Abstract
Introduction
We investigated the role of oxidative stress in the pathogenesis of reexpansion pulmonary edema (RPE) and effect of alpha-lipoic acid (ALA) in the prevention of RPE.
Material and methods
There were 4 groups consisting of 10 rats in each group; control group (CG), α-lipoic acid group (ALAG), reexpansion pulmonary edema group (RPEG), reexpansion pulmonary edema plus α-lipoic acid group (RPE + ALAG). In all the groups, all rats were sacrificed 2 hours after the reexpansion of lungs. To indicate oxidative stress malondialdehyde (MDA), and to indicate antioxidant status superoxide dismutase (SOD), catalase (CAT) and glutathione peroxides (GPx) were measured in the lungs of rats.
Results
Mean MDA value was lower in CG (7.02 ±0.14) and in ALAG (6.95 ±0.11) than the other groups (p = 0.001). It was highest in RPEG (8.89 ±0.21) (p = 0.001). It was lower in RPE + ALA G (7.21 ±0.32) than RPEG (p = 0.001). Antioxidant levels: GPx (37.21 ±3.01), CAT (2.87 ±0.14) and SOD (100.12 ±12.39) were lowest in RPEG among all groups (p = 0.001). These values were GPx (45.21 ±3.54), CAT (3.24 ±0.21) and SOD (172.36 ±15.48) in RPE + ALA G and were greater than those of RPEG (p = 0.001). While normal pulmonary parenchyma was seen in 2 rats in RPE + ALAG, it was not seen in RPEG. Pulmonary edema was seen in 1 rat in RPE + ALAG; however, it was seen in 3 in RPEG.
Conclusions
Oxidative stress might have an important role in the pathogenesis of RPE. In addition, ALA treatment might contribute in preventing RPE.
Keywords: reexpansion, lung, edema, oxidative stress, alpha-lipoic acid
Introduction
Reexpansion pulmonary edema (RPE) is an iatrogenic complication which may occur due to rapid expansion of the chronically collapsed lung during the treatment of pleural effusion or pneumothorax [1, 2]. RPE usually develops due to removal of 2000 ml or more volume in the first 24 hours in the lung collapsed for 3 days or more [3]. The pathophysiology of RPE is yet to be determined completely [1, 2]. The most valid hypothesis for the development of RPE is as follows: blood flow decreases in a great amount as a result of hypoxic vasoconstriction in the collapsed lung, varying degrees of hypoxia and ischemia develop depending on the duration of the collapse, reperfusion and reoxygenation occur after correction of the collapse, the amount of lipid and polypeptide mediators and immune complexes increases, together with the increase in the inflammatory response, monocytes, macrophages and polymorphonuclear leucocytes accumulate in the alveolar-capillary membrane, free oxygen radicals (FOR) form, oxidative stress increases, as a result the permeability of alveoli capillary membrane increases and pulmonary edema develops [1-3]. Alveolar fluid, interstitial edema, an increase in the number of macrophages and a considerable amount of thickening in the basement membrane are seen with histopathological examination of RPE [3]. RPE is a rapid, dramatic and fatal clinical condition, the mortality rate of which reaches about 20-30% [2, 3]. The treatment of RPE consists of supportive measures including supplemental oxygen and/or mechanical ventilation [1, 3]. Currently, preventive measures such as removal of small volumes or monitoring of intrapleural pressure during the procedure are considered more important than the treatment [1, 3].
Oxidative stress is defined as an increment in the production of oxidants and/or a decrement in the antioxidant capacity [4, 5]. Increased oxidative stress was considered to have some role in the pathogenesis of various diseases [6, 7]. Biologically important oxidants are free oxygen radicals such as superoxide (O2–), hydrogen peroxide (H2O2), hydroxyl (OH–) and hypochloric acid (HOCI) [16]. FOR may cause cellular damage by mechanisms such as direct cellular injury, generating vasoactive and proinflammatory molecules with lipid peroxidation, release of proteases with protein oxidation, and inactivation of antioxidant and antiprotease enzymes. In the body, there is an antioxidant system including enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) and non-enzymatic antioxidants such as glutathione (GSH) and thioredoxin against the destructive effects of FOR [4, 8]. However, these antioxidants are utilized rapidly in an acute inflammatory response [8]. Measurement of malondialdehyde (MDA) in the blood is an indirect indicator of oxidative stress [5, 9].
