIV (Anstey, 1999; Duchek et al, 2009; Hultsch et al., 2002, 2008; Nesselrode & Salthouse, 2004), with increased variability being linked to poorer cognitive performance (Hultsch & MacDonald, 2004). The increased variability appears to be particularly strong in more complex, attention-demanding tasks (Bielak et al., 2010; Lövdén et al., 2007; MacDonald, Hultsch, & Dixon, 2003). Rabbitt, Osman, and Moore (2001) have argued that higher IIV indicates less effective behavioral and attentional control that may occur via increasingly inefficient neural and network activation, and this perspective is supported by neurocomputational models (Li et al., 2006; Li, von Oertzen, & Lindenberger, 2006). IIV has proven to be a useful marker of cognitive change, and can reliably distinguish between younger and older adults, even after correcting for age-related changes in central tendency (Bielak et al., 2010; Hultsch, Strauss, Hunter, & MacDonald, 2008). Interestingly, Duchek et al. (2009) and Dixon et al. (2007) reported that the CoV from cognitive attention tasks can also discriminate between healthy and pathological aging, and can even identify cognitively normal individuals at risk for developing dementia (i.e., carriers of an Apolipoprotein ε4 allele). Indeed, Duchek et al. (2009) suggested that the increased IIV may reflect impaired attentional control and executive functioning.
There is currently limited research on the association between white matter integrity and RT inconsistency, although the studies that have been reported have indicated significant associations. Higher IIV, as measured by standard deviation, has been linked to smaller cerebral white matter (Walhovd & Fjell, 2007) as well as prefrontal, temporal and parietal white matter (Ullén et al., 2008) volumes, and greater prefrontal white matter hyperintensities in healthy adults (Bunce et al., 2007). Associations have also been reported between volumetric estimates of the corpus callosum and IIV in individuals with mild cognitive disorders (Anstey et al., 2007). However, to our knowledge, this earlier research has not been extended to older adults. Only one past study (Walhovd & Fjell, 2007) has examined adults beyond 64 years of age and this study focused on total cerebral white matter volume, constraining generalizations about the relationships among aging, variability, and white matter integrity. Furthermore, none of the previous studies have examined this relationship in early-stage AD. Finally, previous studies have tended to use non-demanding cognitive tasks, such as visual oddball tasks (Walhovd & Fjell, 2007), simple RT tasks (Anstey et al., 2007), or isochronous tapping (Ullén et al., 2008). In the present study, the use of standard attentional control tasks as a basis for the calculation of RT IIV allowed us to draw more robust conclusions regarding the association between variability, control systems, and white matter integrity in older adults.
The importance of white matter integrity becomes increasingly important in light of recent work on brain networks. For example, connectivity in the default network, a group of frontal and parietal brain regions that become active and spontaneously correlate in the absence of an explicit task (Raichle et al., 2001), has been central in a number of recent studies of aging and early stage AD. Indeed, both aging and AD are associated with alterations of the default network (Andrews-Hanna et al., 2007; Bai et al., 2008; Damoiseaux et al., 2008; Grecius, Srivastava, Reiss, & Menon, 2004). Typically, the default network is anticorrelated with attentional networks, where one network is suppressed while the other is active. Kelly et al. (2008) reported that dysregulation of default and executive networks (i.e., a lack of strong anticorrelations) is associated with increased IIV. A breakdown in white matter integrity may disrupt both within and cross network correlations, thereby producing the observed relation reported in the Kelly et al. (2008) study.
There is a rich history showing that the characteristics of RT distributions are sensitive to various aspects of cognition (Luce, 1986; Ratcliff, 1978, 1979), such as attentional control and/or drift rate in diffusion models (Heathcote, Popiel, & Mewhort, 1991; Ratcliff, 1978, 1979; Spieler, Balota, & Faust, 1996, 2000; Tse et al., 2010). We have chosen to fit the Ex-Gaussian theoretical distribution to capture the characteristics of the RT distributions in the current data. The ex-Gaussian distribution reflects the convolution of a Gaussian and an exponential distribution. There are three parameters obtained from ex-Gaussian analyses: μ and σ, which capture the mean and standard deviation of the Gaussian portion, respectively; and τ, which reflects both the mean of the exponential portion of the distribution. Changes in μ reflect a shifting of the modal portion of the RT distribution, whereas changes in τ typically reflect an increase in the slow tail of the RT distribution. Critically, the algebraic sum of μ and τ equals the mean of the RT distribution, which enables one to make contact with the mean-dominated literature. The Ex-Gaussian distribution has been shown to fit RT data extremely well (Ratcliff, 1979), and has yielded interesting findings on the nature of human cognition (see Balota & Yap, 2011). Interestingly, it is possible to observe two RT distributions with equivalent means, but with opposing distributional parameters that cancel each other out, highlighting the importance of examining the properties of underlying reaction time distributions. For example, consider the congruency effect (the RT difference in naming the color of a congruent word, e.g., red presented in RED, and the color of a neutral word, e.g., deep presented in RED) in the classic Stroop task. This effect is quite small and sometimes nonsignificant at the level of the mean. However, Heathcote, Popiel, and Mewhort (1991) found that the relatively small congruency effect was due to the opposing influences of a shortening of the modal portion of the distribution (μ) in the congruent condition and a lengthening of the tail (τ) relative to the neutral condition (also see Spieler, Balota, & Faust, 2000), both of which would be undetected at the level of the mean.
More recently, distributional analyses have helped to clarify differences between healthy and pathological aging. For example, Tse et al. (2010) recently found that healthy aging had clear effects on both μ and τ in a set of attentional control tasks, whereas early stage AD only had an additional effect on τ. Balota et al. (2010) have recently shown that the slow tail of the RT distribution in the Stroop task may also be useful in predicting convergence from a cognitive normal state to early stage AD across a 12 year longitudinal follow up. Furthermore, τ has been shown to be more strongly associated with working memory measures than μ or σ (Schmiedek et al., 2007; Tse et al., 2010), and thus it is possible that τ may be more related to the white matter integrity that underlies executive/attentional control abilities (see Balota et al., 2010; Breteler et al., 1994; Grieve et al., 2007; Gunning-Dixon et al., 2009; Raz et al., 2008). To our knowledge, no studies have examined the relationship between the parameters of RT distributional analysis and regional volume.
The present study examines a) the relationship between RT IIV/distributional parameters and white matter volume in targeted brain regions, and b) whether there are differential behavioral-brain associations between healthy aging and early-stage AD. Specifically, given the evidence of associations between IIV and white matter integrity observed in younger adult samples using less-engaging cognitive tasks (Anstey et al., 2007; Bunce et al., 2007; Ullén et al., 2008; Walhovd & Fjell, 2007), and the disruption of the default mode network in healthy aging and early stage AD (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008), we examined the CoV and RT distributional parameters in attentional control tasks , and the relation between these estimates with cerebral white matter volume as well as targeted volumes in prefrontal and default-mode white matter regions. Moreover, because cognitive variability and distributional skewing may be particularly associated with frontal regions (see MacDonald, Li, & Bäckman, 2009; MacDonald, Nyberg, & Bäckman, 2006), those frontal regions important in executive function such as ventral/dorsolateral prefrontal cortex, the superior frontal gyrus, and anterior cingulate may show increased sensitivity to fluctuations in CoV and the exponential estimate from ex-Gaussian analyses. In addition, targeted default-mode regions such as the precuneus, posterior cingulate, and the inferior parietal lobule (see Raichle et al., 2001) may show an association with variability and/or distributional parameters because the anticorrelatons between the default and executive networks has also demonstrated sensitivity to IIV in younger adults (Kelly et al., 2008). Finally, we expected stronger associations between regional white matter volume and cognitive performance in early-stage AD, as there is increasing breakdowns in white matter integrity and IIV, consistent with recent work by Anstey and colleagues (2007), in which they observed a stronger relationship between white matter volume and RT variability in MCI patients.