Alpha-lipoic acid (α-lipoic acid, ALA) is a disulfide derivative of octanoic acid [4]. It is considered as a cofactor in mitochondrial multi-enzyme complexes in the cascade of energy production [4, 9, 10]. ALA has antioxidant properties such as regenerating endogen antioxidants, increasing the formation of GSH and neutralizing FOR [4, 9-11].
In this experimental rat study we investigated the role of oxidative stress in the pathogenesis of RPE and the effect of ALA, with its antioxidant properties, in the prevention of RPE.
Material and methods
We used the same method Yucel et al. previously used [12].
This experimental study was done in Gulhane Military Medical Academy (GMMA) Animal Research Laboratory. GMMA Ethics Committee approved the study.
Properties of laboratory animals
We used 40 adult Sprague-Dawley rats, weighing 150 ±20 g, in this study. All rats were fed with comparable foods (2630 kkal/kg metabolic energy, 21% protein, 7% cellulose, and 9% fat) and water before commencement of the experiment under the same conditions for seven days.
Design of the experiment
By using a simple sampling method, 40 adult rats were divided into 4 groups. The groups were as follows: α-lipoic acid group (ALAG), RPE group (RPEG), RPE plus ALA group (RPE + ALAG), and control group (CG). All rats underwent the same experimental conditions during 3 days.
On the fourth day, the RPE procedure was performed for the RPEG and the RPE + ALAG groups. ALA (50 mg/kg/day, divided into two doses) was given to the RPE + ALAG rats 8 hours before pneumothorax application and continued 72 hours by gavage orally. The same dose of ALA was given to ALAG in the same way and duration at the same time. RPE procedure was not performed in CG and ALAG (Table I). By applying xylazine and ketamine at lethal doses, all the rats in the study were sacrificed 2 hours after the lungs had reexpanded. The lungs were removed via median sternotomy for histopathological and histochemical evaluation. Samples for histopathological evaluation were fixed in 10% buffered formaldehyde and samples for histochemical evaluation were stored in liquid nitrogen for determination of the levels of oxidative stress.
Table I.
Characteristics of the experimental study groups
| Group | n | α-lipoic acid | RPE procedure |
|---|---|---|---|
| CG | 10 | not given | not performed |
| ALAG | 10 | given | not performed |
| RPEG | 10 | not given | performed |
| RPE + ALAG | 10 | given | performed |
CG – control group, ALAG – α-lipoic acid group, RPEG – reexpansion pulmonary edema group, RPE + ALAG – reexpansion pulmonary edema plus α-lipoic acid group
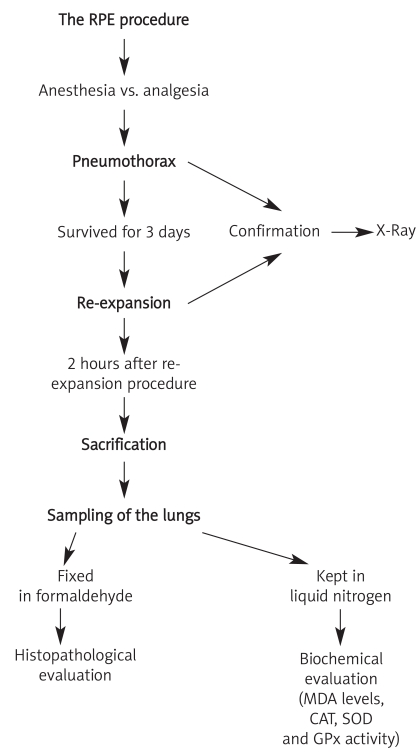
The RPE procedure
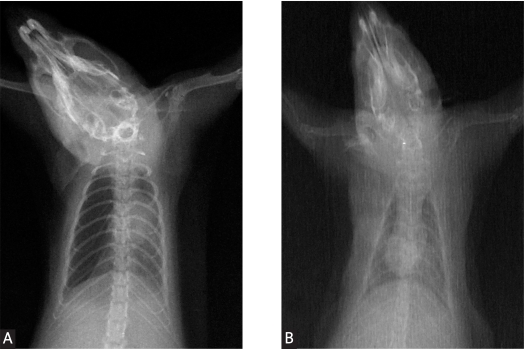
The same RPE procedure used in a previous study [12] was performed (Figure 1). Introducing intraperitoneal ketamine hydrochloride (ketamine hydrochloride solution 5%, Parke–Davis license Eczacibasi Medical Industry, Istanbul) 90 mg/kg and xylazine (xylazine solution 2%, by Parke–Davis license Eczacibasi Medical Industry, Istanbul) 10 mg/kg, all the rats were anesthetized. A mixture of 50 mg α-lipoic acid and 3 ml of 0.9% NaCl was started to be administered to RPE + ALAG, 8 hours before the pneumothorax development, by gavage. Total duration of α-lipoic acid administration was 72 hours. After shaving the right chest walls of all the rats in RPEG and RPE + ALAG, by introducing a 22 gauge cannula, through the fourth intercostal space in the mid-axillary line via a percutaneous route in the right hemithorax, 4 ml of air was injected into the pleural cavity to induce a pneumothorax. X-ray was used to determine the existence of sufficient pneumothorax in all of the rats (Figure 2A). Butorphanol (0.5 mg/kg, subcutaneous) was used for analgesia. After 72 hours, by aspiration with a 22 gauge needle, pneumothoraces disappeared on the chest X-rays of all animals (Figure 2B). All rats were sacrificed by introducing xylazine and ketamine at lethal doses about 2 hours after the reexpansion of the lungs.
Figure 1.
Reexpansion pulmonary edema procedure
Figure 2.
Pneumothorax (A) and reexpansion (B) were confirmed with control chest X-ray in all groups
Histopathological evaluation
First 10% buffered formaldehyde was used to fix the lungs; later on they were embedded in paraffin blocks. Slices of about 4 µm thickness, obtained from paraffin blocks, were stained with hematoxylin-eosin. Existence of a pulmonary edema was investigated by a pathologist.
Determination of oxidative status
To determine oxidative stress and antioxidant enzymes, we used the same method as that used in 2 other studies [12, 13]. Lipid peroxidation was measured with the thiobarbituric acid method and final MDA levels in lung tissue were expressed as mmol/g-protein. Copper/zinc-superoxide dismutase (Cu/Zn-SOD) was measured by the nitroblue tetrazolium method. Measured enzyme activity was shown in the units of U/g-protein. The measurement of GPx activity was performed by using glutathione reductase. U/g-protein was the unit expressing the GPx activity.
Statistical analysis
Since the values were non-parametric, we used Kruskal-Wallis and Mann-Whitney U tests for the comparison of the groups. We also used chi-square test for categorical variables. A p value lower than 0.05 was considered statistically significant.
Results
There were 4 groups consisting of 10 rats in each group: CG, ALAG, RPEG, RPE + ALAG. To indicate oxidative stress MDA, and to indicate antioxidant status CAT, SOD, GPx were measured in the lung tissues of the rats. Mean MDA value was lower in CG (7.02 ±0.14) and in ALAG (6.95 ±0.11) than the other groups (p = 0.001). This value was highest in RPEG (8.89 ±0.21) (p = 0.001). It was lower in RPE + ALAG (7.21 ±0.32) than RPEG (p = 0.001). Antioxidant levels, GPx (37.21 ±3.01), CAT (2.87 ±0.14) and SOD (100.12 ±12.39) were lowest in RPEG among all groups (p = 0.001). These values were GPx (45.21 ±3.54), CAT (3.24 ±0.21) and SOD (172.36 ±15.48) in RPE + ALA G and were greater than those of RPEG (p = 0.001) (Table II).