2. Method
2.1 Participants
One hundred thirty-three cognitively normal individuals (87 females), aged 46 – 96 (M = 68.0; SD = 9.6) and 33 individuals with early-stage AD (17 females), aged 61 – 88 (M = 76.6; SD = 6.1) were recruited from the Knight Alzheimer's Disease Research Center at Washington University. Participants were classified based on the interview-based Clinical Dementia Rating scale (CDR; Berg, 1988), a validated measure highly effective in detecting the earliest stages of dementia (Morris, 1993), as cognitively normal (CDR = 0) or early-stage AD (CDR = 0.5, N = 25; CDR = 1, N = 8). The CDR staging and diagnostic evaluation is based on interviews with the participant and an informed collateral source. All participants were screened for neurological, psychiatric, or medical disorders with the potential to cause dementia. The inclusion and exclusion criteria for diagnosis of AD have been described in detail elsewhere (e.g., Morris, 1993; Morris, McKeel, Fulling, Torack, & Berg, 1988) and conform to those outlined in the criteria of the National Institute of Neurological and Communications Disorders and Stroke– Alzheimer's Disease and Related Disorders Association (McKhann et al., 1984). Dementia severity for each individual was staged in accordance with the Washington University Clinical Dementia Rating (CDR) Scale (Hughes, Berg, Danziger, Coben, & Martin, 1982; Morris, 1993). According to this scale, a CDR of 0 indicates no cognitive impairment, a CDR of 0.5 indicates very mild dementia, a CDR of 1.0 indicates mild dementia a CDR of 2.0 indicates moderate dementia, and a CDR 3.0 indicates severe dementia. At the Knight ADRC, a CDR of 0.5 has been found to accurately indicate the earliest stages of AD (Morris, McKeel, & Storandt, 1991). Both the reliability of the CDR and the validation of the diagnosis (based upon autopsy) by the research team have been excellent (93% diagnostic accuracy) and well-documented (e.g., Berg et al., 1998; Morris et al., 1996; Morris et al., 2001; Storandt et al., 2006). Exclusion criteria included lifetime history of neurological illness or injury (e.g., Parkinson's disease, stroke, seizure, or head trauma resulting in loss of consciousness). Socioeconomic status (SES) was obtained via the Hollingshead four-factor index of social position where 1 refers to high-privilege and 4 refers to low-privilege (Hollingshead, 1975). Hence, this well-characterized sample of early-stage AD individuals provides an excellent group to examine IIV and components of the RT distributions.
Participant demographic information is summarized in Table 1. This study was approved by the Human Research Protection Office at Washington University, and all participants provided their informed consent at the beginning of the study. A subset of these data were previously reported in Duchek et al. (2009) and Tse et al. (2010).
Table 1.
Demographic characteristics of the sample.
| Variable | Cognitively Normal (Mean (SD)) | Early-stage AD (Mean (SD)) |
|---|---|---|
| N | 133 | 33 |
| Age in years* | 68.0 (9.6) | 76.6 (6.5) |
| Gender (F/M) | 87/46 | 17/16 |
| Education in years* | 15.7 (2.7) | 14.6 (2.9) |
| Socioeconomic Status | 2.3 (1.1) | 1.9 (1.0) |
p < .05.
2.2 Cognitive testing
Details of the tasks and the estimates from CoV and ex-Gaussian parameters are presented in Duchek et al. (2009) and Tse et al. (2010).
2.2.1 Stroop task
This task involved the presentation of four color names (red, blue, green, and yellow) and four neutral words (bad, poor, deep, and legal). The task consisted of 36 congruent trials, during which each word appeared in its corresponding color; 36 incongruent trials, during which each word appeared in a nonmatching color; and 32 neutral trials, in which each of the four neutral words appeared in one of the four colors. Participants were instructed to read aloud each word's color.
2.2.2 Simon task
Participants were presented with an arrow pointing either left or right. The arrow would appear on the left half, right half, or center of the screen. Participants were told to ignore the arrow location on the screen and respond according to its direction by pressing a key on either the left (q key) or right side (p key) of the keyboard when the arrow was pointing left or right, respectively. The task consisted of 40 congruent trials, 40 incongruent trials, and 40 neutral trials, during which the arrow appeared at the center of the screen.
2.2.3 Consonant-Vowel Odd-Even (CVOE) switching task
Participants engaged in two different tasks across trials. On each trial, a letter–number pair (e.g., A 3) appeared at the center of the screen with a cue appearing at the top of the screen indicating if it is a letter or number trial (adopted from Minear & Shah, 2008). On a letter trial, participants were told to decide whether the letter was a consonant or vowel (CV). On a number trial, they were told to decide whether the number was odd or even (OE). Participants completed a mixed block of 60 trials in an alternate runs sequence, CV, CV, OE, OE, CV, CV, and so forth, with 30 switch trials (e.g., CV trial followed by OE trial) and 30 nonswitch trials (e.g., CV trial followed by CV trial). Before the switch trials, participants also completed a pure block of CV trials as well as a separate pure block of OE trials. Here we only report results from the mixed block of trials, which places the highest demand on attentional control systems.
2.2.4 Distributional and variability scores
For each of the three tasks, we obtained the ex-Gaussian parameters for each participant for all trials within a task using quantile maximum likelihood estimation procedure in QMPE 2.18 (Cousineau, Brown, & Heathcote, 2004; Heathcote, Brown, & Mewhort, 2002). This procedure provides unbiased parameter estimates and has been shown to be more effective than continuous maximum likelihood estimation for small samples (Heathcote & Brown, 2004; Speckman & Rouder, 2004). All fits successfully converged within 500 iterations.
2.2.5 Derivation of composite scores
As Tse et al. (2010) provided support from a confirmatory factor analysis that composite scores could be reliably created for each of the ex-Gaussian parameters using Stroop, Simon, and switching task performance (μ loadings ≥ .23, σ loadings ≥ .24, τ loadings ≥ .38, where all loadings from all tasks were significant), we calculated a composite μ, σ, τ, and CoV for each of the three attentional tasks. As in Tse et al., these composites were based on the means of the z-scores of each of the parameters based on the overall subject sample. Each Ex-Gaussian parameter and CoV composite variable was created by averaging the z-scores for each task.