Table II.
Mean values and statistical comparison of malondialdehyde and antioxidant enzyme levels
| Parameters | n | MDA [nmol/g] Mean ± SD | GPx [U/g] Mean ± SD | CAT [U/g] Mean ± SD | SOD [U/g] Mean ± SD |
|---|---|---|---|---|---|
| CG | 10 | 7.02 ±0.14a | 44.42 ±3.0 | 3.02 ±0.12 | 237.34 ±17.21 |
| ALAG | 10 | 6.95 ±0.11a | 47.22 ±3.67 | 3.24 ±0.03 | 263.21 ±18.54 |
| RPEG | 10 | 8.89 ±0.21b | 37.21 ±3.01d | 2.87 ±0.14d | 100.12 ±12.39d |
| RPE + ALAG | 10 | 7.21 ±0.32c | 45.21 ±3.54e | 3.24 ±0.21e | 172.36 ±15.48e |
CG – control group, ALAG – α-lipoic acid group, RPEG – reexpansion pulmonary edema group, RPE + ALAG – reexpansion pulmonary edema plus α-lipoic acid group. MDA – malondialdehyde, CAT – catalase, SOD – superoxide dismutase, GPx – glutathione peroxides, SD – standard deviation
p < 0.001 (lower than RPEG and RPE + ALAG),
p < 0.001 (greater than other groups),
p < 0.001 (lower than RPEG),
p < 0.001 (lower than other groups),
p < 0.001 (greater than RPEG)
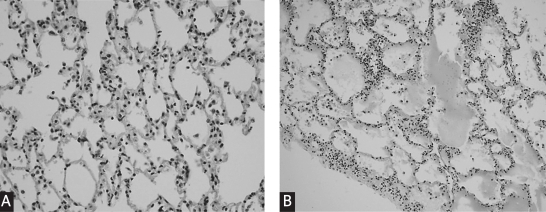
Histopathologically, the lungs were evaluated as follows: normal pulmonary parenchyma, fluid extravasations, fluid extravasations and fluid in the alveoli and pulmonary edema (Figure 3A, B). As expected, all CG and ALAG had normal pulmonary parenchyma since they were not subjected to the RPE procedure. The groups subjected to the RPE procedure, RPE and RPE + ALAG were compared for histopathological findings. Normal pulmonary parenchyma was detected in 2 rats in RPE + ALAG but it was not seen in RPEG. While pulmonary edema was seen in 1 rat in RPE + ALAG, it was seen in 3 in RPEG. While fluid extravasations and fluid in the alveoli were seen in 2 rats in RPE + ALAG, they were seen in 4 in RPEG. Fluid extravasations and fluid in the alveoli and pulmonary edema findings were found lower in the ALA treated group than RPEG. However, these differences did not reach statistical significance, due to the small size of the studied population. On the other hand, while fluid extravasations were found in 3 rats in RPEG, they were found in 5 in RPE + ALA G (Table III).
Figure 3.
Normal pulmonary parenchyma in a rat (hematoxylin-eosin ×400) (A). Intraalveolar eosinophilic fluid accumulation in a rat (hematoxylin-eosin ×200) (B)
Table III.
Histopathological results for lung tissue
| Histological findings | CG, n = 10 | ALAG, n = 10 | RPEG, n = 10 | RPE + ALA G, n = 10 |
|---|---|---|---|---|
| Normal pulmonary parenchyma | 10 | 10 | 0 | 2 |
| Fluid extravasations | 0 | 0 | 3 | 5 |
| Fluid extravasations and fluid in the alveoli | 0 | 0 | 4 | 2 |
| Pulmonary edema | 0 | 0 | 3 | 1 |
CG – control group, ALAG – α-lipoic acid group, RPEG – reexpansion pulmonary edema group, RPE + ALAG – reexpansion pulmonary edema plus α-lipoic acid group
Discussion
In this study, we found that oxidative stress was increased in experimental RPE. This was shown by a remarkable increase in levels of MDA, an indirect indicator of oxidative stress, and a remarkable decrease in antioxidant enzyme levels in the RPE group when compared to CG. Antioxidant enzyme levels might decrease by a great amount in RPE due to their utilization in neutralizing various oxidants formed during the development of RPE [4, 8]. Comparable to this, in our study we found that the RPE group has the lowest antioxidant levels. Our findings were also comparable to the current literature [1, 3, 12]. We also found that ALA treatment significantly increased antioxidant levels in lung parenchyma in healthy rats. This finding suggests that ALA might have a pulmonary protective effect. No other study was found in the current literature indicating this finding. After combining the aforementioned data it may be proposed that ALA treatment has a pulmonary protective effect in RPE. Our findings support this hypothesis that the ALA-treated RPE group had greater antioxidant tissue levels than the RPE group. These findings indicate that ALA treatment may have a role in decreasing oxidative stress in RPE. Our findings also suggest that ALA treatment might contribute to preventing the development of experimental RPE in a rat model. It is indicated in the current literature that oxidative stress might have an important role in the pathogenesis of RPE [1-3]. Parallel to the current literature, in this study mean MDA value, which is supposed to be an indirect indicator of oxidative stress [5, 9], was significantly greater in the RPE group than in the control group. In addition, the mean MDA value was significantly lower in the RPE + ALA group than the RPE group, which suggests an effect of ALA in reducing oxidative stress in RPE, thus contributing to prevention of edema.
Histopathological findings indicating or supporting pulmonary edema were lower in the ALA + RPE group than the RPE group. The numbers of “pulmonary edema” and “fluid extravasations and fluid in the alveoli” in the RPE + ALA group were 1 and 2, and in the RPE group were 3 and 4, respectively. These findings suggest that ALA treatment might have a role in preventing RPE in a rat model. However, the difference between the groups did not reach statistical significance.
ALA treatment has been shown to have protective effects in some experimental studies. In these studies, oxidative stress had an important role such as experimental renal ischemia-reperfusion injury, type 2 diabetes and hemorrhagic shock [11, 14, 15]. In a study performed in diabetic patients, it was also shown that ALA improves endothelial dysfunction induced by acute hyperglycemia during oral glucose tolerance test in impaired glucose tolerance [16].
We could not find any study about ALA treatment in RPE in the current literature. In a previous study, taurine, which has antioxidant properties, was used in a rat model of RPE. In that study, taurine treatment reduced MDA levels, and increased SOD and GPx levels were observed. According to these findings, the authors of that study suggested that taurine might have a protective effect on RPE [12]. Our results, comparable to that study, might suggest preventive effects of ALA treatment in the development of RPE in rats. The small size of the study population and absence of monitoring of intrapleural pressure might be considered among the limitations of our study.
Oxidative stress might have an important role in most pulmonary diseases, and some other anti-oxidants can help to diminish this stress comparable to that seen in RPE. For example, in acute respiratory distress syndrome, overproduction of abundant free radicals, which might cause cell damage, and prevention of this harmful effect to some degree by N-acetylcysteine, a well known antioxidant, was shown in some recent studies [17, 18].
As a result, this study suggests that, similar to previous studies, oxidative stress have an important role in the pathogenesis of RPE. More important than this, ALA treatment might contribute in preventing RPE by reducing oxidative stress. More studies are needed to indicate the exact role of ALA in the treatment of RPE.