2.3 MR image acquisition and processing
Imaging was performed using either a Siemens Vision 1.5T scanner (n = 38) or a Siemens Trio 3T scanner (n = 128). For all scans, cushions reduced head movement during scanning and a scout image was acquired first in order to center the field of view on the brain. For the Vision 1.5 scans, two to four T1-weighted sagittal MP-RAGE scans. (TR = 9.7 ms, TE = 4 ms, flip angle = 10°, TI = 20 ms, 1 × 1 × 1.25 mm resolution) were acquired for each subject. For the Trio 3T scans, two T1-weighted sagittal MP-RAGE scans (TR = 2400 ms, TE = 3.16 ms, flip angle = 8°, TI = 1000 ms, TD = 200 ms, 1 × 1 × 1 mm resolution) were acquired for each subject. The average interval between the MRI scan and the cognitive assessment was ± 8.1 months (SD = 7.1 months). Scanner type was included as a covariate in all analyses, although there were no significant differences in the proportions of gender (χ2 < 1) or CDR status (χ2 < 1), and there was not a significant difference in ICV (t = 1.35, p = .18) between scanner types.
2.4 Regional volumetry
Regional volumes were obtained using Freesurfer software (Desikan et al., 2006; Fischl, Salat et al., 2002; Fischl, van der Kouwe, et al., 2004). During processing, each voxel is assigned a neuroanatomical label based on probabilistic information derived from a manually labeled training set, which included healthy young and older adults. Previous work indicates that this technique generates volumes with a high correspondence to manually generated volumes (Desikan et al., 2006; Fischl, Salat et al., 2002; Fischl, van der Kouwe, et al., 2004). As there were no hypotheses regarding laterality effects, volumes were summed across hemispheres. Total intracranial volume (ICV) was used to adjust regional volumes for body size differences via a formula based on the analysis of covariance approach: Adjusted volume = raw volume – (b × (ICV – mean ICV)), where b is the slope of the regression of the ROI volume on ICV. This covariance approach has been frequently employed to correct for intracranial volume in the investigation of regional volumes (e.g., Buckner et al., 2004; Head et al., 2008; Jack et al., 1989; Mathalon et al., 1993; Raz et al., 2008; Salat et al., 2009). Adjusted regional volume was used as the dependent variable in analysis. White matter regions-of-interest (ROIs) included total cerebral white matter, superior frontal gyrus (SFG), ventral/dorsal-lateral prefrontal cortex (VL/DLPFC; combined middle and inferior frontal gyri), anterior cingulate, posterior cingulate, precuneus, and inferior parietal lobule (IPL). The primary visual cortex was selected as a control ROI.
Although there may be concerns with regard to biases in cross-scanner aggregation, there is evidence of reliability of Freesurfer-derived estimates of cortical thickness and volumes across scanner upgrades, different manufacturers, and number of MP-RAGE acquisitions (e.g., Fennema-Notestine et al., 2007; Han et al., 2006; Jovicich et al., 2009) and cross-scanner aggregation has been successfully used previously (e.g., Desikan et al., 2009; McEvoy et al., 2009; Storandt et al., 2009). Also, as noted above, scanner type was included as a covariate in all analyses.
2.5 Analytic approach
2.5.1 Outliers
The data were first examined for univariate and multivariate outliers. Although no individuals had outliers across multiple regions (as calculated by the Mahalanobis D2; Lattin, Carroll, & Green, 2003), 5 participants had one data point far outside the normal range (i.e., greater than 3 SD). These values (representing 0.12% percent of the total dataset) were considered missing and replaced using regression imputation via likelihood estimation using the Expectation-Maximization algorithm, which replaces the missing data point based on the values of all other variables (i.e., all attention and regional brain volume data).
2.5.2 Covariates
To determine the necessity of inclusion of SES, gender, education, cardiovascular health (i.e., history of heart disease or hypertension), diabetes diagnosis, or depression as covariates in the analyses, we examined the zero-order correlations (or t-tests, in the case of dichotomous variables) between these variables and the predictor and outcome variables (i.e., age, CDR status, ROI volume, σ,μ, τ, CoV). Education, depression and cardiovascular health showed significant associations with some predictors or outcomes and thus we conservatively included these variables as covariates in all analyses. Gender, SES, and diabetes were not significantly associated with any predictors or outcomes (all ps > .085); however, due to some tendency of gender imbalance across groups, gender was included as a covariate in all analyses to be conservative. SES and diabetes were not included in regression models. The scan-cognitive assessment interval was also entered as an additional covariate in all analyses.
2.5.3 Statistical analyses
We next performed a series of hierarchical linear regression analyses to address our primary questions regarding the effects of age, CDR status (i.e., CDR=0 or CDR>0), ROI, and the interaction of these variables on the composite measures of CoV, μ, σ, and τ. Age, ROI, and any continuous covariates were standardized, and the standardized variables were multiplied to create the interaction terms. In each regression model, covariates (i.e., standardized years of education, gender, scan-cognitive assessment interval, scanner type, cardiovascular health, and depression) were entered first, followed by standardized age in the second step, CDR status in the third step, standardized ROI volume in the fourth step, the CDR status × ROI interaction in the fifth step, and finally the Age × CDR status and Age × ROI twoway interaction terms were included in the sixth step. In this way, significant interaction terms contribute model variance above and beyond the effects of individual variables alone. We did not examine higher-order interactions as cell counts created by the crossing of more than two variables were too low for reliable estimates to be made. Separate regression models were created using each cognitive RT measure as a DV with each of the ROIs as an independent variable, and an alpha of .05 was set to indicate significance.
3. Results
3.1 CoV
Descriptive statistics for cognitive and brain structural measures are summarized in Table 2. Age accounted for a significant amount of variance in CoV (ΔR2 = .12, F(1, 158) = 23.04, p <.001), such that older individuals had larger CoV scores. CDR status was also a significant predictor of the CoV composite (ΔR2 = .19, F(1, 157) = 46.79, p < .001), such that early-stage AD individuals had larger CoV composites than cognitively normal individuals.
Table 2.
Descriptive statistics for cognitive and brain structural measures.
| Measure | Cognitively Normal (Mean (SD)) | Early-stage AD (Mean (SD)) |
|---|---|---|
| Cognitive Performance Composites | ||
| Working memory* | 0.16 (0.7) | -0.70 (0.8) |
| Coefficient of variation* (SD/M) | -0.20 (0.5) | 0.80 (0.9) |
| Ex-Gaussian parameters | ||
| μ | -0.04 (0.7) | 0.18 (0.9) |
| σ * | -0.05 (0.5) | 0.27 (0.7) |
| τ * | -0.24 (0.5) | 0.96 (0.9) |
| White Matter Volumes (cm3) | ||
| Total cerebral white matter* | 459.2 (37.0) | 427.5 (39.3) |
| Ventro-/dorsolateral prefrontal cortex* | 27.1 (3.1) | 24.7 (2.9) |
| Superior frontal gyrus* | 32.8 (4.3) | 29.7 (4.2) |
| Anterior cingulate | 7.6 (0.7) | 7.4 (0.8) |
| Posterior cingulate* | 7.9 (0.7) | 7.5 (0.7) |
| Precuneus* | 18.0 (1.8) | 17.0 (2.2) |
| Inferior parietal lobule* | 36.3 (3.5) | 34.5 (3.6) |
| Primary visual cortex* | 4.1 (0.8) | 3.7 (0.8) |
p < .05.
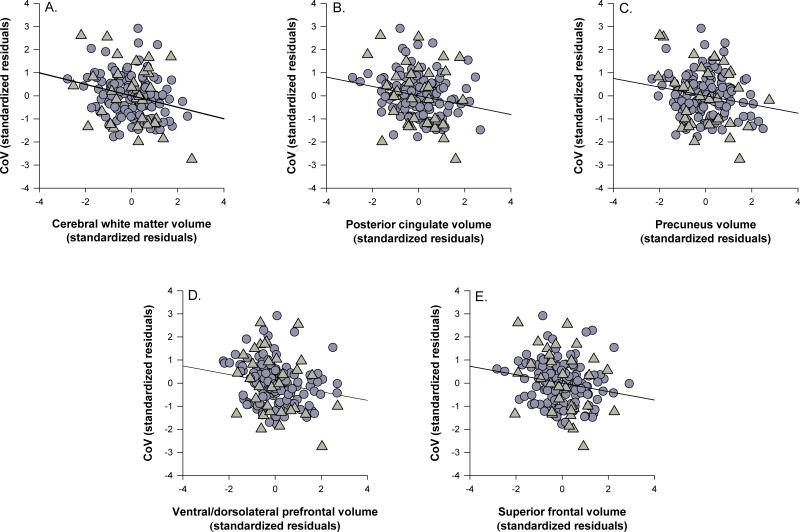
As shown in Figure 1, after controlling for age and CDR status, among the other covariates, larger volumes were associated with a smaller CoV composite for cerebral white matter (Fig. 1a, ΔR2 = .04, F(1, 156) = 10.26, p = .002), posterior cingulate (Fig. 1b, ΔR2 = .03, F(1, 156) = 6.73, p = .010), precuneus (Fig. 1c, ΔR2 = .02, F(1, 156) = 5.71, p = .018), VL/DLPFC (Fig. 1d, ΔR2 = .03, F(1, 156) = 7.78, p = .006), and SFG (Fig. 1e, ΔR2 = .02, F(1, 156) = 5.40, p = .021), but there were no significant main effects of anterior cingulate, IPL, or primary visual cortex volumes (all ps > .21). No significant interactions were observed (all ps > .09).
Figure 1.
Associations between global and regional volumes and the coefficient of variation. A) cerebral white matter, B) posterior cingulate, C) precuneus, D) ventral/dorsolateral prefrontal, E) superior frontal gyrus. Triangles indicate participants with early-stage Alzheimer's and circles indicate healthy older adults. Data are standardized residuals controlling for age, education, gender, scanner type, cardiovascular health, depression, CDR status, and interval between date of scan and date of cognitive assessment.
3.2 Mu
Age accounted for a significant amount of variance in μ (ΔR2 = .08, F(1, 158) = 14.73, p < .001), such that older individuals had larger μ scores. CDR status was not a significant predictor of μ (F < 1).
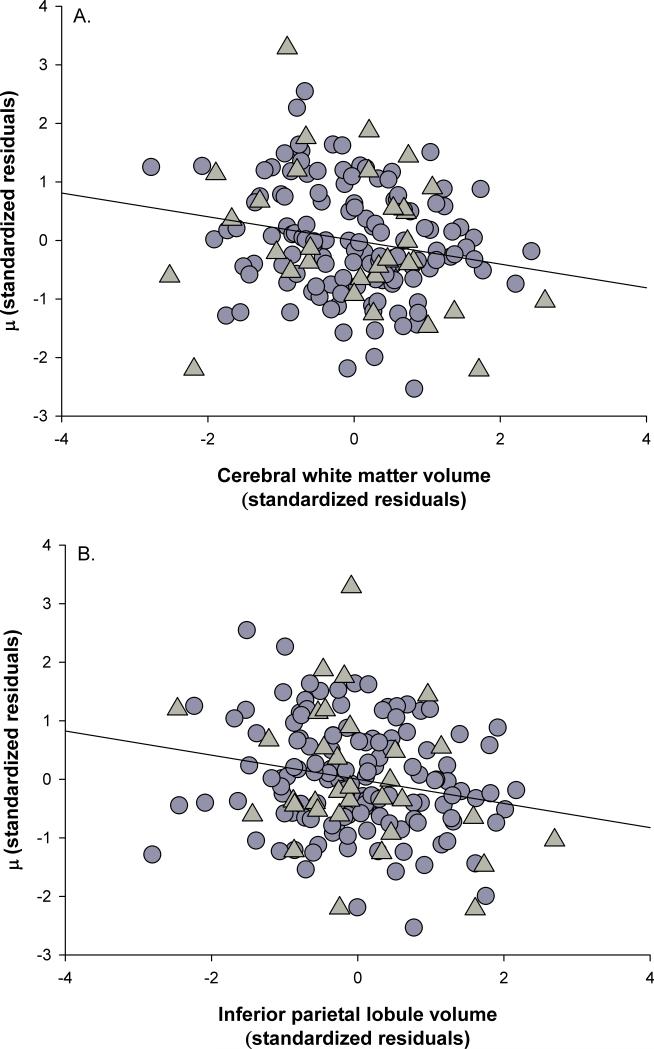
As shown in Figure 2, larger volumes were associated with smaller μ composites for cerebral white matter (Fig. 2a; ΔR2 = .04, F(1, 156) = 6.66, p = .011) and IPL volumes (Fig. 2b; ΔR2 = .04, F(1, 156) = 6.93, p = .009), but there were no significant main effects of VL/DLPFC, SFG, anterior cingulate, posterior cingulate, precuneus, or primary visual cortex volumes (all ps > .30). There were no significant two-way interactions for any of the brain regions in the analyses with μ (all ps > .11).
Figure 2.
Associations between global and regional volumes and μ. A) cerebral white matter, B) inferior parietal lobule. Triangles indicate participants with early-stage Alzheimer's and circles indicate healthy older adults. Data are standardized residuals controlling for age, education, gender, scanner type, cardiovascular health, depression, CDR status, and interval between date of scan and date of cognitive assessment.
3.3 Sigma
Neither age (F(1, 158) = 3.18, p = .077) nor CDR status (F(1, 157) = 3.00, p = .085) accounted for a significant amount of variance in σ. In addition, no other significant main effects or interactions involving any ROIs were observed (all ps > .07).
3.4 Tau
Significant results for τ are summarized in Figure 3. Age accounted for a significant amount of variance in τ (ΔR2 = .19, F(1, 158) = 38.37, p < .001), such that older individuals had larger τ scores. In contrast to μ, CDR status was a significant predictor of the τ composite (ΔR2 = .23, F(1, 157) = 67.95, p < .001), such that early-stage AD individuals had larger τ composites than cognitively normal individuals.