References
- 1.Neustein SM. Reexpansion pulmonary edema. J Cardiothorac Vasc Anesth. 2007;21:887–91. doi: 10.1053/j.jvca.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Trachiotis GD, Vricella LA, Aaron BL, Hix WR. Reexpansion pulmonary edema: updated in 1997. Ann Thorac Surg. 1997;63:1206–7. doi: 10.1016/s0003-4975(97)00193-8. [DOI] [PubMed] [Google Scholar]
- 3.Sohara Y. Reexpansion pulmonary edema. Ann Thorac Cardiovasc Surg. 2008;14:205–9. [PubMed] [Google Scholar]
- 4.Moini H, Packer L, Saris NEL. Antioxidant and prooxidant activities of α-lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90. doi: 10.1006/taap.2002.9437. [DOI] [PubMed] [Google Scholar]
- 5.Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156:341–57. doi: 10.1164/ajrccm.156.2.9611013. [DOI] [PubMed] [Google Scholar]
- 6.Ismail NA, Okasha SH, Dhawan A, Rahman AMOA, Shaker OG, Sadik NAH. Glutathione peroxidase, superoxide dismutase and catalase activities in hepatic tissue from children with glycogen storage disease. Arch Med Sci. 2009;1:86–90. [Google Scholar]
- 7.Feher J, Nemeth E, Nagy V, Lengyel G, Feher J. The preventive role of coenzyme Q10 and other antioxidants in injuries caused by oxidative stres. Arch Med Sci. 2007;4:305–14. [Google Scholar]
- 8.Chow CW, Abreu MTH, Suzuki T, Downey GP. Oxidative stress and acute lung injury. Am J Respir Cell Mol Biol. 2003;29:427–31. doi: 10.1165/rcmb.F278. [DOI] [PubMed] [Google Scholar]
- 9.Wang HL, Chen XT, Yin ST, et al. Opposite effects of α-lipoic acid on antioxidation and long-term potentiation in control and chronically lead-exposed rats. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:303–10. doi: 10.1007/s00210-008-0307-6. [DOI] [PubMed] [Google Scholar]
- 10.Shay KP, Moreau RF, Smith EJ, Hagen TM. Is a-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life. 2008;60:362–7. doi: 10.1002/iub.40. [DOI] [PubMed] [Google Scholar]
- 11.Sena CM, Nunes E, Louro T, et al. Effects of α-lipoic acid on endothelial function in aged diabetic and high-fat fed rats. Br J Pharmacol. 2008;153:894–906. doi: 10.1038/sj.bjp.0707474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yucel O, Kunak ZI, Macit E, et al. Protective efficiacy of taurine against pulmonary edema progression: experimental study. J Cardiothorac Surg. 2008;3:57. doi: 10.1186/1749-8090-3-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ucar M, Korkmaz A, Reiter RJ, et al. Melatonin alleviates lung damage induced by the chemical warfare agent nitrogen mustard. Toxicol Lett. 2007;173:124–31. doi: 10.1016/j.toxlet.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Sehirli O, Sener E, Cetinel S, Yüksel M, Gedik N, Sener G. α-lipoic acid protects against renal ischaemia-reperfusion injury in rats. Clin Exp Pharmacol Physiol. 2008;35:249–55. doi: 10.1111/j.1440-1681.2007.04810.x. [DOI] [PubMed] [Google Scholar]
- 15.Tharakan B, Hunter FA, Smythe WR, Childs EW. Alpha-lipoic acid attenuates hemorrhagic shock-induced apoptotic signaling and vascular hyperpermeability. Shock. 2008;5:571–7. doi: 10.1097/SHK.0b013e31816a7308. [DOI] [PubMed] [Google Scholar]
- 16.Xiang GD, Sun HL, Zhao LS, Hou J, Yue L, Xu L. The antioxidant alpha-lipoic acid improves endothelial dysfunction induced by acute hyperglycaemia during OGTT in impaired glucose tolerance. Clin Endocrinol. 2008;68:716–23. doi: 10.1111/j.1365-2265.2007.03099.x. [DOI] [PubMed] [Google Scholar]
- 17.Soltan-Sharifi MS, Mojtahedzadeh M, Najafi A, et al. Improvement by N-acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti-oxidant power: evidence for underlying toxicological mechanisms. Hum Exp Toxicol. 2007;9:697–703. doi: 10.1177/0960327107083452. [DOI] [PubMed] [Google Scholar]
- 18.Najafi A, Mojtahedzadeh M, Mahmoodpoor A, et al. Effect of N-acetylcysteine on microalbuminuria in patients with acute respiratory distress syndrome. Arch Med Sci. 2009;3:408–14. [Google Scholar]