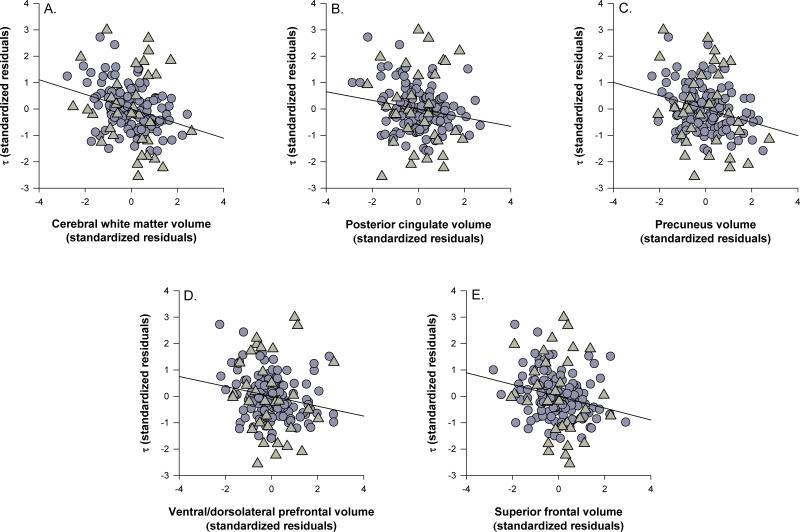
Figure 3.
Associations between global and regional volumes and τ. A) cerebral white matter, B) Posterior cingulate, C) precuneus, D) ventral/dorsolateral prefrontal, E) superior frontal gyrus. Triangles indicate participants with early-stage Alzheimer's and circles indicate healthy older adults. Data are standardized residuals controlling for age, education, gender, scanner type, cardiovascular health, depression, CDR status, and interval between date of scan and date of cognitive assessment.
As shown in Figure 3, larger volumes were associated with a smaller τ composite for cerebral white matter (Fig. 3a, ΔR2 = .04, F(1, 156) = 12.77, p < .001), posterior cingulate (Fig. 3b, ΔR2 = .02, F(1, 156) = 5.35, p = .022), precuneus (Fig. 3c, ΔR2 = .04, F(1, 156) = 11.52, p = .001), VL/DLPFC (Fig. 3d, ΔR2 = .02, F(1, 156) = 5.77, p = .018), and SFG (Fig. 3e, ΔR2 = .02, F(1, 156) = 6.31, p = .013), but there were no significant main effects of inferior parietal lobule, anterior cingulate or primary visual cortex volumes (all ps > .06). There were no significant twoway interactions for any of the brain regions in the analyses with τ (all ps > .053).
The analyses of the τ parameter also yielded a significant interaction between age and CDR status for PVC (p = .045), such that there was a significant relationship between age and τ in the early-stage AD group (r(33) = .411, p = .018) whereas the relationship was not significant in the cognitively normal group (r(133) = -.136, p = .12). No other significant interactions were observed (all ps > .053).
4. Discussion
Recent literature has established important links between cognitive performance and brain structure in healthy and pathological aging, including an emphasis on white matter integrity in executive and default network regions (e.g., Anstey et al., 2007; Bartzokis, 2004; Bartzokis et al., 2003; Bielak et al., 2010; Raz et al., 2008). The current study demonstrated a robust relationship between RT intraindividual variability (IIV) and total cerebral and regional white matter volumes in both healthy older adults and in individuals with early-stage AD. Although expected associations with anterior cingulate did not occur, the general lack of associations with primary visual cortex demonstrated that observed effects are largely restricted to regions important in higher-order cognitive processing. Overall, the present study demonstrates the utility of IIV measures and reaction time distributional analysis in identifying neuroanatomical correlates of cognitive performance in healthy aging and early-stage AD.
4.1 Associations between IIV and white matter volume
The coefficient of variation (CoV) measure proved to be robust in its associations with total cerebral white matter and frontal regions as well as with the posterior cingulate and precuneus. White matter integrity in frontal and parietal regions has been implicated in tasks of attentional control and executive function (Grieve et al., 2007; see Gunning-Dixon et al., 2009, for a review). The present results extend these findings to cognitive inconsistency, a suggested indicator of executive/attentional control function (e.g., Duchek et al., 2009; MacDonald, Li, & Bäckman, 2009; MacDonald, Nyberg, & Bäckman, 2006).
4.1.1 Frontal regions
The significant associations between CoV and VL/DLPFC and SFG are consistent with past studies that have found associations between IIV and frontal regions (Bunce et al., 2007; Murtha, Cismaru, Waechter, & Chertkow, 2002; but see Ullén et al., 2008). Furthermore, the current study supports the idea that disruption of associative pathways in frontal regions as well as across the entire brain may underlie increased IIV (MacDonald, Li, & Bäckman, 2009), and that RT variability may reflect frontally-mediated lapses of attention or fluctuations in executive function (Anstey, 1999; Anstey et al., 2007; Bunce et al., 2007; Bunce, Warr, & Cochrane, 1993). Given that previous research focuses on younger adults, the current study represents a critical extension of the literature to aging and early-stage AD samples.
4.1.2 Default network regions
The findings observed for the posterior cingulate and precuneus extend previous studies of IIV (Ullén et al., 2008), and indicate that regions outside of the frontal lobe must also be considered with regard to cognitive performance and variability. The precuneus has been implicated in self-referential processing (e.g., Cavanna & Trimble, 2006) and one possible explanation of the association with IIV observed here may indicate mind-wandering or other task-unrelated thought across trials in the present attentional control tasks, which leads to increased cross-trial RT inconsistency (Carriere, Cheyne, Solman, & Smilek, 2010; Cheyne, Solman, Carriere, & Smilek, 2009). Anterior and posterior cingulate have also been implicated in self-referential processing (Johnson et al., 2006), and the association between IIV and posterior cingulate may also be indicative of self-directed thought. Of course, the impact of white matter changes on variability beyond frontal regions has not been well-explored. Therefore, these results need to be replicated and non-frontal regions should be further examined to understand the influence of additional regions on variability.
4.1.3 Early-stage AD
Because frontal white matter regions are compromised in early AD (Bartzokis, 2009; Bartzokis et al., 2003) and increases in τ are observed in early stage AD (Tse et al., 2010), τ may serve as a sensitive marker of early white matter decline, particularly as cognitive reserve is depleted (Stern 2002, 2003). However, in contrast to our initial hypotheses based upon the work of Anstey et al. (2007), we did not observe stronger relationships between volume and CoV in the early-stage AD group. Although previous studies have postulated greater cognitive reserve on the part of healthy older adults as a mechanism for group differences in the volume-variability relationship, our specific early-stage AD group may have had sufficient cognitive reserve to compensate for compromised neural structures. Therefore, the relationship between CoV and regional volume may be stronger as the disease pathology advances in the early-stage AD group.
Interestingly, the age-τ association was stronger in the early-stage AD group relative to the cognitively normal older adults for primary visual cortex volume. This pattern of greater decline in τ with advancing calendar age may reflect the combination of age and disease processes in early-stage AD group. However, we did not predict this relationship nor an effect for this brain region a priori and future work should examine changes in τ longitudinally to better determine if there is indeed accelerated decline in τ in AD.
4.2 Associations between RT distributional parameters and white matter volume
Although the importance of characterizing reaction time distributions in understanding cognitive processes is well-documented (Balota & Yap, 2011; Balota et al., 2008; Heathcote, Mewhort, & Popiel, 1991; Spieler, Balota, & Faust, 1996; Tse et al., 2010), to our knowledge no studies to date have attempted to use these methods in exploring white matter correlates of cognitive processes in healthy and pathological aging (but see Madden et al., 1999 for associations with cerebral blood flow). Results showed that τ tracked with CoV in its association with white matter volumes in healthy aging and early-stage AD. Indeed, there was a strong correlation between τ and CoV in our sample (r (166) = .702, p < .001). It is noteworthy, however, that there was some tendency for associations to be somewhat larger for τ relative to CoV. Moreover, τ was also sensitive to the age × CDR interaction which demonstrated stronger age-related increases in τ for early-stage AD relative to cognitively normal individuals. In order to more directly address this, we conducted a post-hoc analysis on the standardized residuals (controlling for covariates, age, and CDR status) of τ and CoV using a one-tailed Steiger's Z test (Steiger, 1980) to determine whether τ was more strongly associated than CoV with the five ROIs that showed associations with τ and CoV (i.e., cerebral white matter, posterior cingulate, precuneus, VL/DLPFC, and SFG). Across all regions, τ was not significantly more sensitive in these regions than CoV (all zs < 1.1). Further research is needed to distinguish the utility of τ from CoV in healthy adults and early-stage AD.
The link between τ and frontal regions as well as posterior cingulate and the precuneus may relate to dysregulation in the anticorrelation of default and executive networks (Kelly et al., 2008), suggesting that a failure of attentional control is associated with impaired functional and structural connectivity. More broadly, the results seen in τ replicate and extend previous work linking distributional skewing to breakdowns in executive function and attentional control (Spieler, Balota, Faust, 1996; 2000). The present study also represents an important extension of the results of Tse et al. (2010), which strongly associated increased τ with reduced working memory capacity, and more firmly associates this impairment with prefrontal and parietal white matter regions.
In contrast,μ only revealed associations with cerebral white matter and IPL volume. Modal reductions tend to be more reflective of generalized decrements, such as general slowing due to aging (Tse et al., 2010), whereas τ may be more associated with impairments in dynamic processes, such as attentional control and working memory (Schmiedek et al., 2007; Spieler, Balota, & Faust, 1996; Tse et al., 2010). Interestingly, τ is also reflected by changes in drift rates in the diffusion model (see Ratcliff, 1978), so one must be cautious in making strong inferences between cognitive operations and ex-Gaussian parameter estimates. Thus, the present results can only be viewed as supportive of this hypothesis with this set of attentional control tasks.
Finally, there were no effects observed in association with σ. The cognitive role of σ is, at present, somewhat poorly understood and needs to be pursued in future studies.
4.3 Limitations and future directions
There are several limitations to the present study. The cross-sectional results presented here cannot determine causality, and a longitudinal follow-up (which we are currently engaged in) is critical to confirm the role of white matter integrity in increases in IIV. In addition, given that demographic factors such as age, gender, education, and SES were statistically controlled where necessary and not strictly matched between healthy older and early-stage AD groups, it is possible that these factors may have an unforeseen impact on the current results. Similarly, the scan-assessment interval was lengthy and may have influenced the current results beyond what could be controlled via including the interval as a covariate. Although it is difficult to anticipate all of the potential influences of these factors, future, larger studies could directly investigate potential higher-order interactions involving age, gender, and SES, and seek to minimize intervals between cognitive assessments and structural scans.
The current report focused on white matter because of the growing evidence for the importance of white matter integrity to IIV (MacDonald, Li, & Bäckman, 2009; MacDonald, Nyberg, & Bäckman, 2006). It is also important to note that there are multiple neural factors contributing to behavioral variability. For example, gray matter lesions, particularly in frontal areas, are associated with increased variability in cognitive tasks (Stuss et al., 2003). There is also evidence that reduced competition between executive and default networks could result in increased IIV (Kelly et al., 2008). MacDonald and colleagues (MacDonald, Li, & Bäckman, 2009; MacDonald, Nyberg, & Bäckman, 2006) also implicate neuromodulatory factors that could impact variability on a neural level, particularly catecholamines such as dopamine. Thus, it is likely that factors at the cellular and systems levels all contribute to increased neural and cognitive variability and future research should focus on the relative contributions of multiple factors to better unravel the dynamic neural underpinnings of IIV.
In sum, there appears to be a robust relationship between white matter integrity and intraindividual variability. Declines in global and regional cerebral white matter were associated with increased RT variability in the current sample. Furthermore, age-related differences in τ were particularly sensitive to CDR status. The present study also demonstrated the utility of RT distributional analysis by revealing differential associations between cerebral and regional white matter volume and parameters of distributions in sensitivity to healthy aging and early stage AD.
Highlights.
>Aging/dementia tied to greater intraindividual variability/distributional skewing.
>We relate variability to total and regional white matter volumes.
>Specific associations observed in frontal and parietal regions.
>Larger volume associated with less variability/skewing in healthy/pathological aging.
Acknowledgments
We thank the Clinical Core of the Washington University Alzheimer Disease Research Center for the clinical assessments and the Imaging Core for the structural MRI data, and Martha Storandt for helpful comments. Supported by NIH grants P50 AG05861, P01 AG 03991, and PO1 AGO26276. Jonathan Jackson was supported by National Institute of General Medical Sciences grant T32-GM81739-02.
The sources of financial support were NIH grants P50 AG05861, P01 AG 03991, PO1 AGO26276 and National Institute of General Medical Sciences grant T32-GM81739-02. The data contained in this manuscript have not previously been published, nor has the manuscript been submitted elsewhere, nor will it be submitted elsewhere while under review. Appropriate ethical guidelines were followed with regard to the treatment of human subjects. All authors have reviewed the manuscript and approve of its contents and validate the accuracy of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors and their institution have no conflicts of interest related to this work.
References
- Amar K, Bucks RS, Lewis T, Scott M, Wilcock GK. The effect of white matter low attenuation on cognitive performance in dementia of the Alzheimer type. Age and Ageing. 1996;25:443–448. doi: 10.1093/ageing/25.6.443. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey KJ. Sensorimotor and forced expiratory volume as correlates of speed, accuracy, and variability in reaction time performance in late adulthood. Aging, Neuropsychology, and Cognition. 1999;6:84–95. [Google Scholar]
- Anstey KJ, Mack HA, Christensen H, Li S-C, Reglade-Meslin C, Maller J, Sachdev P. Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia. 2007;45:1911–1920. doi: 10.1016/j.neuropsychologia.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Bai F, Zhang Z, Watson DR, Yu H, Shi Y, Yuan Y. Abnormal white matter independent of hippocampal atrophy in amnestic type mild cognitive impairment. Neuroscience Letters. 2009;462:147–151. doi: 10.1016/j.neulet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Bai F, Zhang Z, Yu H, Shi Y, Yuan Y, Zhu W, Qian Y. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: A combined structural and resting-state functional MRI study. Neuroscience Letters. 2008;438:111–115. doi: 10.1016/j.neulet.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Balota DA, Tse C-S, Hutchison KA, Spieler DH, Duchek JM, Morris JC. Predicting conversion to dementia of the Alzheimer's type in a healthy control sample: The power of errors in Stroop color naming. Psychology and Aging. 2010;25:208–218. doi: 10.1037/a0017474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Yap MJ. Moving beyond the mean in studies of mental chronometry: The power of response time distributional analyses. Current Directions in Psychological Science. 2011;20:160–166. [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Watson JM. Beyond mean response latency: Response time distributional analysis of semantic priming. Journal of Memory & Language. 2008;59:495–523. [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiology of Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Alzheimer's disaease as homeostatic responses to age-related myelin breakdown. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.08.007. doi:10.1016/j.neurobiolaging.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: A magnetic resonance imaging study. Archives of Neurology. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Berg L. Clinical Dementia Rating (CDR). Psychopharmacol Bulletin. 1988;24:637–639. [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr., Miller JP, Storandt M, Rubin EH, Morris JC, Saunders AM. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: Relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Archives of Neurology. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Bielak AAM, Hultsch DF, Strauss E, MacDonald SWS, Hunter MA. Intraindividual variability is related to cognitive change in older adults: Evidence for within-person coupling. Psychology and Aging. 2010;25:575–586. doi: 10.1037/a0019503. [DOI] [PubMed] [Google Scholar]
- Breteler MM, van Amerongen NM, van Swieten JC, Claus JJ, Grobbee DE, van Harskamp F. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. The Rotterdam Study. Stroke. 1994;25:1109–1115. doi: 10.1161/01.str.25.6.1109. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bunce D, Anstey KJ, Christensen H, Dear K, Wen W, Sachdev P. White matter hyperintensities and within-person variability in community-dwelling adults aged 60–64 years. Neuropsychologia. 2007;45:2009–2015. doi: 10.1016/j.neuropsychologia.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Bunce D, Warr P, Cochrane T. Blocks in choice responding as a function of age and physical fitness. Psychology and Aging. 1993;8:26–33. doi: 10.1037//0882-7974.8.1.26. [DOI] [PubMed] [Google Scholar]
- Carriere JSA, Cheyne JA, Solman JF, Smilek D. Age trends for failures of sustained attention. Psychology and Aging. 2010;25:569–574. doi: 10.1037/a0019363. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cheyne J, Solman G, Carriere J, Smilek D. Anatomy of an error: A bidirectional state model of task engagement/disengagement and attention-related errors. Cognition. 2009;111:98–113. doi: 10.1016/j.cognition.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Cousineau D, Brown S, Heathcote A. Fitting distributions using maximum likelihood: Methods and packages. Behavior Research Methods, Instruments, & Computers. 2004;36:742–756. doi: 10.3758/bf03206555. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Sanz-Arigita EJ, Barkhof F, Schentens P, Stam CJ, Rombouts SARB. Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, Fischl B. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain. 2009;132:2048–2057. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Garrett DD, Lentz TL, MacDonald SWS, Strauss E, Hultsch DF. Neurocognitive markers of cognitive impairment: Exploring the roles of speed and inconsistency. Neuropsychology. 2007;21:381–399. doi: 10.1037/0894-4105.21.3.381. [DOI] [PubMed] [Google Scholar]
- Duchek JM, Balota DA, Tse C-S, Holtzman DM, Fagan AM, Goate A. The utility of intraindividual variability as an early marker for Alzheimer's disease. Neuropsychology. 2009;23:746–758. doi: 10.1037/a0016583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Gamst AC, Quinn BT, Pacheco J, Jernigan TL, Thal L, Gollub RL. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics. 2007;5:235–245. doi: 10.1007/s12021-007-9003-9. [DOI] [PubMed] [Google Scholar]
- Fischl BA, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat D, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Grady C. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grecius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. American Journal of Neuroradiology. 2007;28:226–235. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: A review of MRI findings. Int J Geriatr Psych. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon F, Raz N. The cognitive correlates of white matter abnormalities in normal aging, a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Head D, Rodrigue KM, Kennedy KM. Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychology. 2008;22:491–507. doi: 10.1037/0894-4105.22.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote A, Brown S. Reply to Speckman and Rouder: A theoretical basis for QML. Psychonomic Bulletin & Review. 2004;11:577–578. doi: 10.3758/bf03196613. [DOI] [PubMed] [Google Scholar]
- Heathcote A, Brown S, Mewhort DJK. Quantile maximum likelihood estimation of response time distributions. Psychonomic Bulletin & Review. 2002;9:394–401. doi: 10.3758/bf03196299. [DOI] [PubMed] [Google Scholar]
- Heathcote A, Popiel SJ, Mewhort DJK. Analysis of response time distributions: An example using the Stroop task. Psychological Bulletin. 1991;109:340–347. [Google Scholar]
- Hollingshead A,B. Four factor index of social status. 1975 Unpublished manuscript. [Google Scholar]
- Huang J, Auchus AP. Diffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer's disease. Annals of the New York Academy of Sciences. 2007;1097:259–264. doi: 10.1196/annals.1379.021. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SWS. Intraindividual variability in performance as a theoretical window onto cognitive aging. In: Dixon RA, Bäckman L, Nilsson L-G, editors. New frontiers in cognitive aging. Oxford University Press; New York, NY: 2004. pp. 65–88. [Google Scholar]
- Hultsch DF, MacDonald SWS, Dixon RA. Variability in reaction time performance of younger and older adults. Journals of Gerontology, Series B: Psychological Sciences & Social Sciences. 2002;57B:101–115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SWS, Hunter MA, Levy-Bencheton J, Strauss E. Intraindividual variability in cognitive performance in older adults: Comparison of adults with mild dementia, adults with arthritis, and healthy adults. Neuropsychology. 2000;14:588–598. doi: 10.1037//0894-4105.14.4.588. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Strauss E, Hunter MA, MacDonald SWS. Intraindividual variability, cognition, and aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3rd edition Psychology Press; New York, NY: 2008. pp. 491–556. [Google Scholar]
- Jack CRJ, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Johnson DK, Barrow W, Anderson R, Harsha A, Honea R, Brooks WM, Burns JM. Diagnostic utility of cerebral white matter integrity in early Alzheimer's disease. International Journal of Neuroscience. 2010;120:544–550. doi: 10.3109/00207454.2010.494788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DK, Storandt M, Morris JC, Galvin JE. Longitudinal study of the transition from healthy aging to Alzheimer disease. Archives of Neurology. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive and Affective Neuroscience. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Han X, Salat D, van der Kouwe A, Quinn B, Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. NeuroImage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavcic V, Ni H, Zhu T, Zhong J, Duffy CJ. White matter integrity linked to functional impairments in aging and early Alzheimer's disease. Alzheimer's & Dementia. 2008;4:381–389. doi: 10.1016/j.jalz.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Lattin JM, Carroll JD, Green PE. Analyzing multivariate data. Brooks Cole; Pacific Grove, CA: 2003. [Google Scholar]
- Li S-C, Brehmer Y, Shing YL, Werkle-Bergner M, Lindenberger U. Neuromodulation of associative and organizational plasticity across the life span: Empirical evidence and neurocomputational modeling. Neuroscience and Biobehavioral Reviews. 2006;30:775–790. doi: 10.1016/j.neubiorev.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Li S-C, von Oertzen T, Lindenberger U. A neurocomputational model of stochastic resonance and aging. Neurocomputing. 2006;69:1553–1560. [Google Scholar]
- Lövdén M, Li SC, Shing YL, Lindenberger U. Within-person trial-to-trial variability precedes and predicts cognitive decline in old and very old age: Longitudinal data from the Berlin Aging Study. Neuropsychologia. 2007;45:2827–2838. doi: 10.1016/j.neuropsychologia.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Luce RD. Response times: Their role in inferring elementary mental organization. Oxford University Press; New York, NY: 1986. [Google Scholar]
- MacDonald, Li S-C, Bäckman L. Neural underpinnings of within-person variability in cognitive functioning. Psychology & Aging. 2009;24:792–808. doi: 10.1037/a0017798. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Hultsch DF, Dixon RA. Performance variability is related to change in cognition: Evidence from the Victoria Longitudinal Study. Psychology and Aging. 2003;18:510–523. doi: 10.1037/0882-7974.18.3.510. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Nyberg L, Bäckman L. Intra-individual variability in behavior: Links to brain structure, neurotransmission and neuronal activity. Trends in Neurosciences. 2006;29:474–480. doi: 10.1016/j.tins.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Gottlob LR, Denny LL, Turkington TG, Provenzale JM, Hawk TC, Coleman RE. Aging and recognition memory: Changes in regional cerebral blood flow associated with components of reaction time distribution. Journal of Cognitive Neuroscience. 1999;11:511–520. doi: 10.1162/089892999563571. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Research: Neuroimaging. 1993;50:121–139. doi: 10.1016/0925-4927(93)90016-b. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Roddey JC, Hagler DJ, Jr., Holland D, Karow DS, Dale A,M. Alzheimer disease: Quantitative structural neuroimaging for detection and predictionof clinical and structural changes in mild cognitive impairment. Radiology. 2009;251:195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS–ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Minear M, Shah P. Training and transfer effects in task switching. Memory & Cognition. 2008;36:1470–1483. doi: 10.3758/MC.336.8.1470. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, Storandt M. Very mild Alzheimer's disease: Informant-based clinical, psychometric, and pathologic distinction from normal aging. Neurology. 1991;41:469–478. doi: 10.1212/wnl.41.4.469. [DOI] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, Jr., Fulling K, Torack RM, Berg L. Validation of clinical diagnostic criteria for Alzheimer's disease. Annals of Neurology. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, McKeel DW, Jr., Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Jr., Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer's disease. Archives of Neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Murtha S, Cismaru R, Waechter R, Chertkow H. Increased variability accompanies frontal lobe damage in dementia. Journal of International Neuropsychological Society. 2002;8:360–372. doi: 10.1017/s1355617702813170. [DOI] [PubMed] [Google Scholar]
- Nesselrode JR, Salthouse TA. Methodological and theoretical implications of intraindividual variability in perceptual-motor performance. Journal of Gerontology, Series B: Psychological Sciences and Social Sciences. 2004;59:P49–P55. doi: 10.1093/geronb/59.2.p49. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA. Errors and error correction in choice–response tasks. Journal of Experimental Psychology. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Osman P, Moore B. There are stable individual differences in performance variability, both from moment to moment and from day to day. The Quarterly Journal of Experimental Psychology, A. 2001;54:981–1003. doi: 10.1080/713756013. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R. A theory of memory retrieval. Psychological Review. 1978;85:59–108. [Google Scholar]
- Ratcliff R. Group reaction time distributions and an analysis of distribution statistics. Psychological Bulletin. 1979;86:446–461. [PubMed] [Google Scholar]
- Raz N. The aging brain: Structural changes and their implications for cognitive aging. In: Dixon RA, Bäckman L, Nilsson LG, editors. New frontiers in cognitive aging. Oxford University Press; New York, NY: 2004. pp. 115–130. [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neurosci Biobehav R. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cerebral Cortex. 2008;18:718–726. doi: 10.1093/cercor/bhm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- Salat DH, Greve DN, Pacheco JL, Quinn BT, Helmer KG, Buckner RL, Fischl B. Regional white matter volume differences in nondemented aging and Alzheimer's disease. NeuroImage. 2009;44:1247–1258. doi: 10.1016/j.neuroimage.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedek F, Oberauer K, Wilhelm O, Süβ H-M, Wittmann WW. Individual differences in components of reaction time distributions and their relations to working memory and intelligence. Journal of Experimental Psychology: General. 2007;136:414–429. doi: 10.1037/0096-3445.136.3.414. [DOI] [PubMed] [Google Scholar]
- Speckman PL, Rouder JN. A comment on Heathcote, Brown, and Mewhort's QMLE method for response time distributions. Psychonomic Bulletin & Review. 2004;11:574–576. doi: 10.3758/bf03196613. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Stroop performance in younger adults, healthy older adults and individuals with senile dementia of the Alzheimer's type. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:461–479. doi: 10.1037//0096-1523.22.2.461. [DOI] [PubMed] [Google Scholar]
- Spieler DH, Balota DA, Faust ME. Levels of selective attention revealed through analyses of response time distributions. Journal of Experimental Psychology: Human Perception and Performance. 2000;26:506–526. doi: 10.1037//0096-1523.26.2.506. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y. The concept of cognitive reserve: A catalyst for research. Journal of Clinical and Experimental Neuropsychology. 2003;25:589–593. doi: 10.1076/jcen.25.5.589.14571. [DOI] [PubMed] [Google Scholar]
- Storandt M, Grant EA, Miller JP, Morris JC. Longitudinal course and neuropathologic outcomes in original vs. revised MCI and in pre-MCI. Neurology. 2006;67:467–473. doi: 10.1212/01.wnl.0000228231.26111.6e. [DOI] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh Compound B and cognitive decline associated with A beta deposition. Archives of Neurology. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: The frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- Tse C-S, Balota DA, Yap MJ, Duchek JM, McCabe DP. Effects of healthy aging and early-stage dementia of the Alzheimer's type on components of response time distributions in three attention tasks. Neuropsychology. 2010;24:300–315. doi: 10.1037/a0018274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullén F, Forsman L, Blom Ö, Karabanov A, Madison G. Intelligence and variability in a simple timing task share neural substrates in the prefrontal white matter. The Journal of Neuroscience. 2008;28:4238–4243. doi: 10.1523/JNEUROSCI.0825-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain N, Fouquet M, Baron J-C, Mézenge F, Landeau B, de La Sayette V, Chételat G. Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer's disease. Brain. 2010;133:3301–3314. doi: 10.1093/brain/awq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM. White matter volume predicts reaction time instability. Neuropsychologia. 2007;45:2277–2284. doi: 10.1016/j.neuropsychologia.2007.02.022. [DOI] [PubMed] [Google Scholar]